Abstract
Extreme desiccation and irradiation increase the formation of reactive oxygen species in organisms. Lichens are highly resistant to potential damage, but it is not known whether biochemical interaction between their fungal and algal partners is involved in conferring stress tolerance. Here, we show that antioxidant and photoprotective mechanisms in the lichen Cladonia vulcani are more effective by orders of magnitude than those of its isolated partners. When alone, both alga and fungus suffer oxidative damage during desiccation, but in the lichen, each appears to induce up-regulation of protective systems in the other. Without the fungal contact, the alga tolerates only very dim light and its photoprotective system is only partially effective; without the alga, the glutathione-based antioxidant system of the fungus is slow and ineffective. In the lichen, this mutually enhanced resistance to oxidative stress and, in particular, its desiccation tolerance are essential for life above ground. This lifestyle, in turn, increases the chance of dispersal of reproductive propagules and ensures their joint evolutionary success.
Keywords: glutathione, desiccation tolerance, oxidative stress, xanthophyll cycle
Historically (1), and still today (2), the lichen symbiosis is often regarded as controlled parasitism to the benefit of the fungus. However, lichenization also involved the evolution of a complex above-ground structure that neither fungus nor alga can form by itself. When a fungus undergoes transition to lichenization, it gives up its saprophytic lifestyle and gains carbohydrate from its “photobiont,” one or more green algae or cyanobacteria (3–5). The photobiont may also cease its hidden life in soil (6) or small crevices in rocks (7), and together with a fungus, the “mycobiont,” adopts the new structure (Fig. 1a). This form allows them, as a lichen, to live in habitats that were previously unavailable, but they are then exposed to irradiation and desiccation (8). Indeed, a predominant characteristic of lichens is their ability to survive anhydrobiosis, which is a state of suspended animation in which “desiccation-tolerant” organisms survive indefinite periods until rehydration allows them to resume metabolism. Reactive oxygen species (ROS) (9, 10) are a major cause of damage during desiccation (8, 11, 12), especially in photosynthetic organisms (13, 14). When desiccated in the light, chlorophyll molecules continue to be excited, but the energy not used in carbon fixation will cause formation of singlet oxygen (Fig. 2). Here, we report on biochemical pathways that confer tolerance of anhydrobiosis in a lichen and its algal and fungal symbionts. Our main interests are (i) how the lichen achieves protection against desiccation (8, 11) and irradiation (13, 15, 16) and (ii) whether the extreme stress tolerance of the lichen is simply the sum of the traits of its symbiotic partners or whether it is triggered by the process of lichenization.
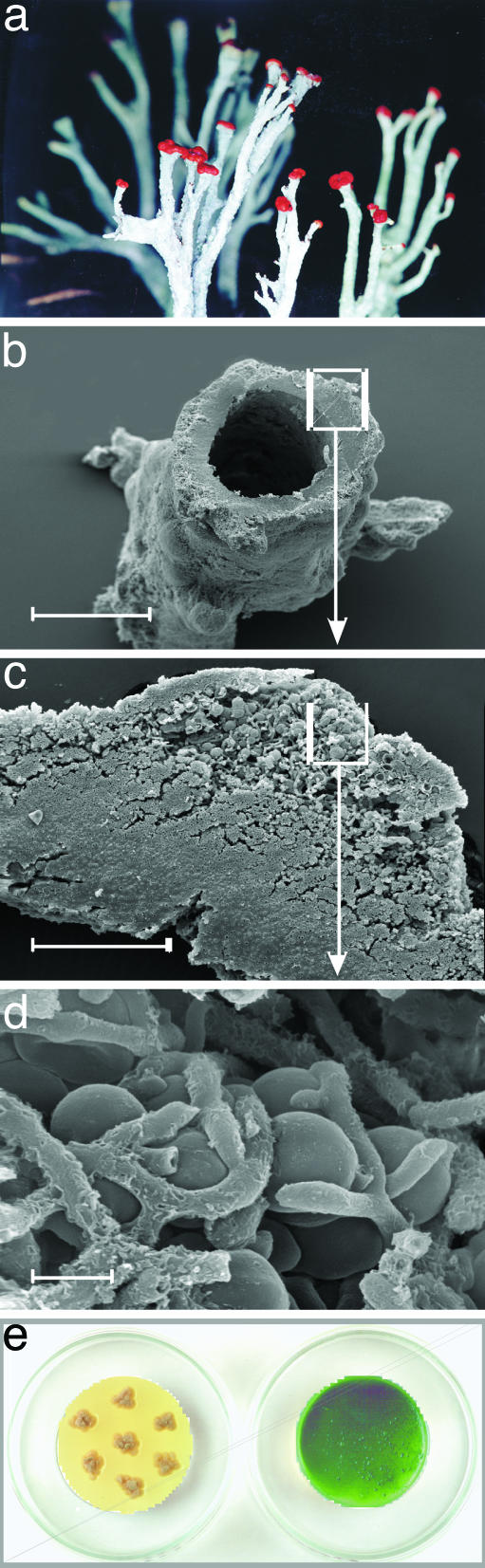
Fig. 1.
The lichen C. vulcani. (a) As collected in the field. (b) Cross section. (c) Detailed view. The algal partner occupies 3.4 ± 1.0% (n = 18) of the lichen volume. (d) Detailed view. Appressoria, fungal hyphae that flatten against algal cells, are the sites of contact between alga and fungus. Scale bars in b–d represent 500, 100, and 5 μm, respectively. (e) The isolated partners, the fungus (Left) and green alga, T. excentrica (Right), growing in axenic culture.
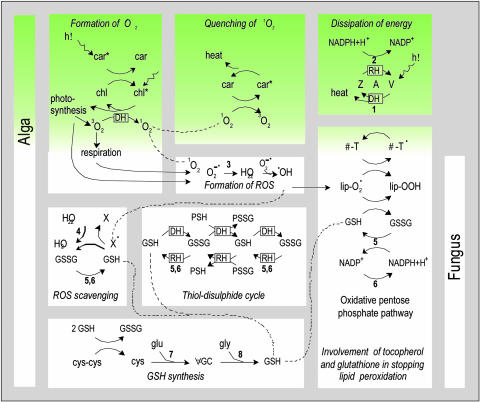
Fig. 2.
Pathways that generate, avoid the formation of, or scavenge free radicals during desiccation and rehydration of C. vulcani and its isolated partners. Green indicates pathways that are present exclusively in the alga. Dots indicate free radicals. DH and RH indicate reactions dominating during dehydration and rehydration, respectively. 1O2 can be formed by transfer of excess excitation energy from chlorophyll (chl) to 3O2 (14, 15). Carotenoids (car) are accessory photosynthetic pigments, but they also dissipate excess light energy and quench 1O2 (14, 15). In particular, in the xanthophyll cycle (13, 16) violaxanthin (V) is converted to antheraxanthin (A) and then zeaxanthin (Z) while solar radiation is dissipated as heat. Free radicals that are formed nevertheless cause damage if not scavenged by antioxidants. The main lipid-soluble free-radical scavengers are α-tocopherol (α-T; vitamin E) (18) and β-carotene (“pro-vitamin A”) (15) and the major water-soluble antioxidant is GSH (γ-GC-glycine) (17, 20, 33). In addition, by binding to protein SH-groups, GSSG protects these from irreversible oxidation (e.g., to sulfonic acids) and irreversible formation of intramolecular disulfide bonds that would cause denaturation of proteins during desiccation (34). Participating enzymes are listed as follows. 1, Violaxanthin deepoxidase; 2, zeaxanthin epoxidase; 3, superoxide dismutase; 4, glutathione peroxidase; 5, GR; 6, G6PDH; 7, γ-GC synthetase; 8, glutathione synthetase. The involvement of ROS (19) and glutathione (20, 21) in signaling is not considered.
We have studied responses of photosynthetic pigments that are the basis of plant life but are sites of ROS formation (15) and of antioxidants (17, 18) that are universally essential for living organisms to scavenge ROS. ROS and antioxidants are also key regulators of metabolic and defense pathways, including hormonal signaling, development, programmed cell death, and stress responses (19–21). Free-radical scavengers include the two major water-soluble antioxidants, glutathione [γ-glutamyl-cysteinyl-glycine; reduced glutathione (GSH)] and ascorbate (“vitamin C”), and the lipid-soluble membrane-bound ROS-scavenger α-tocopherol (“vitamin E”). Lichens also contain phenolic secondary products with alleged antioxidant properties (22). Some of these fungal products are important because they can act as “sun-screen” pigments that protect the alga from irradiation. We have not considered them in this study, because they occur only as extracellular deposits and, therefore, are unlikely to have a major antioxidant function in vivo.
Other researchers have shown that ROS and redox signaling play important roles in the interaction between higher-plant roots and microorganisms, such as the legume Rhizobium symbiosis (23). We chose to investigate the significance of ROS scavengers for establishing and maintaining the symbiosis between a lichen-forming fungus and an alga. In contrast to the legume Rhizobium system, the lichen symbiosis is intricately linked to desiccation tolerance for which a potent ROS scavenging machinery is essential. Fig. 2 shows biochemical interactions of the antioxidants present in Cladonia vulcani in scavenging free radicals, alongside the contribution of photosynthetic pigments to generating, or avoiding the formation of free radicals. In desiccation-tolerant higher plants, ascorbate forms the first line of defense against oxidative damage (24). However, anticipating our results presented below, no ascorbate or (homologues of) erythroascorbate are found in C. vulcani, although they are produced by some fungi (25). Moreover, tocopherol is present in the alga but not in the fungus. In the absence of tocopherol and ascorbate, glutathione plays a pivotal role in oxidative stress prevention for the fungus. Therefore, we have further investigated the activities of two glutathione-related enzymes, glutathione reductase (GR) and glucose-6-phosphate dehydrogenase (G6PDH). The latter enzyme is the key enzyme of the oxidative pentose phosphate pathway, which potentially supplies the NADPH required as a cosubstrate of GR in nonphotosynthesising cells (26).
As a model lichen, we have chosen C. vulcani (Fig. 1a). It consists of a widely distributed genus of lichenized fungi, Cladonia sp., and one photosynthetic partner, Trebouxia excentrica, rather than the two or more that occur in some other lichens, which would complicate the study. Trebouxia sp. is also the most abundant green algal photobiont in lichens (4). The fungus C. vulcani and the alga T. excentrica cannot be collected individually from nature because they occur only rarely, if at all, outside a lichen. Trebouxia sp. may occur free-living (6, 7), but the few cells found occasionally would not suffice for experimentation. Therefore, we separated alga and fungus from the lichen and cultured them. We then measured the responses of the above antioxidants, pigments, and enzymes to desiccation and rehydration in the intact lichen and compared them with those in its isolated partners. We put forward the hypothesis that the enhanced capacity of the lichen to cope with free-radical damage caused by desiccation and irradiation results from mutual stimulation by photobiont and mycobiont, and therefore, that lichenization involves more than nutritional benefit for the fungus.
Methods
Thalli of the lichen C. vulcani Savicz were collected from Mount Yakeyama, Japan, at 1,300 m above sea level. The fungus C. vulcani (which gives its name to the lichen) and the green alga T. excentrica Archibald were isolated as described in ref. 27.
Cultivation and Propagation of Isolated Fungus and Alga. Fungal cultures were grown on Lilly and Barnett's medium containing 2% agar in the dark, and algae were grown on Bold's basal medium with 2% agar on a day/night cycle (12:12 h light/dark), both at 17°C. In contrast to the lichenized alga, isolated Trebouxia sp. grows only in <1% full sunlight (27), consistent with their possible occurrence at low light intensities in rocks and soil. In tissue culture, light intensities >15 μmol m-2·s-1 photosynthetically active radiation stopped growth in T. excentrica. Therefore, we used the light intensity that is optimal for growth: 12 μmol m-2·s-1.
The alga tends to spread over the whole surface of the agar, whereas the fungus forms calli (Fig. 1e). To propagate material, fungal calli were cut into pieces of ≈10 mg of fresh weight (1–2 mg of dry weight). Seven such pieces were transferred to 5-cm plastic Petri dishes containing fresh medium. We diluted ≈50 mg of algal culture in 2–3 ml of distilled, sterile water, and 500-μl aliquots were transferred onto several fresh plates. These procedures were repeated every 2 months for 1.5 years until ≈500 Petri dishes of the notoriously slowly growing fungus and alga were produced, each containing ≈50 mg of dry weight.
Desiccation Treatment. Before desiccation, the intact thalli were equilibrated for 24 h in air of 100% relative humidity in the dark (“controls”). Samples were then placed in a desiccator over silica gel for 9 weeks under a day/night cycle (12:12 h; 300 μmol·m-2·s-1 photosynthetically active radiation/dark) at 17°C. This light intensity is in the optimal range for lichens that grow in the open (28). Two periods of desiccation (3 and 9 weeks) were chosen based on pilot experiments to investigate whether desiccation-induced damage increases with time. After desiccation, material was rehydrated in air of 100% relative humidity.
Desiccation was induced by placing the 5-cm Petri dishes containing the cultures in 10-cm Petri dishes and filling the space between them with silica gel with a moisture indicator. The larger Petri dishes were sealed with parafilm, and the silica gel was renewed when it started to change color. After 3 and 9 weeks of desiccation, rehydration was started by replacing the silica gel with 10 ml of distilled, sterile water. Air humidity within the Petri dishes was checked by humidity sensors (E & E Electronics, Linz, Austria) connected to a data logger (Starlog Macro, Unidata, O'Connor, WA, Australia). The humidity sensors were inserted through holes in the lids of Petri dishes; the holes were sealed, and the sensors were glued to the lid so that they could not touch the cultures underneath. During desiccation, relative humidity was kept at 3–5%, and during rehydration, relative humidity was kept at 95–98%. These conditions were applied also to the intact lichens. Note that, although the intact lichen desiccated within 6–12 h, the agar on which the isolated components grew required 4 days to lose moisture. However, when all water had evaporated from the agar, the speed of water loss in alga and fungus was as fast as in the intact lichen (Fig. 7a, which is published as supporting information on the PNAS web site). Similarly, during rehydration, the isolates recovered water content slower than the intact lichen (Fig. 7b). Scraping the cultures off the agar before desiccation would have minimized the differences in rates of water loss and gain, but it would have caused wounding and additional oxidative stress and would not allow us to draw conclusions about oxidative stress caused by desiccation.
Sampling. After experimental treatment, fungal cultures were sampled by carefully removing the agar attached to the calli. Algal cultures were harvested by scraping them off the medium with a scalpel. Cultures originating from five different Petri dishes were pooled and transferred to humidity-proof plastic vials. For each measurement, at least five replicates (each from five Petri dishes) were analyzed. Vials were frozen immediately in liquid nitrogen and then placed in a freeze-dryer. Freeze-drying, grinding of material to a fine powder, and further sample treatment were performed as described in ref. 29. Similarly, five intact lichen thalli per experimental treatment were frozen in liquid nitrogen and then freeze dried and ground to a fine powder before extraction of analytes.
Plastid Pigments and Antioxidants. Plastid pigments and tocopherols (25), ascorbate and dehydroascorbate (30), and low-molecular-weight thiols [GSH, cysteine, and γ-glutamyl-cysteine (γ-GC)] and their corresponding disulphides (31) were analyzed by HPLC, and GR and G6PDH were analyzed spectrophotometrically (31).
Scanning Electron Microscopy and Image Analysis. Dry lichen samples were rehydrated for 2–3 h and then fixed in buffered glutaraldehyde (2.5% vol/vol). Cross sections of frozen samples (CM3000 cryostat, Leica, Deerfield, IL) were dehydrated with acetone, critical-point-dried, mounted on aluminum stubs, sputter coated with gold (Agar sputter coater; Agar Scientific, Stansted, U.K.) and investigated with a Philips XL30 ESEM at 20 kV by using the high-vacuum mode. The photobiont fraction of the lichen volume was estimated by digital image analyses (optimas, version 6.1, Flir Systems, North Billerica, MA).
Statistics. Data were analyzed for significance by one- or two-way ANOVA in combination with least significant difference post hoc comparisons of means. Unless indicated otherwise, the words “increase,” “decrease,” “differ,” or “change” are used throughout for differences that are significant at a level of P < 0.01.
Results
The lichenized alga makes up 3.4 ± 0.1% of the lichen biomass. It is packed into the outer edge of the lichen (Fig. 1 b–d), where it is partly shielded from irradiation by sun-screen pigments that are deposited in the outer cortex by the fungus (3, 4). In the following section, we describe features of biochemical protection against reactive oxygen formation.
Photoprotective and Antioxidant Properties of Lichenized and Isolated Partners. The lichenized and isolated alga, but not the fungus, contain the photosynthetic pigments chlorophyll a and b, violaxanthin, antheraxanthin, zeaxanthin, lutein, neoxanthin, and β-carotene, as well as the lipid-soluble antioxidant α-tocopherol. The redox couple of GSH and oxidized glutathione (GSSG) were found in fungus, alga, and lichen. In all three, we detected no ascorbate or (homologues of) erythroascorbate.
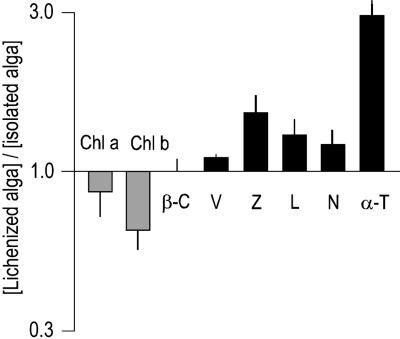
Fig. 3 shows relative pigment and tocopherol concentrations in the lichenized alga (calculated on the basis that the alga occupies 3.4% of the lichen volume) as compared with their concentrations in the isolated alga. These ratios suggest that concentrations change during the loss of, or transition to, lichenization. In the lichen, where it is exposed to higher light intensities, the alga has lower chlorophyll concentrations, as shade plants do in higher light intensities (32). Also, it has higher concentrations of photoprotective pigments involved in nonphotosynthetic quenching of light energy and of the antioxidant α-tocopherol (Fig. 3). Glutathione is present in the lichen at a level 30% greater than the sum of the contents in isolated alga and fungus (calculations are based on the above volume estimates). Hence, in the intact lichen, either alga, fungus, or both, have increased glutathione levels. Overall, as discussed further below, the lichen appears to be better adjusted to cope with oxidative stress than its isolated partners.
Fig. 3.
Photosynthetic pigments and α-tocopherol in the lichenized and the isolated alga. Transition to lichenization results in down-regulation of chlorophyll, but up-regulation of xanthophylls involved in nonphotosynthetic quenching of light energy and of the antioxidant α-tocopherol. chl, chlorophyll; β-C, β-carotene; V, violaxanthin; L, lutein; N, neoxanthin; α-T, α-tocopherol. Data represent mean ± SE (n = 5–10).
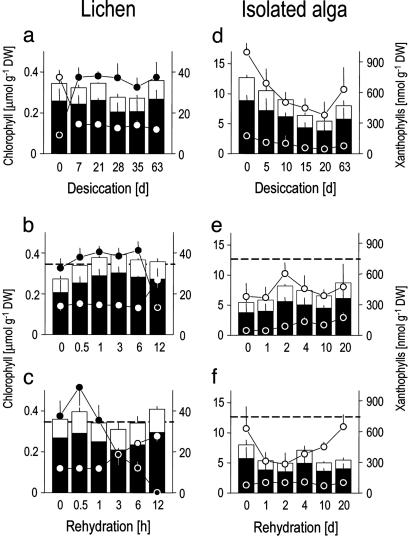
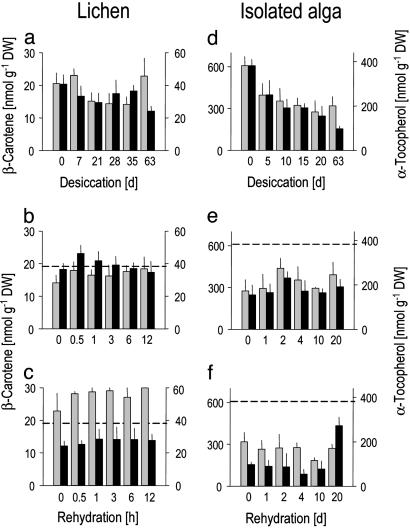
Effects of Desiccation and Rehydration on the Intact Lichen. When desiccated over silica gel, the water content of the lichen drops to 4% (Fig. 7a), but it maintains chlorophyll (Fig. 4a), lutein, neoxanthin (Fig. 8a, which is published as supporting information on the PNAS web site), and β-carotene (Fig. 5a) nearly constant, indicating that desiccation caused no major damage to the photosynthetic apparatus. The lichenized alga makes use of the photoprotective xanthophyll cycle, as shown by the conversion of violaxanthin to zeaxanthin (Fig. 4a). During rehydration, violaxanthin concentration is rapidly reestablished (Fig. 4 b and c). To determine whether longer desiccation causes greater damage, we compared recovery after 3 and 9 weeks. Chlorophyll and carotenoid concentrations did not differ from, or were higher than (for β-carotene), control level during rehydration (Figs. 4 b and c, 5 b and c, and 8 b and c), indicative of a fully functional photosynthetic pigment system up to 9 weeks desiccation. However, α-tocopherol drops by one third during desiccation (Fig. 5a) and is completely reestablished during rehydration after 3, but not 9, weeks of desiccation (Fig. 5 b and c).
Fig. 4.
Effects of desiccation and rehydration on the lichen C. vulcani and its isolated algal partner. (a and d) Desiccation. (b and e) Rehydration after a desiccation period of 3 weeks. (c and f) Rehydration after a desiccation period of 9 weeks. Bars: black and white, chlorophyll a and chlorophyll b, respectively; black and white circles, zeaxanthin and violaxanthin. Dotted lines in rehydration graphs denote total chlorophyll concentrations in undesiccated controls. Data represent mean ± SE (n = 5–10).
Fig. 5.
Effects of desiccation and rehydration on lipid-soluble antioxidants in the lichen C. vulcani and its isolated algal partner. (a and d) Desiccation. (b and e) Rehydration after a desiccation period of 3 weeks. (c and f) Rehydration after 9 weeks of desiccation. Dotted lines in rehydration graphs denote concentrations in undesiccated controls. Data represent mean ± SE (n = 5–10).
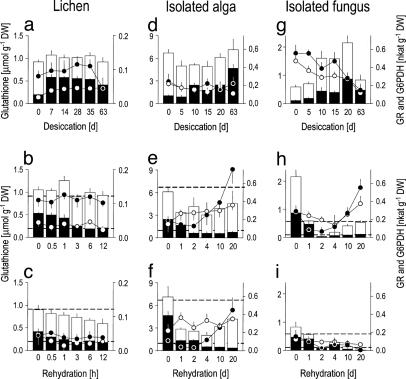
GSH is converted to GSSG during the initial phase of desiccation (Fig. 6a), coinciding with putative ROS attack, but concentrations of total glutathione (GSH plus GSSG) are kept constant. In contrast to the isolated fungus and alga (see below), the enzymes GR and G6PDH initially increase during desiccation in the intact lichen and only G6PDH decreases after 63 days (Fig. 6a). During rehydration, rapid reduction of GSSG ensures reestablishment of the antioxidant GSH. After 3 weeks of desiccation, GSSG is rapidly rereduced to GSH and control concentrations are reestablished (Fig. 6b). After 9 weeks of desiccation, the lichen loses 24% of total glutathione (Fig. 6c) but rapidly regains the high GSH/GSSG ratios found in controls. Previous work showed that the speed of recovery of general physiological function, e.g., of photosynthesis (28), parallels that of ROS scavenging machinery. Therefore, the incomplete recovery of tocopherol and glutathione after 9 weeks of desiccation indicates that some minor damage occurred during longer desiccation periods in C. vulcani.
Fig. 6.
Effects of desiccation and rehydration on the water-soluble antioxidant glutathione and two related enzymes in C. vulcani and in its isolated algal and fungal partners. (a, d, and g) Desiccation. (b, e, and h) Rehydration after 3 weeks of desiccation. (c, f, and i) Rehydration after 9 weeks of desiccation. Black bars, GSSG; white bars, GSH; black cirles, G6PDH; white circles, GR. Dotted lines in rehydration graphs denote the GSH and GSSG concentrations in undesiccated controls. Data represent mean ± SE (n = 5–10).
Effects of Desiccation and Rehydration on the Isolated Alga. The isolated alga loses half of its chlorophylls during desiccation (Fig. 4d), coinciding with losses of two thirds of α-tocopherol (Fig. 5d) and with decreasing photoprotective pigments lutein and neoxanthin (Fig. 8d), as well as β-carotene (Fig. 5d). Lower chlorophyll concentration would decrease potential formation of singlet oxygen (Fig. 2) and be an adjustment to desiccation as discussed for resurrection plants (24) but may also indicate damage. The xanthophyll cycle is not operative because there is no net interconversion of violaxanthin to zeaxanthin (Fig. 4 d–f), but this inactivity is because T. excentrica only grows (27) at light intensities too low to trigger its operation. As in the lichen, GSH is progressively oxidized to GSSG during desiccation (Fig. 6d), indicative of increasing oxidative stress (33). We suggest that the initial drop in total glutathione is caused by the incorporation of GSSG into proteins as soon as it is produced by oxidation of GSH (34) (Fig. 2). GSH is then resynthesized (Fig. 6d), supported by increased concentrations of their precursors cysteine and γ-GC, which increase transiently (Figs. 9d and 10d, which are published as supporting information on the PNAS web site).
The alga is fully rehydrated after 4 days (Fig. 7b), but biochemical recovery is not a linear function of water content. After 3 weeks of desiccation, concentrations of chlorophyll (Fig. 4e), β-carotene and α-tocopherol (Fig. 5e) are still not fully recovered after 20 days of rehydration. In contrast, the high GSH/GSSG ratio is fully recovered after 2 days, although there is an initial loss of total glutathione (Fig. 6e). The enzymes GR and G6PDH are not destroyed during desiccation (Fig. 6d), and their activities increase upon rehydration (Fig. 6e). Starting resynthesis of GSH after 4 days of rehydration is again accompanied by increased concentrations of cysteine (Fig. 10e), whereas γ-GC remains steady (Fig. 9e).
Longer desiccation clearly causes increased damage. After 9 weeks of desiccation, the isolated alga recovers less. Chlorophyll levels fall and remain low (Fig. 4f), α-tocopherol and β-carotene barely recover (Fig. 5f), reduction of GSSG takes 4, rather than 2, days and it loses more total glutathione within the first 4 days of rehydration (Fig. 6f).
Effects of Desiccation and Rehydration on the Isolated Fungus. The fungus also oxidizes GSH to GSSG during desiccation, but at the same time, total glutathione increases 5-fold (Fig. 6g). Synthesis of GSH appears to come from cystine and oxidized γ-GC (Figs. 9g and 10g). Oxidized γ-GC reaches a peak concentration after 10 days of desiccation, just before GSH synthesis is maximal. Cystine serves as a precursor for GSH synthesis in human tumor cells (35), and the fungus appears to use the same unusual pathway (Fig. 2). This route differs from the alga that uses the reduced forms of these thiols and from the lichen that does not produce cystine at all (Figs. 9 and 10). After the initial peak, γ-GC drops to zero (Fig. 9g), and total glutathione then falls (Fig. 6g). After 9 weeks of desiccation, total glutathione concentration is the same as in the undesiccated state, but it is mainly present as GSSG rather than GSH, the active free-radical scavenger.
When the fungus is rehydrated after 3 weeks total glutathione initially drops to zero (Fig. 6h), indicating a temporary block to GSH synthesis despite high concentrations of the substrates cyst(e)ine and γ-GC (Figs. 9h and 10h). The subsequent complete recovery (Fig. 6h) is accompanied by rising levels of GR and G6DPH. By contrast, after 9 weeks of desiccation, the glutathione-based antioxidant system of the fungus does not recover at all. The concentrations of GSH and GSSG and activities of GR and G6PDH all drift down to zero by 20 days (Fig. 6i).
Discussion
Desiccation and Rehydration in the Intact Lichen as Compared with Its Isolated Partners. The intact lichen can survive extremes of desiccation because it operates mechanisms that prevent the formation of, or scavenge, free radicals. In the first stages of desiccation, coinciding with putative ROS attack, GSH is oxidized to GSSG, which accumulates, because desiccation limits its rereduction to GSH. When rehydration allows metabolism to resume (36), the lichen rapidly reduces GSSG, reestablishing its initial GSH concentrations. Fast removal of GSSG and reestablishment of a reducing intra-cellular redox environment (20) are also essential because high GSSG concentrations (37–39) and oxidative conditions (40), respectively, inhibit protein synthesis and induce programmed cell death (20). Also, maintenance of the photosynthetic pigments during desiccation and the rapid recovery of GSH and xanthophyll cycle pigments during rehydration are central to the ability of the lichen to tolerate desiccation and to resume metabolism immediately upon wetting.
When taken apart, alga and fungus are much more susceptible to desiccation-induced stress. The isolated alga can withstand desiccation, but its capacity for photo-protection is limited and it thrives only at very low light intensities (27). During desiccation, it oxidizes GSH as the lichen does, indicative of similar free-radical-scavenging activity, but the alga loses major parts of photosynthetic pigments and tocopherol. In contrast to the lichen, longer desiccation clearly causes increased damage. Although 3 weeks of desiccation do not cause major damage to antioxidants and pigments, after 9 weeks they recover only partially.
The isolated fungus responds even less well to desiccation than the alga. Longer desiccation causes extensive damage, as shown by the depletion of glutathione after 9 weeks of desiccation. A complete loss of antioxidative machinery would be lethal. Because no other “classic” antioxidants appear to be present, the breakdown of the glutathione-based system has detrimental consequences for the isolated fungus and indicates that it is much less able to survive extensive desiccation than is the isolated alga.
The Lichen C. vulcani: More Than the Sum of Its Parts. The fundamental question posed initially was whether the extraordinary desiccation tolerance of the intact lichen is simply the result of the high tolerance of its isolated partners. Our results show that both the isolated alga and fungus can survive desiccation, but whereas the biochemical ROS scavenging and photoprotective machinery of the lichen is not damaged by desiccation, those of both the isolated alga and fungus are. In the undesiccated state, the lichen is already provided with an increased photoprotective and antioxidative machinery, relative to photoassimilatory. These changes ensure that the lichenized alga has enhanced protection from the higher light intensities and desiccation. The lichen also contains higher glutathione concentrations than the weighted sum of the concentrations found in isolated alga and fungus. Thus, transition to lichenization appears to produce, from two less tolerant species, a symbiotic organism able to cope with the enhanced oxidative stress resulting from desiccation (8, 11) and irradiance (8, 16) when living above ground.
Does the Greater Tolerance of the Lichen to Desiccation Result from Mutual Interaction by Its Partners? The observed differences in the behavior of the isolates as compared with the intact lichen might possibly be associated with some modifications in algal and fungal metabolism in tissue culture. However, as discussed above, the lichenized alga up-regulates its photoprotective and antioxidant systems relative to photoassimilatory ones, and in turn, when isolated, the alga loses this enhanced protection. Such improved protection against the stresses that accompany transition to the lichen state and losses because of the isolation process are coherent, not random. Moreover, although tissue culture technique will presumably alter the metabolism of cultured cells after separation from an intact vascular plant, such an alteration is less likely to occur in unicellular algae and fungi. Therefore, we argue that the observed differences between responses of the lichen and its isolated partners are the result of physical separation of the symbionts, rather than effects of tissue culture.
It then follows that the decrease of antioxidant and photoprotective mechanisms as demonstrated for the isolated alga and fungus results from loss of mutual interaction. Without the fungal contact the alga tolerates only very dim light, and its photoprotective system is only partially effective; without the alga, the glutathione-based antioxidant system of the fungus is slow and ineffective. In turn, these inadequate responses suggest that formation of the C. vulcani symbiosis involves mutual signals not only for creating an above-ground structure but also to ensure, as a necessary consequence, greater speed and effectiveness of defense against oxidative damage. Indeed, the lichen suffers none of the damage seen in the isolated alga and fungus during desiccation and rehydration, despite the 25-fold-higher light intensity, which is sufficient to kill the free alga.
Lichens: More Than Enslaving an Alga. The classic view of the lichen symbiosis is of nutritional advantage, with the fungus benefiting from the photosynthate of the alga. Indeed, Schwendener (1), who discovered the lichen symbiosis in 1869, described it as controlled parasitism in which the photobiont is “enslaved by the fungus and compelled into its service.” One fifth of all fungi have chosen lichenization as their lifestyle (41, 42). But would gaining carbohydrates be benefit enough for a fungus to risk the accompanying hazards of light and desiccation? However, the green alga Trebouxia sp. appears to have adopted lichenization almost exclusively. Why does it not remain shielded in soil or cracks in rocks? This article addresses the stressful consequences that the lichen structure inevitably generates for both partners. We put forward the hypothesis that the hazard of increased oxidative stress is overcome by the mutual stimulation by alga and fungus to enhance their photoprotective and antioxidant capacities. Consequently, the lichen has far greater resistance to desiccation than its isolated partners. Most importantly, it recovers very rapidly and completely upon rehydration, allowing sufficient time for growth within a short period of wetting. However, the cost of enhanced antioxidative activity must be outweighed by evolutionary benefit. Following this line of reasoning, for both alga and fungus, the mutually enhanced tolerance of oxidative stress is essential for life above ground, and this resistance increases the chance of dispersal of reproductive propagules and, therefore, evolutionary success.
Supplementary Material
Acknowledgments
We thank M. Inoue (Akita University, Akita, Japan) for collecting lichens; Y. Yamamoto (Akita Prefectural University, Akita, Japan), Y. Kinoshita (Nippon Paint, Tokyo), and T. Kurokawa (Kochi Gakuen College, Kochi, Japan) for their generous help with isolation and maintenance of fungal and algal cultures; G. Kastberger, M. Grube (University of Graz, Graz, Austria), R. Beckett (University of Natal, Pietermaritzburg, South Africa), and H. W. Pritchard (Royal Botanic Gardens, Kew) for helpful comments on the manuscript; and G. Kastberger for photography. This work was supported by Austrian Academy of Science Research Fellowship APART 428 and Austrian Science Foundation FWF Project P12690-BIO (to I.K.). I.K. thanks R. Roth (University of Graz) for encouragement and support.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: γ-GC, γ-glutamyl-cysteine; G6PDH, glucose-6-phosphate dehydrogenase; GR, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized glutathione; ROS, reactive oxygen species.
References
- 1.Schwendener, S. (1869) Die Algentypen der Flechtengonidien (Schultze, Basel).
- 2.Kappen, L. (1994) Crypt. Bot. 4, 193-202. [Google Scholar]
- 3.Ahmadjian, V. (1993) The Lichen Symbiosis (Springer, Berlin).
- 4.Honegger, R. (1991) Annu. Rev. Plant Physiol. Mol. Biol. 42, 553-578. [Google Scholar]
- 5.Smith, D. C. & Douglas, A. E. (1987) The Biology of Symbiosis (Edward Arnold, London).
- 6.Mukhtar, A., Garty, J. & Galun, M. (1994) Symbiosis 17, 247-253. [Google Scholar]
- 7.Ascaso, C., Wierzchos, J. & de los Rios, A. (1995) Bot. Acta 108, 474-481. [Google Scholar]
- 8.Kranner, I. & Lutzoni, F. (1999) in Plant Response to Environmental Stress: From Phytohormones to Genome Reorganisation, ed. Lerner, H. R. (Dekker, New York), pp. 591-628.
- 9.Abele, D. (2002) Nature 420, 27. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell, B. & Gutteridge, J. M. C. (1989) Free Radicals in Biology and Medicine (Oxford Univ. Press, Oxford).
- 11.Smirnoff, N. (1993) New Phytol. 125, 27-58. [DOI] [PubMed] [Google Scholar]
- 12.Rothschild, L. J. & Mancinelli, R. L. (2001) Nature 409, 1092-1101. [DOI] [PubMed] [Google Scholar]
- 13.Demmig-Adams, B. & Adams, W. W. (2000) Nature 403, 371. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell, B. (1984) Chloroplast Metabolism (Clarendon Press, Oxford).
- 15.Frank, H., Young, A., Britton, G. & Cogdell, R. (1999) The Photochemistry of Carotenoids (Advances in Photosynthesis) (Kluwer, Dordrecht), Vol. 8.
- 16.Demmig-Adams, B. & Adams, W. W. (1996) Trends Plant Sci. 1, 21-27. [Google Scholar]
- 17.Noctor, G., Foyer & C. (1998) Annu. Rev. Plant Physiol. Mol. Biol. 49, 249-279. [DOI] [PubMed] [Google Scholar]
- 18.Munne-Bosch, S. & Alegre, L. (2002) Crit. Rev. Plant Sci. 21, 31-57. [Google Scholar]
- 19.Bolwell, G. P. (1999) Curr. Opin. Plant Biol. 2, 287-294. [DOI] [PubMed] [Google Scholar]
- 20.Schafer, F. Q. & Buettner, G. R. (2001) Free Rad. Biol. Med. 30, 1191-1212. [DOI] [PubMed] [Google Scholar]
- 21.Noctor, G, Gomez, L., Vanaker H. & Foyer, C. H. (2002) J. Exp. Bot. 53, 1283-1304. [DOI] [PubMed] [Google Scholar]
- 22.Hidalgo, M. E., Fernandez, E., Quilhot, W. & Lissi, E. (1994) Phytochemistry 37, 1585-1587. [DOI] [PubMed] [Google Scholar]
- 23.Hérouart, H., Baudouin, E., Frendo, P., Harrison, J., Santos, R., Jamet, A., Van de Sype G., Touati, D. & Puppo, A. (2002) Plant. Physiol. Biochem. 40, 619-624. [Google Scholar]
- 24.Kranner, I., Beckett, R. P., Wornik, S., Zorn, M. & Pfeifhofer, H. W. (2002) Plant J. 32, 13-24. [DOI] [PubMed] [Google Scholar]
- 25.Loewus, F. A. (1999) Phytochemistry 52, 193-210. [Google Scholar]
- 26.Kranner, I. (2002) New Phytol. 154, 451-460. [DOI] [PubMed] [Google Scholar]
- 27.Yoshimura, I., Yamamoto, Y. & Nakano, T. (2002) in Protocols in Lichenology. Culturing, Biochemistry, Ecophysiology and Use in Biomonitoring, eds. Kranner, I., Beckett, R. & Varma, A. (Springer, Berlin), pp. 3-33.
- 28.Kranner, I., Zorn, M., Turk, B., Wornik, S., Beckett, R. P. & Batíc, F. (2003) New Phytol. 160, 167-176. [DOI] [PubMed] [Google Scholar]
- 29.Pfeifhofer, H. W., Willfurth, R., Zorn, M. & Kranner, I. (2002) in Protocols in Lichenology. Culturing, Biochemistry, Ecophysiology and Use in Biomonitoring, eds. Kranner, I., Beckett, R. P. & Varma, A. (Springer, Berlin), pp. 363-378.
- 30.Tausz, M., Kranner, I. & Grill, D. (1996) Phytochem. Analysis 7, 69-72. [Google Scholar]
- 31.Kranner, I. (1998) in Mycorrhiza Manual, ed. Varma, A. (Springer, Berlin), pp. 227-241.
- 32.Grumbach, K. H. & Lichtenthaler, H. K. (1981) Photochem. Photobiol. 35, 209-212. [Google Scholar]
- 33.Noctor, G., Arisi, A. C. M., Jouanin, L., Kunert, K. J., Rennenberg, H. & Foyer, C. (1998) J. Exp. Bot. 49, 623-647. [Google Scholar]
- 34.Kranner, I. & Grill, D. (1996) Bot. Acta 109, 8-14. [Google Scholar]
- 35.Gamcsik, M. P., Dubay, G. R. & Cox, B. R. (2002) Biochem. Pharmacol. 63, 843-851. [DOI] [PubMed] [Google Scholar]
- 36.Richardson, D. H. S. (1993) in Stress Tolerance of Fungi, ed. Jennings, D. H. (Academic, London), pp. 275-296.
- 37.Ernst, D., Levin, S. & London, I. (1978) Proc. Natl. Acad. Sci. USA 75, 4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dhindsa, R. S. (1987) Plant Physiol. 83, 816-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fahey, R. C., Di Stefano, D. L., Meier, G. P. & Bryan, R. N. (1980) Plant Physiol. 65, 1062-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shenton, D. & Grant, C. M. (2003) Biochem. J. 374, 513-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawksworth, D. L., Kirk, P. M., Sutton, B. C. & Pegler, D. N. (1995) Dictionary of the Fungi (CAB, Wallingford, United Kingdom).
- 42.Lutzoni, F., Reeb, V. & Pagel, M. (2001) Nature 411, 937-940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.