Abstract
A positive correlation between telomere length and mitochondrial DNA (mtDNA) copy number has previously been observed in healthy individuals, and in patients with psychiatric disorders. In the present study, telomere length and mtDNA copy number were evaluated in gastric cancer (GC) tissue samples. DNA was extracted from 109 GC samples (including 82 intestinal, and 27 diffuse cases), and the telomere length and mtDNA copy number were analyzed using a quantitative-polymerase chain reaction assay. The relative telomere length and mtDNA copy number in tumor tissue, as compared with in normal tissue, (mean ± standard deviation) in all GC samples were 11.48±1.14 and 14.86±1.35, respectively. Telomere length and mtDNA copy number were not identified as exhibiting clinical or prognostic value for GC. However, positive correlations between telomere length and mitochondrial DNA copy number were identified in GC (r=0.408, P<0.001) and in the adjacent normal mucosa (r=0.363; P<0.001). When stratified by Lauren classification, the correlation was identified in intestinal type GC samples (r=0.461; P<0.001), but not in diffuse type GC samples (r=0.225; P=0.260). This result indicated that loss of the correlation of telomeres and mitochondrial function may induce the initiation or progression of GC pathogenesis.
Keywords: gastric cancer, mitochondria DNA copy number, telomere, intestinal type, diffuse type
Introduction
Gastric cancer (GC) is highly prevalent in Asia, and is the one of the leading causes of mortality, following lung cancer and liver cancer, in Korea (1). Its development has been revealed to be a multi-step process, ranging from chronic gastritis to atrophy, intestinal metaplasia, dysplasia and, finally, invasive cancer (2). The major histological type of GCs are adenocarcinomas, which are subdivided into intestinal type, diffuse type and mixed/unclassifiable type by Lauren classification (3). Previous studies have demonstrated that intestinal and diffuse types of GC evolve via distinct genetic pathways (3–6).
Telomeres, which contain TTAGGG repeat sequences in humans, are nucleoprotein complexes that cap each end of a eukaryotic chromosome (7). Mitochondrial DNA (mtDNA) differs from nuclear DNA, and multiple copies of mtDNA are present in each mitochondrion (8). Previous studies have demonstrated that telomere length (TL) and the mtDNA copy number (mtCN) are associated with numerous diseases, particularly specific types of cancer (9–12). In a number of examined cancer types, TL and mtCN changes were significantly associated with clinical and prognostic characteristics, suggesting an early and important effect on carcinogenesis (10–12). Previous studies have demonstrated a sequential accumulation of mitochondrial genetic changes during the progression from chronic gastritis to cancer via intestinal metaplasia and dysplasia (11–14).
Recent studies have indicated that TL and mtCN are positively correlated in healthy individuals and in pregnant females (15–17). Genetic changes in the telomeres and mtDNA independently serve important roles in cellular senescence (18). mtDNA damage caused by cellular senescence may contribute to the production of reactive oxygen species (ROS), resulting in telomere shortening (18). Past reviews have emphasized the importance of the telomere-p53-mitochondrion axis for cancer, suggesting that this may be targeted in future cancer therapy (19,20). Our recent studies (21,22) have also investigated these genetic changes in GC; however, their association has yet to be studied in cancer tissue samples.
In the present study, TL and mtCN were evaluated in GC, including 27 diffuse and 82 intestinal type tissue samples. The results of the present study may aid improvements in the current understanding of GC, through identifying the role of mtDNA and TL in GC pathogenesis.
Materials and methods
Patients and DNA extraction
Tissue samples from a total of 109 patients (57.97±11.74 years old; 82 male and 27 female patients), who underwent gastrectomy to treat gastric adenocarcinoma between October 1999 and December 2001, were selected from an archive of paraffin blocks at Keimyung University Dongsan Hospital (Daegu, Korea). Two experienced pathologists reviewed all cases, and the tumor and adjacent normal mucosa tissue areas were defined according to hematoxylin and eosin stained sections. Tissue sections were deparaffinized with 500 µl 100% xylene, and left in a 65°C water bath for 15 min. To remove the residual xylene, the samples were washed five times with ethanol. The selected areas from the paraffin-embedded tissues then underwent DNA extraction. DNA was isolated using an Absolute™ DNA Extraction kit (BioSewoom, Inc., Seoul, Korea), according to the manufacturer's protocol. DNA quantity and quality were measured using NanoDrop 1000 (Thermo Fisher Scientific, Inc., Pittsburgh, PA, USA).
Determination of TL and mtCN
TL and mtCN were analyzed by quantitative-polymerase chain reaction (q-PCR). For the quantitative determination of TL and mtCN (T) relative to β-globin (a control from nucleic DNA, S), primers for the specific amplification of telomeric repeats and cytochrome c oxidase subunit I (COX I; a gene in mtDNA) were selected (Table I). q-PCR was then performed using a LightCycler® 480 II system (Roche Diagnostics GmbH, Mannheim Germany). The PCR conditions were: 95°C denaturation for 1 min; 40 cycles of 95°C for 10 sec; and 60°C for 30 sec. Relative TL and mtCN were determined by calculating T/S values using the following formula: T/S=2−∆Cq, where ∆Cq=(mean Cq telomere or COX I)-(mean Cq β-globin) (23,24). Each measurement was repeated in triplicate; five serially diluted control samples were also included in each experiment.
Table I.
Primers used in this study.
| Primer | Sequence |
|---|---|
| Telomere | |
| Forward | 5′-CGGTTTGTTTGGGTTTGGGTTTGGGTTTGGGTTTGGGTT-3′ |
| Reverse | 5′-GGCTTGCCTTACCCTTACCCTTACCC-TTACCCTTACCCT-3′ |
| COX I | |
| Forward | 5′-TTCGCCGACCGTTGACTATTCTCT-3′ |
| Reverse | 5′-AAGATTATTACAAATGCATGGGC-3′ |
| β-globin | |
| Forward | 5′-TGTGCTGGCCCATCACTTTG-3′ |
| Reverse | 5′-ACCAGCCA-CCACTTTCTGATAGG-3′ |
Statistical analysis
The SPSS statistical package version 19.0 for Windows (IBM SPSS, Armonk, NY, USA) was used for all statistical analyses. TL and mtCN are presented as the mean ± standard deviation (SD). Pearson correlation coefficients were calculated to evaluate the association between TL and mtCN. To further explore the correlation between these markers and the prognosis of GC, a fold change in the TL or mtCN in tumors (T), compared with that in paired normal tissues (N), was calculated (T/N). Patients were categorized into two subgroups, ‘longer’ and ‘shorter’ for TL, and ‘low’ and ‘high’ for mtCN, according to their median TL and mtCN T/N values. Survival curves, estimated using the Kaplan-Meier method (univariate analysis), were compared with a log-rank test. Overall survival (OS) time was defined as the time from diagnosis to mortality from cancer or other causes. A two-tailed P<0.05 was considered to indicate a statistically significant difference.
Results
TL and mtCN do not significantly differ between intestinal and diffuse GC
TL and mtCN were analyzed in samples obtained from 109 patients with GC (60.0±11.8 years of age) using q-PCR. TL and mtCN T/N values were 11.48±1.14 and 14.86±1.35, respectively (fold change; mean ± SD; Table I). When stratified by Lauren classification, the mtCN was similar in intestinal (14.39±1.61) and diffuse (16.34±2.44) types of GC (P=0.540). TL was shorter in intestinal type samples (10.36±1.27), as compared with in diffuse type samples (15.02±2.45); however, this was not to a statistically significant extent (P=0.074).
TL and mtCN are not associated with clinicopathological characteristics in patients with GC
For the consideration of the association between clinicopathological characteristics and the TL and mtCN in GC, two groups, ‘longer’ and ‘shorter’ for TL, and ‘low’ and ‘high’ for mtCN, were assigned based on the median TL and mtCN T/N fold change of the tissue samples. The clinicopathological characteristics of each group are presented in Table II. A shorter TL was more frequent in intestinal GC (64.6%) compared with in diffuse GC (46.2%), although there was no statistically significant difference (P=0.093). TL was identified to be shorter in females (70.4%) than in males (57.3%); this was also not statistically significant (P=0.229). Additionally, age and other clinicopathological parameters were not determined to have any significant associations with TL and mtCN.
Table II.
Telomere length and mitochondrial DNA copy number in gastric cancer.
| Lauren classification | ||||
|---|---|---|---|---|
| Variables | Total | Intestinal | Diffuse | P-value |
| TL (mean ± SD) | 11.48±1.14 | 10.36±1.27 | 15.02±2.45 | 0.074 |
| MtCN (mean ± SD) | 14.86±1.35 | 14.39±1.61 | 16.34±2.44 | 0.540 |
SD, standard deviation; MtCN, mitochondrial DNA copy number; TL, telomere length.
TL and mtCN are correlated in intestinal, but not diffuse, GC
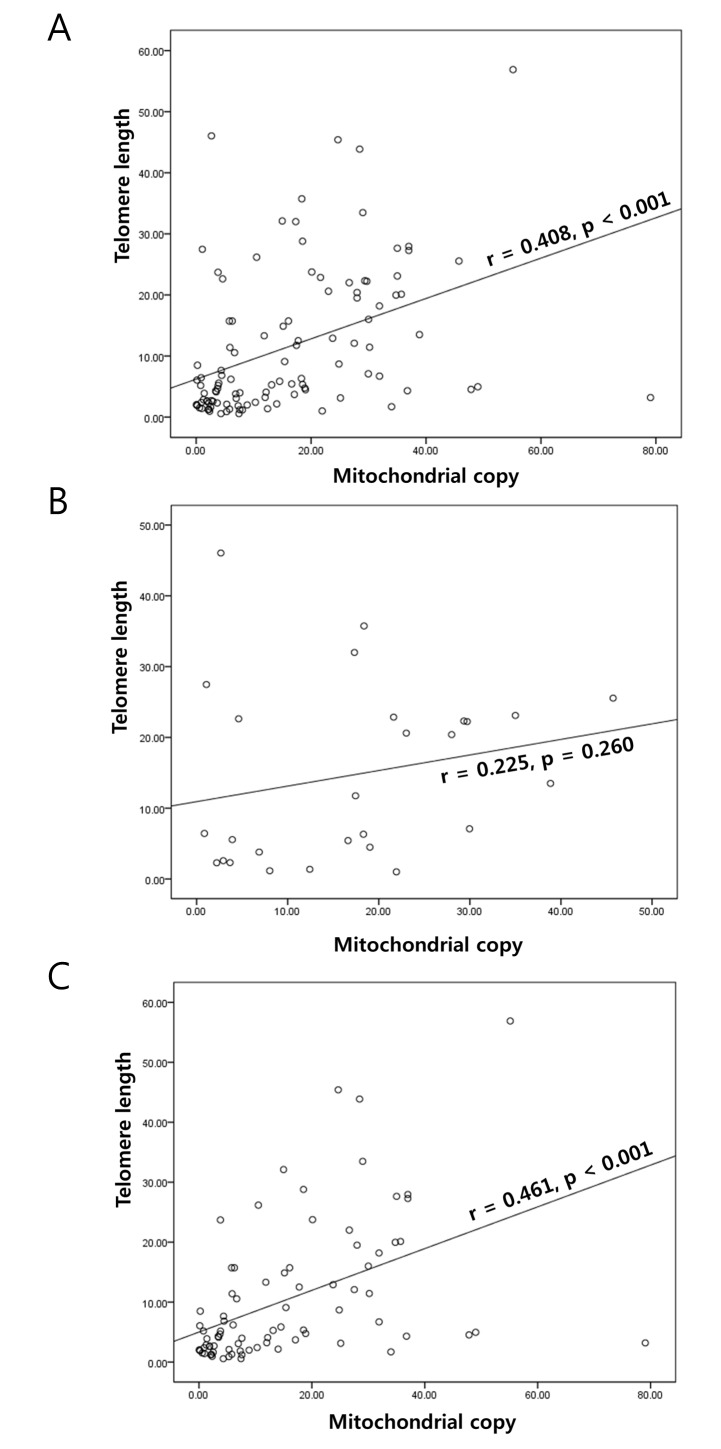
A significant positive correlation in the T/N fold change of TL and mtCN was identified in GC (r=0.408; P<0.001; Fig. 1A). This correlation was also apparent in the adjacent normal mucosa (r=0.363; P<0.001) obtained from the patients with GC. When stratified by Lauren classification, a significant correlation was not identified in diffuse GC (r=0.225; P=0.260; Fig. 1B); however, there was a correlation in the intestinal type samples (r=0.461; P<0.001; Fig. 1C). The stratification did not reveal any significant associations with other parameters (Table III).
Figure 1.
Association between mitochondrial DNA copy number and telomere length. (A) Telomere length and mitochondrial DNA copy number were positively correlated in gastric cancer. When stratified by Lauren classification, positive correlation was (B) not identified in the diffuse type samples; however, (C) it was identified in the intestinal type samples.
Table III.
Clinicopathological characteristics of TL and mtCN in gastric cancer.
| TL (%, n) | mtCN (%, n) | |||
|---|---|---|---|---|
| Group | Longer | Shorter | Low | High |
| All patients | 39.5 (43) | 60.5 (66) | 69.7 (76) | 30.3 (33) |
| Age | ||||
| <60 | 37.0 (20) | 63.0 (34) | 72.2 (39) | 27.8 (15) |
| ≥60 | 41.8 (23) | 58.2 (32) | 67.3 (37) | 32.7 (18) |
| Gender | ||||
| Male | 42.7 (35) | 57.3 (47) | 68.3 (56) | 31.7 (26) |
| Female | 29.6 (8) | 70.4 (19) | 74.1 (20) | 25.9 (7) |
| pT stage | ||||
| 1/2 | 42.3 (30) | 57.7 (41) | 68.1 (49) | 31.9 (23) |
| 3/4 | 34.2 (13) | 65.8 (25) | 73.0 (27) | 27.0 (10) |
| pN stage | ||||
| 0/1 | 40.3 (27) | 59.7 (40) | 68.2 (60) | 31.8 (28) |
| 2/3 | 38.1 (16) | 61.9 (26) | 76.2 (16) | 83.8 (5) |
| Lauren classification | ||||
| Diffuse | 53.8 (14) | 46.2 (12) | 65.4 (17) | 34.6 (9) |
| Intestinal | 35.4 (29) | 64.6 (53) | 70.7 (58) | 29.3 (24) |
| Depth of invasion | ||||
| Early | 39.1 (18) | 60.9 (28) | 71.7 (33) | 28.3 (13) |
| Advanced | 39.7 (25) | 60.3 (38) | 68.3 (43) | 31.7 (20) |
mtCN, mitochondrial DNA copy number; TL, telomere length; pT, primary tumor; pN, regional lymph nodes.
TL and mtCN are not associated with the rate of OS
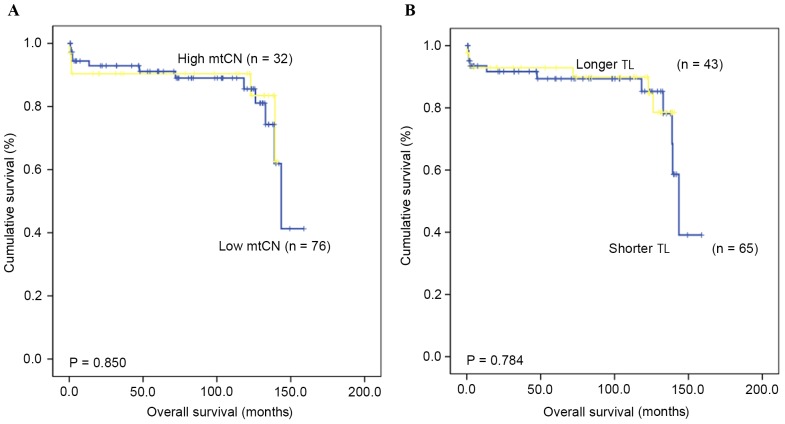
The OS time and rate for patients with GC were assessed in order to identify if there was a prognostic value for TL and mtCN. The median follow-up duration was 82.2 months (range, 3.7–158.8 months), and the 5-year OS rate was 79.3%. The results indicated no prognostic value of the mtCN (P=0.850) and TL (P=0.784) in GC, based on comparing those patients with ‘high’ and ‘low’ mtCN and TL values (Fig. 2).
Figure 2.
Kaplan-Meier plots depicting an overall survival rate analysis of gastric cancer according to (A) mtCN and (B) TL. No significant difference was identified. mtCN, mitochondrial DNA copy number; TL, telomere length.
Discussion
The present study identified that TL is positively correlated with mtCN in GC tissues and paired normal tissues; this was consistent with the results of previous studies conducted on healthy volunteers (15–17). A correlation between TL and mtCN was initially identified in a study of 129 healthy elderly females (15); in a further study, it was also demonstrated in healthy adults and pregnant females (16,17). Furthermore, certain neuropsychiatric conditions were also associated with TL shortening and mtCN increase, although detailed data concerning their association were not presented (25). Taken together, there is a positive correlation between TL alteration and mtCN not only in healthy individuals, but also in cancer tissues.
The clinicopathological significance of TL and mitochondrial genetic change has been previously studied in GC by a number of groups (11,12,14,21,22). TL was previously demonstrated to differ in GC according to Helicobacter pylori infection status, microsatellite instability (MSI), and non-steroidal anti-inflammatory drug use (26–29). Although TL change was identified in gastric carcinogenesis, it was not associated with clinicopathological features or prognosis in the present study. However, Pascua et al (27) reported that a combination of TL with MSI status has prognostic value. Mitochondrial genetic change has been frequently studied in GC, and our previous study (30) identified mtMSI to be a novel genetic marker for GC susceptibility. It was demonstrated that mtMSI was present in 10.2% of GC samples and 12.5% of gastric dysplasia samples, and that mtMSI was associated with a poor prognosis and an increased potential for progression.
In the present study, TL was marginally shorter in intestinal type GC, as compared with in diffuse type GC, whereas TL and mtCN did not have any significant association with clinical characteristics. However, a notable association was identified in GC tissues; in accordance with previous studies on healthy individuals (15–17,25), mtCN and TL were identified to be correlated with each other in normal and cancerous tissues obtained from patients with GC. A positive association was also identified in intestinal type GC, but not in the diffuse type. These results suggest that the normal positive correlation between telomeres and mitochondrial function is disrupted during the carcinogenesis of diffuse type GC, inducing TL change. Therefore, loss of the co-regulation of telomere length and mitochondrial copy number may serve a pivotal initiating role in gastric carcinogenesis. However, the survival analysis from the present study did not observe that this affected the clinical characteristics and further progression of GC.
Mitochondria and telomeres are considered to be key instigators of natural ageing (18). Mitochondrial dysfunction or genetic change during aging may be associated with the aging process through increased ROS production and decreased adenosine triphosphate generation (18). A previous study demonstrated that mitochondrial biogenesis and energy production were decreased in telomerase-deficient mice with severe telomere dysfunction (17). It has been hypothesized that telomere change influences not only oxidative defense mechanisms but also mitochondrial functions, including biogenesis and metabolism, in transcriptomic, molecular, genetic and functional analyses of various cells and organs, including proliferative and post-mitotic tissues (16,17). Therefore, this telomere-mitochondria axis may explain how shortened telomeres can cause mitochondrial change. The results of the present study support this hypothesis, suggesting that deregulation of the telomere-mitochondria axis, as caused by aging or other physiological factors, triggers the carcinogenesis of diffuse type GC.
In summary, TL and mtCN are correlated in normal and cancerous stomach tissues. Additionally, abnormal regulation of the telomere-mitochondria axis was identified in diffuse type GC, although the mechanism underlying this process remains unclear. Therefore, a change in the regulation of mitochondria and telomeres may be essential for diffuse GC carcinogenesis, suggesting that this signaling pathway could be targeted for cancer prevention. Furthermore, this result improves current understanding of how telomere change may contribute not only to age-associated disorders, but also to tumorigenesis.
Acknowledgements
The present study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2014R1A6A3A04058057) and by the Korean Government (grant no. 2014R1A5A2010008).
References
- 1.Jung KW, Won YJ, Oh CM, Kong HJ, Cho H, Lee JK, Lee DH, Lee KH. Prediction of cancer incidence and mortality in Korea, 2016. Cancer Res Treat. 2016;48:451–457. doi: 10.4143/crt.2016.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa P, Shiao YH. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994;54(7 Suppl):1941s–1943s. [PubMed] [Google Scholar]
- 3.Grabsch HI, Tan P. Gastric cancer pathology and underlying molecular mechanisms. Dig Surg. 2013;30:150–158. doi: 10.1159/000350876. [DOI] [PubMed] [Google Scholar]
- 4.Yakirevich E, Resnick MB. Pathology of gastric cancer and its precursor lesions. Gastroenterol Clin North Am. 2013;42:261–284. doi: 10.1016/j.gtc.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Akhavan-Niaki H, Samadani AA. Molecular insight in gastric cancer induction: An overview of cancer stemness genes. Cell Biochem Biophy. 2014;68:463–473. doi: 10.1007/s12013-013-9749-7. [DOI] [PubMed] [Google Scholar]
- 6.Carcas LP. Gastric cancer review. J Carcinog. 2014;13:14. doi: 10.4103/1477-3163.146506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 8.Anderson S, Bankier AT, Barrell BG, De Bruijn MH, Coulson AR, Drouin J, Eperon IC, Nierlich DP, Roe BA, Sanger F, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 9.Blackburn EH, Greider CW, Szostak JW. Telomeres and telomerase: The path from maize, Tetrahymena and yeast to human cancer and aging. Nat Med. 2006;12:1133–1138. doi: 10.1038/nm1006-1133. [DOI] [PubMed] [Google Scholar]
- 10.Blasco MA. Telomeres and human disease: Ageing, cancer and beyond. Nat Rev Genet. 2005;6:611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 11.Cui H, Huang P, Wang Z, Zhang Y, Zhang Z, Xu W, Wang X, Han Y, Guo X. Association of decreased mitochondrial DNA content with the progression of colorectal cancer. BMC Cancer. 2013;13:110. doi: 10.1186/1471-2407-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HC, Li SH, Lin JC, Wu CC, Yeh DC, Wei YH. Somatic mutations in the D-loop and decrease in the copy number of mitochondrial DNA in human hepatocellular carcinoma. Mut Res. 2004;547:71–78. doi: 10.1016/j.mrfmmm.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Liao LM, Baccarelli A, Shu XO, Gao YT, Ji BT, Yang G, Li HL, Hoxha M, Dioni L, Rothman N, et al. Mitochondrial DNA copy number and risk of gastric cancer: A report from the Shanghai Women's Health Study. Cancer Epidemiol Biomarkers Prev. 2011;20:1944–1949. doi: 10.1158/1055-9965.EPI-11-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, Luo S, Hong WK, Spitz MR. Telomere dysfunction: A potential cancer predisposition factor. J Natl Cancer Inst. 2003;95:1211–1218. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Kim HK, Ko JH, Bang H, Lee DC. The relationship between leukocyte mitochondrial DNA copy number and telomere length in community-dwelling elderly women. PloS one. 2013;8:e67227. doi: 10.1371/journal.pone.0067227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu C, Enquobahrie DA, Gelaye B, Hevner K, Williams MA. The association between leukocyte telomere length and mitochondrial DNA copy number in pregnant women: A pilot study. Clin Lab. 2015;61:363–369. doi: 10.7754/Clin.Lab.2014.140313. [DOI] [PubMed] [Google Scholar]
- 17.Tyrka AR, Carpenter LL, Kao HT, Porton B, Philip NS, Ridout SJ, Ridout KK, Price LH. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp Gerontol. 2015;66:17–20. doi: 10.1016/j.exger.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Passos JF, Saretzki G, von Zglinicki T. DNA damage in telomeres and mitochondria during cellular senescence: Is there a connection? Nucleic Acids Res. 2007;35:7505–7513. doi: 10.1093/nar/gkm893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J, Hwang SS, Liesa M, Gan B, Sahin E, Jaskelioff M, Ding Z, Ying H, Boutin AT, Zhang H, et al. Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell. 2012;148:651–663. doi: 10.1016/j.cell.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sahin E, Colla S, Liesa M, Moslehi J, Müller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee H, Lee JH, Kim DC, Hwang I, Kang YN, Gwon GJ, Choi IJ, Kim S. Is mitochondrial DNA copy number associated with clinical characteristics and prognosis in gastric cancer? Asian Pac J Cancer Prev. 2015;16:87–90. doi: 10.7314/APJCP.2015.16.1.87. [DOI] [PubMed] [Google Scholar]
- 22.Lee H, Jin JD, La BM, Park WJ, Choi IJ, Lee JH. TERT promoter mutation, telomere length and TERT expression in gastric cancer. Int J Clin Exp Pathol. 2016;9:1758–1763. [Google Scholar]
- 23.Gil ME, Coetzer TL. Real-time quantitative PCR of telomere length. Mol Biotechnol. 2004;27:169–172. doi: 10.1385/MB:27:2:169. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Hwang I, Kang YN, Choi IJ, Kim DK. Genetic characteristics of mitochondrial DNA was associated with colorectal carcinogenesis and its prognosis. PloS One. 2015;10:e0118612. doi: 10.1371/journal.pone.0118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyrka AR, Parade SH, Price LH, Kao HT, Porton B, Philip NS, Welch ES, Carpenter LL. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiatry. 2016;79:78–86. doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aslan R, Bektas A, Bedir A, Alacam H, Aslan MS, Nar R, Yildirim B, Goren I, Ecemis O, Ustaoglu M, et al. Helicobacter pylori eradication increases telomere length in gastric mucosa. Hepatogastroenterology. 2013;60:601–604. doi: 10.5754/hge12691. [DOI] [PubMed] [Google Scholar]
- 27.Pascua I, Fernández-Marcelo T, Sánchez-Pernaute A, De Juan C, Head J, Torres-García AJ, Iniesta P. Prognostic value of telomere function in gastric cancers with and without microsatellite instability. Eur J Gastroenterol Hepatol. 2015;27:162–169. doi: 10.1097/MEG.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 28.Tahara T, Shibata T, Kawamura T, Ishizuka T, Okubo M, Nagasaka M, Nakagawa Y, Arisawa T, Ohmiya N, Hirata I. Telomere length in non-neoplastic gastric mucosa and its relationship to H. pylori infection, degree of gastritis and NSAID use. Clin Exp Med. 2016;16:65–71. doi: 10.1007/s10238-014-0335-0. [DOI] [PubMed] [Google Scholar]
- 29.Tahara T, Shibata T, Okubo M, Kawamura T, Horiguchi N, Ishizuka T, Nakani N, Nagasaka M, Nakagawa Y, Ohmiya N. Demonstration of potential link between helicobacter pylori related promoter CpG island methylation and telomere shortening in human gastric mucosa. Oncotarget. 2016;7:43989–43996. doi: 10.18632/oncotarget.9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeong CW, Lee JH, Sohn SS, Ryu SW, Kim DK. Mitochondrial microsatellite instability in gastric cancer and gastric epithelial dysplasia as a precancerous lesion. Cancer Epidemiol. 2010;34:323–327. doi: 10.1016/j.canep.2010.03.015. [DOI] [PubMed] [Google Scholar]