Abstract
Multiple myeloma (MM) is a malignancy characterized by plasma cell hyperplasia. The majority of patients with MM suffer from mortality due to tumor recurrence and metastasis, which has become an emerging clinical problem. Transforming growth factor β1 (TGFβ1) has been implicated in tumor metastasis; however, its role in RPMI 8226 cells remains to be elucidated. In the present study, RPMI 8226 cells were treated with various concentrations of SB431542, a TGFβ1 inhibitor, for 12, 24 and 48 h. RPMI 8226 cells were transfected with lentiviral-TGFβ1 vectors to overexpress TGFβ1. Cell proliferation rate was subsequently determined by cell-counting kit-8 assay and cell invasion was assessed by Transwell assay. Expression of TGFβ1, SMAD family member 2 (Smad2) and matrix metallopeptidase 3 (MMP3) were analyzed by western blotting. The results demonstrated that cell proliferation and invasion of RPMI 8226 cells was significantly inhibited by SB431542 (P<0.05). SB431542 was able to significantly downregulate the expression of TGFβ1, phosphorylated (p)-Smad2 and MMP3; however, the overexpression of TGFβ1 significantly upregulated the expression of TGFβ1, p-Smad2 and MMP3. In conclusion, SB431542 reduced cell invasion in RPMI 8226 cells, and this effect may be mediated via the TGFβ1/Smad2/MMP3 signaling pathway.
Keywords: multiple myeloma, transforming growth factor β1, SMAD family member 2, matrix metallopeptidase 3, cell invasion
Introduction
Multiple myeloma (MM) is a malignancy, which is characterized by plasma cell hyperplasia in the bone marrow and strain integrity of monoclonal immunoglobulin (IgG, IgA, IgD or IgE) or Bence Jones protein (free monoclonal light chains κ or γ) overexpression (1). Despite active chemotherapy regimes and autologous stem cell transplantation, the majority of MM cases result in mortalities due to tumor recurrence and metastasis, with the incidence and mortality rate on the increase year by year (2). Invasion is a prerequisite of metastasis, which is a leading cause of mortality from tumors and MM (3,4). Therefore, inhibiting cell invasion may benefit patients who have relapsed or have metastatic MM. However, the precise molecular mechanisms of cell invasion in MM remain to be elucidated.
Transforming growth factor β1 (TGFβ1) is ubiquitously expressed. TGFβ1 has been reported to stimulate the production of angiogenic factors and has a critical role in the development and progression of MM (5). In a previous study of aggressive myeloma, cells secreting TGFβ1 induced tumor epithelial-mesenchymal transition (EMT) and promoted tumor metastasis (6). SMAD family member 2 (Smad2), which mediates TGFβ1 signaling, has also been reported to be involved in the migration of tumor cells (7).
The role of matrix metalloproteinases (MMPs) in tumor cell metastasis has been well studied, and they been demonstrated to degrade the basement membrane and extracellular matrix (ECM), which is associated with tumor invasion and metastasis (8). MMP3, a member of the family of matrix metalloproteinases, promotes tumor cell invasion and metastasis along the basement membrane via degradation of the ECM (9). It has also been reported that TGFβ1 stimulates the expression of MMP3 in human corneal epithelial cells (10); however, to the best of our knowledge, this has not been documented in MM cells.
In the present study, the role of SB431542 in the inhibition of cell invasion in multiple myeloma cells and the mechanism of this process were investigated. Furthermore, this present study provided evidence for the involvement of the TGFβ1/Smad2/MMP3 signaling pathway in the SB431542-induced reduction in cell invasion in multiple myeloma RPMI 8226 cells.
Materials and methods
Cell culture and reagents
RPMI 8226 cells were purchased from American Type Culture Collection (Manassas, VA, USA). Cells were cultured with RPMI 1640 medium with 15% fetal bovine serum (both Hyclone; GE Healthcare Life Sciences, Logan, USA), in a humidified incubator at 37°C with 5% CO2 for 2 to 3 days. Cells in the logarithmic growth phase were used for subsequent experiments. The primary antibodies anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; cat. no. sc-47724; dilution, 1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), anti-TGFβ1 (cat. no. sc-146; dilution, 1:500; Santa Cruz Biotechnology, Inc.), anti-smad2 (cat. no. 12,052), anti-p-smad2 (cat. no. 11,958) and anti-MMP3 (cat. no. 14351S) (both dilution, 1:1,000) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). The bovine anti-goat IgG horseradish peroxidase-conjugated secondary antibody (cat. no. sc-2352; dilution, 1:1,000) was purchased from Santa Cruz Biotechnology, Inc.
Cell counting kit-8 (CCK-8) cell proliferation assay
RPMI 8226 cells (1×104/100 µl) were seeded in a 96-well plate for 24 h and subsequently incubated at 37°C in the presence and absence of SB431542 (1.0, 10, 100 and 1,000 nmol/ml; Med Chem Express Co., Monmouth Junction, NJ, USA) for 12, 24 and 48 h. CCK-8 solution (10%; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added, and the plate was incubated at 37°C for 3 h. The optical density (OD) of each well was measured at 450 nm using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The OD values were calculated based on the formula: Proliferation inhibition rate=(1-mean OD experimental value/mean OD control value)x100%.
Cell motility assay
Cell invasion was assessed by Transwell assay (pore size, 8 µm; Corning Inc., Corning, NY, USA). In brief, cells (1×104/well) were treated with SB431542 or lentiviral-TGFβ1 vectors and the untreated cells (6×104) were seeded in the upper chamber. The cells were attached to porous polycarbonate membranes, which were coated with Matrigel basement membrane matrix. The lower chamber was filled with RPMI 1640 medium with 15% fetal bovine serum. Cells that failed to attach to polycarbonate membranes were removed by washing with PBS following 30 h incubation at 37°C, whilst invaded cells were fixed with 4% paraformaldehyde for 30 min and subsequently stained with crystal violet dissolved in 1% SDS for 30 min at 20°C. The number of cells in 5 randomly selected visual fields at ×200 magnification under a Olympus CX23 light microscope (Olympus Corporation, Tokyo, Japan) was recorded.
Western blot analysis
Cell lysis buffer [20 mmol/l Tris (pH 7.5), 150 mmol/l NaCl, 1% Triton X-100, protease and phosphate inhibitors (0.7 µg/ml) was added to the cells, which were incubated on ice for 30 min. The cells were subsequently centrifuged at 6,720 × g at 4°C for 12 min, and the supernatants were obtained. The concentration of the total protein was measured using the BCA protein assay (Thermo Fisher Scientific, Inc., Waltham, MA, USA), and protein was heated to 100°C with 4 µl loading buffer for 8 min. Protein (40 µg) was subsequently separated using SDS-PAGE (8% gel) and transferred to polyvinylidene difluoride membranes. The membranes were blocked with 5% fat-free milk or 5% bovine serum albumin (Beyotime Institute of Biotechnology, Haimen, China) for 2 h. The membranes were subsequently incubated with the previously mentioned primary antibodies overnight at 4°C and secondary antibodies for 2 h at room temperature. The blots were visualized using the Millipore-Immobilon ECL kit (EMD Millipore, Billerica, MA, USA) and the blots were quantified with Quantity One software (version 4.62; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Preparation and transfection of the lentiviral-TGFβ1 vector
Double digests of the PLVX-IRES-mcherry plasmid (Laboratory of Neuron Biotechnology Company, Shanghai, China) and vector (GV287), containing the TGFβ1 gene, with AgeI (5 U/µl) and EcoRI (20 U/µl) (both Beyotime Institute of Biotechnology) were performed. The product was recovered, purified and mixed with 1 µl T4 DNA ligase at 16°C for 6 h. The transformation of competent DH5α cells (Takara Bio, Inc., Otsu, Japan) was performed. Briefly, the plasmids 1 ng (5 ul) were cooled in ice for 2 min and 100 µl of competent cells were added to the plasmids. The cells were kept in an ice bath for 5 min and then plated onto agar plates containing X-Gal, IPTG and ampicillin. White colonies were selected for. Recombinant positive clones were identified by polymerase chain reaction and restriction analysis. The PCR thermocycling conditions used were as follows: Denaturation step, 95°C for 5 min; annealing step, 55°C for 30 sec; elongation step, 72°C for 1 min for 35 cycles; 72°C for 10 min; and held at 4°C. A Taq DNA polymerase kit (Takara Bio, Inc.) was used for PCR. Primer sequences were as follows: TGFβ1 forward, 5′-ATAAGCTTGATATCGAATTCCACAGAGCCTTCTCGG−3′ and reverse, 5′-GAGGATCCCCGGGTACCGGTCGCCACCATGGCGCTCTTCGTGCGGC-3′. Plasmids were sent to Biotechnology Co. Ltd. (Shanghai, China) for sequencing. RPMI 8226 cells, in the logarithmic growth phase, were seeded in 6-well plates (2×105 cells/ml) and cultured for 24 h. The lentiviral-TGFβ1 vector was added with a multiplicity of infection value of 80 to RPMI 8226 cells. RPMI 8226 cells were also transfected with GV287 vectors. Non-transfected RPMI 8226 cells served as negative controls. Transfection efficiency was detected by fluorescence microscopy and western blotting at 6 days post-transfection.
Statistical analysis
All experiments were independently performed in triplicate. Student's t-test was performed to compare between two independent sample groups. All statistical analyses were performed using GraphPad Prism software (version 5.01; GraphPad Software, Inc., La Jolla, CA, USA). One-way analysis of variance (ANOVA) was used in conjunction with Fisher's least significant difference post-hoc test to analyze the difference between the concentration groups, and one-way ANOVA with Tukey's test was performed to compare the differences among different treatment groups. P<0.05 was considered to indicate a statistically significant difference.
Results
SB431542 reduces cell proliferation and invasion in RPMI 8226 cells
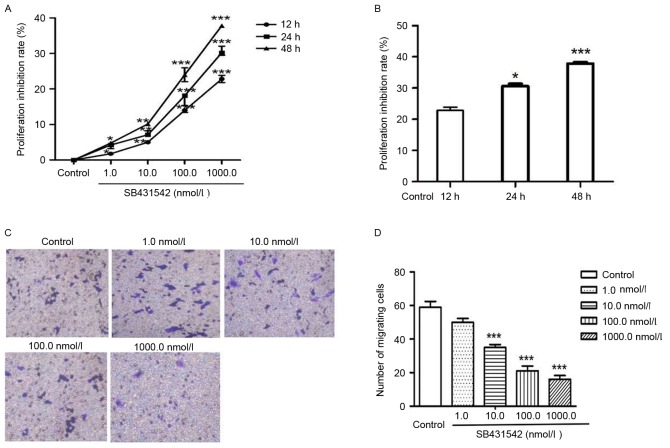
To investigate the function of SB431542 on cell proliferation and invasion, RPMI 8226 cells were treated with 1, 10, 100 and 1,000 nmol/ml SB431542 (Fig. 1A). A significant dose-dependent decrease in cell proliferation was observed between 1 and 1,000 nmol/l SB431542. The cells were subsequently treated with 1,000 nmol/ml SB431542 for 12, 24 and 48 h (Fig. 1B). Cell proliferation was also inhibited by SB431542 in a dose- and time-dependent manner. Transwell assay was performed to assess cell invasion (Fig. 1C). The number of migrating cells in the control and SB431542 treatment groups (1, 10, 100 and 1,000 nmol/ml) was 59±6, 50±4, 35±3, 21±5 and 16±4, respectively (Fig. 1D). A significant difference was observed between the control and the 10, 100 and 1,000 nmol/ml SB431542 treatment groups (P<0.05). No significant difference was observed between the control and the lowest SB431542 dosage group (1 nmol/ml).
Figure 1.
(A) RPMI 8226 cells were treated with SB431542 (1, 10, 100 and 1,000 nmol/l) for 12, 24 and 48 h. The proliferation inhibition rate was measured using a cell-counting kit-8 assay. (B) The cells were treated with 1,000 nmol/ml SB431542 for 12, 24 and 48 h. The proliferation inhibition rate was measured using a cell-counting kit-8 assay. (C) Cell invasion was measured using a Transwell assay. (D) The number of migrating cells in 10 random fields was counted and the average was calculated. Values are expressed as the mean ± standard deviation of 3 independent experiments. *P<0.05, **P<0.01, ***P<0.001 vs. the control.
SB431542 reduces TGFβ1 accumulation, Smad2 activation and MMP3 expression
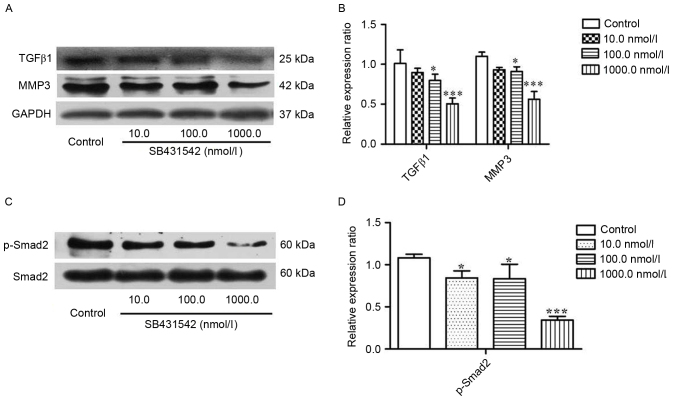
To investigate the effect of SB431542 on the accumulation of TGFβ1 and MMP3 in MM cells, RPMI 8226 cells were treated with 10, 100 and 1,000 nmol/ml SB431542 for 48 h. Western blotting was performed. The results demonstrated that SB431542 significantly decreased the accumulation of TGFβ1 and MMP3 at 100 and 1,000 nmol/ml when compared with the control (Fig. 2A and B). Smad2, as a signaling molecule, can be initially activated by TGFβ1. Therefore, the effect of SB431542 on the expression of Smad2 and phosphorylated (p)-Smad2 in RPMI 8226 cells was investigated. There was no difference in the levels of Smad2 between the different SB431542 treatment groups and the control, but a significant increase in the level of p-Smad2 was demonstrated (Fig. 2C and D), indicating that SB431542 was able to repress the TGFβ1/Smad2 signaling pathway in RPMI 8226 cells.
Figure 2.
RPMI 8226 cells were treated with SB431542 (10, 100 and 1,000 nmol/l) for 48 h. (A) Representative image and (B) quantification of expression of TGFβ1, MMP3 and GAPDH as determined by western blotting. (C) Representative image and (D) quantification of expression of Smad2 and p-Smad2 as determined by western blotting. Values are expressed as the mean ± standard deviation of 3 independent experiments. *P<0.05, ***P<0.001 vs. the control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MMP3, matrix metallopeptidase 3; p, phosphorylated; Smad2, SMAD family member 2; TGFβ1, transforming growth factor β1.
Increased expression of p-smad2 and MMP3 in RPMI 8226 cells overexpressing TGFβ1
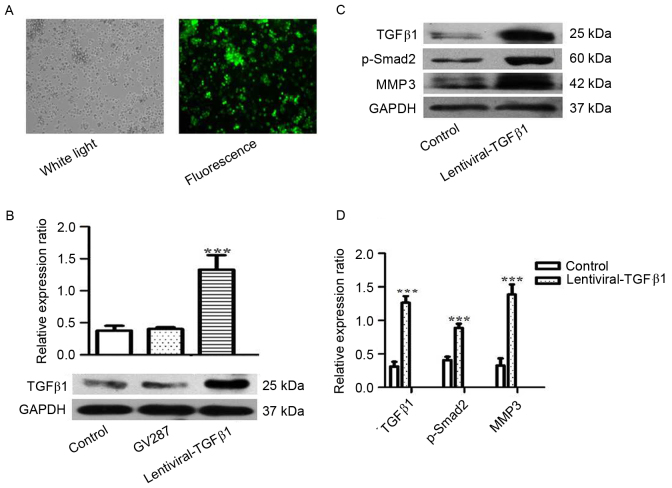
To investigate the role of TGFβ1 in the metastasis of MM, RPMI 8226 cells were treated with lentiviral-TGFβ1 vectors to stably express TGFβ1. Transfection efficiency was observed under fluorescence microscopy and detected by western blotting (Fig. 3A and B). The expression of TGFβ1, p-Smad2 and MMP3 was analyzed, and increased expression of p-smad2 and MMP3 was observed in the cells overexpressing TGFβ1 compared with the control (Fig. 3C and D).
Figure 3.
RPMI 8226 cells were transfected with lentiviral-TGFβ1 vectors and (A) the transfection efficiency was assessed by fluorescence microscopy. (B) The expression of TGFβ1 was assessed by western blotting in RPMI 8226 cells transfected with lentiviral-TGFβ1 vectors, GV287 and non-transfected cells (control). (C) The expression of TGFβ1, p-Smad2 and MMP3 was analyzed by western blotting in RPMI 8226 cells transfected with lentiviral-TGFβ1 vectors and in non-transfected cells (control). (D) The ratios of TGFβ1, p-Smad2 and MMP3 relative to GAPDH were calculated. Values are expressed as the mean ± standard deviation of 3 independent experiments. ***P<0.05 vs. the control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MMP3, matrix metallopeptidase 3; p-Smad2, phosphorylated SMAD family member 2; TGFβ1, transforming growth factor β1.
Cell invasion and MMP3 expression in TGFβ1 overexpressing RPMI 8226 cells is partly inhibited by SB431542
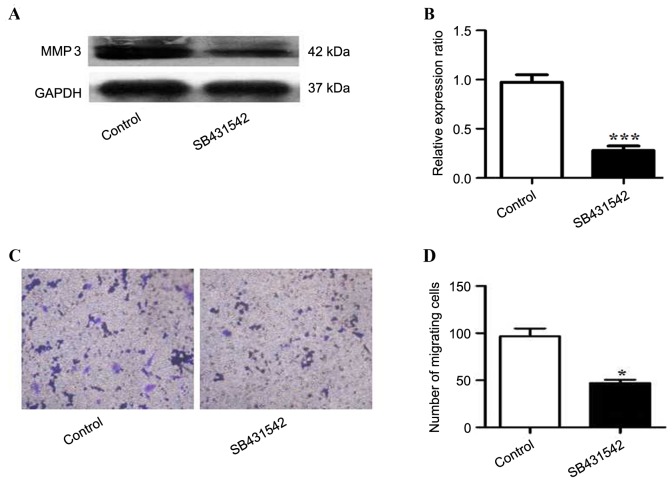
To investigate the role of SB431542 in RPMI 8226 cells overexpressing TGFβ1, the expression of MMP3 in the cells treated with SB431542 was analyzed. A significant decrease in MMP3 expression was observed in SB431542 treated group when compare to the control (Fig 4A and B). Cell invasion was assessed by Transwell assay, and a decrease in the number of migrating cells was observed in the SB431542 treated group (Fig. 4C and D).
Figure 4.
Expression of matrix metallopeptidase 3 in TGFβ1 overexpressing RPMI 8226 cells treated in the presence and absence of SB431542. (A) Western blotting was performed and (B) ratios relative to GAPDH were calculated. (C) Cell invasion was assessed by Transwell assay and (D) the number of migrating cells was counted. Values are expressed as the mean ± standard deviation of 3 independent experiments. *P<0.05, ***P<0.001 vs. the control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MMP3, matrix metallopeptidase 3; TGFβ1, transforming growth factor β1.
Discussion
Human MM is a hematological malignancy, and at present there is limited treatment available (11), with the majority of cases leading to mortality due to metastasis. A requirement of the metastatic process is for cells to acquire invasive capability (12). Therefore, it is a priority to elucidate the mechanism of metastasis in MM. In the present study, the mechanism of metastasis in MM was investigated. It was demonstrated that SB431542 significantly inhibited proliferation and cell invasion in RPMI 8226 cells. Additionally, it was demonstrated that treating RPMI 8226 cells with SB431542 significantly decreased TGFβ1 expression, Smad2 phosphorylation and MMP3 expression. Furthermore, overexpression of TGFβ1 resulted in increased expression of p-Smad2 and MMP3, which could be partly inhibited by SB431542. Taken together, the results demonstrated that SB431542 reduced cell invasion in RPMI 8226 cells via the TGFβ1/Smad2/MMP3 signaling pathway.
SB431542 is a small molecular inhibitor of TGFβ1 receptor, and its role in inducing anti-tumor immunity has been previously reported (13). Matsuyama et al (14) demonstrated that SB431542 exerts an anti-tumor effect primarily by inhibiting the proliferation of human osteosarcoma. Likewise, the present study demonstrated that the proliferation of MM cells was inhibited by SB431542 in a dose- and time-dependent manner. A number of studies have reported that TGFβ1 has growth-stimulating effects on several cell lines, including hepatic stellate cells (15), skin fibroblast (16) and bone marrow-derived mesenchymal stem cells (17). In the present study, TGFβ1 expression was inhibited by SB431542 in RPMI 8226 cells, leading to an increase in cell proliferation.
The role of TGFβ1 in promoting tumor metastasis has also been reported in a number of studies (18–20). Likewise, the results of the present study also demonstrated the role of TGFβ1 in promoting tumor metastasis in RPMI 8226 cells. When the expression of TGFβ1 was inhibited by SB431542, the number of migrating cells decreased; however, the reverse effect was observed following overexpression of TGFβ1. The mechanism of TGFβ1 in regulating cell invasion was also investigated, and this present study demonstrated that MMP3 may be involved and act as a downstream target of TGFβ1/Smad2 signaling.
MMP3 is a member of the MMP family and is a type of proteolytic enzyme involved in the degradation of structural components of the ECM (21), and in the release of precursor forms of growth factors in order to degrade cell-cell and cell-ECM adhesion structure (22). This mechanism contributes to tumor invasion and metastasis. In the present study it was observed that SB431542 was able to significantly decrease MMP3 expression, indicating that SB432542 is able to inhibit cell invasion in RPMI 8226 cells. The role of MMPs in promoting cell invasion and tumor metastasis has been previously reported (23–25), and is associated with EMT. It has also been reported that MMP3 is a target and regulator of EMT (26,27). Furthermore, in the present study, it was demonstrated that overexpression of TGFβ1 increased MMP3 expression, which may be partly inhibited by SB431542. Therefore, it was concluded that MMP3 expression was regulated by TGFβ1, and that MMP3 is downstream of TGFβ1/Smad2 signaling.
In conclusion, overcoming cell invasion and tumor metastasis is a clinical challenge that requires to be addressed and SB431542 may be a potential and valuable drug.
Acknowledgements
The present study was supported by the Medical research project of the Health Bureau of Chongqing (Chongqing, China; grant no. 2012-2-390).
References
- 1.Solomon A, Frangione B, Franklin EC. Bence Jones proteins and light chains of immunoglobulins. Preferential association of the V lambda VI subgroup of human light chains with amyloidosis AL (lambda) J Clin Invest. 1982;70:453–460. doi: 10.1172/JCI110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, Kapoor P, Dingli D, Hayman SR, Leung N, et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–1128. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta GP, Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal R, Ghobrial IM, Roodman GD. Chemokines in multiple myeloma. Exp Hematol. 2006;34:1289–1295. doi: 10.1016/j.exphem.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jurczyszyn A, Czepiel J, Biesiada G, Gdula-Argasińska J, Cibor D, Owczarek D, Perucki W, Skotnicki AB. HGF, sIL-6R and TGF-β1 play a significant role in the progression of multiple myeloma. J Cancer. 2014;5:518–524. doi: 10.7150/jca.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011;9:e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ungefroren H, Groth S, Sebens S, Lehnert H, Gieseler F, Fändrich F. Differential roles of Smad2 and Smad3 in the regulation of TGF-β1-mediated growth inhibition and cell migration in pancreatic ductal adenocarcinoma cells: Control by Rac1. Mol Cancer. 2011;10:67. doi: 10.1186/1476-4598-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 10.Kim HS, Shang T, Chen Z, Pflugfelder SC, Li DQ. TGF-beta1 stimulates production of gelatinase (MMP-9), collagenases (MMP-1, -13) and stromelysins (MMP-3, -10, -11) by human corneal epithelial cells. Exp Eye Res. 2004;79:263–274. doi: 10.1016/j.exer.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth, induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61:3071–3076. [PubMed] [Google Scholar]
- 12.Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15–33. doi: 10.1007/s10555-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka H, Shinto O, Yashiro M, Yamazoe S, Iwauchi T, Muguruma K, Kubo N, Ohira M, Hirakawa K. Transforming growth factor β signaling inhibitor, SB-431542, induces maturation of dendritic cells and enhances anti-tumor activity. Oncol Rep. 2010;24:1637–1643. doi: 10.3892/or_00001028. [DOI] [PubMed] [Google Scholar]
- 14.Matsuyama S, Iwadate M, Kondo M, Saitoh M, Hanyu A, Shimizu K, Aburatani H, Mishima HK, Imamura T, Miyazono K, Miyazawa K. SB-431542 and Gleevec inhibit transforming growth factor-beta-induced proliferation of human osteosarcoma cells. Cancer Res. 2003;63:7791–7798. [PubMed] [Google Scholar]
- 15.He Y, Huang C, Sun X, Long XR, Lv XW, Li J. MicroRNA-146a modulates TGF-beta1-induced hepatic stellate cell proliferation by targeting SMAD4. Cell Signal. 2012;24:1923–1930. doi: 10.1016/j.cellsig.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Li P, Liu P, Xiong R, Zhang E, Chen X, Gu D, Zhao Y, Wang Z, Zhou Y. The essential role for c-Ski in mediating TGF-beta1-induced bi-directional effects on skin fibroblast proliferation through a feedback loop. Biochem J. 2008;409:289–297. doi: 10.1042/BJ20070545. [DOI] [PubMed] [Google Scholar]
- 17.Bergfeld SA, DeClerck YA. Bone marrow-derived mesenchymal stem cells and the tumor microenvironment. Cancer Metastasis Rev. 2010;29:249–261. doi: 10.1007/s10555-010-9222-7. [DOI] [PubMed] [Google Scholar]
- 18.Sawada T, Kimura K, Nishihara T, Onoda N, Teraoka H, Yamashita Y, Yamada N, Yashiro M, Ohira M, Hirakawa K. TGF-beta1 down-regulates ICAM-1 expression and enhances liver metastasis of pancreatic cancer. Adv Med Sci. 2006;51:60–65. [PubMed] [Google Scholar]
- 19.Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, Arteaga CL. Inhibition of TGF-β with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2017;127:1116. doi: 10.1172/JCI93333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacman D, Merkel S, Croner R, Papadopoulos T, Brueckl W, Dimmler A. TGF-beta receptor 2 downregulation in tumour-associated stroma worsens prognosis and high-grade tumours show more tumour-associated macrophages and lower TGF-beta1 expression in colon carcinoma: A retrospective study. BMC Cancer. 2007;7:156. doi: 10.1186/1471-2407-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 23.Casey TM, Eneman J, Crocker A, White J, Tessitore J, Stanley M, Harlow S, Bunn JY, Weaver D, Muss H, Plaut K. Cancer associated fibroblasts stimulated by transforming growth factor beta1 (TGF-beta 1) increase invasion rate of tumor cells: A population study. Breast Cancer Res Treat. 2008;110:39–49. doi: 10.1007/s10549-007-9684-7. [DOI] [PubMed] [Google Scholar]
- 24.Fuxe J, Vincent T, de Herreros A Garcia. Transcriptional crosstalk between TGF-β and stem cell pathways in tumor cell invasion: Role of EMT promoting Smad complexes. Cell Cycle. 2010;9:2363–2374. doi: 10.4161/cc.9.12.12050. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Zhu J, Zuo S, Ma J, Zhang J, Chen G, Wang X, Pan Y, Liu Y, Wang P. 1,25(OH)2D3 attenuates TGF-β1/β2-induced increased migration and invasion via inhibiting epithelial-mesenchymal transition in colon cancer cells. Biochem Biophys Res Commun. 2015;468:130–135. doi: 10.1016/j.bbrc.2015.10.146. [DOI] [PubMed] [Google Scholar]
- 26.Blavier L, Lazaryev A, Shi XH, Dorey FJ, Shackleford GM, DeClerck YA. Stromelysin-1 (MMP-3) is a target and a regulator of Wnt1-induced epithelial-mesenchymal transition (EMT) Cancer Biol Ther. 2010;10:198–208. doi: 10.4161/cbt.10.2.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robichaud N, Del Rincon SV, Huor B, Alain T, Petruccelli LA, Hearnden J, Goncalves C, Grotegut S, Spruck CH, Furic L, et al. Phosphorylation of eIF4E promotes EMT and metastasis via translational control of SNAIL and MMP-3. Oncogene. 2015;34:2032–2042. doi: 10.1038/onc.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]