Abstract
Although the available treatment options have expanded, the survival of patients with metastatic renal cell carcinoma (RCC) remains poor. As patients with RCC lack responsiveness to chemotherapy or radiation, therapeutic options predominantly include surgical interventions and immunomodulatory approaches, including the administration of tyrosine kinase inhibitors (TKIs) such as sunitinib. Natural killer (NK) cells have been reported to be key players in TKI-mediated off-target effects on the immune system. However, only limited information is available regarding the possible impact of sunitinib on the function of NK cells of individual patients. The present study reports on the immunomonitoring results of three patients with metastatic RCC who underwent sunitinib treatment. These results were compared with age-matched, healthy controls in terms of the immune status of T, B and NK cells, focusing on functional in vitro analyses of NK cells. In all three patients, NK cell number, subset distribution and function, as measured by cluster of differentiation 107a degranulation, did not exhibit any significant alterations as a result of sunitinib treatment. These results indicate that sunitinib does not negatively affect NK cell function, which supports the pursuit of therapeutic modalities that combine immunomodulation and NK cell-stimulating approaches.
Keywords: natural killer cells, renal cell carcinoma, tyrosine kinase inhibitor, tumor immunology, sunitinib, immunomonitoring
Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of cancer cases worldwide (1). The majority of patients with RCC are aged >60 years, and the known risk factors for the disease include cigarette smoking, obesity, high blood pressure, advanced renal disease that requires dialysis, male gender and certain germ line mutations; however, hormonal changes, analgesic abuse, viral hepatitis or exposure to trichloroethylene, cadmium and other potential carcinogens are controversial with respect to their role in RCC risk (2–5). The majority of cases of RCC are sporadic, whereas hereditary forms comprise only 2–3% of all cases (6). The most common subtypes of RCC are clear cell RCC (70–75% of cases), papillary RCC (10–16%) and chromophobe RCC (5%), which primarily metastasize to the lung, liver, bones and brain (7). Patients with RCC are usually asymptomatic and are often coincidentally diagnosed during an imaging procedure; as a result, 25–30% of patients already have metastatic RCC (mRCC) at the time of diagnosis (8). Although the number of available treatment options has increased, the survival of patients with mRCC remains poor. As RCCs rarely respond to chemotherapy or radiation, the therapeutic options predominantly include surgical interventions and immunomodulatory approaches. These include treatment with cytokines, such as interferon (IFN)-α and interleukin-2(IL-2). Novel treatments, referred to as targeted therapies, include various tyrosine kinase inhibitors (TKIs), such as sunitinib, sorafenib, pazopanib and axitinib, as well as the monoclonal antibody bevacizumab, which each act on different pathways that inhibit tumor angiogenesis (9). Alternatively, drugs that inhibit mammalian target of rapamycin, such as temsirolimus or everolimus, may be used as targeted therapies in patients with mRCC.
In their treatment guidelines for RCC, the European Association of Urology concluded that metastasectomy remains the most adequate local treatment for mRCC, with the exception of cases of brain and bone metastases, which are more likely to benefit from radiotherapy in terms of symptomatic relief (2). In cases in which systemic treatment is necessary, the TKI sunitinib is one of the first-line treatments (10–15).
Little is known about the specific effects of sunitinib on the immune system in different individuals. However, the interaction of sunitinib with the natural killer (NK) cells in the patient is of particular interest (16). NK cells are able to mediate antitumor effects and have thus been used for NK cell adoptive therapy in recent clinical trials (17). NK cells have further been reported as key players in TKI-mediated off-target effects on the immune system (18–20).
In the current study, blood samples from three patients with mRCC who underwent treatment with sunitinib were analyzed. In addition, blood samples from age-matched, healthy controls were analyzed, and a comparison of leukocyte counts and immune statuses of T, B and NK cells was conducted. Furthermore, functional in vitro analyses were performed to investigate whether NK cells may be affected by sunitinib treatment.
Materials and methods
Patients and sample preparation
The current immunomonitoring study was approved by the ethics committee of the Friedrich-Alexander University Erlangen-Nuremberg (Erlangen, Germany), according to the Declaration of Helsinki (approval no. 4146). All patients provided written informed consent.
Samples (10–20 ml heparinized peripheral blood) were collected from 3 patients between June 2010 and June 2011. The inclusion criteria for patients included a minimum age of 18 years and a confirmed mRCC diagnosis. The samples were collected prior to TKI administration (day 0) and then at 3–5, 8–10, 17–23 and 48 (1 patient only) weeks after the start of TKI treatment. Of the 3 patients, 2 were followed-up subsequent to the termination of TKI therapy. Peripheral blood mononuclear cells (PBMCs) were isolated from the samples by density gradient centrifugation (Pancoll human; Pan-Biotech GmbH, Aidenbach, Germany).
Control group characteristics
The control group consisted of four healthy individuals with an age range of 44–60 years. These individuals were monitored over 91 days. Blood samples were obtained on days 0, 60 and 91.
Flow cytometry
The following mouse anti-human monoclonal antibodies and antibody conjugates were used in different panels: Anti-T-cell receptor α/β-allophycocyanin (APC; dilution, 1:100; cat. no., 130-091-237) and anti-cluster of differentiation (CD) 335-APC (dilution, 1:30; cat. no., 130-092-609; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany); anti-CD4-phycoerythrin (PE) -Cy7 (dilution, 1:100; cat. no., 557852), streptavidin-APC-Cy7 (dilution, 1:500; cat. no., 554063), anti-CD314-biotin (dilution, 1:50;cat. no., 552866) andanti-CD127-PE (dilution, 1:300;cat. no., 557938; BD Pharmingen, San Diego, CA, USA);anti-CD25-PE (dilution, 1:60; cat. no., 341,011), anti-CD336-PE (dilution, 1:50; cat. no., 558563), anti-CD8-peridinin chlorophyll (PerCP; dilution, 1:100; cat. no., 345774), anti-CD117-PerCP-Cy5.5 (dilution, 1:50; cat. no., 333950), anti-CD19-V450 (dilution, 1:300; cat. no., 560353) and anti-CD3-V450 (dilution, 1:200;cat. no., 560366;BD Biosciences, Franklin Lakes, NJ, USA), anti-CD3-APC-Cy7 (dilution, 1:300; cat. no., 300318), anti-CD16-PE-Cy7 (dilution, 1:300; cat. no., 302016), anti-CD19-PerCP (dilution, 1:50; cat. no., 302228), anti-CD107a-PE (dilution, 1:10; cat. no., 328608), anti-CD56-fluorescein isothiocyanate (FITC; dilution, 1:50; cat. no., 318304) andanti-CD19-PE-Cy7 (dilution, 1:100; cat. no., 302216; Biolegend, Inc., San Diego, CA, USA); anti-CD27-FITC (dilution, 1:70; cat. no. MHCD2701) and anti-CD45-PE (dilution, 1:200; cat no., MHCD4504; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) andanti-CD159a-biotin (dilution, 1:30; cat. no., PNIM2750; Beckman Coulter, Inc., Brea, CA, USA).
A total of 2×106 PBMCs with antibodies were incubated for 10 min at 4°C in the dark for each staining procedure. The PBMCs were then washed with phosphate-buffered saline (PBS) for 4 min and then suspended in PBS. Staining with DAPI was performed directly prior to cytometric analysis (dilution, 1:6,000, 4°C, 2 min) to identify dead cells and isotype controls (IgG1-PE, IgG2a-FITC, IgG1-PerCP) were performed prior to analyzing the samples with fluorescence-activated cell sorting (FACS). The samples were examined using a BDFACSCanto II™ (BD Biosciences) with three lasers (488, 633 and 405 nm). The experiment was repeated at least five times before the start of analyzing patients' data, and then performed once with each patient sample. Data were analyzed by FlowJo 7.6.5 software (FlowJo, LLC, Ashland, OR, USA).
Functional in vitro assays
To determine the activity of NK cells, the extent of degranulation was evaluated by CD107a staining following the incubation of whole blood samples with target cells. Cells from the human MHC-I deficient erythroleukaemic cell line K562 (American Type Culture Collection, Manassas, VA, USA) were used as the target cells. The cells were maintained in Iscove's modified Dulbecco's medium (Invitrogen; Thermo Fisher Scientific, Inc.) containing 10% FCS (Invitrogen; Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin.
A total of 200 µl from the heparinized whole blood samples was incubated with 2×105 K562 target cells for 3 h at 37°C, as previously described by Claus et al (21). Erythrocyte lysis was then performed in an ammonium-chloride-potassium buffer for 10 min at 4°C; the lysis procedure was repeated 2–3 times for each sample. In order to stain the cells for FACS, PBMC were incubated with anti-CD107a-PE, anti-CD3-APC-Cy7, anti-CD19-PerCP, anti-CD56-FITC, anti-CD16-PE-Cy7 or anti-CD335-APC (as described in the Flow Cytometry section) for 10 min at 4°C. Staining with DAPI was performed, as previously described, to identify dead cells. For each patient at each time point, the extent of CD107a degranulation was measured in triplicate. To serve as a control for spontaneous degranulation, PBMCs from the samples were incubated without target cells. The FACS data were analyzed using FlowJo 7.6.5 software; the NK cell population was characterized as CD56+, CD3− and CD19−, once dead cells and doublets had been excluded. The mean CD56+ and CD107a+values of three repetitions of the experiment and the control were then presented in the figures.
To measure IFN-γ production, 2×105 freshly isolated PBMC were treated with 100 U/ml or 1,000 U/ml IL-2 (Novartis International AG, Basel, Switzerland) for 24 h in a 96-well round bottom microplate (BD Biosciences) with 200 µl cell culture medium. Supernatants were removed to determine IFN-γ concentration with an ELISA kit (BD OptEIA™ Set; BD Biosciences). Control samples were incubated without IL-2. For each condition (control, 100 U/ml IL2 and 1,000 U/ml IL2) triplicates were performed.
Statistical methods
All analyses were performed using Prism software (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance was calculated with the Mann-Whitney U-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
Three patients with advanced mRCC were selected for a case study; these patients were patients A, B and C (Table I). Patient A, a 60-year-old male, presented with clear cell RCC of stage T3b-N2-M1, with peritoneal carcinomatosis and pulmonary, osseous and muscular metastases. Patient B, a 63-year-old male, experienced multiple osseous, pulmonary, and chest wall metastases from a clear cell RCC. Patient C, a 68-year-old female, presented with a stage pT3a-pNx-cM1 clear cell RCC with sarcomatoid features, as well as multiple pulmonary and mediastinal lymph node metastases.
Table I.
Characteristics of three selected patients with metastatic RCC.
| Patient | Gender | Age, years | Diagnosis | TNM stage | Metastasis | First-line TKI |
|---|---|---|---|---|---|---|
| A | Male | 60 | RCC, clear cell | T3b-N2-M1 | Osseous, peritoneal, pulmonary, muscular | Sunitinib |
| B | Male | 63 | RCC, clear cell | No primary tumor detectable | Osseous, pulmonary, chest wall | Sunitinib |
| C | Female | 68 | RCC, clear cell, sarcomatoid | pT3a-pNx-cM1 | Pulmonary, mediastinal lymph nodes | Sunitinib |
RCC, renal cell carcinoma; TNM, tumor-node-metastasis; TKI, tyrosine kinase inhibitor.
All three patients were treated orally with 50 mg of sunitinib (Sutent®; Pfizer, Inc., New York, NY, USA) once daily for 4 weeks, followed by a 2-week break, in accordance with prescribing information. Patient B underwent a dosage reduction to 37.5 mg daily after 8 weeks due to adverse effects. Patients B and C experienced disease progression, which led to a change from sunitinib to sorafenib for patient C on day 98 and to everolimus for patient B on day 126.
Patients A and B received sunitinib as a first-line therapy prior to surgery, whereas patient C received sunitinib as a first-line therapy immediately subsequent to nephrectomy.
Immunomonitoring during treatment with sunitinib
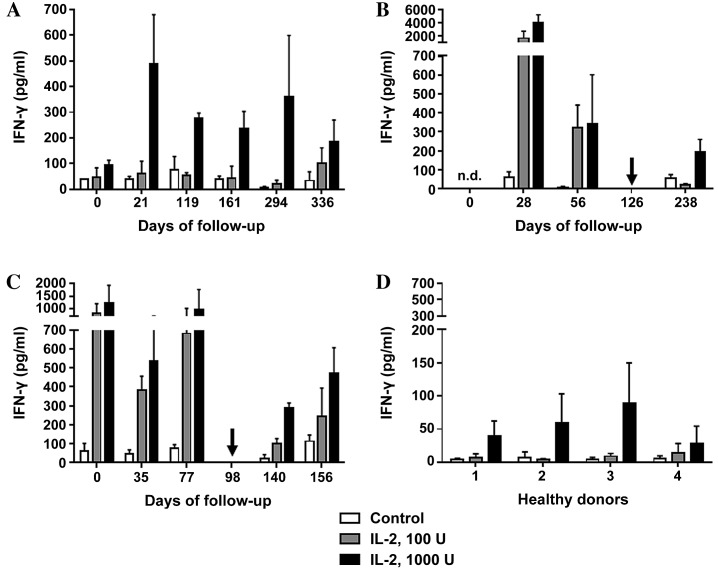
To evaluate the effects of sunitinib treatment on the immune system of patients with mRCC, blood samples were collected at various time points prior to, during and subsequent to TKI treatment. These samples were analyzed and the following parameters were compared with those of the control group: Leukocyte count (Fig. 1); immunomonitoring of T, B, and NK cells (Fig. 2); ratio of NK cell subsets (CD16bright:CD16dim; Fig. 3); NK cell function, as measured by degranulation capacity (Fig. 4) and IFN-γ production by PBMCs (Fig. 5).
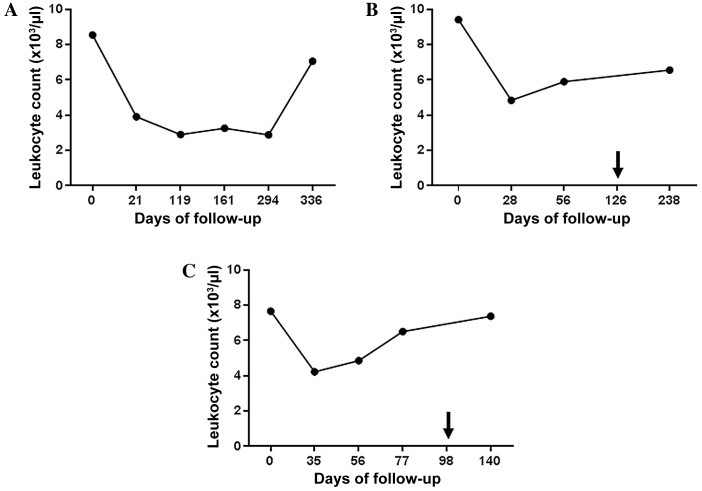
Figure 1.
Immunomonitoring of leukocyte count. Graphs show the leukocyte counts of (A) patient A, (B) patient B and (C) patient C, prior to, during and subsequent to therapy with the tyrosine kinase inhibitor sunitinib. Arrows indicate the time points when the treatment regimen was changed (fromsunitinib to everolimus in patient B, and from sunitinib to sorafenib in patient C).
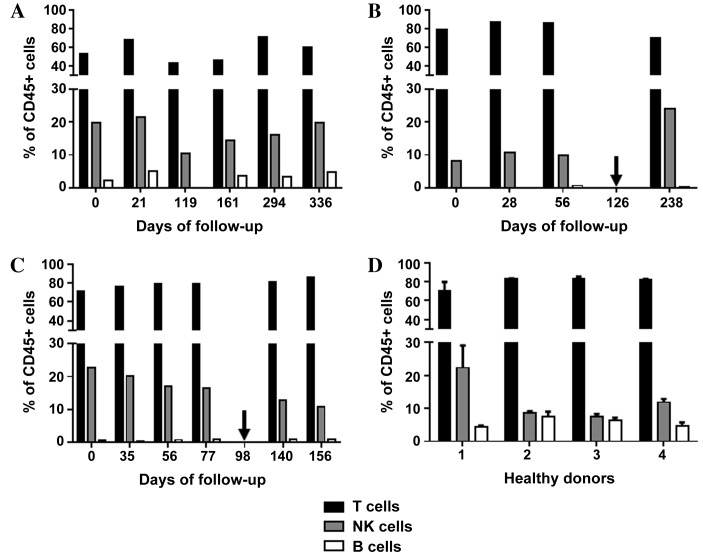
Figure 2.
Immunomonitoring of T, NK, and B cells. Graphs show the levels of T cells, NK cells and B cells as a percentage of all CD45+ cells in (A) patient A, (B) patient B and (C) patient C, prior to, during and subsequent to therapy with the tyrosine kinase inhibitor sunitinib. Arrows indicate the time points when the treatment regimen was changed (from sunitinib to everolimus in patient B, and from sunitinib to sorafenib in patient C). (D) The means+ standard deviations of three measurements are included for each of the healthy donor controls (nos. 1–4). NK cells, natural killer cells; CD45, cluster of differentiation 45.
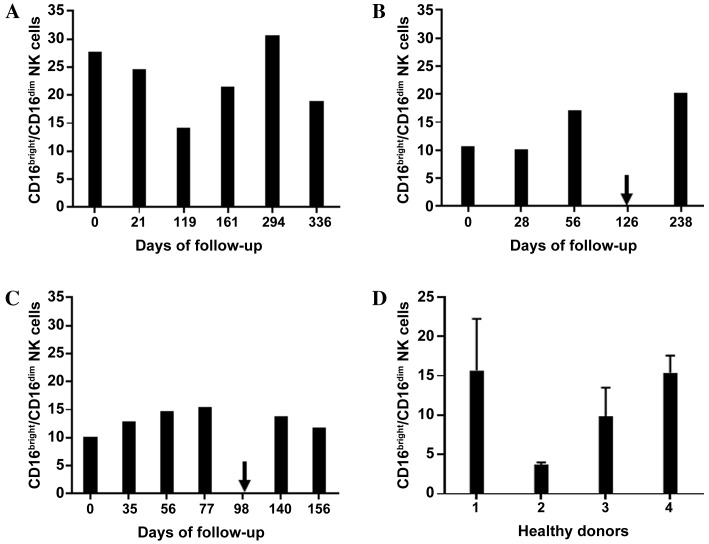
Figure 3.
Ratio of subsets of NK cells. Graphs show the ratios of CD16bright:CD16dim NK cells in (A) patient A, (B) patient B and (C) patient C prior to, during and subsequent to therapy with the tyrosine kinase inhibitor sunitinib. Arrows indicate the time points when the treatment regimen was changed (from sunitinibto everolimus in patient B, and from sunitinibto sorafenib in patient C). (D) The mean + standard deviation of three measurements are shown for each of the healthy donor controls (nos. 1–4). NK cells, natural killer cells; CD16, cluster of differentiation 16.
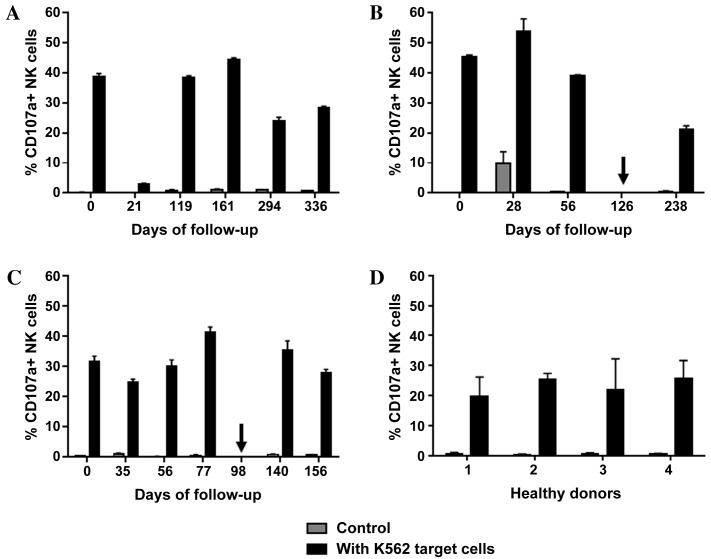
Figure 4.
NK cell degranulation. For each patient and time point, triplicate experiments with and without (control) K562 target cells were performed. The data presented includes mean + standard deviation. The proportion of CD107a+ NK cells in (A) patient A, (B) patient B and (C) patient C prior to, during and subsequent to therapy with the tyrosine kinase inhibitor sunitinib are presented. Arrows indicate the time points when the treatment regimen was changed (from sunitinib to everolimus in patient B, and from sunitinib to sorafenib in patient C). (D) Measurements of CD107a+ proportion from day 0, 60 and 91 are included for each of the healthy donor controls (nos. 1–4). NK cells, natural killer cells; CD16, cluster of differentiation 16.
Figure 5.
IFN-γ production by peripheral blood mononuclear cells. Cells were incubated with 0 U (control), 100 U or 1,000 U of IL-2, and the concentration of IFN-γ produced was measured in (A) patient A, (B) patient B and (C) patient C prior to, during and subsequent to therapy with the tyrosine kinase inhibitor sunitinib. All data are presented as the mean + standard deviation from triplicate experiments. Arrows indicate the time points when the treatment regimen was changed (from sunitinib to everolimus in patient B, and from sunitinib to sorafenib in patient C). (D) Measurements of IFN-γ concentration on day 0, 60 and 91 are included for each of the healthy donor controls (nos. 1–4). IFN-γ, interferon γ; IL-2, interleukin 2; n.d., no data.
Patient A
A significant decrease in total leukocyte count from 8.5×103/µl to 3.9×103/µl was observed in patient A within the first four months of sunitinib therapy, which then stabilized and began to rise marginally between days 294 and 336 (Fig. 1A). Out of the total number of lymphocytes, the percentage of T cells fluctuated between 43 and 71% during follow-up. The percentage of B cells marginally increased between days 0 and 21, and then stabilized slightly higher than the baseline level from day 161 until the end of the study. The percentage of NK cells decreased by 50% (from 21.5 to 10.4%) between days 21 and 119 and then recovered to baseline (Fig. 2A). As shown in Fig. 3A, the ratio of CD16bright:CD16dim NK cell subsets decreased between days 0 and 119; however, by day 294, it increased again to a level higher than that on day 0. NK cell degranulation, which serves as an indication of NK cell activity, appeared to fluctuate, although the percentage of CD107a+ cells of the total NK cell count remained at a higher level than that in the healthy donor group, with the exception of the values at day 21 (Fig. 4A). The production of IFN-γ by PBMCs following in vitro stimulation with 1,000 U/ml IL-2 exhibited a significant increase at all time points compared with the starting point (Fig. 5A).
Patient B
Patient B exhibited a decrease in the total leukocyte count from 9.4×103/µl to 4.8×103/µl within the first month of sunitinib treatment. Subsequently, the total leukocyte count increased during continued sunitinib treatment; however, it did not reach the initial level measured at day 0 (Fig. 1B). Out of the total number of lymphocytes, the percentages of T and NK cells during sunitinib treatment remained relatively stable at 79–87 and 8–10%, respectively. However, the percentage of NK cells increased to 24% when sunitinib treatment ended. The percentage of B cells in this patient remained low (~0.5%) at all time points (Fig. 2B) compared with the percentages of B cells in individuals from the healthy donor group (Fig. 2D). The ratio of CD16bright:CD16dim NK cell subsets increased from 10.4 on day 0 to 16.9 on day 56, and continued to increase following the discontinuation of sunitinib treatment (Fig. 3B). NK cell degranulation, as shown in Fig. 4B, marginally decreased from 45% of CD107a+of the total NK cell count on day 0 to 39% on day 56, and further decreased to 21% following the discontinuation of sunitinib treatment and the commencement of everolimus treatment on day 126. No data are available regarding IFN-γ production on day 0 as an insufficient number of cells were obtained to allow for the isolation of PBMCs; however, on day 28, the production of IFN-γ following in vitro stimulation with 1,000 U/ml IL-2 reached an extremely high level of 3,980 pg/ml. This value decreased under continued sunitinib treatment and further decreased following the change in TKI therapy (Fig. 5B).
Patient C
In patient C, a decrease in the total leukocyte count from 7.6×103/µl to 4.2×103/µl on day 35 of sunitinib treatment was observed. Subsequently, the number increased, reaching 6.5×103/µl on day 77, which was the last follow-up time point prior to the end of sunitinib treatment and the initiation of sorafenib treatment on day 98 (Fig. 1C). Out of the total number of lymphocytes, the percentage of T cells during and following sunitinib treatment remained stable at 71–79%, while the percentage of B cells also remained stable at a low level during the entire observation period. The percentage of NK cells during treatment decreased marginally from 22% on day 0 to 16% on day 77. At 5 months after the change in treatment from sunitinib to sorafenib, the percentage of NK cells had further decreased to 10% (Fig. 2C). The ratio of CD16bright:CD16dim NK cell subsets increased continuously from 9.9 on day 0 to 15.3 on day 77, and marginally decreased again following the change in TKI treatment (Fig. 3C). NK cell degranulation in this patient, measured as the percentage of CD107a+ NK cells, reached various values, ranging from 24% (day 35) to 40% (day 77); this value decreased marginally, compared with that on day 77, following the change in TKI treatment (Fig. 4C). PBMC-associated IFN-γ production following in vitro stimulation with 1,000 U/ml IL-2 led to high IFN-γ values (>200 pg/ml) at baseline and throughout the sunitinib treatment period (Fig. 5C).
Comparison of patients A, B, and C with the healthy control group
There was a decrease in the total leukocyte count from 8.5±0.87×103/µl on day 0 to 4.3±0.48×103/µl after the first month of treatment within the patient population. Thereafter, leukocyte counts stabilized and increased again, but did not return to the initial levels by the end of the observation period (Fig. 1).
In order to assess the relative abundance of different lymphocyte populations, the means ± SD of all data throughout the whole observation period were evaluated. The proportion of T cells in the total number of lymphocytes remained relatively stable in all patients (patient A, 57.1%±11.5; patient B, 80.7%±7.6; patient C, 78.9%±5.1) and in the control group (79.5%±7.4). The proportion of B cells was also relatively stable in the patient group (patient A, 3.83%±1.1; patient B, 0.5%±0.3; patient C, 0.8%±0.2). Compared with the control group (5.6%±1.5), the proportion of B cells was observed to be lower in patient samples at all time points, including prior to the initiation of sunitinib treatment. The relative abundance of NK cells was 12.9%±6.9 in the control group and 7–21% in the mRCC group (patient A, 16.9%±4.1; patient B, 13.2%±7.2; patient C, 16.7%±4.4). There was a wide range of NK proportions in patients and controls, but no significant alteration in NK proportion was observed following sunitinib treatment (Fig. 2).
The ratio of CD16bright:CD16dim NK cell subsets varied within the mRCC and the control groups. No trend was identified in terms of alterations in the NK cell subset ratio during sunitinib treatment (Fig. 3).
NK cell degranulation, as measured by the percentage of CD107a-expressing NK cells, was efficiently stimulated by the incubation with K562 target cells in the mRCC and healthy donor groups. There was a significant increase of CD107a surface expression in the mRCC patient and healthy donor samples compared with the control specimens of the same source incubated without target cells (co-culture with K562 target cells vs. control without K562 target cells; P<0.0001; Fig. 4). Within the mRCC group, no significant trend was observed with regard to NK cell degranulation during sunitinib treatment. The expression of CD107a after stimulation with K562 cells was significantly higher at all time points in patients compared with the healthy donor group (P=0.011). The mean ± SD of all time points were as follows: Patient A, 34.8%±8.4; patient B, 39.9%±13.8; patient C, 31.9%±5.8 compared with the healthy donor group 23.2%±2.8 (P=0.011).
PBMC-associated IFN-γ production was efficiently induced by 24 h of incubation following in vitro stimulation with 1,000 U/ml IL-2 in the patient and healthy donor groups. Notably, higher mean values were measured in the patient group (mean ± SD of all data measured at different time points, as follows: Patient A, 273±137 pg/ml; patient B, 506±2,144 pg/ml; patient C, 699±385 pg/ml) compared with the control group (54±27 pg/ml; P<0.001, patients vs. healthy donors treated with 1000 U/ml IL-2; Fig. 5).
Discussion
Within the last two decades, numerous new strategies have been developed to improve overall survival, quality of life and disease regression in patients with advanced RCC (1,4–6,16,19), and TKI therapy has been shown to be an important treatment modality in addressing these objectives (10,12,13). Nevertheless, data are limited on how TKIs may interact with the immune system of an individual patient (16,19). The present study analyzed the influence of the TKI sunitinib on the cellular immunity of three patients with mRCC, focusing specifically on NK cell phenotype and functionality as potential targets for novel immunomodulatory treatment options.
A decrease in the total leukocyte count was observed in all patients within the first month of sunitinib therapy; however, this stabilized and increased again during continuous TKI treatment. Although patients with mRCC, as compared with healthy controls, appeared to have a lower percentage of B cells even prior to treatment, the percentages of T and B cells out of the total number of lymphocytes remained relatively stable over the observation period. A decline in the total number of T cells, as previously demonstrated by Powles et al (22), could not be confirmed in the present study. The percentages of NK cells were variable within the patient and control groups. These variations maybe the result of several factors that inhibit or activate NK cells, such as different pre-treatment regimens, states of disease or concomitant diseases. Notably, no elevated infection rate was observed in the sunitinib-treated patients.
Importantly, in all three patients, NK cell number, subset distribution, and extent of degranulation did not exhibit any significant changes that could be associated with sunitinib treatment. The present study has confirmed in vivo what was previously demonstrated by Krusch et al (16) in vitro; specifically, there is no negative influence to NK cells CD107a expression and no decline in cytokine production, including of IFN-γ, by PBMC when treated with sunitinib. The enhanced activity rate of PBMC and NK cells during and prior to therapy with sunitinib may be associated with the underlying disease mechanics of mRCC. Notably, an increased activity of the immune system such as elevated secretion of IFN-γ, IL-2 and IL4, by CD4+ cells in patients with renal cancer has been described (23–26).
In conclusion, the current case series revealed no negative impact on NK cell number or function under sunitinib treatment. B cell percentages appeared to be reduced in the mRCC patients independently of the sunitinib therapy. B and T cell counts were not observed to be negatively affected by continued sunitinib treatment. The limitations of the current study include the small numbers of mRCC patients and controls. Nevertheless, the results suggest that treatment strategies involving NK cells, such as adoptive NK cell transfer (17,19), may potentially be used in combination with sunitinib and may offer a feasible treatment option for patients with mRCC in the future. Further trials are required to evaluate the effects of TKIs in larger cohorts.
Acknowledgements
The authors would like to thank Dr Irena Kroeger, Dr Stephanie Gerstner and Ms. Julia Schneider (Department of Internal Medicine 5, Hematology and Oncology, University Hospital Erlangen, Friedrich-Alexander University Erlangen-Nuremberg, D-91054 Erlangen, Germany) for their experimental and technical support. They are also grateful to Dr Sara Tognarelli (LOEWE Center for Cell and Gene Therapy, Goethe University Frankfurt, Germany) for proof reading and discussing the manuscript. The authors were supported by German Cancer Aid (Max Eder Nachwuchsgruppe, Deutsche Krebshilfe) and by the LOEWE Center for Cell and Gene Therapy (Frankfurt, Germany), funded by the Hessian Ministry of Higher Education, Research and the Arts, Germany (grant no. III L 4-518/17.004).
Glossary
Abbreviations
- APC
allophycocyanin
- CD
cluster of differentiation
- FACS
fluorescence-activated cell sorting
- FITC
fluorescein isothiocyanate
- IFN
interferon
- IL-2
interleukin 2
- NK cells
natural killer cells
- mRCC
metastatic renal cell carcinoma
- PBMC
peripheral blood mononuclear cell
- PE
phycoerythrin
- PerCP
peridinin chlorophyll
- RCC
renal cell carcinoma
- SD
standard deviation
- TKI
tyrosine kinase inhibitor
References
- 1.Lyon: Eurocim version 4.0. European incidence database V2.3, 730 entity dictionary. European Network of Cancer Registries. 2001 [Google Scholar]
- 2.Ljungberg B, Bensalah K, Bex A, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, et al. Guidelines on Renal Cell Carcinoma. Eur Assoc Urol. 2014 doi: 10.1016/j.eururo.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Ljungberg B, Campbell SC, Choi HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. doi: 10.1016/S0140-6736(09)60229-4. [DOI] [PubMed] [Google Scholar]
- 5.Macleod LC, Hotaling JM, Wright JL, Davenport MT, Gore JL, Harper J, White E. Risk factors for renal cell carcinoma in the VITAL study. J Urol. 2013;190:1657–1661. doi: 10.1016/j.juro.2013.04.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67:519–530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Shuch B, Amin A, Armstrong AJ, Eble JN, Ficarra V, Lopez-Beltran A, Martignoni G, Rini BI, Kutikov A. Understanding pathologic variants of renal cell carcinoma: Distilling therapeutic opportunities from biologic complexity. Eur Urol. 2015;67:85–97. doi: 10.1016/j.eururo.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Gupta K, Miller JD, Li JZ, Russell MW, Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): A literature review. Cancer Treat Rev. 2008;34:193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Schmidinger M. Improving outcomes in metastatic clear cell renal cell carcinoma by sequencing therapy. American Society of Clinical Oncology educational book/ASCO. American Society of Clinical Oncology; Meeting; 2014; pp. e228–e238. [DOI] [PubMed]
- 10.Dranitsaris G, Schmitz S, Broom RJ. Small molecule targeted therapies for the second-line treatment for metastatic renal cell carcinoma: A systematic review and indirect comparison of safety and efficacy. J Cancer Res Clin Oncol. 2013;139:1917–1926. doi: 10.1007/s00432-013-1510-5. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Albiges L, Sonpavde G. Optimal management of metastatic renal cell carcinoma: Current status. Drugs. 2013;73:427–438. doi: 10.1007/s40265-013-0043-1. [DOI] [PubMed] [Google Scholar]
- 12.Schnadig ID, Hutson TE, Chung H, Dhanda R, Halm M, Forsyth M, Vogelzang NJ. Dosing patterns, toxicity, and outcomes in patients treated with first-line sunitinib for advanced renal cell carcinoma in community-based practices. Clin Genitourin Cancer. 2014;12:413–421. doi: 10.1016/j.clgc.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 13.Kim S, Ding W, Zhang L, Tian W, Chen S. Clinical response to sunitinib as a multitargeted tyrosine-kinase inhibitor (TKI) in solid cancers: A review of clinical trials. Onco Targets Ther. 2014;7:719–728. doi: 10.2147/OTT.S61388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poprach A, Pavlik T, Melichar B, Kubackova K, Bortlicek Z, Svoboda M, Lakomy R, Vyzula R, Kiss I, Dusek L, et al. Clinical and laboratory prognostic factors in patients with metastatic renal cell carcinoma treated with sunitinib and sorafenib after progression on cytokines. Urol Oncol. 2014;32:488–495. doi: 10.1016/j.urolonc.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Dudek AZ, Zolnierek J, Dham A, Lindgren BR, Szczylik C. Sequential therapy with sorafenib and sunitinib in renal cell carcinoma. Cancer. 2009;115:61–67. doi: 10.1002/cncr.24009. [DOI] [PubMed] [Google Scholar]
- 16.Krusch M, Salih J, Schlicke M, Baessler T, Kampa KM, Mayer F, Salih HR. The kinase inhibitors sunitinib and sorafenib differentially affect NK cell antitumor reactivity in vitro. J Immunol. 2009;183:8286–8294. doi: 10.4049/jimmunol.0902404. [DOI] [PubMed] [Google Scholar]
- 17.Terme M, Ullrich E, Delahaye NF, Chaput N, Zitvogel L. Natural killer cell-directed therapies: Moving from unexpected results to successful strategies. Nat Immunol. 2008;9:486–494. doi: 10.1038/ni1580. [DOI] [PubMed] [Google Scholar]
- 18.Geisler K, Reischer A, Kroeger I, Jacobs B, Meinhardt K, Bauer R, Ryffel B, Mackensen A, Ullrich E. Nilotinib combined with interleukin-2 mediates antitumor and immunological effects in a B16 melanoma model. Oncol Rep. 2014;31:2015–2020. doi: 10.3892/or.2014.3070. [DOI] [PubMed] [Google Scholar]
- 19.Krieg S, Ullrich E. Novel immune modulators used in hematology: Impact on NK cells. Front Immunol. 2013;3:388. doi: 10.3389/fimmu.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mignot G, Ullrich E, Bonmort M, Ménard C, Apetoh L, Taieb J, Bosisio D, Sozzani S, Ferrantini M, Schmitz J, et al. The critical role of IL-15 in the antitumor effects mediated by the combination therapy imatinib and IL-2. J Immunol. 2008;180:6477–6483. doi: 10.4049/jimmunol.180.10.6477. [DOI] [PubMed] [Google Scholar]
- 21.Claus M, Greil J, Watzl C. Comprehensive analysis of NK cell function in whole blood samples. J Immunol Methods. 2009;341:154–164. doi: 10.1016/j.jim.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Powles T, Chowdhury S, Bower M, Saunders N, Lim L, Shamash J, Sarwar N, Sadev A, Peters J, Green J. The effect of sunitinib on immune subsets in metastatic clear cell renal cancer. Urol Int. 2011;86:53–59. doi: 10.1159/000319498. [DOI] [PubMed] [Google Scholar]
- 23.Kondo T, Nakazawa H, Ito F, Hashimoto Y, Osaka Y, Futatsuyama K, Toma H, Tanabe K. Favorable prognosis of renal cell carcinoma with increased expression of chemokines associated with a Th1-type immune response. Cancer Sci. 2006;97:780–786. doi: 10.1111/j.1349-7006.2006.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olive C, Cheung C, Nicol D, Falk MC. Expression of cytokine mRNA transcripts in renal cell carcinoma. Immunol Cell Biol. 1998;76:357–362. doi: 10.1046/j.1440-1711.1998.00759.x. [DOI] [PubMed] [Google Scholar]
- 25.Onishi T, Ohishi Y, Goto H, Tomita M, Abe K. An assessment of the immunological status of patients with renal cell carcinoma based on the relative abundance of T-helper 1- and -2 cytokine-producing CD4+ cells in peripheral blood. BJU Int. 2001;87:755–759. doi: 10.1046/j.1464-410x.2001.02210.x. [DOI] [PubMed] [Google Scholar]
- 26.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: A potential new link between adaptive and innate immunity. Blood. 2003;101:3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]