Abstract
A 48-kDa β-N-acetylglucosamine (GlcNAc)-binding protein was isolated from mouse brain by GlcNAc-agarose column chromatography. The N-terminal amino acid residues showed the protein to be a mouse Na+/K+-ATPase β1-subunit. When the recombinant FLAG-β1-subunit expressed in Sf-9 cells was applied to a GlcNAc-agarose column, only the glycosylated 38- and 40-kDa proteins bound to the column. In the absence of KCl, little of the proteins bound to a GlcNAc-agarose column, but the 38- and 40-kDa proteins bound in the presence of KCl at concentrations above 1 mM. Immunohistochemical study showed that the β1-subunit and GlcNAc-terminating oligosaccharides are at the cell contact sites. Inclusion of anti-β1-subunit antibody or chitobiose in cell aggregation assays using mouse neural cells resulted in inhibition of cell aggregation. These results indicate that the Na+/K+-ATPase β1-subunit is a potassium-dependent lectin that binds to GlcNAc-terminating oligosaccharides: it may be involved in neural cell interactions.
Keywords: GlcNAc-binding lectin, cell surface, mouse brain
N-linked glycosylation is the most frequent protein modification in eukaryotes, and a variety of functions are associated with the oligosaccharide structures (1, 2). High mannose-type oligosaccharides, an initial N-glycosylation form, are involved in the quality control of nascent proteins (3). Some of the oligosaccharides are then processed to mature complex-type oligosaccharides by the trimming of mannose residues by mannosidases I and II followed by the addition of GlcNAc residues by β-N-acetylglucosaminyltransferases I, II, IV, and V. In most cases, the GlcNAc residues of the outer branches are galactosylated and then sialylated. However, glycoproteins from mammalian brains contain N-linked oligosaccharides terminating with GlcNAc residues in relatively higher proportions (4, 5) in contrast to those from other tissues. Although the mechanism to bear the GlcNAc-terminating oligosaccharides is unknown, this is partly due to the different expression of β-1,4-galactosyltransferases in the brain from other tissues (6, 7). The presence of GlcNAc residues at the nonreducing termini of N-linked oligosaccharides may have some biological functions in the brain. In an effort to elucidate the biological functions of such unique oligosaccharides, we have demonstrated that the growth and neurite extension of mouse primary cultured neural cells and mouse and human neuroblastoma cells are regulated through their GlcNAc-terminating oligosaccharides expressed at the cell surface by culturing them in dishes coated with Psathyrella velutina lectin (PVL), a β-GlcNAc-binding lectin (8). These results suggest that GlcNAc-binding proteins are present at the cell surface and/or extracellular matrices and are involved in cellular interactions. In the present study, we have isolated and identified a major GlcNAc-binding protein from mouse brain and shown its involvement in cellular interactions.
Materials and Methods
Isolation and Identification of a GlcNAc-Binding Protein from Mouse Brain. The plasma membranes were prepared from 8-week-old BALB/c male mouse brains by the conventional method (9), suspended in phosphate buffered-saline (pH 7.2) (PBS) containing 1% Nonidet P-40, and stirred overnight. The solubilized membrane proteins were applied to an agarose column to eliminate those that bind nonspecifically to agarose. The remaining proteins were applied to a column containing GlcNAc-agarose (Sigma), and the column was washed with PBS containing 0.1% Nonidet P-40 and 0.5 M NaCl, and then PBS containing 0.1% Nonidet P-40 and 20 mM chitobiose. The fractions thus obtained were dialyzed against water, and the retentates were lyophilized. They were subjected to SDS/polyacrylamide gel electrophoresis (SDS/PAGE), and separated proteins were transferred to poly(vinylidene difluoride) (PVDF) filters and stained with Coomassie brilliant blue R250. The stained band in the bound fraction was excised and used for protein sequence analysis (Shimazu PPSQ-23). The N-terminal sequence obtained was subjected to blast homology search to identify the protein. Asialo (As)-, As and agalacto (AsAg)-, and As, Ag, and a-N-acetylglucosamino (AsAgAgn)-human transferrin, which contains two biantennary complex-type oligosaccharides, were prepared by the method described in ref. 10 and immobilized on Sepharose 4B beads. Affinity chromatography using these columns was conducted as described above except that an appropriate haptenic sugar was used to elute bound proteins from each column.
Immunoblot and lectin blot analyses using chicken anti-canine Na+/K+-ATPase antibody (Biomol, Plymouth Meeting, PA) and rabbit anti-rat Na+/K+-ATPase β1-subunit antibody (Upstate Biotechnology, Lake Placid, NY), anti-FLAG M2 monoclonal antibody (Chemicon, Temecula, CA), and concanavalin A (Con A) were performed as described previously (11). The anti-mouse neural cell adhesion molecule (N-CAM) antibody and biotinylated PVL were gifts from H. Asou at the Tokyo Metropolitan Institute of Gerontology and from N. Kochibe at Gunma University, respectively.
Purification of Na+/K+-ATPase from Mouse Brain. Na+/K+-ATPase (EC 3.6.1.3) was purified from mouse brain, and it showed two main protein bands (48 and 110 kDa) as resolved by SDS/PAGE followed by staining with Coomassie brilliant blue. It possessed an ATP-hydrolase activity of >150 μmol of phosphate released per mg of protein per hr, relatively higher activity than reported previously (12). The purified proteins were incubated with 0.4 mM biotinyl diazirine-conjugated chitobiose as a photoaffinity probe in the presence or absence of 20 mM chitobiose at room temperature for 60 min without light, and then at 4°C for 60 min under short-wave UV light according to the method described in ref. 13. The photoaffinity-labeled protein was detected by Western blot analysis using alkaline phosphatase-labeled streptavidin.
Expression of the Recombinant FLAG-β1-Subunit. The mouse Na+/K+-ATPase β1-subunit cDNA was amplified by reverse transcription–PCR (RT-PCR). The cDNA encoding the full-length mouse β1-subunit was then amplified by PCR using the mouse brain single-strand cDNAs as a template, and 5′ and 3′ primers (5′-CGGAATTCAATGGCCCGCGGAAAAGCCAAG-3′ and 5′-CGGGATCCTTTGTGCTTGTGATCAGCTC-3′) specific to the β1-subunit gene were designed on the basis of the mouse Na+/K+-ATPase β1-subunit gene (GenBank accession no. X16646) (14). For amplification of the cDNA encoding the fused FLAG-β1-subunit, 5′-CGGCATGCATGGACTACAAAGACGATGACGACAAGATGGCCCGCGGAAAAGCC-3′ and 5′-GGAGATCTCAGTCTTCTGGA-3′) were used for PCR as 5′ and 3′ primers, respectively. The nucleotide sequence corresponding to the FLAG sequence, which was introduced in the 5′-terminal region of the β1-subunit cDNA, is underlined.
To amplify the recombinant bacmid DNA, the FLAG-β1-subunit cDNA was ligated into a pFASTBAC 1 expression vector according to the BAC-TO-BAC baculovirus expression system protocols (GIBCO/BRL), and the pFASTBAC/FLAG-β1-subunit was transfected into DH10Bac competent Escherichia coli cells. The recombinant bacmid DNA was then transfected into Sf-9 cells with a FuGENE6 transfection reagent (Roche Applied Science, Indianapolis). The virus stocks with high titers were obtained after two successive amplifications in Sf-9 cells. For protein expression, 2.0 × 107 Sf-9 cells in 15-cm tissue culture dishes were infected with the viral stocks. After 3 days of culture, the cells were harvested, centrifuged, and washed with PBS.
Neural Cell Aggregation Assay. Mouse neural cells established from 17.5-day gestation ICR mouse embryos and mouse neuroblastoma neuro-2a cells were harvested by treatment with 0.2% trypsin and 1 mM EDTA, suspended in culture medium containing 1 mM CaCl2 at 3 × 105 cells per ml, placed in 96-well plates precoated with 1% BSA, and rotated on a gyratory shaker at room temperature for up to 2 hr. Aggregation was terminated by adding 2% glutaraldehyde. The extent of cell aggregation was defined as the ratio of the total particle number (Nt) after incubation to the initial particle number (N0) before incubation according to the method described previously (15), and evaluated by a National Institutes of Health image-analyzing system. To examine the involvement of the β1-subunit and GlcNAc-terminating oligosaccharides in cellular interactions, rabbit anti-rat β1-subunit IgG antibody, chitobiose, galactose, mannose, or normal rabbit IgG was included in the assays before incubation, and their effects on cell aggregation were evaluated by counting the particles.
For cytochemical experiments, cell aggregates fixed with 4% paraformaldehyde were permeabilized with 0.3% Triton X-100 and then preincubated with 5% normal goat serum. The pellets were incubated with rabbit anti-rat β1-subunit IgG antibody and then with Alexa Fluor 568-labeled goat anti-rabbit IgG antibody (Molecular Probes) or with mouse anti-human β-tubulin class III antibody (BMA Biochemicals, Augst, Switzerland) and then with Alexa Fluor 488-conjugated goat anti-mouse IgG antibody (Molecular Probes). Similarly, the aggregated cells were incubated with biotinylated PVL and then with Cy3-labeled streptavidin (Jackson ImmunoResearch). The specimens were observed under a laser scanning confocal microscope.
Results
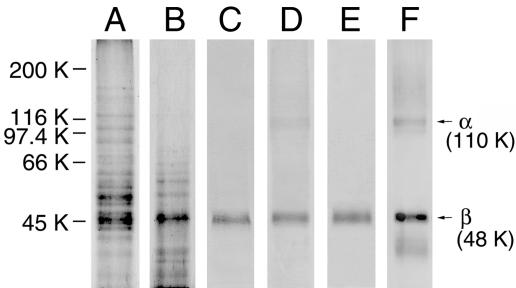
Isolation of a GlcNAc-Binding Protein from Mouse Brain. To isolate GlcNAc-binding proteins, solubilized plasma membrane proteins from mouse brain were applied to a GlcNAc-agarose column, and the bound proteins were eluted with PBS containing 20 mM chitobiose and 0.1% Nonidet P-40. When proteins in the bound fraction were subjected to Western blot analysis followed by staining with Coomassie brilliant blue, a band with an approximate molecular mass of 48 kDa was detected as a major protein (Fig. 1, lane B). The 48-kDa band was excised from the blot and subjected to protein sequence analysis. The N-terminal sequence, MARGKAKEEG, was revealed by blast homology search to be the first 10 amino acid residues of the mouse Na+/K+-ATPase β1-subunit (herein abbreviated as the β1-subunit, unless necessary). To confirm this identification, the blot was incubated with anti-canine Na+/K+-ATPase antibody, which has been shown to crossreact with the mouse protein, and the 48-kDa protein band reacted with the antibody (Fig. 1, lane C). Although the Na+/K+-ATPase consists of α1- and β1-subunits in neural cells (16), less immunoreactivity was detected for the band corresponding to the α-subunit in the bound fraction (Fig. 1, lane C). Therefore, Na+/K+-ATPase was isolated from mouse brain and applied to a GlcNAc-agarose column. Immunoblot analysis showed that only a small amount of the α1-subunit is detectable in addition to the β1-subunit in the bound fraction (Fig. 1, lane D). When the blot containing mouse brain plasma membrane proteins was incubated with anti-Na+/K+-ATPase antibody, two major protein bands with molecular masses of 48 and 110 kDa were detected (Fig. 1, lane F). These results suggest that most of the α1-subunit may dissociate from the β1-subunit upon binding to GlcNAc-agarose. When the solubilized plasma membrane proteins were applied to an AsAg-transferrin-Sepharose column, which contained GlcNAc-terminating N-linked oligosaccharides, the β1-subunit was detected in the bound fraction (Fig. 1, lane E), similar to that of lane C in Fig. 1.
Fig. 1.
Isolation of GlcNAc-binding proteins from mouse brain by GlcNAc-agarose column chromatography. The blots containing proteins in the pass-through (lane A) and bound (lane B) fractions were stained with Coomassie brilliant blue. The blots containing proteins in the bound fraction (lane C), purified mouse brain Na+/K+-ATPase that bound to a GlcNAc-agarose column (lane D), mouse brain plasma membrane proteins that bound to an AsAg-transferrin-Sepharose column (lane E), and mouse brain plasma membrane proteins (lane F) were incubated with anti-canine Na+/K+-ATPase antibody. The positions of the α- and β-subunits are indicated with arrows and molecular masses.
To confirm the above observations, the purified Na+/K+-ATPase was incubated with a chitobiose (GlcNAcβ1→4GlcNAc)-conjugated photoprobe in the presence or absence of 20 mM chitobiose, and then the chitobiose-bound protein was photoaffinity-labeled by UV-irradiation. When the products were subjected to Western blot analysis, only a 48-kDa protein was detected, and it reacted with anti-Na+/K+-ATPase antibody (data not shown). In the presence of haptenic sugar, no protein was photoaffinity-labeled (data not shown). These results indicate that the β1-subunit, but not the α1-subunit, binds to GlcNAc-terminating N-linked oligosaccharides.
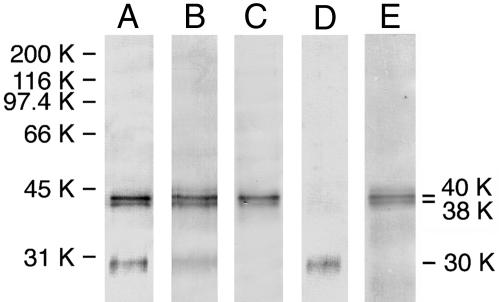
Expression of the FLAG-β1-Subunit in Sf-9 Cells. To determine the lectin properties of the β1-subunit, the FLAG-β1-subunit expressed in Sf-9 cells was isolated from cell lysate by anti-FLAG antibody-agarose column chromatography. Immunoblot analysis showed that protein bands with approximate molecular masses of 30, 38, and 40 kDa reacted with the anti-FLAG antibody (Fig. 2, lane A), and with the anti-rat β1-subunit antibody, although the 30-kDa band reacted only weakly (Fig. 2, lane B). Because the 30-kDa protein appeared to be unglycosylated on the basis of the estimated molecular mass, and high mannose-type oligosaccharides are expressed predominantly in Sf-9 cell glycoproteins (17), the blot was incubated with Con A, which interacts mainly with high mannose-type oligosaccharides. The results showed that Con A binds to the 38- and 40-kDa proteins but not to the 30-kDa protein (Fig. 2, lane C), indicating that the 30-kDa protein is unglycosylated and the 38- and 40-kDa proteins are glycosylated. Digestion of the 38- and 40-kDa proteins with N-glycanase produced a shift in their apparent molecular masses to 30 kDa (data not shown), indicating that the 38- and 40-kDa proteins are differently N-glycosylated.
Fig. 2.
Immunoblot and lectin blot analyses of the FLAG-β1-subunit expressed in Sf-9 cells. The blot containing purified FLAG-β1-subunit proteins was incubated with anti-FLAG antibody (lane A), anti-rat β1-subunit antibody (lane B), or Con A (lane C). The purified FLAG-β1-subunit proteins were subjected to GlcNAc-agarose column chromatography, and blots containing proteins in the pass-through (lane D) and bound (lane E) fractions were incubated with anti-rat β1-subunit antibody.
Requirement of N-glycosylation of the FLAG-β1-subunit for binding to GlcNAc-agarose. The purified 30-, 38-, and 40-kDa proteins were applied to a GlcNAc-agarose column, and proteins in the pass-through and bound fractions were subjected to immunoblot analysis. When the blots were incubated with an anti-FLAG antibody, the 30-kDa protein was detected in the pass-through fraction, whereas the 38- and 40-kDa proteins were in the bound fraction (Fig. 2, lanes D and E, respectively). Furthermore, the recombinant proteins treated with N-glycanase failed to bind to GlcNAc-agarose (data not shown). These results indicate that only a glycosylated form of the FLAG-β1-subunit can bind to GlcNAc-agarose.
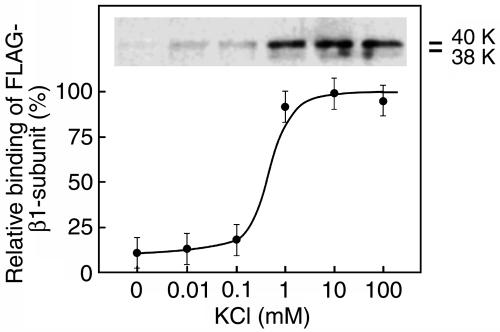
Requirement of K+ for binding of the FLAG-β1-subunit to GlcNAc-agarose. When the purified recombinant proteins were dissolved in 50 mM Tris·HCl buffer (pH 7.2) containing 0.15 M NaCl instead of PBS and applied to a GlcNAc-agarose column, little protein bound to the column (Fig. 3, 0 mM KCl), suggesting that the K+ is important for the binding of the β1-subunit to GlcNAc-agarose. The optimal K+ concentration for the binding of the 38- and 40-kDa proteins to GlcNAc-agarose was determined by changing the K+ concentrations in the buffer used to prepare the proteins from the cell lysate and for column chromatography. The results showed that a concentration as high as 1 mM K+ is enough for the FLAG-β1-subunit to bind to GlcNAc-agarose (Fig. 3).
Fig. 3.
Effect of K+ concentration in the buffer on the binding of the 38- and 40-kDa FLAG-β1-subunit proteins to GlcNAc-agarose. Purified FLAG-β1-subunit proteins were applied to a GlcNAc-agarose column in the presence of increasing concentrations of KCl. Relative bindings of the FLAG-β1-subunit to the column were determined by anti-rat β1-subunit antibody reactivity toward the 38- and 40-kDa proteins in the bound fractions. Three independent experiments were conducted, and the mean values are shown with standard errors.
Binding specificity of the FLAG-β1-subunit. To examine the carbohydrate-binding specificity of the β1-subunit, the purified FLAG-β1-subunit proteins were applied to columns with immobilized glycoproteins or carbohydrates, and the relative amounts of the 38- and 40-kDa proteins bound and eluted from each column with PBS containing various haptenic sugars are shown in Table 1. The relative bindings of the 38- and 40-kDa proteins to each column were scored by quantifying the antibody reactivities toward the 38- and 40-kDa proteins in the bound fractions, with the reactivities toward the proteins eluted from the GlcNAc-agarose column taken as +++ by using an nih image analyzing system. The results showed that the 38- and 40-kDa proteins bind strongly to AsAg-transferrin-Sepharose and GlcNAc-agarose and weakly to N-acetylgalactosamine (GalNAc)-agarose, and they show no significant binding to AsAgAgn-transferrin-Sepharose, As-transferrin-Sepharose, transferrin-Sepharose, mannose-agarose, and lactose-agarose (Table 1).
Table 1. Relative binding specificities of the FLAG-β1-subunit toward carbohydrates.
| Glycoprotein-Sepharose or carbohydrate-agarose | Relative binding |
|---|---|
| AsAg-transferrin-Sepharose | +++ |
| AsAgAgn-transferrin-Sepharose | - |
| As-transferrin-Sepharose | - |
| Transferrin-Sepharose | - |
| GlcNAc-agarose | +++ |
| GalNAc-agarose | + |
| Mannose-agarose | - |
| Lactose-agarose | - |
The relative bindings are shown by scoring band intensities of the proteins recovered from the GlcNAc-agarose column as +++ by nih image analysis after detection with the antibody.
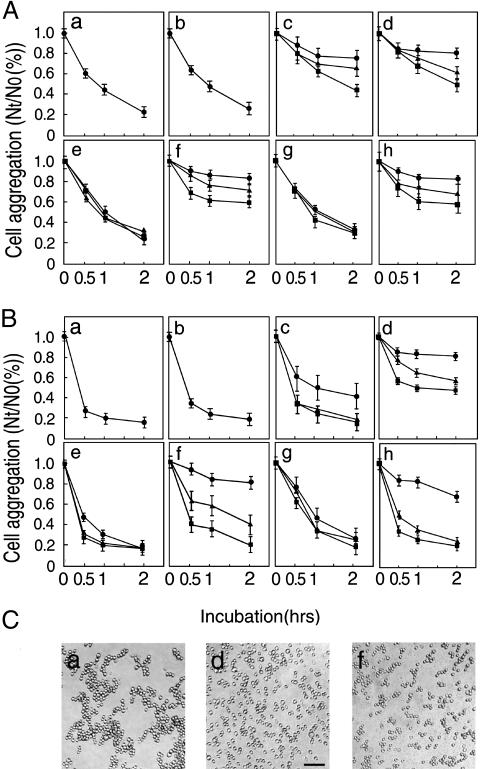
Involvement of the β1-Subunit in Cellular Interactions. Although the β1-subunit has been shown to have the lectin activity, it is unclear whether the activity is really involved in cellular interaction. To address this possibility, an aggregation assay was performed with mouse embryonic neural cells and neuroblastoma neuro-2a cells. Both cells contained a time-dependent cell aggregation activity (Fig. 4 Aa and Ba). Inclusion of 5 mM EDTA in the medium did not change cell aggregation activity (Fig. 4 Ab and Bb), indicating that cadherins are not involved in the aggregate formation. Because N-CAM has been shown to be involved in neural cell aggregation (18), an antibody against mouse N-CAM was added to the assay, where it strongly inhibited embryonic neural cell aggregation (Fig. 4Ac) and weakly inhibited neuro-2a cell aggregation (Fig. 4Bc), both in a dose-dependent manner. When the anti-β1-subunit antibody was included in the assay, the aggregation of both cell types was strongly inhibited in a dose-dependent manner (Fig. 4 Ad and Bd). No inhibition of cell aggregation was found in either case when normal IgG was added to the medium (Fig. 4 Ae and Be). A strong dose-dependent inhibition of aggregation of both cell types was also observed in the presence of chitobiose (Fig. 4 Af and Bf). However, no inhibition of aggregation was induced by lactose in either cell type (Fig. 4 Ag and Bg). In the case of mannose, a stronger inhibition of aggregation was observed in embryonic neural cells (Fig. 4Ah) than in neuro-2a cells (Fig. 4Bh) despite the lack of binding of the β1-subunit to mannose-agarose (Table 1). When neural cells were incubated in PBS without K+, the viability of cells decreased to 70% at the end of incubation, and the significance of K+ to the aggregate formation was not established. Fig. 4C shows cell aggregates after 2-hr incubation in the absence (a) and presence of the anti-β1-subunit antibody (10 μg/ml) (d) or chitobiose (20 mM) (f). Incubation of neuro-2a cells with GlcNAc-agarose beads showed cell-to-bead aggregation in addition to cell-to-cell aggregation (data not shown). These results indicate that the β1-subunit and GlcNAc-terminating oligosaccharides are potentially involved in neural cell adhesion in a trans-binding fashion.
Fig. 4.
Cell aggregation activity of the β1-subunit. The extent of cell aggregation is represented as the ratio of the total particle number at indicated incubation time (Nt) to the initial particle number (N0). The Nt/N0 values represent the means ± standard errors of three independent experiments. Effects of antibodies and carbohydrates on aggregate formation of mouse embryonic neural cells and neuro-2a cells are shown in A and B, respectively. Cells were incubated in the absence of additions (a) or in the presence of 5 mM EDTA (b), anti-N-CAM antibody (c), rabbit anti-rat β1-subunit antibody (d), normal rabbit IgG (e), chitobiose (f), lactose (g), or mannose (h). ▪, ▴, and • indicate concentrations of 0.4, 2.0, and 10.0 μg/ml antibody, or 2, 10, and 20 mM carbohydrate, respectively. (C) Aggregates of control neuro-2a cells as a representative (a), and neuro-2a cells in the presence of 10.0 μg/ml anti-rat β1-subunit antibody (d) and 20 mM chitobiose (f) after 2-hr incubation. (Bar, 50 μm.)
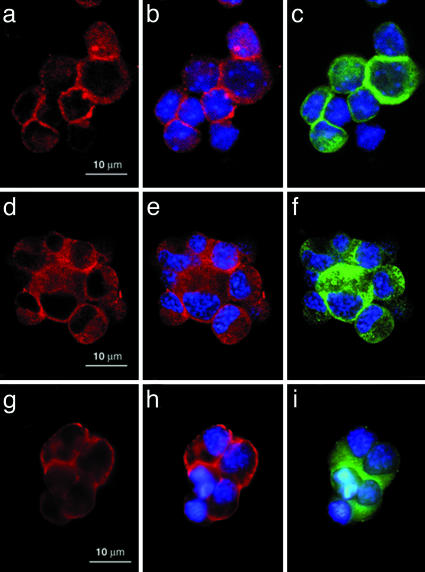
Immunocytochemical Observation of the β1-Subunit. Fixed cell aggregates were incubated with anti-rat β1-subunit antibody to show the localization of the β1-subunit at the cell contact sites immunocytochemically. The cell aggregates were also incubated with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) and a mouse anti-human β-tubulin class III antibody, which reacts with the mouse protein, to stain nuclei blue (Fig. 5 b, c, e, f, h and i) and β-tubulin, specific to neural cells, green (Fig. 5 c, f, and i), respectively. The β1-subunit was detected not only at the cell contact sites but also in the free cell surface of mouse embryonic neural cells (Fig. 5 a and b) and neuro-2a cells (Fig. 5 d and e). Because no antibody against the mouse Na+/K+-ATPase α1-subunit is available, whether the β1-subunit localizes with the α1-subunit at the cell contact sites remains to be elucidated. Furthermore, to show the presence of GlcNAc-terminating oligosaccharides at the cell contact sites, aggregated fixed neuro-2a cells were incubated with PVL, which binds to GlcNAc-terminating oligosaccharides (19). The immunocytochemical observations showed that they are also present not only at the cell contact sites but also in the free cell surface (Fig. 5 g and h). Similar results were obtained by using mouse embryonic neural cells (data not shown). As shown in our previous studies (8), there are many PVL-positive proteins at the neural cell surface. By using a column with immobilized anti-FLAG antibody to which the FLAG-β1-subunit is bound, we isolated a 74-kDa protein as a major PVL-positive band that bound to and was eluted from the column with 20 mM chitobiose, indicating that only a few rather than all glycoproteins carry ligand activity for the β1-subunit.
Fig. 5.
Localization of the β1-subunit and GlcNAc-terminating oligosaccharides in cell aggregates. Fixed aggregates of mouse embryonic neural cells (a–c) and neuro-2a cells (d–f) were incubated with anti-rat β1-subunit antibody and visualized (a, b, d, and e). Fixed aggregates of neuro-2a cells were incubated with PVL and visualized (g and h). Nuclei and β-tubulin were stained blue and green, respectively (b, c, e, f, h, and i).
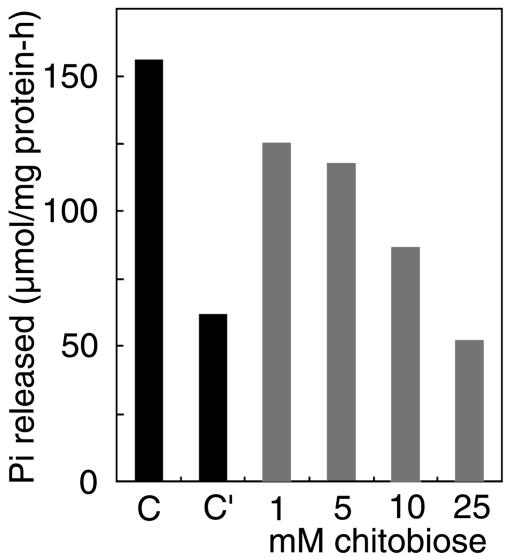
Effect of Chitobiose on Na+/K+-ATPase Activity. Because most of the β1-subunit is associated with the α1-subunit in mouse neural cells, whether the binding of the β1-subunit to oligosaccharides affects the pump activity was investigated by determining the effect of chitobiose rather than the anti-β1-subunit antibody on the ATPase activity. When 1–25 mM chitobiose was included in the reaction mixture, a dose-dependent inhibition of ATPase activity took place; 25 mM chitobiose inhibited the activity by almost 70% (Fig. 6). Without K+ in the assay mixture, the ATP hydrolytic activity was inhibited by 60%, indicating the requirement of K+ binding to the α-subunit and/or β-subunit for catalytic activity because both subunits contain K+-binding sites (20). The results indicate that the Na+/K+-ATPase activity is inhibited upon binding of the β1-subunit to oligosaccharides.
Fig. 6.
Effect of chitobiose on Na+/K+-ATPase activity. Na+/K+-ATPase was purified from mouse brain, and its activity was determined in the presence of chitobiose. C and C′ indicate the activities obtained in the presence and absence of KCl, respectively.
Discussion
Na+/K+-ATPase is a transmembrane protein that keeps the osmotic pressure of animal cells constant by the active transport of Na+ and K+ in conjunction with ATP hydrolysis. The enzyme is composed of an α-subunit and a β-subunit, both of which contain isoforms (α1-, α2-, and α3-subunits and β1-, β2-, and β3-subunits), the combination of which differs among cell types and tissues and at various developmental stages (21). The α-subunit plays a crucial role in active transport, but the role of the β-subunit remains unclear (20). However, it has been suggested that the β-subunit plays a role in the plasma membrane targeting of the α-subunit and in stability of the Na+/K+-ATPase complex (22–24).
The present study shows that the β1-subunit expressed predominantly in neural cells is a lectin that binds to GlcNAc-terminating oligosaccharides in the presence of K+. To our knowledge, no lectins that require K+ for ligand binding have been described until now. In fact, the β1-subunit appears to have two K+-binding sites, one in the extracellular domain and the other in the cytoplasmic domain, and the subunit can change its conformation by binding to K+ (20). Although the ligand-binding site in the β1-subunit remains to be elucidated, the amino acid sequence 16–265 of the mouse β1-subunit shows 28% identity to conserved amino acid sequences of fungus chitin synthases in Aspergillus fumigatus and Exophiala dermatitis (25), as analyzed by a multiple sequence analysis (clustal w 1.74). Because this enzyme binds to UDP-GlcNAc and GlcNAc oligomers with the conserved domain (26), such a sequence included in the mouse β1-subunit might be involved in ligand binding.
Cell aggregation assays using mouse embryonic neural cells and neuro-2a cells indicated that the β1-subunit is involved in cell-to-cell interaction, most probably by binding to GlcNAc-terminating oligosaccharide carried by some glycoprotein expressed at the cell surface of the opponent cells. It has been shown that several cell adhesion molecules, including N-CAM, are involved in neural cell interactions. In fact, an anti-N-CAM antibody inhibited cell aggregation strongly in embryonic neural cells but weakly in neuro-2a cells, a difference that could be due to the different expression levels of N-CAM between the two cell types. Similarly, anti-rat β1-subunit antibody and chitobiose produced strong dose-dependent inhibitions of aggregation of both cell types, even though other cell adhesion molecules were participating in the aggregate formation. Furthermore, mannose but not lactose also strongly inhibited aggregate formation. This observation is probably due to the participation of high mannose-type oligosaccharides on the myelin-associated glycoprotein and L1 in neural cell adhesion by recognition with N-CAM and basigin (27, 28), which is independent of the present β1-subunit-mediated cellular interaction.
Immunohistochemical studies of the cell aggregates revealed that the β1-subunit is present not only at cell-to-cell contact sites but also on the free cell surface. However, the antibody staining at the cell-to-cell contact sites was more intense, indicating that the β1-subunit accumulates at the contact sites. Similarly, GlcNAc-terminating oligosaccharides were also shown to be localized more at the cell contact site. The 74-kDa oligosaccharide-carrier protein may play a role here. Although the physiological relevance of this cellular interaction is not clear at present, it can be investigated by raising mice deficient in the gene for the 74-kDa protein. However, mice deficient in the β1-subunit gene may not contribute to this issue because abrogation of the β1-subunit may cause primarily osmotic abnormality leading to death as observed in the β2-subunit-knockout mice (29). In support of the present observation, mice that fail to synthesize GlcNAc-terminating N-linked oligosaccharides show abnormal growth of neural epithelial cells and suffer a defect in neural tube formation (30, 31), suggesting that the binding of GlcNAc-terminating oligosaccharides to the β1-subunit is requisite for brain development. Further studies are necessary to elucidate the developmental significance of this cellular interaction.
The binding of ligand oligosaccharides to the β1-subunit may affect Na+/K+-ATPase activity, because the inclusion of chitobiose in a Na+/K+-ATPase preparation inhibited its activity. This inhibition suggests that the cellular interaction couples with regulation of the ionic environment. However, in the case of Drosophila melanogaster, Nervana, a protein homologous to the β1-subunit, is a known neural cell antigen and acts independently of the α-subunit-corresponding molecule at the cell surface (32, 33). Therefore, in mice, some of the β1-subunit proteins may remain as single molecules, if such molecules occur at the cell surface, and function in cell adhesion without the regulation of the ionic environment.
Interestingly, the mouse β2-subunit, which shares 52% identity with the β1-subunit and is expressed predominantly in glial cells, has been shown to be an adhesion molecule on glia and to be involved in the interaction between neural and glial cells (34). Recently, this interaction has been shown to be mediated by the binding of N-CAM/L1 molecules on neural cells to oligosaccharides expressed on the β2-subunit on glial cells (27). Furthermore, because the neonatal lethality of a β2-subunit gene deficiency in mice can be restored by the β1-subunit-knock-in method (21), the β1-subunit can take the place of the β2-subunit. Therefore, it is of interest to examine whether the β2-subunit itself has a lectin activity similar to the β1-subunit.
Acknowledgments
We are grateful to Drs. Yoshitaka Nagai, Koichi Suzuki, Masaki Saito, and Akira Kobata for their encouragement. This work was supported by Grants-in-Aid for Scientific Research 10680696, 09240104, and 14580707 (to K.F.) from the Ministry of Education, Science, Culture and Sports of Japan and by the Narishige Zoological Science Award (to K.F.).
Abbreviations: Ag, agalacto; Agn, a-N-acetylglucosamino; As, asialo; N-CAM, neural cell adhesion molecule; PVL, Psathyrella velutina lectin.
References
- 1.Furukawa, K., Takamiya, K., Okada, M., Inoue, M., Fukumoto, S. & Furukawa, K. (2001) Biochim. Biophys. Acta 1525, 1–12.11342247 [Google Scholar]
- 2.Varki, A. (1993) Glycobiology 3, 97–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Helenius, A. & Aebi, M. (2004) Annu. Rev. Biochem. 73, 1019–1049. [DOI] [PubMed] [Google Scholar]
- 4.Chen, Y.-J., Wing, D. R., Guile, G. R., Dwek, R. A., Harvey, D. J. & Zamze, S. (1998) Eur. J. Biochem. 251, 691–703. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu, H., Ochi, K., Ikenaka, K., Mikoshiba, K. & Hase, S. (1993) J. Biochem. (Tokyo) 114, 334–338. [DOI] [PubMed] [Google Scholar]
- 6.Nakamura, N., Yamakawa, N., Sato, T., Tojo, H., Tachi, C. & Furukawa, K. (2001) J. Neurochem. 76, 29–38. [DOI] [PubMed] [Google Scholar]
- 7.Zhou, D. P., Chen, C., Jiang, S. M., Shen, Z. H., Chi, Z. W. & Gu, J. X. (1998) Biochim. Biophys. Acta 1425, 204–208. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura, N., Ikekita, M., Hayakawa, S., Funahashi, H. & Furukawa, K. (2004) J. Neurosci. Res. 175, 384–390. [DOI] [PubMed] [Google Scholar]
- 9.Ray, T. K. (1970) Biochim. Biophys. Acta 196, 1–9. [DOI] [PubMed] [Google Scholar]
- 10.Furukawa, K., Matsuta, K., Takeuchi, F., Kosuge, E., Miyamoto, T. & Kobata, A. (1990) Int. Immunol. 2, 105–112. [DOI] [PubMed] [Google Scholar]
- 11.Sato, T., Furukawa, K., Greenwalt, D. E. & Kobata, A. (1993) J. Biochem. 117, 890–900. [DOI] [PubMed] [Google Scholar]
- 12.Webb, M. R. A. (1992) Proc. Natl. Acad. Sci. USA 89, 4884–4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatanaka, Y., Kempin, U. & Park, J.-J. (2000) J. Org. Chem. 65, 5639–5643. [DOI] [PubMed] [Google Scholar]
- 14.Gloor, S. (1989) Nucleic Acids Res. 17, 10117. [PMC free article] [PubMed] [Google Scholar]
- 15.Hirabayashi, S., Tajima, M., Yao, I., Nishimura, W., Mori, H. & Hata, Y. (2003) Mol. Cell. Biol. 23, 4267–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lecuona, E., Luquin, S., Avila, J., Garcia-Segura, L. M. & Martin-Vasallo, P. (1996) Brain Res. Bull. 40, 167–174. [DOI] [PubMed] [Google Scholar]
- 17.Kubelka, V., Altmann, F., Kornfeld, G. & Marz, L. (1994) Arch. Biochem. Biophys. 308, 148–157. [DOI] [PubMed] [Google Scholar]
- 18.Ranheim, T. S., Edelman, G. M. & Cunningham, B. A. (1996) Proc. Natl. Acad. Sci. USA 93, 4071–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endo, T., Ohbayashi, H., Kanazawa, K., Kochibe, N. & Kobata, A. (1992) J. Biol. Chem. 267, 707–713. [PubMed] [Google Scholar]
- 20.Chow, D. C. & Forte, J. G. (1995) J. Exp. Biol. 198, 1–17. [DOI] [PubMed] [Google Scholar]
- 21.Weber, P., Bartsch, U., Schachner, M. & Montag, D. (1998) J. Neurosci. 18, 9192–9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eakle, K. A., Kabalin, M. A., Wang, S. G. & Farley, R. A. (1994) J. Biol. Chem. 269, 6550–6557. [PubMed] [Google Scholar]
- 23.Geering, K., Beggah, A., Good, P., Girardet, S. Roy, S., Schaer, D. & Jaunin, P. (1996) J. Cell Biol. 133, 1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonough, A. A., Geering, K. & Farley, R. A. (1990) FASEB J. 4, 1598–1605. [DOI] [PubMed] [Google Scholar]
- 25.Bowen, A. R., Chen-Wu, J. L., Momany, M., Young, R., Szaniszlo, P. J. & Robbins, P. W. (1992) Proc. Natl. Acad. Sci. USA 89, 519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagahashi, S., Sudoh, M., Ono, N., Sawada, R., Yamaguchi, E., Uchida, Y., Mio, T., Takagi, M., Arisawa, K. & Yamada-Okabe, H. (1995) J. Biol. Chem. 270, 13961–13967. [DOI] [PubMed] [Google Scholar]
- 27.Heller, M., von der Ohe, M., Kleene, R., Mohajeri, M. H. & Schachner, M. (2003) J. Neurochem. 84, 557–565. [DOI] [PubMed] [Google Scholar]
- 28.Kleene, R. & Schachner, M. (2004) Nature Rev. Neurosci. 5, 195–208. [DOI] [PubMed] [Google Scholar]
- 29.Magyar, J. P., Bartsch, U., Wang, Z.-Q., Howells, N., Aguzzi, A., Wagner, E. F. & Schachner, M. (1994) J. Cell Biol. 127, 835–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ioffe, E. & Stanley, P. (1994) Proc. Natl. Acad. Sci. USA 91, 728–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metzler, M., Gertz, A., Sarkar, M., Schachter, H., Schrader, J. W. & Marth, J. D. (1994) EMBO J. 13, 2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun, B. & Alvaterra, S. P. M. (1995) Proc. Natl. Acad. Sci. USA 92, 5396–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeyasu, K., Okamura, H., Yasuhara, J. C., Ogita, Y. & Yoshimura, S. H. (2001) Cell. Mol. Biol. 47, 325–333. [PubMed] [Google Scholar]
- 34.Gloor, S., Antonicek, H., Sweadner, K. J., Pagliusi, S., Frank, R., Moos, M. & Schachner, M. (1990) J. Cell Biol. 110, 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]