Abstract
Neurofibromatosis type 1 (NF1) is an inherited neurocutaneous disorder associated with neurodevelopmental disorders including autism spectrum disorder (ASD). The frequency of ASD/NF1 co-occurrence has been subject to debate since the 1980s. This relationship was investigated in a large population-based sample of 8-year-old children identified with ASD (N = 12,271) by the Centers for Disease Control and Prevention’s Autism and Developmental Disabilities Monitoring (ADDM) Network. Twenty-two (1-in-558) children with ASD had diagnosed NF1, exceeding NF1 general population estimates by four to five fold. Children with ASD/NF1 versus ASD without NF1 were significantly less likely to receive a community-based ASD diagnosis (p = 0.04) and understand non-verbal communication (p = 0.001). These findings underscore the importance of including social-communication ability among relevant developmental concerns in children with NF1.
Keywords: Autism, Neurofibromatosis, Developmental disabilities, Children, Non-verbal communications
Introduction
Neurofibromatosis type 1 (NF1) is an autosomal dominant neurocutaneous disorder characterized by café-au-lait spots, neurofibromas, optic nerve pathway gliomas, and distinctive bone abnormalities (National Institutes of Health Consensus Development Conference 1988). NF1 occurs in about 1 in 3000 individuals in the general population (Lammert et al. 2005). NF1 is associated with neurodevelopmental disorders such as learning disabilities, speech and language impairments, attention-deficit/hyperactivity disorder (ADHD), and intellectual disability (ID; Brei et al. 2014; Hyman et al.2006; Hyman et al. 2005; Huijbregts and de Sonneville 2011; Dilts et al. 1996; Ozonoff 1999). The co-occurrence of NF1 and autism spectrum disorder (ASD), referred to hereafter as “ASD/NF1” has been a subject of debate since the 1980s. Interestingly, the mean age of diagnosis for NF1 (4.6 years) and age at which most children with NF1 are identified (8 years) mirror that of ASD (ADDM Network Surveillance Year 2010 Principal Investigators 2014; DeBella et al. 2000; McKeever et al. 2008). Results from previous studies of the association of ASD with NF1 vary significantly. Investigations that focused on the frequency of NF1 in strictly-defined ASD cohorts based on criteria from the Diagnostic and Statistical Manual of Mental Disorders Third Edition (DSM-III) or earlier editions [American Psychiatric Association (APA), 1980] reported NF1 prevalence rates ranging from 0 to 6 % (Gillberg and Forsell 1984; Fombonne et al. 1997; Mouridsen et al. 1992; Williams and Hersh 1998). The spectrum of impairment recognized as ASD has broadened over time and now includes individuals with greater clinical variability (Miller et al. 2013; Volkmar et al. 1988); thus, more individuals with NF1 may now qualify for an ASD diagnosis, and ASD/NF1 prevalence may be higher than previously recognized.
Recent investigations of ASD/NF1 used ASD questionnaires to screen clinical populations or registries of individuals with NF1 for ASD characteristics (Garg et al. 2013; Garg et al. 2013; Huijbregts and de Sonneville 2011; Tinker et al. 2014; Walsh et al. 2013). Tinker et al. (2014) found that the frequency of positive ASD screens among pediatric patients served in a university-based pediatric NF1 clinic were similar to the frequency of positive screens in the general population [0 % on the Modified Checklist for Autism in Toddlers (M-CHAT) and 12.5 % on the Childhood Autism Spectrum Test (CAST)].
However, multiple studies that used the Social Responsiveness Scale (SRS) as a screening tool reported higher than expected frequencies of positive ASD screens among children with NF1 (Garg et al. 2013; Plasschaert et al. 2014; Van Eeghen et al. 2012; Walsh et al. 2013). Van Eeghen et al. (2012) found, in their clinic-based cohort of NF1 patients, the prevalence of children who scored in the clinically severe range (T scores at or above 75) was 18 %. Two other studies, one population-based (Garg et al. 2013) and one clinic-based (Walsh et al. 2013) used similar SRS clinical severity ranges and found 14–30 % of children met screening criteria for ASD. Furthermore, Garg et al. (2013) conducted second-phase, structured, in-person clinical evaluations among a subset of children who screened positive for ASD, and found that the frequency of ASD in that population-based cohort was elevated in comparison to the general population. A similarly structured study by Plasschaert et al. (2014) using the SRS on all participants and a detailed in-person evaluation on a participant subgroup showed that children with NF1 tend to have more social problems than children without NF1, and found an ASD prevalence estimate of 26 % in their outpatient clinic cohort.
We found no reports in public databases that describe both the prevalence and clinical characteristics of children with ASD/NF1 in a population-based sample. Clarifying the strength of the association between ASD and NF1 may inform screening practices in both ASD and NF1 clinical settings. If a positive association is present, it would support the investigation of a potential shared etiology between these disorders. To investigate this relationship, we sought to 1) determine the prevalence of NF1 in a large, population-based sample of 8-year-old children identified with ASD and 2) compare the patterns of ASD diagnostic criteria and related characteristics of children with ASD/NF1 to those with ASD who did not have co-occurring NF1.
Methods
Participants
The Centers for Disease Control and Prevention (CDC) Autism and Developmental Disabilities Monitoring (ADDM) Network identified 8-year-old children with ASD (N = 12,271) through a population-based, multi-source, records review methodology during surveillance years 2000, 2002, 2004, 2006, and 2008. ADDM sites that participated in one or more surveillance years include Alabama, Arizona, Arkansas, Colorado, Florida, Georgia, Maryland, Missouri, New Jersey, North Carolina, Pennsylvania, South Carolina, Utah, West Virginia, and Wisconsin. A full description of ADDM methods has been published elsewhere (ADDM Network Surveillance Year 2002 Principal Investigators 2007; ADDM Network Surveillance Year 2006 Principal Investigators 2009; ADDM Network Surveillance Year 2008 Principal Investigators 2012; ADDM Network Surveillance Year 2010 Principal Investigators 2014; Yeargin-Allsopp et al. 2003). Multiple-source, records-based screening is conducted at health sources for all sites, and at educational sources for most (Arizona, Arkansas, Colorado, Georgia, Maryland, New Jersey, North Carolina, South Carolina, Utah, West Virginia sites only), but not all sites within defined geographic areas. Educational sources include public schools, and health sources include public health and private clinics, hospitals, diagnostic centers, and individual providers specializing in services for children with disabilities. Health records selected for abstractor review are identified through an electronic query of approximately 200 International Classification of Diseases Ninth Revision (ICD-9) codes (National Center for Health Statistics 2002), that include a spectrum of childhood neurodevelopmental disorders and mental health diagnoses. The ICD-9 code for NF1 (237.71) is included on this list; however, it is not uniformly queried across all ADDM sites for every study year. For ADDM sites with access to education sources, records of 8-year-old children receiving special education services during the study year are reviewed by abstractors. Based on the presence of pre-defined “trigger” words that indicate likelihood that a child may have ASD, health and educational records are abstracted from one or more sources at each site and combined into a single composite record for each child. Abstracted information includes demographic data, behavioral descriptions, diagnostic summaries, psychometric test results, and presence of comorbid medical/psychiatric diagnoses and disabilities. Trained clinician reviewers examine composite records and code key information, including the presence or absence of Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, Text Revision (DSM-IV-TR; APA 2000) behaviors, ASD and non-ASD diagnoses in the records, and other behavioral features, using standardized methodology developed by the Metropolitan Atlanta Developmental Disabilities Surveillance Program (Yeargin-Allsopp et al. 2003). Final case status is determined based on whether the child meets the ADDM ASD case definition, which is based on the DSM-IV-TR criteria for autistic disorder, Asperger disorder, and pervasive developmental disorder, not otherwise specified (PDD NOS, including atypical autism) (ADDM Network Surveillance Year 2002 Principal Investigators 2007; ADDM Network Surveillance Year 2006 Principal Investigators 2009; ADDM Network Surveillance Year 2008 Principal Investigators 2012). ADDM methodology quality assurance occurs in two phases: (1) records screening and abstraction are checked periodically for accuracy and (2) clinician reviewers maintain coding reliability using a blinded, random 10 % sample of abstracted records that are scored independently by two reviewers (ADDM Network Surveillance Year 2010 Principal Investigators 2014). The presence of NF1 does not influence the ASD case status algorithm.
Each ADDM site functions as a public health authority under the Health Insurance Portability and Accountability Act (HIPAA) of 1996 Privacy Rule and meets applicable local Institutional Review Board privacy and confidentiality requirements under 45 CFR 46 (US Department of Health and Human Services 2009).
Measures
Children who met the case definition for ASD were identified, and the presence of co-occurring medical conditions was based on either a description of the condition in the record or documentation of an ICD-9 code for that condition. A child was considered to have a previous community-based ASD diagnosis if they had an ASD diagnosis recorded in an abstracted evaluation, an ICD-9 code for ASD on record (299.XX), or was eligible for ASD special education services. Children in the ASD/NF1 group met the ASD case definition and also had an educational or health record with a diagnosis of NF1 and/or a NF1 ICD-9 code (237.71). Of note, neither a “rule out” NF1 diagnosis in the records nor an ICD-9 code for café-au-lait spots (709.09) contributed to NF1 case status. The comparison group included all other children meeting ASD case status that did not have NF1 documented in their records. A child was classified as having co-morbid ASD and ID if their most recent documented IQ score was at or below 70, or, in the absence of an IQ score, a statement was present in the child’s record describing the child’s functioning level as being in the ID range during previous psychometric testing provided that this statement was made by a qualified examiner and based on intellectual testing attempted or previously completed. Co-occurring ADHD and/or epilepsy were identified through the systematic abstraction and coding of the presence of these specific diagnoses as a standard component in the ADDM methodology. Sociodemographic and other selected characteristics of children with ASD included the following: sex, race/ethnicity, presence (and age) of developmental regression, presence (and earliest age) of a previous community-based ASD diagnosis, ASD classification as determined by clinician review (autistic disorder versus ASD-NOS), and the DSM-IV-TR diagnostic criteria met by the child as documented by clinician review.
Analysis
Differences in selected sociodemographic and other characteristics between ASD cases with and without NF1 were tested using χ2 goodness-of-fit tests for categorical variables and t-tests for continuous variables. Tests were first conducted within each site to look for evidence of heterogeneity before combining data. Two sites (West Virginia and Florida) identified no children with NF1. A second analysis after removing data from these two sites did not demonstrate any substantive differences in the results. For co-occurring ID, data were restricted to sites that collected IQ test results at the frequency threshold (ranging from 70–85 %) required by the ADDM Network for reporting for the respective study year. During the 2002 and 2004 surveillance years, most participating sites used an abbreviated abstraction and clinician review process for records of children who already had a documented ASD diagnosis or special education autism eligibility, limiting the availability of data for these children. For DSM-IV-TR criteria and pre-existing ASD community diagnosis comparisons, samples were therefore restricted to surveillance years 2000, 2006, 2008 for which complete data on each child were available. The prevalence and 95 % confidence intervals of NF1 were estimated among children with ASD in the ADDM network. All statistical analyses were conducted using SAS software version 9.3 (2011: SAS Institute, Cary NC, USA) and statistical significance was assessed at alpha = 0.05.
Results
Among the 12,271 children who met ASD case definition, 22 (0.18 %; 95 % confidence interval (CI): 0.12–0.27 %) had ASD/NF1. Of these 22 children, 7 were identified with NF1 from both ICD-9 codes and records review, 9 from records review only, 2 from multiple ICD-9 codes only, and 4 from a single ICD-9 code only. There was no difference in the distribution of sex or race/ethnicity between children with ASD/NF1 and children with ASD without NF1. The co-occurrence of ID, ADHD, or seizure disorder was similar between groups; although a lower frequency of co-occurring ID was found in children with ASD/NF1 compared to children with ASD without NF1 (17 vs. 41 %, respectively, p = 0.09). Significantly fewer (p = 0.04) children with ASD/NF1 had a previous ASD community diagnosis (53 %), compared to children with ASD without NF1 (76 %) (Table 1). The mean age at which groups received a previous ASD community diagnosis was similar (data not shown).
Table 1.
Characteristics of children with a final case status of autism spectrum disorder (ASD) with and without neurofibromatosis type 1 (NF1)
| Characteristics | All ASD cases (N=12,271)
|
χ2 p value | |
|---|---|---|---|
| With NF1 (N = 22) n (%) |
Without NF1 (N = 12,249)n (%) |
||
| Sex | 0.56 | ||
| Male | 17 (77) | 10,050 (82) | |
| Ethnicity | 0.62 | ||
| Non-hispanic white | 15 (68) | 7185 (59) | |
| Non-hispanic black | 3 (14) | 2760 (23) | |
| Hispanic | 3 (14) | 1253 (10) | |
| Other/unknown | 1 (5) | 1051 (9) | |
| Co-occurring condition | |||
| Intellectual disabilitya | 2 (17) | 2428 (41) | 0.09 |
| ADHDb | 5 (23) | 2340 (19) | 0.67 |
| Seizure disorder | 1 (5) | 514 (4) | 0.77 |
| History of regression | 3 (14) | 2433 (20) | 0.46 |
| Final case definition | 0.90 | ||
| Autism | 15 (68) | 7799 (64) | |
| ASD-NOSc | 4 (18) | 2655 (22) | |
| Streamlined ASDd | 3 (14) | 1780 (15) | |
| Previous ASD diagnosise | 8 (53) | 6100 (76) | 0.04 |
Data were restricted to sites that collected IQ test results among children with ASD at the frequency threshold (ranging from 70–85 %) required by the Autism and Developmental Disabilities Monitoring Network for reporting for the respective study year (with NF1, n = 12; without NF1, n = 5895)
Attention-deficit/hyperactivity disorder
Autism spectrum disorder not otherwise specified (e.g. Asperger disorder and pervasive developmental disorder, not otherwise specified)
For the 2002 surveillance year, the records of children who already had a documented ASD diagnosis or special education autism eligibility underwent an abbreviated abstraction and clinician review process
Only includes children identified with ASD in study years 2000, 2006, and 2008 (with NF1, n = 19; without NF1, n = 10,455)
Children with ASD/NF1 were significantly less likely than those with ASD without NF1 (58 and 84 % respectively, p = 0.001) to meet DSM-IV-TR diagnostic criterion 1a (difficulty using or understanding non-verbal communication; Table 2 and Fig. 1). No statistically significant difference was observed between the ASD/NF1 and ASD without NF1 groups in the mean number of DSM-IV-TR criteria met (8.4 vs. 8.9, respectively, p = 0.43), presence of regression, mean age at regression, and mean age at earliest ASD diagnosis (22.0 vs. 24.3 months, p = 0.80, and 60.6 vs. 56.7 months, p = 0.56, respectively). A sensitivity analysis performed after removing the four children exclusively identified with NF1 through a single ICD-9 code indicated that results did not vary substantively from the original results.
Table 2.
Diagnostic and Statistical Manual of Mental Disorders Fourth Edition, Text Revision criteria and definitions
| Criteria | Definition |
|---|---|
| 1a | Difficulty using or understanding non-verbal communication |
| 1b | Difficulty making friends |
| 1c | Difficulty sharing their interests with other appropriately |
| 1d | Difficulty with emotional/social reciprocity |
| 2a | Delayed language development |
| 2b | Marked impairment in the ability to initiate or sustain a conversation with others |
| 2c | Unusual language |
| 2d | Lack of imaginary play |
| 3a | Preoccupation or narrow interests |
| 3b | Difficulty with change, insistence on non-functional routines |
| 3c | Repetitive body movements |
| 3d | Persistent preoccupation with parts of objects |
Fig. 1.
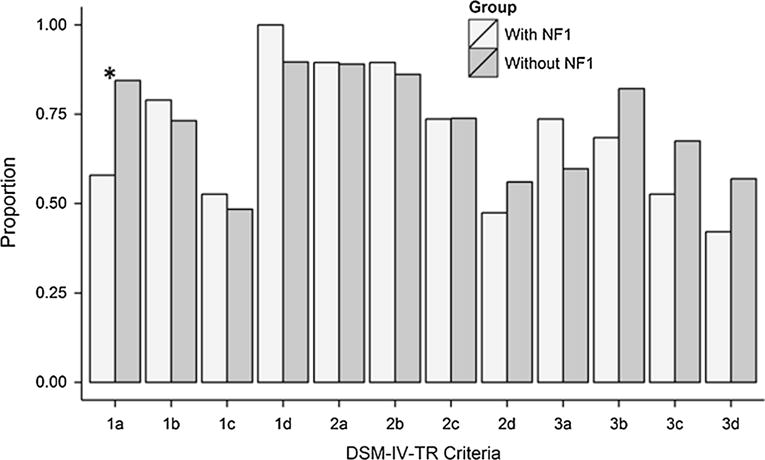
Percentage of children with autism spectrum disorder (ASD) and neurofibromatosis type 1 (NF1) (n = 19) and ASD without NF1 (n = 10,455) meeting DSM-IV-TR diagnostic criteria described in Table 2. Sample includes children with a final case status of ASD identified by the Autism and Developmental Disabilities Monitoring Network in study years 2000, 2006 or 2008. *Indicates significant difference between NF1/ASD and ASD without NF1 groups at p = 0.001 level
Discussion
Our estimated NF1 prevalence among a population-based sample of 8-year-old children with ASD (1-in-558) is 4.4 fold higher (95 % confidence interval: 3.9 to 5.1) than the prevalence of NF1 found among a German school-based cohort of 6-year-old children (Lammert et al. 2005), which is the most comparable ASD/NF1 prevalence estimate to use for comparison with ASD/NF1 prevalence among 8-year-old children identified by the ADDM Network. The prevalence of NF1 in Lammert et al.’s study provides a meaningful context in which to evaluate NF1 prevalence among children with ASD in the ADDM Network because of the similarities in methodologies between these studies in regards to participant age; large, population-based ascertainment; and inclusive of schools as ascertainment sites. Our findings are consistent with recent literature that described higher than expected frequencies of ASD characteristics (based on positive ASD screening and/or structural clinical evaluations) among children with NF1 (Garg et al. 2013; Garg et al. 2013; Plasschaert et al. 2014; Van Eeghen et al. 2012; Walsh et al. 2013).
The lower prevalence of ID among children with ASD/NF1 suggests that overall functional impairment experienced by children with ASD/NF1 may be less than in those children with ASD without NF1. Likewise, the relatively low presence of a previously established community ASD diagnosis may indicate milder ASD symptoms in children with NF1 compared to children with ASD without NF1. Although differences in ASD severity may contribute to these findings, we also found that the percentage of children with ASD/NF1 who met full criteria for autistic disorder (as opposed to Asperger disorder or PDD-NOS) was similar to that of children with ASD without NF1 (68 and 64 %, respectively).
Health supervision guidelines by American Academy of Pediatrics (AAP) for children with NF1 include routine developmental screening for learning disabilities, speech and language impairment, ID, and ADHD (Hersh and Committee on Genetics 2008). These guidelines also recommend review of children’s social adjustment. Social impairment noted in the records of children identified with ASD/NF1 by ADDM may have been attributed by community-based clinicians to one or more of these other common comorbid conditions in children without a previous community-based ASD diagnosis. Evidence from this study is mixed in regard to this hypothesis as we found no difference in the frequencies of co-occurring ADHD between children with ASD/NF1 and children in the ASD without NF1 group but found that the frequency of co-occurring ID in the ASD/NF1 group was lower, although not statistically, than in the ASD without NF1 group. Hence, the results of this study highlight the need to consider ASD within the differential diagnosis of any child with NF1 who exhibits deficits in social-communication skills.
This study is subject to at least four limitations. First, it is limited by its ascertainment methods for identifying children with NF1 living within the ADDM Network surveillance area. Because NF1 surveillance was not a primary goal of ADDM surveillance, sites varied in the extent to which they included the NF1 ICD-9 code in the initial records request to health sources. The reliance on a single ICD-9 code to identify four children with NF1 also raises the possibility that these children’s NF1 diagnoses may have been miscoded. However, repeating the analysis when these children were removed from the ASD/NF1 group demonstrated that study findings were robust to any potential misdiagnoses. For most sites during the 2002 and 2004 surveillance years, records of children who already had a documented ASD diagnosis or special education autism eligibility underwent an abbreviated abstraction and clinician review process. For ASD cases for whom only education records were available for review, the presence of NF1 may have been missed because schools do not commonly reference medical diagnoses such as NF1 in their records. Finally, the presence of a previous ASD diagnosis by a qualified community provider is incorporated into the ADDM algorithm for determining ASD case status. Consequently, the lower frequency of prior ASD diagnosis among children with ASD/NF1 likely contributes to an underestimate of ASD/NF1. These limitations collectively suggest that the prevalence of ASD/NF1 in this report represents a minimum estimate. Alternatively, the presentation of ASD characteristics in children classified in this study with ASD/NF1 may be more subtle than that required to meet diagnostic thresholds assessed during in person assessments.
The impact of the new DSM-5 (APA 2013) criteria on children with ASD/NF1 has not yet been established. In the general population, Maenner et al. (2014) reported a 19 % percent reduction in the number of children meeting DSM-5 ASD criteria compared to DSM-IV-TR criteria. Children with ASD/NF1 in the current study were significantly less likely (p = 0.001) than those with ASD without NF1 (58 vs. 84 %, respectively) to demonstrate impairment in the use or understanding of non-verbal communication. Because this criterion is now required to establish a DSM-5 ASD diagnosis, implementation of DSM-5 may have a disproportionately greater effect on children with ASD/NF1. However, Maenner et al. (2014) suggest that the DSM-5 implementation may increase clinical awareness of the ASD signs and symptoms required for establishing an ASD diagnosis; this practice may counterbalance the proposed effects of changing criteria on ASD prevalence. The impact of DSM-5 on ASD diagnosis in children with NF1 merits further investigation.
Conclusion
Our estimated NF1 prevalence among a population-based sample of 8-year-old children with ASD (1-in-558) is approximately 4 to 5 times higher than the NF1 prevalence in a comparable population-based German cohort. Children with NF1/ASD share many similarities to the general population of children with ASD, but they may experience a lower co-occurrence of ID, a relative absence of a previous community-based ASD diagnosis, and less frequent impairment in the use or understanding of non-verbal communication. These data suggest that clinicians caring for children with NF1 should be aware of this association and perform periodic AAP-recommended screening for ASD and follow up with diagnostic assessments and intervention. Additional research into the pattern of developmental impairment in children with NF1 may also provide insights into underlying pathogenic mechanisms of ASD.
Acknowledgments
Case identification was funded through a grant from the Centers for Disease Control and Prevention under Cooperative Agreement UR3 DD000685-04/DD10-1002 and the Utah Department of Health. This research was funded by the Centers for Disease Control and Prevention under Cooperative Agreement UR3 DD000685-04/dd10-1102 and the Utah Department of Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. We thank the Utah Department of Health and Utah State Office of Education for their support of the Utah Registry of Autism and Developmental Disabilities.
Footnotes
Author Contributions Dr. Bilder assisted with the conceptualization of the study through her review of the relevant literature, wrote the initial draft of the introduction, methods, and discussion sections, reviewed and revised the manuscript, and approved the final manuscript as submitted; Dr. Bakian assisted with the conceptualization of the study, performed the data analysis, wrote the results section, reviewed and revised the manuscript, and approved the final manuscript as submitted; Dr. Stevenson reviewed and revised the manuscript, and approved the final manuscript as submitted; Dr. Carbone reviewed and revised the manuscript, and approved the final manuscript as submitted; Dr. Cunniff wrote the initial draft of the conclusion section, reviewed and revised the manuscript, and approved the final manuscript as submitted; Dr. Goodman informed sub-analyses of the data, reviewed and revised the manuscript, and approved the final manuscript as submitted; Dr. McMahon reviewed and revised the manuscript, and approved the final manuscript as submitted; Ms. Fisher reviewed, revised, formatted, and approved the final manuscript as submitted; Dr. Viskochil assisted with the conceptualization of the study, critiqued the review of the relevant literature, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Compliance with Ethical Standards
Ethical Approval All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd. Washington: American Psychiatric Association; 1980. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington: American Psychiatric Association; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th. Washington: American Psychiatric Association; 2013. [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2002 Principal Investigators. Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2002. Morbidity and Mortality Weekly Report. 2007;56:12–28. [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2006 Principal Investigators. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, United States, 2006. Morbidity and Mortality Weekly Report. 2009;58(SS10):1–20. [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators. Prevalence of autism spectrum disorders—Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. Morbidity and Mortality Weekly Report. 2012;61(3):1–19. [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2010. Morbidity and Mortality Weekly Report. 2014;63(2):1–21. [PubMed] [Google Scholar]
- Brei NG, Klein-Tasman BP, Schwarz NG, Casnar CL. Language in young children with neurofibromatosis-1: Relations to functional communication, attention, and social functioning. Research in Developmental Disabilities. 2014;35:2495–2504. doi: 10.1016/j.ridd.2014.06.016. [DOI] [PubMed] [Google Scholar]
- DeBella K, Szudek J, Friedman JM. Use of the national institutes of health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000;105:608–614. doi: 10.1542/peds.105.3.608. [DOI] [PubMed] [Google Scholar]
- Dilts CV, Carey JC, Kircher JC, Hoffman RO, Creel D, Ward K, et al. Children and adolescents with neurofibromatosis 1: A behavioral phenotype. Journal of Developmental and Behavioral Pediatrics. 1996;17:229–239. [PubMed] [Google Scholar]
- Fombonne E, Du Mazaubrun C, Cans C, Grandjean H. Autism and associated medical disorders in a French epidemiological survey. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(11):1569–1591. doi: 10.1016/S0890-8567(09)66566-7. [DOI] [PubMed] [Google Scholar]
- Garg S, Green J, Leadbitter K, Emsley R, Lehtonen A, Evans EG, et al. Neurofibromatosis type 1 and autism spectrum disorder. Pediatrics. 2013a;132:e1642–e1648. doi: 10.1542/peds.2013-1868. [DOI] [PubMed] [Google Scholar]
- Garg S, Lehtonen A, Huson SM, Emsley R, Trump D, Evans DG, et al. Autism and other psychiatric comorbidity in neurofibromatosis type 1: Evidence from a population-based study. Developmental Medicine and Child Neurology. 2013b;55:139–145. doi: 10.1111/dmcn.12043. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Forsell C. Childhood psychosis and neurofibromatosis—more than a coincidence? Journal of Autism and Developmental Disorders. 1984;14(1):1–8. doi: 10.1007/BF02408551. [DOI] [PubMed] [Google Scholar]
- Hersh JH, Committee on Genetics Health supervision for children with neurofibromatosis. Pediatrics. 2008;121:633–642. doi: 10.1542/peds.2007-3364. [DOI] [PubMed] [Google Scholar]
- Huijbregts SCJ, de Sonneville LMJ. Does cognitive impairment explain behavioral and social problems of children with neurofibromatosis type 1? Behavior Genetics. 2011;41:430–436. doi: 10.1007/s10519-010-9430-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SL, Arthur SE, North KN. Learning disabilities in children with neurofibromatosis type 1: Subtypes, cognitive profile, and attention-deficit-hyperactivity disorder. Developmental Medicine and Child Neurology. 2006;48:973–977. doi: 10.1017/S0012162206002131. [DOI] [PubMed] [Google Scholar]
- Hyman SL, Shores A, North KN. The nature and frequency of cognitive deficits in children with neurofibromatosis type 1. Neurology. 2005;65:1037–1044. doi: 10.1212/01.wnl.0000179303.72345.ce. [DOI] [PubMed] [Google Scholar]
- Lammert M, Friedman JM, Kluwe L, Mautner VF. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Archives of Dermatology. 2005;141:71–74. doi: 10.1001/archderm.141.1.71. [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Rice CE, Arneson CL, Cunniff C, Schieve LA, Carpenter LA, et al. Potential impact of DSM-5 criteria on autism spectrum disorder prevalence estimates. Journal of the American Medical Association Psychiatry. 2014;71(3):292–300. doi: 10.1001/jamapsychiatry.2013.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeever K, Shepherd CW, Crawford H, Morrison PJ. An epidemiological, clinical and genetic survey of neurofibromatosis type 1 in children under sixteen years of age. Ulster Medical Journal. 2008;77:160–163. [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Bilder D, Farley M, Coon H, Pinborough-Zimmerman J, Jenson W, et al. Autism spectrum disorders reclassified: A second look at the 1980’s Utah/UCLA autism epidemiologic study. Journal of Autism and Developmental Disorders. 2013;43(1):200–210. doi: 10.1007/s10803-012-1566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouridsen SE, Andersen LB, Sorensen SA, Rich B, Isager T. Neurofibromatosis in infantile autism and other types of childhood psychoses. Acta Paedopsychiatrica. 1992;55(1):15–18. [PubMed] [Google Scholar]
- National Center for Health Statistics. Classification of diseases and injuries. 2002 Retrieved from ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/Publications/ICD-9/ucod.txt.
- National Institutes of Health Consensus Development Conference. Neurofibromatosis conference statement. Archives of Neurology. 1988;45:575–578. [PubMed] [Google Scholar]
- Ozonoff S. Cognitive impairment in neurofibromatosis type 1. American Journal of Medical Genetics. 1999;89:45–52. [PubMed] [Google Scholar]
- Plasschaert E, Descheemaeker M, Van Eylen L, Noens I, Steyaert J, Legius E. Prevalence of autism spectrum disorder symptoms in children with neurofibromatosis type 1. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2014;168(1):72–80. doi: 10.1002/ajmg.b.32280. [DOI] [PubMed] [Google Scholar]
- Computer software. Cary, NC: SAS Institute Inc; 2011. Statistical Analysis System (Version 9.3) [Google Scholar]
- Tinker J, Carbone PS, Viskochil D, Mathiesen A, Ma KN, Stevenson DA. Screening children with neurofibromatosis type 1 for autism spectrum disorder. American Journal of Medical Genetics. Part A. 2014;164(7):1706–1712. doi: 10.1002/ajmg.a.36549. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Code of federal regulations. Title 45. Public Welfare CFR 46. 2009 Retrieved from http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html.
- van Eeghen AM, Pulsifer MB, Merker VL, Neumeyer AM, van Eeghen EE, Thibert RL, et al. Understanding relationships between autism, intelligence, and epilepsy: A cross-disorder approach. Developmental Medicine and Child Neurology. 2012;55(2):146–153. doi: 10.1111/dmcn.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmar FR, Bregman J, Cohen DJ, Cicchetti DV. DSM-III and DSM-III-R diagnoses of autism. American Journal of Psychiatry. 1988;145:1404–1408. doi: 10.1176/ajp.145.11.1404. [DOI] [PubMed] [Google Scholar]
- Walsh KS, Velez JI, Kardel PG, Imas DM, Muenke M, Packer RJ, et al. Symptomatology of autism spectrum disorder in a population with neurofibromatosis type 1. Developmental Medicine and Child Neurology. 2013;55:131–138. doi: 10.1111/dmcn.12038. [DOI] [PubMed] [Google Scholar]
- Williams PG, Hersh JH. Brief report: the association of neurofibromatosis type 1 and autism. Journal of Autism and Developmental Disorders. 1998;28(6):567–571. doi: 10.1023/a:1026012414193. [DOI] [PubMed] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. Journal of the American Medical Association. 2003;289:49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]


