Abstract
Neuroblastoma (NB) is the most common type of extracranial solid tumor in children with a high prevalence in toddlers. For childhood cancer survivors, preservation of reproductive potential is an important factor for quality of life. The optimization of NB minimal residual disease (MRD) detection in testicular tissue is crucial to evaluate the risk of malignant cell reintroduction. The first step in the present study was to assess the accuracy of reverse transcription-quantitative polymerase chain reaction (RT-qPCR) to detect tyrosine hydroxylase (TH), paired-like homeobox 2b (PHOX2B) and doublecortin (DCX) mRNA expression in frozen/thawed testicular tissues of patients with non-obstructive azoospermia (NOA) contaminated (in vitro model) with an increasing number of IMR-32 and SK-N-SH NB cells. Testicular tissues were frozen by slow or snap freezing. The second step was to determine the expression levels of these markers in testicular samples from 4 pre-pubertal males (2 with stage IV NB and 2 with non-NB malignancy). The yield of extracted RNA was similar in testicular samples frozen by slow or snap freezing. In the in vitro model, TH and DCX transcripts were detected in uncontaminated testicular tissues, whereas PHOX2B mRNA was not detected. There was a strong positive association between the number of NB cells used for contamination and PHOX2B transcript levels. For IMR-32 and SK-N-SH NB cell lines, specificity and sensitivity rates of detection were 100% for PHOX2B following in vitro contamination with 10 tumor cells. In testicular samples from pre-pubertal males with and without NB, PHOX2B mRNA expression was not observed, but high expression levels of TH and DCX mRNA were detected, which were similar to expression detected in the in vitro model. Among the markers used in blood and bone marrow for NB MRD studies, the detection of PHOX2B transcripts by RT-qPCR may provide an accurate assessment of NB cells in testicular tissues from males who require fertility preservation.
Keywords: neuroblastoma, minimal residual disease, reverse transcription quantitative polymerase chain reaction, testicular tissue, fertility preservation
Introduction
Neuroblastoma (NB) is the most common type of extracranial solid tumor in children, with a high prevalence in toddlers (1). At diagnosis, a high proportion of patients have stage IV metastatic disease (2). Intensive treatments for this type of cancer improved the long-term survival rate, including in children with high-risk NB (3). However, since dividing cells are the target of chemo- and radiotherapy, these treatments act not only on cancer cells but also on germ cells. Therefore, they may affect reproductive function, exposing patients to a high risk of infertility (4). Therefore, fertility preservation for boys with NB is currently recommended (5,6). For childhood cancer survivors, preservation of reproductive potential is an important issue for quality of life (7).
When spermatogenesis is effective in pubertal males, sperm cryopreservation should be proposed (8). In pre-pubertal males, since spermatogenesis has not yet started, the preferred strategy is cryopreservation of testicular tissue (9–11). To date, no restoration of human fertility has been reported by the use of frozen/thawed testicular tissue. However, the animal data are promising, and births have been reported following spermatogonial stem cell transplantation or testicular tissue grafting in rodents or pigs (12–14). Furthermore, in vitro culture of testicular stem cells has been studied in mice for its potential to generate post-meiotic male gametes (15,16). These preliminary results may offer the potential for fertility restoration in young males in the future. However, fertility restoration using cryopreserved testicular samples needs to be safe, without any risk of reintroducing cancer cells.
As a number of cases of metastatic testicular NB have previously been reported (17–19), the possible presence of malignant cells in cryopreserved testicular tissue involves a risk of recurrence of the primary disease following fertility restoration by germ cell transplantation. The detection of NB minimal residual disease (MRD) in blood and bone marrow of patients with metastatic NB was developed using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) for tyrosine hydroxylase (TH), paired-like homeobox 2b (PHOX2B) and doublecortin (DCX) transcripts (20–22). These transcripts represent useful and clinically significant biomarkers of MRD in the blood and bone marrow of metastatic NB cells. However, these biomarkers have not been assessed for NB MRD detection in testicular tissue. The optimization of NB MRD detection in testicular tissue is crucial to evaluate the risk of malignant cell reintroduction. This detection method needs to be applicable for testicular samples frozen by slow freezing and for testicular tissues immediately following surgical collection, which could subsequently be frozen by snap freezing until RNA extraction is performed. This would allow detection of MRD for previously cryopreserved testicular tissues and for future testicular sample collections.
Therefore, the first objective of the present study was to assess the accuracy of NB MRD detection. This was performed by quantification of TH, DCX and PHOX2B transcripts in human testicular tissues, cryopreserved by slow or snap freezing and contaminated in vitro with increasing number of tumor cells from two NB cell lines. Once accuracy was assessed in this in vitro model, the expression levels of these three biomarkers were evaluated in frozen testicular samples from pre-pubertal males with and without NB.
Materials and methods
Experimental design
The specificity and sensitivity of NB MRD detection were assessed by RT-qPCR quantification of TH, PHOX2B and DCX mRNA expression in thawed testicular tissues that were contaminated with IMR-32 and SK-N-SH human NB cell lines (CCL-127 and HTB-11; American Type Culture Collection, Manassas, VA, USA). Prior to these experiments, IMR-32 and SK-N-SH cells were investigated for positivity and stability of TH, PHOX2B and DCX mRNA expression in the subclones.
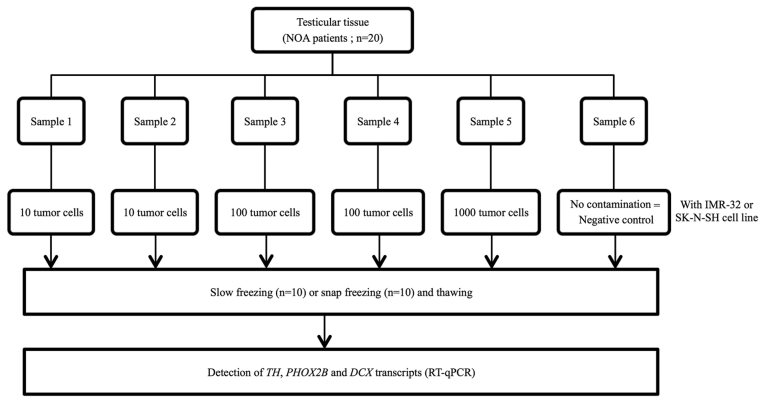
Subsequently, thawed testicular tissue from patients with non-obstructive azoospermia (NOA) were contaminated with 0 (negative control), 10, 100 and 1,000 IMR-32 and SK-N-SH tumor cells from human NB cell lines. The contamination procedure was performed at room temperature and took ~20 min. RNA extraction was performed immediately following contamination, without any additional incubation of the samples. Experiments involving contamination with 10 and 100 cells were performed in duplicate for each sample (Fig. 1). Once contamination was achieved, RNA extraction and RT-qPCR were performed. Testicular tissues frozen either by snap or slow freezing methods were used to investigate whether the freezing method may interfere with RT-qPCR analysis.
Figure 1.
Experimental design for detection of TH, PHOX2B and DCX transcripts in thawed testicular tissues following NB cell contamination by IMR-32 and SK-N-SH cell lines. Each testicular sample from patients with NOA was sectioned into 6 thin samples of equal size (~1 mm3). Each testicular sample was contaminated with an increasing number of IMR-32 and SK-N-SH NB cells prior to slow or snap freezing and detection of TH, Phox2B and DCX transcripts by RT-qPCR. NOA, non-obstructive azoospermia; NB, neuroblastoma; TH, tyrosine hydroxylase; PHOX2B, paired-like homeobox 2b; DCX, doublecortin; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Following assessment of MRD detection accuracy in the in vitro model, thawed testicular samples obtained from pre-pubertal males with stage IV NB and with non-NB malignancy (Ewing tumor) were analyzed in order to expand the method to include pre-pubertal testicular tissues.
Patients and samples
Testicular tissues were obtained from 20 males (mean age, 32.4 years; range, 25–38 years) with NOA between November 2014 and September 2015 at the University Hospital of Clermont-Ferrand (Clermont-Ferrand, France). The patients underwent testicular sperm extraction for intra-cytoplasmic sperm injection. Following sperm retrieval and freezing, the remaining testicular tissue sample would usually be destroyed, but in this instance it was used for the present study.
In addition, testicular tissues obtained from 2 pre-pubertal patients with stage IV NB in January 2012 (patient A, aged 3 years) and in April 2014 (patient B, aged 2 years) and 2 pre-pubertal patients with Ewing sarcoma in January 2016 (patients C and D, aged 6 years) were analyzed. Sample A was obtained at the University Hospital of Rouen (Rouen, France). Samples B, C and D were obtained at the University Hospital of Clermont-Ferrand (Clermont-Ferrand, France). Bilateral testis samples from patients A, B and C were evaluated. For patient D, only one sample of the right testis was available and evaluated. The median weight of the testicular samples for these 4 patients was 12 mg.
The present study was approved by the Committee for Personal Protection (DC 2008 558). Written informed consent was obtained from all patients prior to enrollment in the present study and for inclusion of the testicular samples in the GERMETHEQUE biobank (NFS 96900, www.chu-toulouse.fr/germetheque-centre-de-ressources-biologiques). In the case of two males with NB who succumbed to disease prior to the study, informed and written consent was obtained from their parents. The study was declared on the clinicaltrial.gov website (no. NCT02400970).
Freezing and thawing
For the testicular tissues obtained from adult patients with NOA, the samples were first cut into 6 thin fragments of equal size (~1 mm3) following weighing. Subsequently, the testicular fragments (equally sized samples) were frozen by using the slow (n=10 patients) or snap (n=10 patients) freezing method. The testicular tissues obtained from pre-pubertal males had been previously frozen by slow freezing for the two males with NB and by snap freezing for the males with Ewing sarcoma.
The slow freezing method was performed as previously described and validated by Rives et al (10). For the snap freezing method, testicular samples were put in cryogenic vials and immediately frozen by immersion in liquid nitrogen. For both freezing procedures, the samples were stored in cryogenic vials (CRYO BIO SYSTEM, L'Aigle, France). The same thawing procedure was used for testicular tissues frozen by slow or snap freezing. The cryogenic vials were thawed at 37°C for 3 min and placed into three baths of Leibovitz's L-15 medium (Eurobio, Courtaboeuf, France) for 5 min for each step at room temperature.
Culture of IMR-32 and SK-N-SH subclones
IMR-32 and SK-N-SH are well-established subclones of human cell NB. The IMR-32 cell line was maintained in Dulbecco's modified Eagle's medium (PAN-Biotech, Aidenbach, Germany), supplemented with 10% fetal calf serum (PAN-Biotech), 4 mmol/l L-glutamine, 10 UI/ml penicillin and 10 µg/ml streptomycin (PAN-Biotech) (23). The SK-N-SH cell line was maintained in Iscove's modified Dulbecco's Medium (PAN-Biotech), supplemented with 10% fetal calf serum, 4 mmol/l L-glutamine, 10 UI/ml penicillin and 10 µg/ml streptomycin. Cell counting analysis was performed twice prior to each contamination using the Malassez cell counting chamber. Blue trypan staining (diluted 1/20 with phosphate buffered saline) for 10 min at room temperature was used to assess cell viability. For IMR-32 and SK-N-SH cell lines, the viability was always ≥85%. The cell counting analysis and cell viability test were performed with a light microscope by an experienced observer prior to each contamination test.
Detection of TH, DCX and PHOX2B mRNA expression by RT-qPCR
RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's instructions. RNA was treated with DNAse I (Roche Diagnostics, Meylan, France) to remove any contaminating DNA. A total of 1 µg RNA was reverse-transcribed using SuperScript II (Invitrogen; Thermo Fisher Scientific, Inc.). The target TH, DCX and PHOX2B gene transcripts and the reference β-2-microglobulin (β2M) housekeeping gene transcript were measured using the LightCycler 480 RT-PCR system (Roche Diagnostics), using previously reported primers and probes (24). The sequences are provided in supplementary methods of the publication (24). The cycling conditions were as follows: 10 min at 95°C, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Absolute quantification analysis with the LightCycler software was performed to determine transcripts numbers, as described previously (20). Briefly, standard curves were generated using serial dilutions of plasmid containing a known number of molecules of each transcript and used to calculate the number of transcripts in the samples. The number of the reference β2M gene transcripts was used for the normalization of data. Normalized data were expressed as the copy numbers of target transcripts per 106 copies of β2M transcripts. The PCR reactions were performed in triplicate.
Statistical analysis
Statistical analysis was performed using Stata software (version 13; StataCorp LP, College Station, TX, US). The results for quantitative parameters were presented as the mean ± standard deviation, according to statistical distribution (assumption of normality assessed by Shapiro-Wilk's test). The investigation of associations between quantitative parameters was based on the estimation of correlation coefficients (Pearson's or Spearman's rho according to statistical distribution, noted r). Subsequently, in order to take into account differences between and within subject variability (due to several measurements for a given patient), random-effects for correlated measures were performed instead of the usual statistical tests, which would not be appropriate as the hypothesis of independence of data was not possible. The assumption of normality of residuals was studied using the Shapiro-Wilk test. When appropriate (data not exhibiting normal distributions), a logarithmic transformation was performed in order to achieve normality assumption and to ensure the correct use of previous analyses. Finally, receiver operating characteristic curve analysis was performed to evaluate sensitivity and specificity of RT-qPCR to detect the expression of TH, PHOX2B and DCX mRNAs in testicular tissue. All tests described in this section are two-sided, with a type I error set at α=0.05: P<0.05 was considered to indicate a statistically significant difference.
Results
Yield of RNA extraction from testicular tissue frozen using snap and slow freezing methods
The median weight of testicular samples from 20 men with NOA was 96 mg (33–210 mg). Each testicular sample was sectioned into six equal size samples with a median weight of 14 mg (6–46 mg). The median RNA yield from these samples was 15 µg (0.6–36.6 µg). The amount of RNA obtained per mg of tissue did not differ between the testicular samples frozen by snap (0.87 µg/mg) or slow freezing (0.80 µg/mg) methods (P>0.05).
Expression of TH, PHOX2B and DCX transcripts in IMR-32 and SK-N-SH cell lines
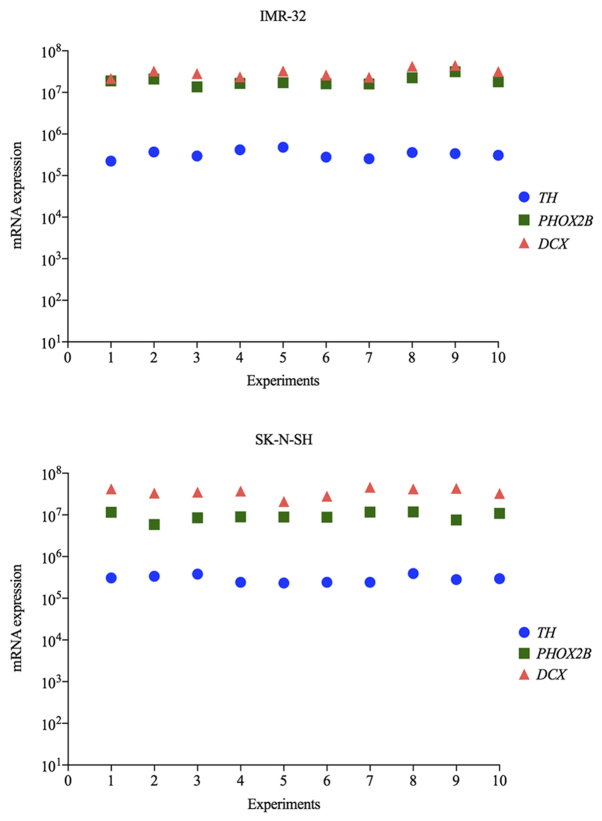
The expression levels of TH, PHOX2B and DCX transcripts in IMR-32 and SK-N-SH cell lines were evaluated for each artificial tumor contamination experiment (Fig. 2). Overall, the cell lines exhibited high levels of expression of the three markers, with the mean normalized values of 3.3×105 for TH, 1.9×107 for PHOX2B and 3.1×107 for DCX in IMR-32 cells and 3.0×105 for TH, 9.5×106 for PHOX2B and 3.6×107 for DCX in SK-N-SH cells. The levels of transcripts indicated minimal variations between different experiments, which were <1.3 fold. The average fold changes in transcript levels in IMR-32 and SK-N-SH cells were 1.26 and 1.22 for TH, 1.26 and 1.25 for PHOX2B and 1.28 and 1.27 for DCX, respectively. These data indicated the high reproducibility of the cell line models in terms of marker mRNA expression.
Figure 2.
Normalized expression of TH, PHOX2B and DCX in IMR-32 and SK-N-SH cell lines (per 106 β2M transcripts). For each cell line, 10 different subcultures were used for tumor contamination experiments. These subcultures exhibited minimal variations in the levels of the three mRNAs (<1.3 fold), indicating a good reproducibility of contamination experiments. TH, tyrosine hydroxylase; PHOX2B, paired-like homeobox 2b; DCX, doublecortin; β2M, β-2-microglobulin.
Detection of TH, PHOX2B and DCX transcripts in frozen testicular samples contaminated with IMR-32 and SK-N-SH NB cells
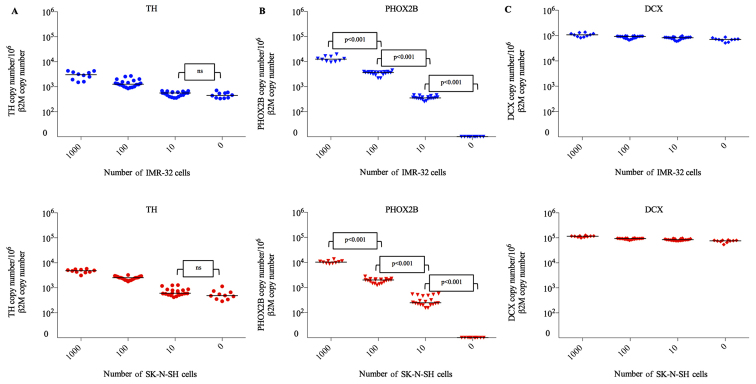
In the present study, expression of TH transcripts was observed in negative control testicular samples (n=10). Expression of TH transcripts was detected in samples frozen by snap freezing (IMR-32, 537 copy number/106 β2M copy number; SK-N-SH, 475 copy number/106 β2M copy number) and by slow freezing (IMR-32, 363 copy number/106 β2M copy number; SK-N-SH, 490 copy number/106 β2M copy number; Fig. 3A). Compared with the negative control (uncontaminated testicular samples), the level of TH mRNA was significantly higher in testicular samples contaminated with 100 and 1,000 NB cells from IMR-32 and SK-N-SH cell lines, respectively (P<0.001). There was no significant difference between the levels of TH mRNA detected in testicular samples contaminated with 10 NB cells and uncontaminated samples (IMR-32, P=0.18; SK-N-SH, P=0.24). For determination of TH expression, the specificity and sensitivity rates were 100% when 100 NB cells were used for contamination.
Figure 3.
Relative expression of (A) TH, (B) PHOX2B and (C) DCX in thawed testicular tissues following contamination by neuroblastoma cell lines (0, 10, 100 and 1,000 cells). Each symbol represents one testicular tissue fragment. Blue, IMR-32 cells; red, SK-N-SH cells. ns, no significant; TH, tyrosine hydroxylase; PHOX2B, paired-like homeobox 2b; DCX, doublecortin.
PHOX2B mRNA was not detected in the uncontaminated testicular samples (Fig. 3B) frozen either by the snap or slow freezing method (n=10). PHOX2B transcripts were detected at significantly different levels following contamination of testicular samples with 10, 100 and 1,000 NB cells (P<0.001 for IMR-32 and SK-N-SH). Furthermore, there was a strong linear correlation between the number of NB cells used for contamination (10, 100 and 1,000 NB cells) and the levels of PHOX2B mRNA (r=0.96 for IMR-32 and SK-N-SH). The specificity and sensitivity rates for PHOX2B mRNA detection were 100% following contamination by 10 NB IMR-32 or SK-N-SH cells.
High expression levels of DCX mRNA were detected in negative controls (n=10) frozen by snap freezing (IMR-32, 73,231 copy number/106 β2M copy number; SK-N-SH, 68,386 copy number/106 β2M copy number) and by slow freezing (IMR-32, 64,811 copy number/106 β2M copy number; SK-N-SH, 78,810 copy number/106 β2M copy number) methods (Fig. 3C). Furthermore, there was no significant difference in DCX mRNA levels between the negative controls and samples contaminated with 10, 100 and 1,000 NB cells.
Analysis of TH, DCX and PHOX2B mRNA expression levels in testicular tissues of pre-pubertal males
In the testicular samples obtained from two pre-pubertal NB stage IV males, the levels of TH mRNA were similar in the right (patient A, 431 copy number/106 β2M copy number; patient B, 469 copy number/106 β2M copy number) and left (patient A, 420 copy number/106 β2M; patient B, 371 copy number/106 β2M copy number) testis tissue samples. PHOX2B mRNA was undetectable in the testis samples of patient A and B. Similar high levels of DCX mRNA were observed in the right (patient A, 58,700 copy number/106 β2M; patient B: 48,297 copy number/106 β2M) and left (patient A: 51,280 copy number/106 β2M; patient B: 44,738 copy number/106 β2M) testis samples.
In the samples obtained from two pre-pubertal males with no-NB malignancy (Ewing sarcoma; patients C and D), mRNA TH levels were similar in the right (patient C, 684 copy number/106 β2M; patient D, 500 copy number/106 β2M) and left (patient C, 736 copy number/106 β2M copy number) testis. PHOX2B mRNA was not detected in testis samples obtained from either male. High levels of DCX mRNA were observed in the right (patient C, 53,690 copy number/106 β2M; patient D, 59,380 copy number/106 β2M copy number) and left (patient C, 59,380 copy number/106 β2M copy number) testis samples.
High expression levels of TH and DCX observed in the testicular tissues of the four pre-pubertal males were similar to the high expression levels detected in uncontaminated testicular samples (negative controls) in the in vitro model described previously in the results section.
Discussion
Given the increased survival rate of patients with NB and the cytotoxic effects of therapies used for treatment of this cancer (25), fertility preservation is highly recommended for these children. Therefore, for a pre-pubertal male facing sterilizing chemotherapy, testicular biopsy may be performed as a preventive strategy. Freezing testicular tissue allows subsequent transplantation either by infusion of a testicular cell suspension into the seminiferous tubules (26) or intra-testicular grafting of tissue (27). However, >60% of children with metastatic NB have circulating tumor cells at the time of diagnosis (28). Although testis is not the most frequent NB invasion site, numerous cases of testicular NB metastasis have been described previously. Simon et al (19) reported testicular metastasis in 10/1,076 male patients with NB using the data of the Cooperative German Neuroblastoma trials. Kushner et al (29) demonstrated 11 positive cases in a series of 289 male NB autopsies. Nistal et al (30) also reported two cases of testicular metastases confirmed by histological examination in a total of 216 NB patients. Therefore, the possible presence of NB cells in testicular tissue collected from NB males should be taken into account to avoid reintroducing cancer cells when the thawed testicular sample is used to restore fertility. However, in all of the previous studies mentioned, only histological analyses were performed. As the sensitivity of MRD detection by histological analysis is lower compared with RT-qPCR, the actual frequency of NB infiltration in testis may be higher.
In the present study, sufficient amounts of RNA were extracted from small testicular samples to perform RT-qPCR in optimal conditions and to analyze the level of three transcripts associated with NB. The use of a small quantity of testicular tissue to detect NB MRD ensures that a sufficient amount of sample remains for fertility preservation in pre-pubertal males, in whom large surgical retrieval is not always possible due to age and small testis size. The present study did not observe any effect of the freezing method on the yield of RNA extracted. Therefore, the RT-qPCR method may be performed on testis samples that have already been cryopreserved using slow freezing and on samples, which are cryopreserved by snap freezing at the time of surgical retrieval for subsequent analysis.
In the present study, an in vitro model of NB dissemination in human testicular tissue was established to evaluate the sensitivity and specificity for the detection of NB mRNAs. The SK-N-SH and IMR-32 tumor cell lines used for contaminations are well known and were established from human NB. SK-N-SH cells were established from the bone marrow metastasis from a 4 year-old Caucasian child (31). IMR-32 cells were obtained from a metastatic site in an abdominal mass in a 13 month-old Caucasian male with NB. The tumor was diagnosed as NB with rare areas of organoid differentiation (32). Therefore, SK-N-SH and IMR-32 human cell lines are highly representative of metastatic NB. In the present study, TH, DCX and PHOX2B mRNAs were expressed at high levels in SK-N-SH and IMR-32 cell lines. The testicular samples that were used to validate the in vitro contamination model were obtained from adult males with NOA, as it was unethical to perform these preclinical tests with healthy pre-pubertal testicular samples. Similar to pre-pubertal patients, testicular biopsies from patients with NOA contain low numbers or absence of mature germ cells.
In children with NB, metastatic tumor cells were previously detected in the bone marrow and peripheral blood by RT-qPCR for NB-specific target genes, including TH, DCX and PHOX2B (24,33,34). To the best of our knowledge, this is the first study to quantify the expression level of these transcripts in human testicular tissues to detect MRD. TH encodes for the key enzyme involved in the synthesis of catecholamines, which serves a functional role in the testis. A previous study provided evidence that Leydig cells exhibited close similarity to cathecholaminergic nerve cells with high expression of TH (35). This may be explained by a developmental arrest of Leydig cells at the immature state (36). In addition, Leydig cells are characterized as non-dividing cells with characteristics of early stem cell-like progenitors with endocrine, neuronal and glial cell features (37). Although TH is one of the most commonly used targets for MRD detection in peripheral blood and bone marrow of patients with NB (20,21), the use of this transcript as an MRD marker in testicular samples is hindered by its background expression detected in uncontaminated samples of patients with NOA and pre-pubertal males with and without NB. Therefore, it is hypothesized that the background expression of TH may be due to the presence of Leydig cells (35,37). Furthermore, 100% sensitivity and specificity rates of TH detection were only observed when 100 NB cells were used for contamination, which may suggest that the accuracy of this marker for NB MRD detection is insufficient for detection in human testicular samples.
Previously, the expression level of other transcripts, including DCX and PHOX2B, were demonstrated to be useful for NB MRD detection in bone marrow and peripheral blood from patients with metastasis (24). DCX is involved in the signaling pathway that regulates microtubule in migrating neurons (38). A previous study reported a high expression of a DCX homolog, DCD1, in human testis (39). This may explain the lack of sensitivity and specificity that was observed for analysis of DCX expression in the in vitro model in the present study, and the high expression of DCX in pre-pubertal males with or without NB. PHOX2B encodes a transcription factor involved in the development of the autonomous nervous system (34,40). It was previously revealed that PHOX2B is a highly specific marker for sensitive MRD detection of NB in the bone marrow, peripheral blood and harvested hematopoietic stem cells (40). To the best of our knowledge, the present study demonstrated for the first time a high sensitivity and specificity of RT-qPCR analysis for PHOX2B mRNA, enabling the detection of as few as 10 NB cells in a testis sample. The absence of background PHOX2B expression in uncontaminated testicular samples underlines the high specificity of this marker. Furthermore, PHOX2B expression was not detected in testicular samples of pre-pubertal males without NB in the present study. The present study demonstrated that NB MRD assessment in human testicular tissue by the detection of PHOX2B by RT-qPCR is highly accurate. It is unknown whether MRD marker genes of NB are up- or downregulated during treatment (23,24), so a panel of PCR targets may be required to overcome tumor heterogeneity (41). Since TH and DCX transcripts are not sensitive and specific NB markers in testicular tissues, it will be necessary to continue the evaluation of other known NB-specific transcripts. Analysis of NB mRNA is able to detect the presence of malignant cells but cannot predict their vitality or invasive potential. Therefore, further studies are required to investigate the risk of tumor dissemination on MRD-positive pre-pubertal testicular samples by xenotransplantation in rete testis of nude or severe combined immunodeficiency (SCID) mice.
In conclusion, the present study provides evidence that among the established NB markers for MRD analysis of blood and bone marrow using RT-qPCR, PHOX2B mRNA may provide an accurate assessment of MRD in testicular tissues for males that require fertility preservation. These preliminary results require confirmation by a further study of testicular tissues from a large cohort of pre-pubertal males with NB and additional studies are warranted to evaluate the safety threshold by xenotransplantation in nude or SCID mice.
Acknowledgments
The present authors would like to thank the Ligue contre le Cancer for supporting the project. The authors also thank Mrs. Camille Meindre and Mrs. Lucie Vivier (Centre de Biothérapie d'Auvergne, CHU Clermont-Ferrand, Clermont-Ferrand, France), Mrs. Sandra Carlet-Dollet and Mr. Cyril Bouche (Assistance Médicale à la Procréation, CECOS, CHU Clermont-Ferrand, Clermont-Ferrand, France), Mrs. Marine Nervi and Mrs. Farida Godeau (Cytogénétique Médicale, CHU Clermont-Ferrand, Clermont-Ferrand, France) and Mrs. Alexandra Usclade (Unité CRECHE, INSERM-CIC 1405, Université Clermont Auvergne, Clermont-Ferrand, France) for their excellent technical assistance.
References
- 1.Kaatsch P. Epidemiology of childhood cancer. Cancer Treat Rev. 2010;36:277–285. doi: 10.1016/j.ctrv.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Haase GM, Perez C, Atkinson JB. Current aspects of biology, risk assessment, and treatment of neuroblastoma. Semin Surg Oncol. 1999;16:91–104. doi: 10.1002/(SICI)1098-2388(199903)16:2<91::AID-SSU3>3.3.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 3.Oberthuer A, Hero B, Berthold F, Juraeva D, Faldum A, Kahlert Y, Asgharzadeh S, Seeger R, Scaruffi P, Tonini GP, et al. Prognostic impact of gene expression-based classification for neuroblastoma. J Clin Oncol. 2010;28:3506–3515. doi: 10.1200/JCO.2009.27.3367. [DOI] [PubMed] [Google Scholar]
- 4.Brougham MF, Wallace WH. Subfertility in children and young people treated for solid and haematological malignancies. Br J Haematol. 2005;131:143–155. doi: 10.1111/j.1365-2141.2005.05740.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, Beck LN, Brennan LV, Oktay K. American Society of Clinical Oncology: American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 6.Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, Quinn G, Wallace WH, Oktay K, American Society of Clinical Oncology Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31:2500–2510. doi: 10.1200/JCO.2013.49.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudour-Bonnange H, Tabone MD, Thomas-Teinturier C, Pacquement H, Oberlin O, Marec-Berard P, Laurence V, Aubier F, Duranteau L, Bernier-Chastagner V, et al. Fertility preservation in children and teenagers with cancer. Bull Cancer. 2013;100:727–735. doi: 10.1684/bdc.2013.1790. (In French) [DOI] [PubMed] [Google Scholar]
- 8.Daudin M, Rives N, Walschaerts M, Drouineaud V, Szerman E, Koscinski I, Eustache F, Saïas-Magnan J, Papaxanthos-Roche A, Cabry-Goubet R, et al. Sperm cryopreservation in adolescents and young adults with cancer: Results of the French national sperm banking network (CECOS) Fertil Steril. 2015;103:478–486.e1. doi: 10.1016/j.fertnstert.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Wyns C, Curaba M, Vanabelle B, Van Langendonckt A, Donnez J. Options for fertility preservation in prepubertal boys. Hum Reprod Update. 2010;16:312–328. doi: 10.1093/humupd/dmp054. [DOI] [PubMed] [Google Scholar]
- 10.Rives N, Milazzo JP, Travers A, Arkoun B, Bironneau A, Sibert L, Liard-Zmuda A, Marie-Cardine A, Schneider P, Vannier JP, Macé B. Cryopreservation of testicular tissue in children. Bull Acad Natl Med. 2013;197:877–886. (In French) [PubMed] [Google Scholar]
- 11.Goossens E, Van Saen D, Tournaye H. Spermatogonial stem cell preservation and transplantation: From research to clinic. Hum Reprod. 2013;28:897–907. doi: 10.1093/humrep/det039. [DOI] [PubMed] [Google Scholar]
- 12.Shinohara T, Inoue K, Ogonuki N, Kanatsu-Shinohara M, Miki H, Nakata K, Kurome M, Nagashima H, Toyokuni S, Kogishi K, et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum Reprod. 2002;17:3039–3045. doi: 10.1093/humrep/17.12.3039. [DOI] [PubMed] [Google Scholar]
- 13.Kanatsu-Shinohara M, Ogonuki N, Inoue K, Ogura A, Toyokuni S, Shinohara T. Restoration of fertility in infertile mice by transplantation of cryopreserved male germline stem cells. Hum Reprod. 2003;18:2660–2667. doi: 10.1093/humrep/deg483. [DOI] [PubMed] [Google Scholar]
- 14.Nakai M, Kaneko H, Somfai T, Maedomari N, Ozawa M, Noguchi J, Ito J, Kashiwazaki N, Kikuchi K. Production of viable piglets for the first time using sperm derived from ectopic testicular xenografts. Reproduction. 2010;139:331–335. doi: 10.1530/REP-09-0509. [DOI] [PubMed] [Google Scholar]
- 15.Stukenborg JB, Schlatt S, Simoni M, Yeung CH, Elhija MA, Luetjens CM, Huleihel M, Wistuba J. New horizons for in vitro spermatogenesis? An update on novel three-dimensional culture systems as tools for meiotic and post-meiotic differentiation of testicular germ cells. Mol Hum Reprod. 2009;15:521–529. doi: 10.1093/molehr/gap052. [DOI] [PubMed] [Google Scholar]
- 16.Reuter K, Ehmcke J, Stukenborg JB, Simoni M, Damm OS, Redmann K, Schlatt S, Wistuba J. Reassembly of somatic cells and testicular organogenesis in vitro. Tissue Cell. 2014;46:86–96. doi: 10.1016/j.tice.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Kumari PK, Surendran N, Chellam VG, Pillai GR, Ramachandran K. Neuroblastoma with testicular metastasis. Review of literature and report of a case. Indian J Cancer. 1994;31:52–55. [PubMed] [Google Scholar]
- 18.Casola G, Scheible W, Leopold GR. Neuroblastoma metastatic to the testis: ultrasonographic screening as an aid to clinical staging. Radiology. 1984;151:475–476. doi: 10.1148/radiology.151.2.6709923. [DOI] [PubMed] [Google Scholar]
- 19.Simon T, Hero B, Berthold F. Testicular and paratesticular involvement by metastatic neuroblastoma. Cancer. 2000;88:2636–2641. doi: 10.1002/1097-0142(20000601)88:11<2636::AID-CNCR28>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 20.Tchirkov A, Paillard C, Halle P, Bernard F, Bordigoni P, Vago P, Deméocq F, Kanold J. Significance of molecular quantification of minimal residual disease in metastatic neuroblastoma. J Hematother Stem Cell Res. 2003;12:435–442. doi: 10.1089/152581603322286060. [DOI] [PubMed] [Google Scholar]
- 21.Burchill SA, Lewis IJ, Abrams KR, Riley R, Imeson J, Pearson AD, Pinkerton R, Selby P. Circulating neuroblastoma cells detected by reverse transcriptase polymerase chain reaction for tyrosine hydroxylase mRNA are an independent poor prognostic indicator in stage 4 neuroblastoma in children over 1 year. J Clin Oncol. 2001;19:1795–1801. doi: 10.1200/JCO.2001.19.6.1795. [DOI] [PubMed] [Google Scholar]
- 22.Chambon F, Tchirkov A, Pereira B, Rochette E, Deméocq F, Kanold J. Molecular assessment of minimal residual disease in PBSC harvests provides prognostic information in neuroblastoma. Pediatr Blood Cancer. 2013;60:E109–E112. doi: 10.1002/pbc.24538. [DOI] [PubMed] [Google Scholar]
- 23.Stutterheim J, Zappeij-Kannegieter L, Ora I, van Sluis PG, Bras J, den Ouden E, Versteeg R, Caron HN, van der Schoot CE, Tytgat GA. Stability of PCR targets for monitoring minimal residual disease in neuroblastoma. J Mol Diagn. 2012;14:168–175. doi: 10.1016/j.jmoldx.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Viprey VF, Gregory WM, Corrias MV, Tchirkov A, Swerts K, Vicha A, Dallorso S, Brock P, Luksch R, Valteau-Couanet D, et al. Neuroblastoma mRNAs predict outcome in children with stage 4 neuroblastoma: A European HR-NBL1/SIOPEN study. J Clin Oncol. 2014;32:1074–1083. doi: 10.1200/JCO.2013.53.3604. [DOI] [PubMed] [Google Scholar]
- 25.Pinto NR, Applebaum MA, Volchenboum SL, Matthay KK, London WB, Ambros PF, Nakagawara A, Berthold F, Schleiermacher G, Park JR, et al. Advances in risk classification and treatment strategies for neuroblastoma. J Clin Oncol. 2015;33:3008–3017. doi: 10.1200/JCO.2014.59.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brünsten RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation; Proc Natl Acad Sci USA; 1994; pp. 11298–11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baert Y, van Saen D, Haentjens P, In't Veld P, Tournaye H, Goossens E. What is the best cryopreservation protocol for human testicular tissue banking? Hum Reprod. 2013;28:1816–1826. doi: 10.1093/humrep/det100. [DOI] [PubMed] [Google Scholar]
- 28.Yáñez Y, Hervás D, Grau E, Oltra S, Pérez G, Palanca S, Bermúdez M, Márquez C, Cañete A, Castel V. TH and DCX mRNAs in peripheral blood and bone marrow predict outcome in metastatic neuroblastoma patients. J Cancer Res Clin Oncol. 2016;142:573–580. doi: 10.1007/s00432-015-2054-7. [DOI] [PubMed] [Google Scholar]
- 29.Kushner BH, Vogel R, Hajdu SI, Helson L. Metastatic neuroblastoma and testicular involvement. Cancer. 1985;56:1730–1732. doi: 10.1002/1097-0142(19851001)56:7<1730::AID-CNCR2820560744>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 30.Nistal M, González-Peramato P, Paniagua R. Secondary testicular tumors. Eur Urol. 1989;16:185–188. doi: 10.1159/000471566. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert LC, Wachsman JT. Characterization and partial purification of the plasminogen activator from human neuroblastoma cell line, SK-N-SH. A comparison with human urokinase. Biochim Biophys Acta. 1982;704:450–460. doi: 10.1016/0167-4838(82)90067-X. [DOI] [PubMed] [Google Scholar]
- 32.Tumilowicz JJ, Nichols WW, Cholon JJ, Greene AE. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970;30:2110–2118. [PubMed] [Google Scholar]
- 33.Viprey VF, Corrias MV, Kagedal B, Oltra S, Swerts K, Vicha A, Ladenstein R, Burchill SA. Standardisation of operating procedures for the detection of minimal disease by QRT-PCR in children with neuroblastoma: Quality assurance on behalf of SIOPEN-R-NET. Eur J Cancer. 2007;43:341–350. doi: 10.1016/j.ejca.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Viprey VF, Lastowska MA, Corrias MV, Swerts K, Jackson MS, Burchill SA. Minimal disease monitoring by QRT-PCR: Guidelines for identification and systematic validation of molecular markers prior to evaluation in prospective clinical trials. J Pathol. 2008;216:245–252. doi: 10.1002/path.2406. [DOI] [PubMed] [Google Scholar]
- 35.Davidoff MS, Ungefroren H, Middendorff R, Koeva Y, Bakalska M, Atanassova N, Holstein AF, Jezek D, Pusch W, Müller D. Catecholamine-synthesizing enzymes in the adult and prenatal human testis. Histochem Cell Biol. 2005;124:313–323. doi: 10.1007/s00418-005-0024-x. [DOI] [PubMed] [Google Scholar]
- 36.Seil FJ, Johnson ML, Nishi R, Nilaver G. Tyrosine hydroxylase expression in non-catecholaminergic cells in cerebellar cultures. Brain Res. 1992;569:164–168. doi: 10.1016/0006-8993(92)90385-M. [DOI] [PubMed] [Google Scholar]
- 37.Schulze W, Davidoff MS, Holstein AF. Are Leydig cells of neural origin? Substance P-like immunoreactivity in human testicular tissue. Acta Endocrinol (Copenh) 1987;115:373–377. doi: 10.1530/acta.0.1150373. [DOI] [PubMed] [Google Scholar]
- 38.Oltra S, Martinez F, Orellana C, Grau E, Fernandez JM, Cañete A, Castel V. The doublecortin gene, a new molecular marker to detect minimal residual disease in neuroblastoma. Diagn Mol Pathol. 2005;14:53–57. doi: 10.1097/01.pas.0000149876.32376.c0. [DOI] [PubMed] [Google Scholar]
- 39.Zeng L, Gu S, Li Y, Zhao E, Xu J, Ye X, Wu Q, Wang L, Xie Y, Mao Y. Identification of a novel human doublecortin-domain-containing gene (DCDC1) expressed mainly in testis. J Hum Genet. 2003;48:393–396. doi: 10.1007/s10038-003-0033-3. [DOI] [PubMed] [Google Scholar]
- 40.Stutterheim J, Gerritsen A, Zappeij-Kannegieter L, Kleijn I, Dee R, Hooft L, van Noesel MM, Bierings M, Berthold F, Versteeg R, et al. PHOX2B is a novel and specific marker for minimal residual disease testing in neuroblastoma. J Clin Oncol. 2008;26:5443–5449. doi: 10.1200/JCO.2007.13.6531. [DOI] [PubMed] [Google Scholar]
- 41.Corrias MV, Haupt R, Carlini B, Cappelli E, Giardino S, Tripodi G, Tonini GP, Garaventa A, Pistoia V, Pistorio A. Multiple target molecular monitoring of bone marrow and peripheral blood samples from patients with localized neuroblastoma and healthy donors. Pediatr Blood Cancer. 2012;58:43–49. doi: 10.1002/pbc.22960. [DOI] [PubMed] [Google Scholar]