Abstract
The case of a 35-year old female patient with a diagnosis of metastatic mixed acinar-endocrine carcinoma (MAEC) is investigated in the present study. The patient was believed to have a well-differentiated neuroendocrine tumor (NET) with a high Ki-67 index and uptake on 68Gallium-DOTATOC positron emission tomography-computed tomography for 9 years, and was treated accordingly. The patient had long lasting disease control by treatment with sunitinib, and a response was observed in numerous lesions with peptide receptor radionuclide therapy (PRRT). Following treatment for metastatic disease for >4 years, liver transplantation was performed, as an exception to normal recommendations, at the time of progression of a centrally located liver lesion inducing obstructive jaundice. Following transplantation, the diagnosis of a Grade 3 NET, as defined by the WHO 2010 classification, was challenged and changed to MAEC. MAEC is a rare type of tumor of the pancreas, exhibiting endocrine and acinar differentiation. It is difficult to diagnose, often being misidentified as acinar cell carcinoma or predominantly as neuroendocrine neoplasms. Immunohistochemical labelling provides the only evidence for the dual differentiation of neuroendocrine (synaptophysin and chromogranin) and acinar (lipase, trypsin and chymotrypsin) cell markers. Studies investigating MAECs with a clear histopathological diagnosis are scarce, in addition to evidence of disease behaviour and treatment options. It is generally agreed that surgery is the primary treatment in patients with resectable tumors. The responses to sunitinib and PRRT suggested that treatments considered or developed for NETs may be beneficial in MAEC cases.
Keywords: mixed acinar-endocrine carcinoma, neuroendocrine carcinoma, pancreas, 68Gallium-Dotatoc, positron emission tomography-computed tomography, peptide receptor radionuclide therapy, liver transplantation
Introduction
Acinar cell carcinoma (ACC) is a rare pancreatic neoplasm that may contain scattered endocrine cells in ≤40% of cases (1). In addition, unusual tumors exist in which the endocrine component constitutes a significant proportion (>25–30%) of the neoplasm; these tumors are called mixed acinar-endocrine carcinoma (MAEC) (2–4). These tumors are thought to behave more similarly to typical pancreatic ACCs compared with well-differentiated pancreatic endocrine neoplasms, and certain authors have suggested they represent part of the spectrum of ACCs (3). However, the existence of cases with a predominant endocrine tumor cell component challenges this notion. MAEC is rare and, thus, evidence on disease behaviour and treatment options is scarce. MAECs are usually regarded as tumors with a poor prognosis and are treated with surgery and/or chemotherapy (4). The diagnosis of MAEC remains a challenge; therefore, cases may be underreported and misidentified as ACC, solid-pseudopapillary neoplasms, neuroendocrine tumors (NETs) or neuroendocrine carcinomas (NECs) (1,4). Here, a case of a female with a diagnosis of metastatic MAEC is presented. During the course of the patient's disease it was believed for 9 years to be a well-differentiated NET with a high Ki-67 index, a Grade 3 (G3) pancreatic neuroendocrine tumor (NET) (5), with uptake on 68Gallium-labelled somatostatin analogs (68Ga-SMA)-positron emission tomography-computed tomography (PET/CT). Treatment proposals were provided accordingly. The patient had long-lasting disease control by treatment with sunitinib and a response was observed in numerous lesions due to peptide receptor radionuclide therapy (PRRT). Following the last surgery, seven years after initial presentation, the diagnosis of G3 NET was challenged and changed to MAEC. Written informed consent from the patient was obtained.
Case report
In August 2007, a 35-year old female underwent an enucleation of a 2.5 cm cystic tumoral lesion located in the head of the pancreas. Histopathological examination resulted in identification of a NET with a Ki-67 index of 40%; however, diagnosis was later updated to solid-pseudopapillary neoplasm as focal β-catenin positivity was revealed. No complementary surgery was performed.
In March 2010, a novel lesion was observed in the pancreas, along with multiple bilobar liver metastases. The patient was expected to undergo a pancreaticoduodenectomy and two-stage hepatectomy. The patient underwent the resection of the local recurrence of the primary tumor together with the first stage of the hepatectomy, with a resection of the metastases in the left liver lobe in December 2010. Subsequently, histopathology revealed that the tumor was a well-differentiated NET, displaying a higher than usual Ki-67 index (40%).
Re-evaluation in January 2011 revealed the presence of novel liver lesions, indicating that a repeat hepatectomy would be futile (Fig. 1). Despite the high Ki-67 index and the short period until relapse, treatment with sunitinib was selected rather than chemotherapy, as the tumor did not demonstrate the morphology of poorly differentiated NEC. The lesions remained stable on 3-monthly interval 18FDG-PET CT (Siemens Biograph mCT 20; Siemens AG, Munich, Germany) during 17 months of sunitinib treatment (Fig. 2). The dose of sunitinib was reduced from 37.5 to 25 mg daily from May 2012 onwards, due to the emergence of hand-foot skin reaction. During treatment with sunitinib a 68Ga-Dotatoc PET-CT was performed in April 2012, which demonstrated uptake, and the patient was then referred for PRRT; however, treatment was denied due to the high proliferation index.
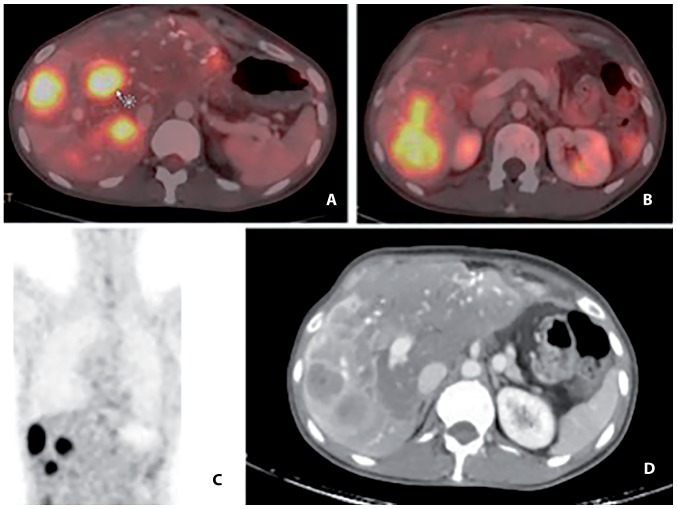
Figure 1.
18Fludeoxyglucose PET-CT scan at the commencement of sunitinib treatment. (A and B) The bright spots indicate liver metastases with high 18FDG-uptake (arrow) on combined PET-CT-scan in 2 cross-sectional images. (C) high 18FDG-uptake on PET-scan in 1 coronal plane image. (D) liver metastases on CT scan in 1 cross-sectional image. PET-CT, positron emission tomography-computed tomography.
Figure 2.
18F-FDG PET-CT in March 2012. (A) Metabolic response (no 18FDG-uptake) on PET scan in 1 coronal plane image. (B) liver metastases on CT scan in 1 cross-sectional image. An increased volume of the liver metastases with minimal 18F-FDG uptake on PET suggests a metabolic response with necrosis. 18F-FDG, 18Fludeoxyglucose; PET-CT, positron emission tomography-computed tomography.
In June 2012, chemotherapy supplemented with cisplatin and etoposide was initiated due to progression of the disease (Fig. 3). The chemotherapy was moderately tolerated by the patient and stopped in February 2013 when the disease was stable following three cycles of treatment. In September 2013, the patient was referred and accepted for PRRT (Fig. 4A and B). The patient underwent 3/4 planned treatment sessions; delays were often experienced due to bone marrow toxicity. Therapy with PRRT was completed in January 2014 and a good response in all but one lesion was observed (Fig. 4C). This lesion demonstrated a high metabolic activity on 18Fludeoxyglucose (18F-FDG) PET/CT and fine needle aspiration revealed a Ki-67 index of 40%.
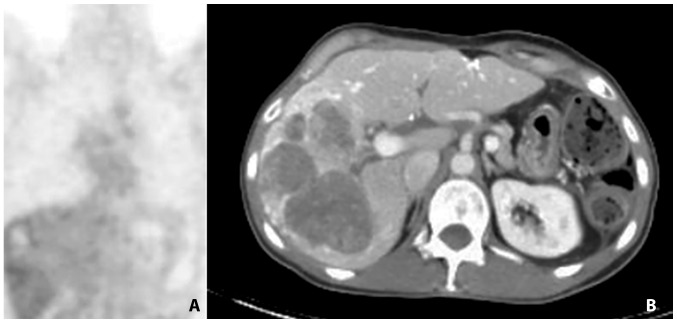
Figure 3.
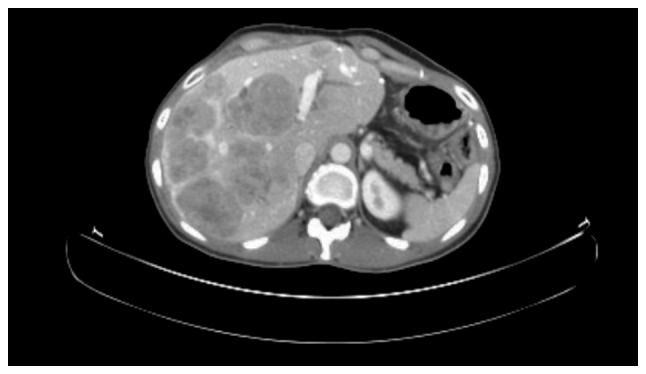
Computed tomography scan at the commencement of treatment with platinum-based chemotherapy illustrating liver metastases.
Figure 4.
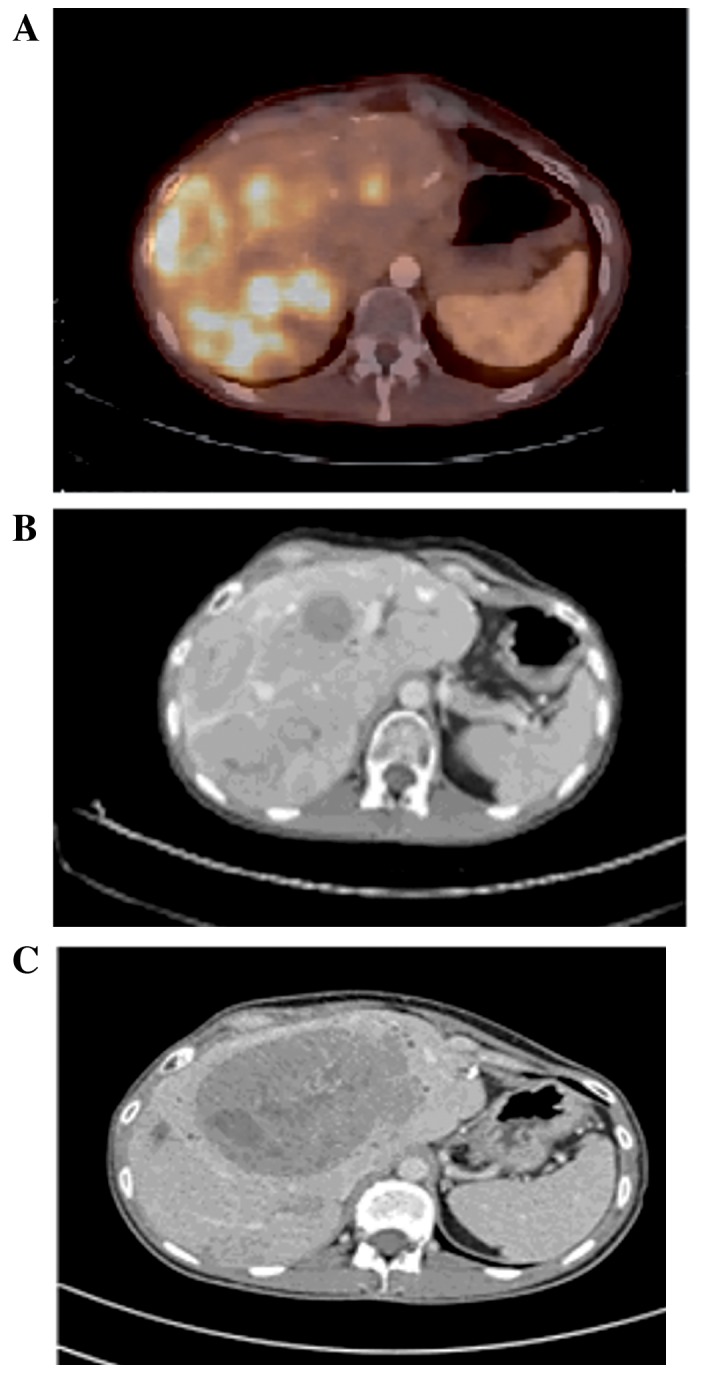
(A) 68Gallium-DOTATOC positron emission tomography-computed tomography prior to PRRT commencement: Liver metastases demonstrating high uptake of 68Gallium-DOTATOC (September 2013). (B) CT scan prior to PRRT commencement demonstrating the same liver metastases (September 2013). (C) CT scan following PRRT demonstrating one progressive metastasis and response in the lesions that exhibited a high uptake of 68Gallium-DOTATOC (January 2014). CT, computed tomography; PRRT, peptide receptor radionuclide therapy.
In April 2014, therapy with temozolomide-capecitabine was initiated, and fast progression was observed. The patient developed jaundice due to biliary obstruction at the hilus of the liver (Fig. 5). It was technically impossible to perform adequate biliary internal drainage. External drainage was performed, leading to amelioration, but no resolution of the jaundice was achieved, limiting the treatment options.
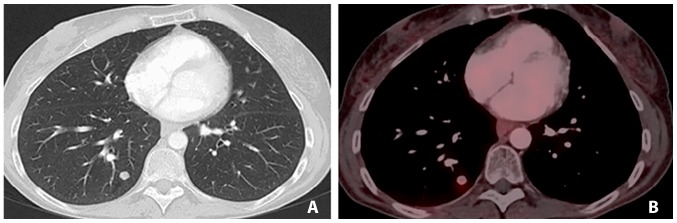
Figure 5.
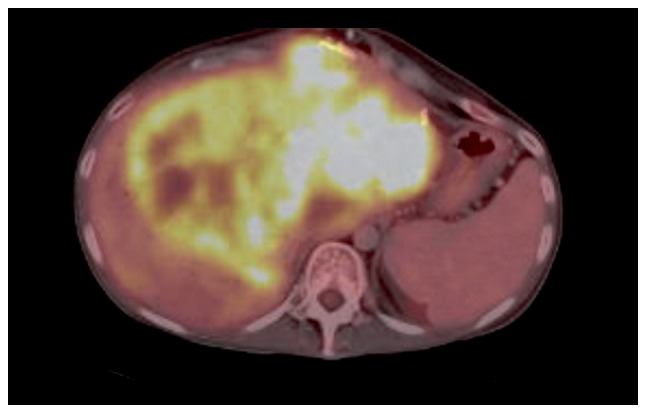
18Fludeoxyglucose positron emission tomography-computed tomography in April 2014 illustrating progression of the biggest liver metastasis leading to biliary obstruction.
At this time, the patient had had a diagnosis of metastatic G3 NET for four years, and the disease had been well controlled for long periods previously. Alternative treatment options could have been discussed again if the level of bilirubin was improved. During the clinical course and disease evolution, despite the high Ki-67 index, it was agreed to perform a liver transplantation in the patient using a marginal graft. A suitable graft from a 68-year old brain-dead female donor with a history of 15 years of breast cancer became available, leading to a successful transplantation in October 2014. In the explant liver, all lesions had similar pathological features and a diagnosis of a well-differentiated NET with Ki-67 index ≤40%. Immunosuppression was based on tacrolimus and mycophenolate mofetil, with a switch to sirolimus from tacrolimus after four weeks. Restaging in March 2015 revealed no signs of tumor recurrence; however, a novel re-evaluation in October 2015, one year following transplantation, identified numerous metastatic long nodules, demonstrating uptake on 68Ga-Dotatoc PET-CT (Fig. 6). Therapy with octreotide analogues was initiated. The patient is currently (January 2017) asymptomatic and has a good quality of life.
Figure 6.
(A) CT scan and (B) 68Ga-DOTATOC positron emission tomography-computed tomography demonstrating identical lesions, which are exhibiting numerous long nodules one year following transplantation.
Review of the pathology specimens at this time challenged the diagnosis. Following revision of all previous resection specimens and the inclusion of markers for pancreatic lipase, trypsin and chymotrypsin, a diagnosis of MAEC was determined (Fig. 7).
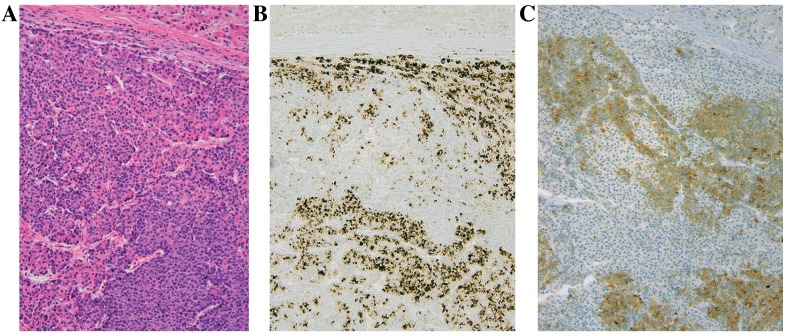
Figure 7.
(A) Microphotographs captured using an Olympus BX upright microscope illustrating the liver metastasis demonstrating (A) solid areas alternating with tumor cells arranged into small acinar structures (hematoxylin and eosin stain, with magnification, ×200). Immunohistochemistry revealed that the acinar cells stained positively with antibodies directed against (B) chymotrypsin at magnification, ×200 and (C) the endocrine cells label with antibodies against synaptophysin, at magnification, ×200.
Discussion
MAEC are rare tumors of the pancreas. They are malignant epithelial neoplasms exhibiting both endocrine and acinar differentiation. (2) By arbitrary definition, each component must compromise ≥25% of the neoplasm for a diagnosis of MAEC (2,4). Immunohistochemical labelling is performed in order to diagnose the disease. This revealed regions with acinar differentiation displaying positivity for lipase, trypsin and/or chymotrypsin, and regions with endocrine differentiation demonstrated positivity for synaptophysin and chromogranin (2–4).
MAEC is challenging for pathologists to diagnose and easily confused with ACC, solid pseudopapillary neoplasms and predominantly with neuroendocrine neoplasms (1,4). In the majority of cases there is a close intermingling of the acinar and endocrine component without clear segregation of the two cell types, thus it is not possible to identify with certainty the two lines of differentiation by routine histology (2). Immunohistochemical labelling for neuroendocrine and acinar cell markers provides the only evidence for dual differentiation. In contrast to neuroendocrine immunohistochemical markers, markers for pancreatic enzymes are not widely available outside of large hospitals, including at the time of diagnosis of the patient in the present case. In order to achieve the correct diagnosis, referral to a specialized department with access to these markers is indicated.
MAEC is thought to behave more akin to typical pancreatic ACCs, compared with well-differentiated pancreatic endocrine neoplasms (3,4). Pancreatic ACC is commonly treated in the same way as adenocarcinomas, although the disease may have a more indolent course, as suggested by increased survival rates demonstrated in the Surveillance, Epidemiology, and End Results Programme database for unresected (5-year survival rate, 22%) and resected ACC (5-year survival rate, 72%) (1). In the present study analysis, it is confirmed that the diagnosis of ACC can be difficult to make and that incorrect classification of NETs and MAEC may favourably bias the survival rate.
Reports on MAECs with a clear histopathological diagnosis are scarce, in addition to evidence on disease behaviour and treatment options. Recently, a review on ~30 presented cases in literature was published (4). The general consensus is that surgery is the primary treatment method for patients with resectable tumors, and reports of patients benefiting from tumor debulking surgery have been revealed (4). The median overall survival time following surgery was indicated to be ~12 months; however, this assumption was made based on a small number of patients with various tumor loads and lengths of follow-up (4). The patient in the present case relapsed 2.5 years following resection of the primary tumor and exhibited local recurrence in the pancreas and liver metastases.
To the best of our knowledge, no previous studies have been published investigating the use of functional imaging techniques in MAEC, although in a certain case report the use of an octreoscan following resection was discussed (6). Patients with neuroendocrine neoplasms may have lesions with various uptakes of 18F-FDG PET/CT and 68Ga-SMA PET/CT. Typically there is a higher uptake of 68Ga-SMA in well differentiated NETs compared with in poorly differentiated NECs, and a higher uptake of 18F-FDG in poorly differentiated NEC compared with in well differentiated NETs (7,8). Particularly in patients with multiple metastases, tracer uptake can be variable at different lesion sites (8). Furthermore, certain lesions within the same patient may be apparent on 68Ga-SMA PET/CT and not on 18F-FDG PET/CT, or vice versa: this reflects tumor heterogeneity (8). This case study presented a patient who had distinct lesions on 68Ga-Dotatoc PET-CT, in accordance with the histopathological diagnosis of a component of well-differentiated NET. The patient also exhibited uptake on 18F-FDG PET/CT in certain lesions, which may be associated with the acinar cell component.
In NETs, an higher uptake on functional imaging scans has been suggested to be associated with increased of overall survival, and treatment decisions have been challenged based on the results of functional scans (7,9–11). Treatment decisions in tumor-node-metastasis stage IV NETs were previously determined based on the grade of differentiation, prior to the introduction of the Rindi grading system in 2006 (12), which was later integrated into the World Health Organisation (WHO) 2010 classification system (5). It is becoming more accepted that the current WHO 2010 G3 category includes neuroendocrine neoplasms of two distinct types: A highly proliferative group of well-differentiated NETs (WD-NETs) and the poorly-differentiated NECs, divided into small cell and large cell NECs (5,12–17). The pattern of uptake in functional imaging provided evidence supporting the decision to administer the patient in the present study, who was thought to have a diagnosis of a well-differentiated G3 neuroendocrine neoplasm with a Ki-67 index of 40% maximum, chemotherapy (cisplatin-etoposide and temozolomide-capecitabine), as recommended for NEC, and a tyrosine kinase inhibitor (TKI), sunitinib, PRRT and liver transplantation (5,12–17).
For a rare disease such as MAEC, it is not possible to conduct trials for robust evidence of therapeutic effects and case reports are important sources of information. The patient in the present study had stable disease with treatment of sunitinib for 17 months, and no objective response to cisplatin-etoposide, but control of the disease was obtained for 15 months. Conversely, the response to PRRT was clearly documented in all but one lesion; however, time to tumor progression (TTP) was only four months due to one rapidly progressive lesion. It was observed that the combination of temozolomide-capecitabine in the fourth line of treatment did not demonstrate any therapeutic benefit to the patient; however, the subsequent therapeutic interventions may have altered the properties of the tumor. Selection pressure may have exhibited an impact on slower growing parts of the tumor, leaving more aggressive cells behind. However, the tumor lesions in the explant liver were extensively sampled and were not considered to differ histopathologically. More specifically, the rapidly progressive lesion was not revealed to have a higher Ki-67 index or a differing cell population.
Upon revision at one year, performed for academic reasons, and the application of immunohistochemical markers for pancreatic enzymes, the diagnosis was changed to an MAEC. Revision of older tissue specimens concurred with this finding. Liver transplantation is not a standard treatment in neuroendocrine neoplasms with a high Ki-67 index, and was performed exceptionally as the only lifesaving option at the time of progression of a centrally located liver lesion that had induced obstructive jaundice in a young patient treated for metastatic disease for >4 years. Relapse occurred 13 months following this and the novel lesions again demonstrated uptake on 68Ga-Dotatoc PET-CT, suggesting a component of well-differentiated NET. The absence of fast recurrence following this surgical intervention in spite of therapy with immune suppression may add to the suggestion that surgery should be performed when possible in patients with MAEC (1,4).
The response to the active sunitinib and PRRT treatments suggested that treatment of the endocrine component within the MAEC in addition to surgery may be beneficial. In conclusion, a case of MAEC of the pancreas that demonstrated uptake on 68Ga-Dotatoc PET-CT was presented, challenging the suggestion of using these active treatments for the endocrine component of the tumor. The majority of the lesions responded to PRRT and a durable disease control was suggested with TKIs and chemotherapy. However, the present case report also revealed that surgical options must be considered in MAEC.
References
- 1.Wisnoski NC, Townsend CM, Jr, Nealon WH, Freeman JL, Riall TS. 672 Patients with acinar cell carcinoma of the pancreas: A population-based comparison to pancreatic adenocarcinoma. Surgery. 2008;144:141–148. doi: 10.1016/j.surg.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Klimstra DS, Rosai J, Heffess CS. Mixed acinar-endocrine carcinomas of the pancreas. Am J Surg Pathol. 1994;18:765–778. doi: 10.1097/00000478-199408000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Ohike N, Kosmahl M, Klöppel G. Mixed acinar-endocrine carcinoma of the pancreas. A clinicopathological study and comparison with acinar-cell carcinoma. Virchows Arch. 2004;445:231–235. doi: 10.1007/s00428-004-1037-x. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Dong C, Wang C, Liu Q, Sun D, Wang L. Mixed acinar-endocrine carcinoma of pancreas: A case report and brief review of the literature. Onco Targets Ther. 2015;8:1633–1642. doi: 10.2147/OTT.S87406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasaoglu E, Dursun N, Ozyalvacli G, Hacihasanoglu E, Behzatoglu K, Calay O. Comparison of world health organization 2000/2004 and world health organization 2010 classifications for gastrointestinal and pancreatic neuroendocrine tumors. Ann Diagn Pathol. 2015;19:81–87. doi: 10.1016/j.anndiagpath.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Ogbonna OH, Garcon MC, Syrigos KN, Saif MW. Mixed acinar-neuroendocrine carcinoma of the pancreas with neuroendocrine predominance. Case Rep Med. 20132013:705092. doi: 10.1155/2013/705092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Has Simsek D, Kuyumcu S, Turkmen C, Sanlı Y, Aykan F, Unal S, Adalet I. Can complementary 68 Ga-DOTATATE and 18F-FDG PET/CT establish the missing link between histopathology and therapeutic approach in gastroentero-pancreatic neuroendocrine tumors? J Nucl Med. 2014;55:1811–1817. doi: 10.2967/jnumed.114.142224. [DOI] [PubMed] [Google Scholar]
- 8.Kayani I, Bomanji JB, Groves A, Conway G, Gacinovic S, Win T, Dickson J, Caplin M, Ell PJ. Functional imaging of neuroendocrine tumors with combined PET/CT using 68Ga-DOTATATE (DOTA-DPhe1, Tyr3-octreotate) and 18F-FDG. Cancer. 2008;112:2447–2455. doi: 10.1002/cncr.23469. [DOI] [PubMed] [Google Scholar]
- 9.Panagiotidis E, Bomanji J. Role of 18F-fluorodeoxyglucose PET in the study of neuroendocrine tumors. PET Clin. 2014;9:43–55. doi: 10.1016/j.cpet.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 10.Kartalis N, Mucelli RM, Sundin A. Recent developments in imaging of pancreatic neuroendocrine tumors. Ann Gastroenterol. 2015;28:193–202. [PMC free article] [PubMed] [Google Scholar]
- 11.Basu S, Ranade R, Thapa P. Correlation and discordance of tumour proliferation index and molecular imaging characteristics and their implications for treatment decisions and outcome pertaining to peptide receptor radionuclide therapy in patients with advanced neuroendocrine tumour: Developing a personalized model? Nucl Med Commun. 2015;36:766–774. doi: 10.1097/MNM.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 12.Rindi G, Klöppel G, Ahlman H, Caplin M, Couvelard A, De Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P, et al. TNM staging of foregut (neuro)endocrine tumors: A consensus proposal including a grading system. Virchows Arch. 2006;449:395–401. doi: 10.1007/s00428-006-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C, Anlauf M, Cwikla JB, et al. ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology. 2016;103:186–194. doi: 10.1159/000443172. [DOI] [PubMed] [Google Scholar]
- 14.Vélayoudom-Céphise FL, Duvillard P, Foucan L, Hadoux J, Chougnet CN, Leboulleux S, Malka D, Guigay J, Goere D, Debaere T, et al. Are G3 ENETS neuroendocrine neoplasms heterogeneous? Endocr Relat Cancer. 2013;20:649–657. doi: 10.1530/ERC-13-0027. [DOI] [PubMed] [Google Scholar]
- 15.Basturk O, Yang Z, Tang LH, Hruban RH, Adsay V, McCall CM, Krasinskas AM, Jang KT, Frankel WL, Balci S, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol. 2015;39:683–690. doi: 10.1097/PAS.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang LH, Untch BR, Reidy DL, O'Reilly E, Dhall D, Jih L, Basturk O, Allen PJ, Klimstra DS. Well differentiated Neuroendocrine tumors with a morphologically apparent high-grade component: A pathway distinct from poorly differentiated neuroendocrine Carcinomas. Clin Cancer Res. 2016;22:1011–1017. doi: 10.1158/1078-0432.CCR-15-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann Oncol. 2013;24:152–160. doi: 10.1093/annonc/mds276. [DOI] [PubMed] [Google Scholar]