ABSTRACT
The formation of robust resting cysts enables Acanthamoeba to resist harsh environmental conditions. This study investigated to what extent these cysts are resistant to physical and chemical stresses as applied in food industry cleaning and disinfection procedures. Moreover, it was assessed whether certain intracystic meat-borne bacterial pathogens are more stress resistant than free-living bacterial monocultures and if intracystic passage and subsequent association with trophozoites induces cross-tolerance toward other stressors. Several physical and chemical stressors (NaCl, H2O2, benzalkonium chloride, 55°C, heating until boiling, ethanol, dishwashing detergent, and sodium hypochlorite) frequently used in domestic and industrial food-related environments were tested against (i) Acanthamoeba castellanii cysts, (ii) single strains of bacterial monocultures, (iii) intracystic bacteria, and (iv) bacteria after intracystic passage (cyst-primed bacteria). Only heating until boiling and hypochlorite treatment were cysticidal. After boiling, no viable trophozoites could be recovered from the cysts, and hypochlorite treatment caused a 1.34- to 4.72-log10 cells/ml reduction in cyst viability. All treatments were effective in reducing or even eliminating the tested bacterial monocultures, whereas bacteria residing inside cysts were more tolerant toward these stressors. All cyst-primed bacteria exhibited an increased tolerance toward subsequent H2O2 (>92% decrease in median log10 CFU/ml reduction) and 70% ethanol (>99% decrease) treatments. Moreover, intracystic passage significantly increased the survival of Yersinia enterocolitica (74% decrease in median log10 reduction), Escherichia coli (58%), and Salmonella enterica (48%) after NaCl treatment and of E. coli (96%), S. enterica (99%), and Listeria monocytogenes (99%) after sodium hypochlorite treatment compared with that of nonprimed bacteria.
IMPORTANCE The results from this study demonstrated that both viable and nonviable amoebal cysts can protect internalized bacteria against stressful conditions. Moreover, cyst passage can induce cross-tolerance in bacteria, increasing their survival when exposed to selected stressors. These findings underscore the potential importance of free-living amoebae in food-related environments and their impact on the persistence of meat-borne bacterial pathogens.
KEYWORDS: Acanthamoeba, bacteria, cyst, resistance, stress, survival
INTRODUCTION
Free-living amoebae are ubiquitous in soil, air, and water and are part of the in-house microbiota of food-related environments (1). Besides being bacterial predators, free-living amoebae, such as Acanthamoeba, can also host grazing-resistant bacteria and are therefore regarded as a potential reservoir, vector, shelter, and virulence training ground for pathogenic bacteria. It is hypothesized that these free-living amoebae can facilitate the persistence of bacterial pathogens in food-related environments.
Most free-living amoebae have two forms: an active trophozoite and a dormant cyst. Nutrient depletion or other environmental stress conditions such as desiccation or changes in pH, temperature, and oxygen level promote the conversion from trophozoites to cysts, a process called encystment (2). During encystment, excess food and water are expelled, and cellular levels of RNA, protein, triacylglycerides, and glycogen diminish and the cell volume decreases (2). The cyst structure of Acanthamoeba, an amoebal model species and one of the most prevalent free-living protozoa, has been described in detail. The outer wrinkled ectocyst wall is mainly composed of proteins and polysaccharides, while the inner endocyst layer consists of glycans, proteins, fibrils, and cellulose (3). Although it has been suggested that cellulose is present in both layers of the cyst wall (3), other studies propose that cellulose is present only in the endocyst (4, 5). The morphology and exact composition of the cyst wall can vary between species and strains and, under in vitro conditions, also depends on the encystation medium used (2). Amoebal cysts are resistant to adverse physical and chemical conditions, such as desiccation (6), freezing-thawing cycles, radiation (7), heat (10 min at 80°C, 30 min at 70°C, or 60 min at 60°C [8]), and various biocides (9–11). It has been shown that mature Acanthamoeba cysts are more resistant than trophozoites to disinfectants and biocides (10, 12).
Amoebal cyst resistance to adverse environmental conditions can be due to the thick cyst wall, which represents a permeability barrier, and/or to the metabolically inactive nature of the cysts, which renders the action of certain biocides ineffective (2).
Resistance to harsh environmental conditions plays an important role in the persistence and dispersal of free-living amoebae itself. Furthermore, there is an increasing concern that free-living amoebae might shelter grazing-resistant internalized bacteria. A pilot-scale study on domestic water systems suggested that Acanthamoeba cysts protect internalized Legionella pneumophila from disinfectants and were the source of recolonization after treatment (13).
Several studies investigated the survival of protozoan trophozoites and cysts after exposure to chemical compounds, such as biocides used in water treatment systems and lens disinfectant solutions (10, 14–17). Although these studies are relevant for the prevention and treatment of infections by pathogenic or opportunistic free-living protozoa, they do not take into account the fate of the internalized (pathogenic) bacteria. Only a few studies so far have focused on the effect of chemical treatments on intracystic bacteria. Mycobacterium has been shown to survive in Acanthamoeba polyphaga cysts when exposed to free chlorine (15 ppm [18]). In addition, intracystic foodborne pathogens appeared to be better protected against low pH and gentamicin treatment than free-living bacteria (19).
In this study, we examined the effect of eight stressors, frequently occurring in food-related environments, on the survival capacities of Acanthamoeba cysts and on single strains of Salmonella enterica, Yersinia enterocolitica, Escherichia coli, and Listeria monocytogenes. Furthermore, it was assessed if intracystic meat-borne pathogenic bacteria were better protected against stressful chemical and physical conditions than non-cyst-associated bacteria and if cyst-primed meat-borne pathogenic bacteria, after their release back into the extra-amoebal environment, were more resistant to the various stressors than nonprimed bacteria.
RESULTS
Cysticidal effect of chemical and physical stress treatments.
Results from cysts of amoebal monocultures treated with various chemical and physical stress factors are shown in Table 1. Only heating until boiling and sodium hypochlorite treatment caused cysticidal activity that was significantly different from the reductions in the nontreated controls. After heating until boiling, no viable trophozoites could be recovered. Sodium hypochlorite treatment caused a 1.34-log10 cells/ml reduction in cyst viability by day 14. For all other treatments, no significant reduction in amoebal viability could be observed by day 14. However, 55°C and 70% ethanol treatment caused a delay in excystment, as after 2 days, 2.22-log10 and 4.47-log10 cells/ml reductions were observed, respectively, which were significantly higher than that of the nontreated controls. This significant delay was no longer present on day 7. Cysts challenged with the stressors did not differ morphologically (by light microscopy) from the controls.
TABLE 1.
Reduction in cyst viability after treatment with various stressors
| Treatment condition | Viability reduction (log10 cells/ml)a |
|||||
|---|---|---|---|---|---|---|
| Day 2 |
Day 7 |
Day 14 |
||||
| Median | IQR | Median | IQR | Median | IQR | |
| NaCl (5% for 120 min) | 2.02 | 2.02–3.37 | 1.10 | 1.01–1.7 | 0.46 | 0.43–0.60 |
| H2O2 (0.3% for 15 min) | 1.47 | 1.37–4.72 | 1.18 | 1.18–1.18 | 0.46 | 0.41–0.75 |
| Benzalkonium chloride (10 mg/liter for 15 min) | 1.60 | 1.54–1.60 | 1.18 | 0.82–1.18 | 0.46 | 0.41–0.60 |
| 55°C (15 min) | 2.22 | 2.22–2.37 | 0.51 | 0.51–1.39 | 0.46 | 0.41–0.60 |
| Heating until boiling (1 min) | 4.72 | 4.72–4.72 | 4.72 | 4.42–4.72 | 4.78 | 4.66–4.86 |
| Ethanol (70% for 5 min) | 4.47 | 4.47–4.47 | 2.27 | 2.20–2.39 | 0.48 | 0.41–1.11 |
| Dishwashing detergent (0.8 ml/liter for 5 min) | 1.37 | 1.32–1.47 | 1.18 | 1.18–1.22 | 0.46 | 0.41–0.96 |
| Sodium hypochlorite (2.5% for 15 min) | 4.72 | 4.72–4.72 | 2.27 | 1.80–2.2 | 1.34 | 1.28–1.50 |
| Nontreated controls | 0.91 | 0.87–0.98 | 0.52 | 0.41–0.60 | 0.46 | 0.39–0.51 |
IQR, interquartile range; n = 4; shaded boxes indicate significant reduction (P < 0.05) compared with that of nontreated controls.
Bactericidal effect of various stressors.
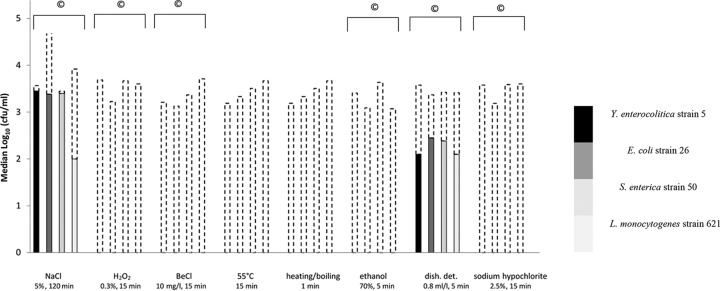
In general, for each bacterial strain, all treatments were effective in reducing or even eliminating the monocultured bacteria (Fig. 1). Treatment with 0.3% H2O2, 10 mg/liter benzalkonium chloride, incubation at 55°C, heating until boiling, 70% ethanol, and 2.5% hypochlorite led to total bacterial elimination (i.e., no viable bacteria could be detected; detection limit, 1 CFU/ml) for all tested strains. Dishwashing detergent and 5% NaCl were less effective in reducing bacterial numbers, and interspecific differences were observed. Yersinia enterocolitica (0.12-log10 CFU/ml reduction) and S. enterica (0.05-log10 CFU/ml reduction) were significantly more resistant toward 5% NaCl than the other tested pathogens (ca. 1.5- to 2-log10 CFU/ml reduction). Dishwashing detergent caused a significant reduction (ca. 1- to 1.5-log10 CFU/ml reduction) for all tested strains.
FIG 1.
Efficacy of various stress treatments on foodborne pathogens. Median numbers of monocultured bacteria without treatment (controls, dashed lines) and of monocultured bacteria after stress treatment (shaded boxes). BeCl, benzalkonium chloride; dish det, dishwashing detergent; ©, viable bacteria could be recovered from cysts after treatment; n = 4.
Amoebal cysts shelter internalized bacteria against various stressors.
Bacteria inside amoebal cysts were protected against the majority of the stressors (Fig. 1). Except for cyst-associated bacteria incubated at 55°C or heated until boiling, all tested bacterial strains could be recovered from the cysts after the various stress treatments. After the induction of excystment, trophozoites and extracellular bacteria started to appear after 24 to 48 h. Released bacteria were able to grow in the presence of the trophozoites.
Priming inside amoebal cysts and subsequent association with trophozoites provides higher bacterial stress tolerance after release.
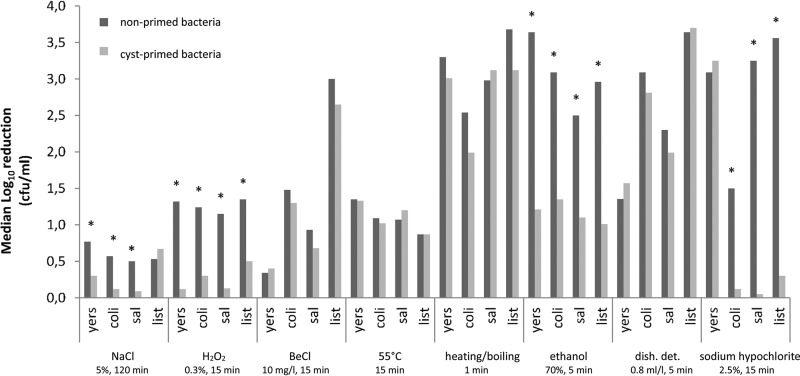
To evaluate if intracystic passage and subsequent association with excysted trophozoites (cyst priming) induces enhanced tolerance to other stressors once the bacteria are released back into the environment, cyst-induced resistance assays were performed. Before treatment, the bacterial concentrations of primed and nonprimed bacteria were between ca. 108 and 5 × 108 CFU/ml. Cyst-primed bacteria acquired a higher tolerance toward multiple stressors than the nonprimed cells (Fig. 2). For all tested bacteria, a lower reduction in bacterial numbers was observed in primed bacteria when exposed to 0.3% H2O2 (>1.1 log10 CFU/ml or >92% increased survival, P < 0.05) and 70% ethanol (>2.5 log10 or >99% increased survival, P < 0.05) treatments. Moreover, cyst passage significantly increased (P < 0.05) the number of viable Y. enterocolitica (0.60 log10 or 73% increased survival), E. coli (0.37 log10 or 58% survival), and S. enterica (0.29 log10, >48% survival) after NaCl treatment and of E. coli (1.48 log10 or 96% survival), S. enterica (3.25 log10 or 96% survival), and L. monocytogenes (3.56 log10 or 99% survival) after sodium hypochlorite treatment in comparison to that of nonprimed bacteria. No significant differences in bacterial viability were observed between primed and nonprimed bacteria after treatment with benzalkonium chloride, incubation at 55°C, heating until boiling, and dishwashing detergent.
FIG 2.
Effect of stressors on the survival of monocultured bacteria (nonprimed) and primed bacteria after intracystic passage. yers, Yersinia enterocolitica strain 5; coli, Escherichia coli strain 26; sal, Salmonella enterica strain 50; list, Listeria monocytogenes strain 621; BeCl, benzalkonium chloride; dish det, dishwashing detergent; *, P < 0.05 between primed and nonprimed bacteria, detergent; n = 4.
DISCUSSION
The aim of the present study was to determine if intracystic and amoeba-primed meat-borne pathogenic bacteria are better protected against stressful treatments than free-living bacteria. Therefore, meat-borne pathogens (as monocultures, located within amoebal cysts or passaged through cysts) were subjected to various stressors.
Results of the bacterial resistance assay clearly illustrate that the tested monocultured bacterial strains are vulnerable toward the tested stressors (NaCl, H2O2, benzalkonium chloride, 55°C, heating until boiling, ethanol, dishwashing detergent, and sodium hypochlorite), confirming the usefulness of those treatments in food-related environments.
However, the results of the cyst resistance assay demonstrated that only heating until boiling and treatment with 2.5% sodium hypochlorite for 15 min were effective in killing amoebal cysts. Heat causes denaturation of the protein constituents as well as liquefaction of the microbial membranes (20). The effectiveness of boiling has already been documented by Coulon et al. (21), who observed a >4-log10 cells/ml cyst reduction in all tested Acanthamoeba strains, which is confirmed in the present study (median log10 reduction between 4.72 log10 and 4.78 log10 cells/ml). Moreover, in accordance with our results, these authors demonstrated that exposure at 55°C for 10 min is inefficient in reducing cyst viability. Although heating until boiling seems to be an easy, cheap, and effective procedure to eliminate amoebal cysts, it should be noted that there are reports of some thermotolerant strains which could resist exposure to 80°C for 10 min (8). Sodium hypochlorite, or household bleach, is known to have a broad spectrum of antimicrobial activity and is already widely used in the food industry, e.g., for sanitizing food contact surfaces. The effectiveness of hypochlorite in killing Acanthamoeba cysts (17, 21, 22) has been confirmed in the present study. The microbiocidal activity of chlorine is mainly attributed to undissociated hypochlorous acid, but the exact action mechanisms are still unknown. Inactivation could result from a number of factors, including nucleic acid denaturation, the oxidation of respiratory components, and an impairment of enzyme and protein functions (23). Hypochlorite can cause swelling of the cyst wall due to interactions of the biocide with the inner cyst wall (21). However, in the experiments reported here, no swelling of the cysts was observed by light microscopy. Despite its effectiveness in eliminating bacteria and cysts, hypochlorite treatment has some disadvantages, including a corrosiveness to metals, inactivation by organic matter, and the release of toxic chlorine gas when mixed with ammonia or acid.
Remarkably, a small reduction in amoebal cyst numbers was also observed for the nontreated control cysts. This can probably be explained by the loosening and subsequent loss of cysts during the various washing steps. The other tested stress treatments (NaCl, H2O2, benzalkonium chloride, 55°C, 70% ethanol, and dishwashing detergent) did not have a significant cyst-reducing effect compared with that of the controls, confirming that Acanthamoeba cysts are robust entities. A study performed with Acanthamoeba cysts revealed strain differences among various treatments, with the most resistant strains exhibiting thicker ectocyst structures (21). Dupuy and colleagues observed that Acanthamoeba cysts were less sensitive to chlorine than Hartmannella cysts (10). The authors stated that this might reflect the difference in cellulose content between those species, as Hartmannella has only a small quantity of cellulose in the envelope (4.2%) compared with that of Acanthamoeba (30%).
Kilvington and Anger showed there was an effect of cyst age on disinfectant efficiency, with mature cysts being more resistant than immature cysts (15). Moreover, it should be underlined that resistance to treatments can depend on the applied encystment induction method (24, 25). These findings make it difficult to compare cyst resistance values between different studies.
The tested bacteria inside amoebal cysts were more tolerant to stressful conditions. Whereas monocultured bacteria were killed after H2O2, 10 mg/liter benzalkonium chloride, 70% ethanol, and 2.5% hypochlorite treatments, those that had been inside cysts survived these treatments. Hypochlorite treatment was demonstrated to be cysticidal, suggesting that nonviable cysts could still protect internalized bacteria against this treatment. Resistance to chlorine might be strain dependent. King et al. demonstrated that coliforms, including E. coli, and pathogenic bacteria have an increased resistance (>50-fold) to free chlorine residuals (0.13 to 1 mg/liter) when ingested by Acanthamoeba trophozoites (26). Cysts failed to protect internalized bacteria when incubated at 55°C or heated until boiling, indicating that cysts mainly provide a physical barrier against chemicals.
Before excystment of the amoebae (i.e., 24 to 48 h after inoculation in peptone yeast extract glucose [PYG] medium), no extracellular bacteria were observed, indicating that bacteria recovered at time points after excystment were indeed from inside the cysts. The exact amount of bacteria inside the cysts was not determined, as the robust cyst wall hampers the staining of viable intracellular bacteria. The presence of viable bacteria after excystment implies that at least some bacteria were also viable inside the cysts.
The results of the amoeba-induced resistance assay revealed that bacterial passage through cysts and the subsequent association with trophozoites may induce tolerance to various stressors. It was described that exposure to one stressor renders the bacteria resistant to other subsequent stress factors (i.e., cross-resistance [27, 28]). The intracystic environment can be considered a stressful condition for intracystic organisms, as cysts dehydrate and expel nutrients during encystment (2). However, bacteria, including foodborne pathogens, have evolved complex interacting systems to tolerate desiccation and osmotic stresses (29, 30). Gruzdev proved that desiccated Salmonella acquired higher tolerance to subsequent ethanol, hypochlorite, hydrogen peroxide, and NaCl treatments (29). This is in accordance with the results obtained in the present study. Indeed, cross-protection against several stressors was observed for the tested Y. enterocolitica, L. monocytogenes, S. enterica, and E. coli strains, suggesting the presence of a common stress response mechanism for these bacteria that is responsible for the development of cross-tolerance to other stressors. Further research is needed to reveal (i) how long this increased tolerance lasts, (ii) the range of stressors this cross-resistance may apply to, (iii) the mechanisms behind this cross-resistance, and (iv) how it can be counteracted/prevented.
It was not in the scope of this study to determine the minimal effective dose and treatment time to kill cysts and bacteria. This would involve testing different durations and concentrations of stressors on various bacterial strains and in interaction with multiple amoebal strains. Others have already demonstrated that inhibitory concentrations are highly variable among amoebal strains and culture conditions (25). To fully assess the risk of free-living protozoa in food-related environments, standardized stress-tolerance protocols and improved isolation and cultivation techniques for “in-house-specific” amoebae, ciliates, flagellates, and cysts need to be developed.
Moreover, this study included only a restricted set of meat-borne pathogenic strains, and therefore may not formally reflect all possible forms of interactions between amoebae and bacteria. The interactions we report in our study demonstrate concepts, but it remains to be shown whether they also take place in natural settings. The strong protective association between free-living amoebae and foodborne pathogens demonstrated in this study underscores that reducing or eliminating foodborne pathogens is a complex event, also requiring a better inventory and control of free-living amoebae in food-related systems.
MATERIALS AND METHODS
Amoebal strain and culture conditions.
Acanthamoeba castellanii (ATCC 30234) was maintained and grown axenically in proteose PYG medium (ATCC recipe) at 22°C in 75-cm2 culture flasks. To induce cyst formation, the amoebal trophozoites (3.5 days old, forming a confluent monolayer) were resuspended in high saline (HS) buffer (0.1 M KCl, 8 mM MgSO4·7H2O, 0.02 M Tris, 0.4 mM CaCl2, 1 mM NaHCO3, pH 9) and incubated at 22°C for 6 days (19). Encystment was verified daily by light microscopy. After 6 days, the amoebae were treated for 2 h with 3% HCl to kill immature cysts and any remaining trophozoites. Mature cysts were then resuspended in Page's amoeba saline (PAS) (ATCC recipe) and were enumerated with a Füchs-Rosenthal counting chamber (Blaubrand, Wertheim, Germany) and adjusted to 105 cysts/ml.
Bacterial strains and culture conditions.
Single strains of four meat-borne bacterial species, which are able to survive inside amoebal cysts (19), were used, including Salmonella enterica serovar Typhimurium (strain identification number [ID], 50; origin, pig carcass), Yersinia enterocolitica 4/O:3, pYV+ (ID, 5; origin, minced meat), Escherichia coli O:26 verotoxin 2 positive (VT2+) eae+ (ID, 26; origin, cattle carcass), and Listeria monocytogenes 1/2a (ID, 621; origin, salami). All strains were previously isolated from meat or animal carcasses and stored in glycerol at −20°C. Bacteria were grown to stationary phase in tryptone soy broth at 37°C. On the basis of growth curve parameters, bacteria were suspended and diluted in peptone water ([PW] 0.1% peptone, 0.85% NaCl) to obtain the required concentration (ca. 2 × 103 CFU/ml for the bacterial resistance assay and ca. 5 × 107 CFU/ml for the cyst shelter assay). To determine the exact number of viable cultivable bacteria used at the start of the experiments, serial dilutions were plated on plate count agar (PCA; Bio-Rad, Hercules, California, USA) and incubated 48 h at 30°C.
Cyst resistance assay.
Eight stressors frequently applied in domestic and industrial food-related environments (Table 2) were tested for their cysticidal effect on A. castellanii cysts using the most probable number (MPN) technique for amoebic enumeration. This technique is simple and reproducible and was performed as described by Beattie et al. (17) with some modifications. In short, 1-ml aliquots of cyst cultures (105 cysts/ml) were added to 9 ml of each chemical stressor (final concentrations as shown in Table 2) or in 9 ml PAS for the physical stress treatments and the controls. All tested solutions were freshly prepared. After exposure to the stress treatments (Table 2), various chemical stressors were neutralized by adding a 1-ml suspension of stressed cysts to 9 ml PAS or an appropriate neutralizer (1:10 dilution). Benzalkonium chloride was neutralized in 30 g/liter Tween 80 and 3 g/liter soy lecithin dissolved in deionized water (31). Sodium hypochlorite was neutralized in 30 g/liter Tween 80 and 5 g/liter sodium thiosulfate in deionized water (31). Directly after exposure or after the neutralization step (depending on the stressor), cysts were washed with PAS and resuspended in the same amount of PYG medium. This 1:10 suspension was serially diluted in PYG medium to obtain a 1:102, 1:103, and 1:104 dilution. Five replicates of 1 ml of each dilution were inoculated in 24-well plates. The plates were incubated under aerobic conditions at 22°C in the dark and were examined for the presence of viable trophozoites after 2, 7, and 14 days of incubation. Plates were considered positive (score 1) when adherent moving amoebal trophozoites with distinct vacuoles and nucleus were present. Absence of these trophozoites was considered negative and scored as 0. The score for each 10-fold dilution (i.e., 10−2, 10−3, and 10−4) thus yields a three digit number, which is then entered into the MPN table to give a semiquantitative result on the most probable number of amoebae per ml (17). A cysticidal treatment was defined as a treatment that resulted in significantly lower excystment after 14 days of incubation in excystment buffer (32).
TABLE 2.
Overview of the stressors tested in this study
| Type of stressor | Treatmenta | Final concn | Contact time (min) | Company | Reference(s) for basis |
|---|---|---|---|---|---|
| Chemical | NaCl | 5% | 120 | Sigma | 28 |
| Chemical | H2O2 | 0.3% | 15 | Laboratoires Gilbert | 33 |
| Chemical | Benzalkonium chloride | 10 mg/liter | 15 | Sigma | 31 |
| Physical | 55°C water bath | —b | 15 | — | 21, 34 |
| Physical | Heating until boiling | — | 1 (boiling) | — | — |
| Chemical | Ethanol (Disolol) | 70% | 5 | Chem-Lab | 21 |
| Chemical | Dishwashing detergentc | 0.8 ml/liter | 5 | Procter & Gamble | — |
| Chemical | Sodium hypochlorited | 2.5% dilution; free chlorine, <25,000 ppm | 15 | VWR | 21 |
Unless stated otherwise, treatments were performed at 22°C.
—, not applicable.
Commercial dishwashing detergent containing 15–30% anionogenic surface active molecules, 5–15% nonionogenic surface molecules, methylisothiazolinone, phenoxyethanol, and perfume.
Stock solution of 5% with 50,000 ppm free chlorine, diluted in hard tap water. The use of hard water matches the situation in food processing areas, where often tap water or ground water of drinking quality is used. The addition of sodium hypochlorite to water increases the pH and the OCl− form will be prevalent, but no efforts were made to neutralize the pH as it is not a common practice in industrial procedures. As such the concentration of free chlorine will be <25,000 ppm.
Bacterial resistance assay.
Chemical and physical stressors (Table 2) were also tested for their bactericidal effect on monocultures of meat-borne bacteria. Therefore, bacterial cultures were aliquoted (500 μl of 2 × 103 CFU/ml PW) in Eppendorf tubes and immediately mixed with 500 μl of double-strength chemical stressor solution to achieve the indicated concentration in a final volume of 1 ml with 103 bacteria/ml. This bacterial concentration is representative of the number of intra-amoebal bacteria (32; E. Lambrecht, unpublished data). For physical stressors (heating until boiling and 55°C) and for the nontreated controls, bacteria were mixed with 500 μl PW. The cells were gently vortexed and incubated in the presence of the stressors for the indicated times (Table 2). After exposure, bacterial cells were neutralized by 10-fold serial dilution in PW, and viable bacteria were enumerated by plating on PCA. Benzalkonium chloride and sodium hypochlorite were neutralized with a Tween 80-based neutralizer before serial dilution in PW and plating on PCA. In addition, 100 μl of the neutralized undiluted sample was enriched by inoculation in 9 ml PYG medium (excystment medium) and incubated at 22°C for 3 days before plating on PCA to detect low numbers of viable bacteria. Plates were incubated at 30°C for 48 h to enumerate the bacteria. The identities of the recovered bacteria were confirmed using conventional microbial testing (ISO6579-FDAmd1, ISO10273, ISO10272-1, ISO11290-1/A1, and ISO16654).
Cyst shelter assay.
To assess if amoebal cysts can protect internalized bacteria against chemical and physical stressors, a cyst shelter assay was set up. Briefly, bacteria (5 × 107 CFU/ml) were coincubated with 5 × 105 Acanthamoeba trophozoites/ml (multiplicity of infection, 1:100) for 6 days in HS buffer at 22°C in a 25-cm2 culture flask (19). Under these conditions, amoebal trophozoites will take up bacteria and begin to encyst. Monocultured axenic amoebae were used as controls. The formation and morphology of cysts were checked microscopically. After 6 days, adherent cells were washed with PAS to remove the encystment medium and the majority of extracellular bacteria. A subsequent treatment with 3% HCl was established to eliminate immature cysts, remaining trophozoites, and extracellular bacteria. Next, the mature cysts (ca. 105 cysts/ml) were treated with 100 μg/ml gentamicin for 1 h at 22°C to kill any remaining extracellular bacteria, were washed twice with PAS, and were challenged with the various stressors (Table 2). Afterwards, cells were neutralized where needed as described in “Cyst resistance assay” above and were washed in PAS and resuspended in PYG. The culture flasks were incubated at 22°C and checked daily by light microscopy for the presence of excysted amoebae. Before incubation and on days 1, 2, 3, 5, 7, and 14, fractions of the supernatants were serially diluted, plated on PCA, and incubated at 30°C for 2 days to detect released extracellular bacteria. The identity of the recovered bacteria was confirmed by conventional microbial testing as described above.
Amoeba-induced resistance assay.
To evaluate if bacterial survival inside amoebic cysts and the subsequent association with excysted trophozoites induces enhanced bacterial tolerance to other stressors once the bacteria are released back into the environment, amoeba-induced resistance assays were performed. To obtain intracystic bacteria, cocultures of amoebal trophozoites and bacteria in HS buffer were set up as described above for the cyst shelter assay. After HCl and gentamicin treatments, cysts were washed twice with PAS, resuspended in PYG, and incubated at 22°C. After 24 to 48 h of incubation, excystment occurred, resulting in visible trophozoites and the release of extracellular cyst-primed bacteria (ca. 103 CFU/ml). Bacterial nonprimed control suspensions were set up by inoculating 103 CFU/ml glycerol stock bacteria in PYG. Both primed and nonprimed bacteria were incubated for 3 more days to reach the stationary phase for all bacteria (ca. 108 to 109 CFU/ml) to exclude growth phase differences, as this might cause different stress responses. During this incubation, amoebal trophozoites remained present in the primed bacterial setup. Afterwards, fractions of each suspension were serially diluted and plated on PCA for bacterial enumeration (nbefore treatment). The remaining fractions were treated with the various stressors for the indicated times and neutralized where needed as described above. After treatment, the cells were washed and resuspended in PW. The suspensions were serially diluted, plated on PCA, and incubated at 30°C for 2 days to enumerate bacteria (nafter treatment). The identity of the recovered bacteria was confirmed by conventional microbial testing as described above.
Statistical analysis.
All experiments were repeated at least four times. Quantitative data on bacterial survival and estimated MPN values for cysts were recorded in an Excel spreadsheet, and statistical analysis was performed on the log10-transformed quantitative data using SPSS version 21 (IBM Corp., Armonk, New York). For the cyst and bacterial resistance assays, a Wilcoxon rank-sum test was used to detect significant differences in the median log10 reduction (M) between cells that were treated with stressors and the nontreated controls. For the cyst-induced resistance assay, a Wilcoxon rank-sum test was used to detect differences in median log10 reduction after stress treatment (nbefore treatment − nafter treatment) between cyst-primed bacteria and the monocultured bacterial controls.
REFERENCES
- 1.Vaerewijck M, Baré J, Lambrecht E, Sabbe K, Houf K. 2014. Interactions of foodborne pathogens with free-living protozoa: potential consequences for food safety. Comp Rev Food Sci Food Saf 13:924–944. doi: 10.1111/1541-4337.12100. [DOI] [Google Scholar]
- 2.Khan NA. 2009. Acanthamoeba: biology and pathogenesis. Caister Academic Press, Norfolk, England. [Google Scholar]
- 3.Chavez-Munguia B, Omana-Molina M, Gonzalez-Lazaro M, Gonzalez-Robles A, Bonilla P, Martinez-Palomo A. 2005. Ultrastructural study of encystation and excystation in Acanthamoeba castellanii. J Eukaryot Microbiol 52:153–158. doi: 10.1111/j.1550-7408.2005.04-3273.x. [DOI] [PubMed] [Google Scholar]
- 4.Linder M, Winiecka-Krusnell J, Linder E. 2002. Use of recombinant cellulose-binding domains of Trichoderma reesei cellulase as a selective immunocytochemical marker for cellulose in protozoa. Appl Environ Microbiol 68:2503–2508. doi: 10.1128/AEM.68.5.2503-2508.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weisman RA. 1976. Differentiation in Acanthamoeba castellanii. Annu Rev Microbiol 30:189–219. doi: 10.1146/annurev.mi.30.100176.001201. [DOI] [PubMed] [Google Scholar]
- 6.Sriram R, Shoff M, Booton G, Fuerst P, Visvesvara GS. 2008. Survival of Acanthamoeba cysts after desiccation for more than 20 years. J Clin Microbiol 46:4045–4048. doi: 10.1128/JCM.01903-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aksozek A, McClellan K, Howard K, Niederkorn JY, Alizadeh H. 2002. Resistance of Acanthamoeba castellanii cysts to physical, chemical, and radiological conditions. J Parasitol 88:621–623. doi: 10.1645/0022-3395(2002)088[0621:ROACCT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Storey MV, Winiecka-Krusnell J, Ashbolt NJ, Stenstrom TA. 2004. The efficacy of heat and chlorine treatment against thermotolerant Acanthamoebae and Legionellae. Scand J Infect Dis 36:656–662. doi: 10.1080/00365540410020785. [DOI] [PubMed] [Google Scholar]
- 9.Turner NA, Russell AD, Furr JR, Lloyd D. 2000. Emergence of resistance to biocides during differentiation of Acanthamoeba castellanii. J Antimicrob Chemother 46:27–34. doi: 10.1093/jac/46.1.27. [DOI] [PubMed] [Google Scholar]
- 10.Dupuy M, Berne F, Herbelin P, Binet M, Berthelot N, Rodier MH, Soreau S, Hechard Y. 2014. Sensitivity of free-living amoeba trophozoites and cysts to water disinfectants. Int J Hyg Environ Health 217:335–339. doi: 10.1016/j.ijheh.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Greub G, Raoult D. 2003. Biocides currently used for bronchoscope decontamination are poorly effective against free-living amoebae. Infect Control Hosp Epidemiol 24:784–786. doi: 10.1086/502137. [DOI] [PubMed] [Google Scholar]
- 12.Khunkitti W, Lloyd D, Furr JR, Russell AD. 1998. Acanthamoeba castellanii: growth, encystment, excystment and biocide susceptibility. J Infect 36:43–48. doi: 10.1016/S0163-4453(98)93054-7. [DOI] [PubMed] [Google Scholar]
- 13.Thomas V, McDonnell G, Denyer SP, Maillard JY. 2010. Free-living amoebae and their intracellular pathogenic microorganisms: risks for water quality. FEMS Microbiol Rev 34:231–259. doi: 10.1111/j.1574-6976.2009.00190.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahearn DG, Gabriel MM. 1997. Contact lenses, disinfectants, and Acanthamoeba keratitis. Adv Appl Microbiol 43:35–56. [DOI] [PubMed] [Google Scholar]
- 15.Kilvington S, Anger C. 2001. A comparison of cyst age and assay method of the efficacy of contact lens disinfectants against Acanthamoeba. Br J Ophthalmol 85:336–340. doi: 10.1136/bjo.85.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes R, Kilvington S. 2001. Comparison of hydrogen peroxide contact lens disinfection systems and solutions against Acanthamoeba polyphaga. Antimicrob Agents Chemother 45:2038–2043. doi: 10.1128/AAC.45.7.2038-2043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beattie TK, Seal DV, Tomlinson A, McFadyen AK, Grimason AM. 2003. Determination of amoebicidal activities of multipurpose contact lens solutions by using a most probable number enumeration technique. J Clin Microbiol 41:2992–3000. doi: 10.1128/JCM.41.7.2992-3000.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adekambi T, Ben Salah S, Khlif M, Raoult D, Drancourt M. 2006. Survival of environmental mycobacteria in Acanthamoeba polyphaga. Appl Environ Microbiol 72:5974–5981. doi: 10.1128/AEM.03075-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambrecht E, Baré J, Chavatte N, Bert W, Sabbe K, Houf K. 2015. Protozoan cysts act as a survival niche and protective shelter for foodborne pathogenic bacteria. Appl Environ Microbiol 81:5604–5612. doi: 10.1128/AEM.01031-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackey BM, Miles CA, Parsons SE, Seymour DA. 1991. Thermal denaturation of whole cells and cell components of Escherichia coli examined by differential scanning calorimetry. J Gen Microbiol 137:2361–2374. doi: 10.1099/00221287-137-10-2361. [DOI] [PubMed] [Google Scholar]
- 21.Coulon C, Collignon A, McDonnell G, Thomas V. 2010. Resistance of Acanthamoeba cysts to disinfection treatments used in health care settings. J Clin Microbiol 48:2689–2697. doi: 10.1128/JCM.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Critchley M, Bentham R. 2009. The efficacy of biocides and other chemical additives in cooling water systems in the control of amoebae. J Appl Microbiol 106:784–789. doi: 10.1111/j.1365-2672.2008.04044.x. [DOI] [PubMed] [Google Scholar]
- 23.Stewart M, Olson B. 1996. Bacterial resistance to portable water disinfectants, p 140–192. In Hurst JH. (ed), Modeling disease transmission and its prevention by disinfection. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 24.Coulon C, Dechamps N, Meylheuc T, Collignon A, McDonnell G, Thomas V. 2012. The effect of in vitro growth conditions on the resistance of Acanthamoeba cysts. J Eukaryot Microbiol 59:198–205. doi: 10.1111/j.1550-7408.2012.00612.x. [DOI] [PubMed] [Google Scholar]
- 25.Hughes R, Heaselgrave W, Kilvington S. 2003. Acanthamoeba polyphaga strain age and method of cyst production influence the observed efficacy of therapeutic agents and contact lens disinfectants. Antimicrob Agents Chemother 47:3080–3084. doi: 10.1128/AAC.47.10.3080-3084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King C, Shotts E, Wooley R, Porter K. 1988. Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl Environ Microbiol 54:3023–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vorob'eva LI. 2004. Stressors, stress reactions, and survival of bacteria (a review). Prikl Biokhim Mikrobiol 40:261–269. (In Russian.) [PubMed] [Google Scholar]
- 28.Kultz D. 2005. Molecular and evolutionary basis of the cellular stress response. Annu Rev Physiol 67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- 29.Gruzdev N, Pinto R, Sela S. 2011. Effect of desiccation on tolerance of Salmonella enterica to multiple stresses. Appl Environ Microbiol 77:1667–1673. doi: 10.1128/AEM.02156-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess CM, Gianotti A, Gruzdev N, Holah J, Knochel S, Lehner A, Margas E, Esser SS, Sela Saldinger S, Tresse O. 2016. The response of foodborne pathogens to osmotic and desiccation stresses in the food chain. Int J Food Microbiol 221:37–53. doi: 10.1016/j.ijfoodmicro.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Vaerewijck MJ, Sabbe K, Bare J, Spengler HP, Favoreel HW, Houf K. 2012. Assessment of the efficacy of benzalkonium chloride and sodium hypochlorite against Acanthamoeba polyphaga and Tetrahymena spp. J Food Prot 75:541–546. doi: 10.4315/0362-028X.JFP-11-359. [DOI] [PubMed] [Google Scholar]
- 32.Lambrecht E, Baré J, Van Damme I, Bert W, Sabbe K, Houf K. 2013. Behavior of Yersinia enterocolitica in the presence of the bacterivorous Acanthamoeba castellanii. Appl Environ Microbiol 79:6407–6413. doi: 10.1128/AEM.01915-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linley E, Denyer SP, McDonnell G, Simons C, Maillard JY. 2012. Use of hydrogen peroxide as a biocide: new consideration of its mechanisms of biocidal action. J Antimicrob Chemother 67:1589–1596. doi: 10.1093/jac/dks129. [DOI] [PubMed] [Google Scholar]
- 34.Habib I, Uyttendaele M, De Zutter L. 2010. Survival of poultry-derived Campylobacter jejuni of multilocus sequence type clonal complexes 21 and 45 under freeze, chill, oxidative, acid and heat stresses. Food Microbiol 27:829–834. doi: 10.1016/j.fm.2010.04.009. [DOI] [PubMed] [Google Scholar]