Abstract
Smoking and alcohol consumption are major risk factors for the development of esophageal squamous cell carcinoma (ESCC). Recent studies have demonstrated that smoking and alcohol consumption may be associated with altered DNA methylation in human cancer development. The aim of the present study was to evaluate methylation-modulated protein expression of tumor-related genes (TRGs) in the early stages of esophageal squamous neoplasia (ESN). ESN tissue samples (n=141) comprising 19 cases of low-grade intraepithelial neoplasia (LGIN), 70 of high-grade intraepithelial neoplasia/carcinoma in situ (HGIN/CIS) and 52 of invasive cancer, were endoscopically resected. The methylation-modulated protein expression of 5 TRGs [fragile histidine triad (FHIT), E-cadherin, MutL homolog 1 (MLH1) /MutS homolog 2 (MSH2) and cyclooxygenase-2 (COX-2)] as well as p53 was examined with immunohistochemistry, and their expression was compared with patient clinicopathological characteristics. Reduced or loss of FHIT, E-cadherin, MLH1/MSH2 and COX-2 expression was detected in 26.3 (5/19), 5.3 (1/19), 0 (0/19) and 63.2% (12/19) of LGIN cases, 61.4 (43/70), 18.6 (13/70), 7.1 (5/70) and 65.7% (46/70) of HGIN/CIS cases, and 78.8 (41/52), 50.0 (26/52), 11.5 (6/52) and 59.6% (31/52) of invasive cancer cases, respectively. Reduced or absent expression of FHIT and E-cadherin was significantly associated with neoplastic progression (FHIT, P=0.0007; E-cadherin, P=0.00014). The mean number of TRGs (FHIT, E-cadherin, MLH1/MSH2, and COX-2) that exhibited reduced or absent expression in LGIN, HGIN/CIS and invasive cancer specimens was 1.12±0.61, 1.66±0.93 and 2.09±0.96, respectively, demonstrating a significant stepwise increment from LGIN to HGIN/CIS and then to invasive cancer (P<0.05). p53 overexpression was frequently detected in ESN with head and neck carcinomas. However p53 overexpression was not significantly associated with ESN progression. An increase in the number of the 5 TRG proteins with reduced or loss of expression in the early stages of esophageal tumorigenesis was demonstrated, and their decreased expression was observed to be associated with tumor progression. Therefore, smoking and alcohol drinking may be associated with not only carcinogenesis but also the progression of ESN.
Keywords: esophageal cancer, endoscopic resection, fragile histidine triad, E-cadherin, MutL homolog 1
Introduction
In Japan, esophageal squamous cell carcinoma (ESCC) accounts for >90% of esophageal cancer cases (1). As ESCC is frequently detected at an advanced stage, it has one of the worst prognoses among digestive carcinomas (1). Therefore, early detection of malignant changes in the esophageal epithelium is the most effective way to improve the prognosis for ESCC. Detection at an early stage is gradually becoming more feasible due to advances in endoscopy, and the number of cases that can be potentially treated endoscopically is increasing (2).
A variety of genetic and epigenetic alterations are associated with esophageal carcinoma (3,4), including mutation of the p53 gene (5,6). A number of previous studies have concluded that p53 is the most frequently mutated gene in ESCC (7,8). The role of epigenetic changes, including aberrant DNA methylation, is particularly significant in human cancer development (9,10). An increasing number of tumor-related genes (TRGs) that are inactivated by the hypermethylation of CpG islands have been reported in esophageal cancer, including ESCC (11,12). However, the role of hypermethylation of CpG islands in early neoplastic lesions is not well understood.
In esophageal carcinomas, FHIT, E-cadherin and MLH1/MSH2 have been previously demonstrated to be inactivated through hypermethylation of their promoters (13–17). These genes may be inactivated by a combination of genetic or epigenetic alterations of two alleles. However, epigenetic change is likely to be the predominant mechanism associated with the loss of FHIT, E-cadherin and MLH1/MSH2 function in sporadic esophageal carcinomas (13–17).
Numerous epidemiological studies have identified tobacco smoking and alcohol consumption as the major risk factors for the development of ESCC (18,19). Increasing evidence indicates that smoking and alcohol induce epigenetic alterations, particularly aberrant patterns of DNA methylation. Such alterations to methylation are induced by tobacco and alcohol consumption in the development of ESCC (20,21), and may be important contributing factors in carcinogenesis (20,22).
In the present study, the clinical significance of TRG protein expression was evaluated during the early stages of esophageal carcinogenesis. Previous studies have demonstrated that the DNA methylation status of the TRGs FHIT, E-cadherin, MLH1/MSH2 and COX-2 can be detected by immunohistochemical analysis of tumor specimens (13–17,23). Therefore, the protein expression of these 5 TRGs and p53 was analyzed by immunostaining in 141 early esophageal neoplasia tumor specimens, comprising 19 low-grade intraepithelial neoplasia (LGIN), 70 high-grade intraepithelial neoplasia/carcinoma in situ (HGIN/CIS) and 52 invasive cancer tissue samples extracted during endoscopic resection.
Patients and methods
Patient and tissue samples
A total of 141 esophageal squamous neoplasia (ESN) tumor specimens were obtained from 112 patients (including 102 males and 10 females) who underwent endoscopic resection at Tottori University Hospital (Yonago, Japan) between January 2004 and December 2014. The age of the patients ranged from 43 to 85 years (mean, 68.6 years). None of the patients had received preoperative radiotherapy or chemotherapy. Based on a histological examination, the 141 tumor specimens were classified as 19 cases of low-grade dysplasia, 70 of high-grade dysplasia and 52 of invasive cancer confined to the lamina propria mucosa or submucosa based on the World Health Organization classification (24) (Table I). Among the 112 patients with ESN, 98 (87.5%) had a history of smoking and 99 (88.4%) had a history of drinking. A total of 5 patients (4.4%) were neither smokers nor drinkers. All cases were analyzed anonymously, i.e., all specimens were assigned numbers without any associated personal information. Approval was obtained for the present study from the Institutional Ethics Review Board of Tottori University (grant nos. 314 and 1508A024; Yonago, Japan), which certified that the study complied with the Declaration of Helsinki and written informed consent was obtained from all patients.
Table I.
Clinicopathological characteristics and patient background of patients with early esophageal neoplasia.
| Characteristic | n |
|---|---|
| Gender | |
| Male | 102 |
| Female | 10 |
| Age, mean ± SD, years | 68.6±8.7 |
| Tumor size, mm | |
| <10 | 16 |
| ≥10 to <20 | 51 |
| ≥20 | 74 |
| Tumor location | |
| Upper | 62 |
| Middle | 64 |
| Lower | 15 |
| Macroscopic tumor type | |
| Elevated | 18 |
| Flat | 59 |
| Depressed | 64 |
| Invasion depth | |
| Low-grade intraepithelial neoplasia | 19 |
| High-grade intraepithelial neoplasia/carcinoma in situ | 70 |
| Lamina propria invasion | 27 |
| Muscularis mucosa invasion | 11 |
| Submucosal invasion | 14 |
| Alcohol history | |
| Drinker | 99 |
| Non-drinker | 13 |
| Smoking history | |
| Current/previous smoker | 98 |
| Never smoked | 14 |
| Brinkman indexa, mean ± SD | 791±689 |
| History of head and neck cancer | 9 |
A total of 141 samples were used from 112 patients. SD, standard deviation.
Brinkman index: number of cigarettes/day × years.
Immunohistochemical staining
For immunohistochemical staining, paraffin-embedded, 4-µm-thick tumor sections were first deparaffinized in xylene and rehydrated in ethanol. Subsequently, the sections were immersed in citrate buffer (0.01 M, pH 6.0) and heated in a microwave oven for 20–30 min for antigen retrieval. Endogenous peroxidase activity was blocked by incubation with 3% H2O2 for 30 min at room temperature. The sections were subsequently incubated with a primary antibody overnight at 4°C. The primary antibodies used included rabbit polyclonal anti-FHIT (dilution, 1:100; clone F130; cat. no. 18163; Immuno-Biological Laboratories Co. Ltd., Gunma, Japan), mouse monoclonal anti-p53 (dilution, 1:50; clone DO-7; cat. no. M7001; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA), anti-E-cadherin (dilution, 1:50; clone HECD-1; catalog no. M106; Takara, Bio Inc., Otsu, Japan), anti-MSH2 (dilution, 1:100; clone FE11; cat. no. 33-7900; Oncogene Research Products; EMD Millipore, Billerica, MA, USA), anti-MLH1 (dilution, 1:50; clone G168-15; cat. no. 550838; BD Pharmingen, San Diego, CA, USA) and anti-COX-2 (dilution, 1:100; clone 33/Cox-2; cat. no. 610204; BD Biosciences, Franklin Lakes, NJ, USA) antibodies. As a negative control, the primary antibody was replaced with normal rabbit IgG (cat. no. GTX35035; Gene Tex, Irvine, CA, USA) or normal mouse IgG (cat. no. NC494H; Biocare Medical, Pike Lane Concord, CA, USA) at a similar dilution. Antibody binding was detected by incubating with anti-rabbit (dilution, 1:200; cat. no. BA-1000; Vector Laboratories, Inc., Burlingame, CA, USA) or anti-mouse (dilution, 1:200; cat. no. BA-2000; Vector Laboratories, Inc., Burlingame, CA, USA) with avidin-biotin-peroxidase for 30 min at room temperature, and was then visualized using the chromogen diaminobenzidine tetrahydrochloride (cat. no. SK-4100; Vector Laboratories, Inc., Burlingame, CA, USA). The signal was detected as described in the Vectastain Elite avidin-biotin complex kit protocol (Vector Laboratories, Inc., Burlingame, CA, USA). Hematoxylin was used as a counterstain.
For each specimen, >5 fields were viewed under a light microscope (magnification, ×100) (Olympus Corporation, Tokyo, Japan). Protein expression was evaluated by two independent observers. p53 overexpression was defined as a distinct nuclear immunoreaction in >30% of the cells of the sample.
FHIT expression was graded, based on the intensity of cytoplasmic staining, as ‘reduced’, ‘absent’ or ‘positive’ as described previously (25). FHIT expression in normal esophageal squamous epithelia and normal esophageal glands served as positive controls. The adjacent normal squamous epithelium was used as an internal positive control. FHIT weak positive lesions showed positive reactivity at a level that was weak compared with the level in the normal epithelia, and these lesions were described as reduced.
E-cadherin, MLH1/MSH2 and COX-2 expression were classified as ‘positive’ or ‘decreased’. Cases with staining (E-cadherin, membrane; MLH1/MSH2, nucleus; COX-2, cytoplasm) in <30% of the tumor cells or with a complete absence of staining were categorized as decreased, respectively.
Statistical analysis
Statistical analysis was performed using the χ2 test with the Yates correction or the Newman-Keuls test. P<0.05 was considered to indicate a statistically significant difference. All statistics were calculated using Stat Flex version 6.0 (Artech Co., Ltd, Osaka, Japan).
Results
Immunohistochemical analysis of the expression of TRGs (FHIT, E-cadherin, MLH1/MSH2 and COX-2) in ESN tumors
Representative images of immunohistochemical staining of FHIT, E-cadherin and MLH1/MSH2 in ESN tumors is included in Fig. 1, and the results are quantified in Table II. There was a reduced or loss of FHIT, E-cadherin, MLH1/MSH2 and COX-2 expression in 26.3% (5/19), 5.3% (1/19), 0% (0/19) and 63.2% (12/19) of LGIN cases, in 61.4% (43/70), 18.6% (13/70), 7.1% (5/70) and 65.7% (46/70) of HGIN/CIS cases, and in 78.8% (41/52), 50.0% (26/52), 11.5% (6/52) and 59.6% (31/52) of invasive cancer cases, respectively. The incidence of absent or reduced FHIT or E-cadherin expression was significantly associated with neoplastic progression from LGIN to HGIN/CIS to invasive cancer (FHIT, P=0.0007; E-cadherin, P=0.00014). In the present study, absent or reduced protein expression of any of the 5 TRGs (FHIT, E-cadherin, MLH1, MSH2 and COX-2) in early ESN samples was not associated with the expression of the other TRGs, or with other clinical parameters, including size, age, gender and tumor location (data not shown).
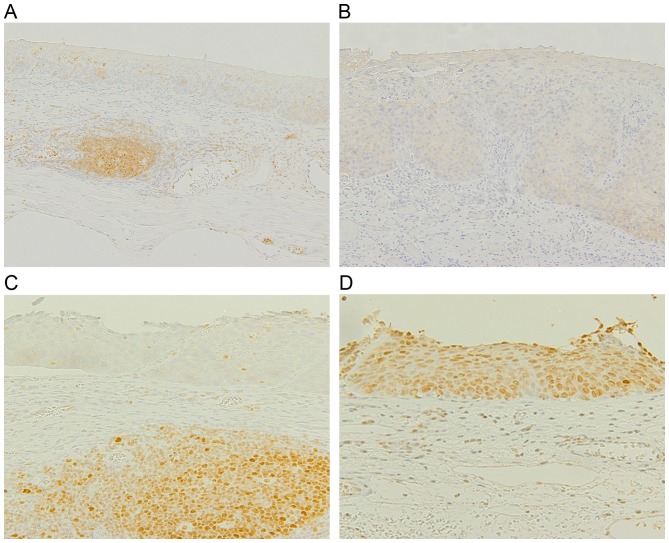
Figure 1.
Representative immunostaining results of FHIT, E-cadherin, MLH1 and MSH2 in neoplastic tissue. Esophageal squamous neoplastic tissue obtained from endoscopic resection was stained with antibodies against FHIT, E-cadherin and MLH1/MSH2. (A) A HGIN/CIS lesion with reduced FHIT immunostaining, with positive staining of the adjacent germinal center and infiltrating lymphocytes. (B) A HGIN/CIS lesion with negative E-cadherin immunostaining. (C) A HGIN/CIS lesion with negative MLH1 immunostaining. (D) A HGIN/CIS lesion with positive MSH1 immunostaining. FHIT, fragile histidine triad; MLH1, MutL homolog 1; MSH1, MutS homolog 1; HGIN/CIS, high-grade intraepithelial neoplasia/carcinoma in situ.
Table II.
Analysis of FHIT, CDH1, MLH1/MSH2 and COX-2 protein expression in patients with early esophageal squamous neoplasia.
| Reduced or loss of expression | LGIN, n (%) | HGIN/CIS | Invasive cancer | Total |
|---|---|---|---|---|
| Total samples, n | 19 | 70 | 52 | 141 |
| FHIT | 5 (26.3)a | 43 (61.4)a | 41 (78.8)a | 89 (63.1) |
| CDH1 | 1 (5.3)b | 13 (18.6)b | 26 (50.0)b | 40 (28.4) |
| MLH1 | 0 (0) | 5 (7.1) | 5 (9.6) | 10 (7.1) |
| MSH2 | 0 (0) | 0 (0) | 2 (3.8) | 2 (1.4) |
| COX-2 | 12 (63.2) | 46 (65.7) | 31 (59.6) | 89 (63.1) |
P=0.0007
P=0.0001; χ2 test with Yates' correction. A total of 1 sample with reduced and loss of MLH1 and MSH2 expression. CDH1, E-cadherin; COX-2, cyclooxygenase-2; FHIT, fragile histidine triad; MLH1, MutL homolog 1; MSH2, MutS homolog 2; LGIN, low-grade intraepithelial neoplasia; HGIN/CIS, high-grade intraepithelial neoplasia/carcinoma in situ.
There was an increase in the mean number of the TRG proteins with reduced or loss of expression with increasing tumor progression from LGIN (mean ± standard deviation, 1.12±0.61) to HGIN/CIS (1.66±0.93) to invasive cancer (2.09±0.96; Fig. 2). The presence of a trend was evaluated using the Newman-Keuls test on the three groups (LGIN, HGIN/CIS and invasive cancer), which identified a significant increase in the number of the TRG proteins with reduced or loss of expression from LGIN to invasive cancer (P<0.05).
Figure 2.
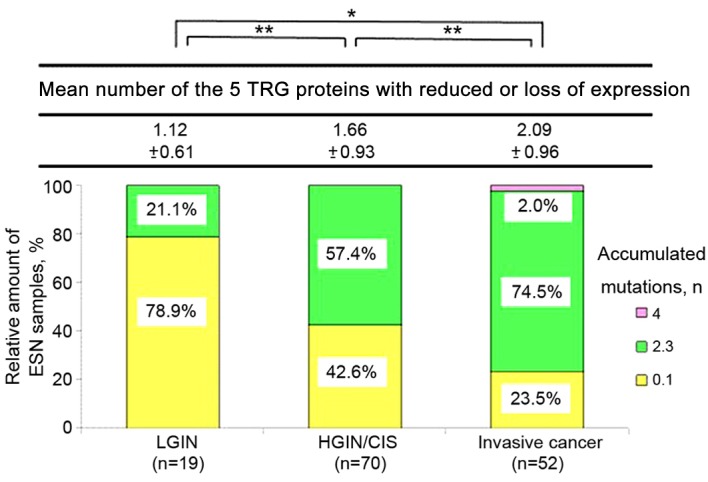
Number of TRGs (FHIT, CDH1, MLH1/MSH2 and COX-2) with negative expression in early esophageal squamous neoplasia. The number of TRG proteins for which the expression was decreased or absent in tumors gradually increased in the progression from LGIN and HGIN/CIS to invasive cancer. *P<0.01, **P<0.05; Newman-Keuls test. Data are presented as the mean ± standard deviation. CDH1, E-cadherin; COX-2, cyclooxygenase-2; TRG, tumor-related genes; FHIT, fragile histidine triad; MLH1, MutL homolog 1; MSH1, MutS homolog 1; LGIN, low-grade intraepithelial neoplasia; HGIN/CIS, high-grade intraepithelial neoplasia/carcinoma in situ.
Immunohistochemical analysis of p53 in ESN tumors
p53 overexpression was detected in 42.1% (8/19) of LGIN cases, in 57.1% (40/70) of HGIN/CIS cases, and in 55.8% (29/52) of invasive cancer cases (data not shown). p53 overexpression was not associated with ESN progression. In addition, p53 overexpression was not associated with absent or reduced protein expression of any of the 5 TRGs or with the number of TRGs expressed. The percentage of tumors with p53 overexpression was higher in ESN with head and neck carcinoma (HNC; 8/9; 88.9%) compared with in ESN with other cancer (25/50; 50.0%; P=0.064).
Discussion
The process of carcinogenesis and the early stage of tumor progression in ESCC are poorly understood. Frequent hypermethylation of CpG islands was previously observed in ESCC (3,4,26). Epigenetic changes, including hypermethylation of the CpG island associated with a TRG, may result in the transcriptional silencing of a gene, with the subsequent loss of protein expression that may contribute to tumorigenesis (27). However, the majority of previous studies on the methylation of TRGs limited to analysis in surgically resected ESCC samples and did not study the status of TRG genes in early neoplastic lesions (25,28). In the present study, IHC staining for 5 TRGs (FHIT, E-cadherin, MLH1, MSH2, and COX-2) was used to analyze methylation status, and it was identified that the expression of all 5 TRGs may be lost or reduced in cases of endoscopically resected early ESN. Additionally, ESN progression was indicated to be associated with the accumulation of methylation-modulated absence or reduced expression of the TRGs.
The expression of FHIT and E-cadherin appeared to be reduced or lost with the progression from LGIN to HGIN/CIS and then to invasive cancer. This finding is consistent with a previous study by the present authors (29) where the average number of the 5 TRG proteins with reduced or loss of expression in ESN showed a significant increase from LGIN to invasive cancer (P<0.05). Ishii et al (30) reported that the accumulation of the DNA methylation of TRGs is associated with tumor progression in ESCC. Therefore, a continuous reduction or loss in the number of the TRG proteins expressed during ESCC progression may be an important mechanism of pathogenesis, not only for initial tumor development but also for the progression of ESCC.
MLH1 and MSH2 are post-replication mismatch repair genes (31). MLH1 and MSH2 promoter hypermethylation has been reported in 3–62% (15,32) and 29–32% (16,17) of ESCCs, respectively. In a previous study (28), the loss of MLH1 or MSH2 expression was detected in 28.7% of primary ESCCs that underwent radical esophagectomy and was significantly associated with increased malignancy as assessed by the increasing metastasis to lymph nodes, extent of invasion and decreasing level of tissue differentiation. There are only few studies in which abnormalities in the expression of MLH1 and MSH2 genes were investigated in the early stage of ESCC, including from endoscopically resected specimens. In the present study, lost or reduced MLH1/MSH2 expression was detected in 0% (0/19) of LGIN cases, 7.1% (5/70) of HGIN/CIS cases, and 11.5% (6/52) of invasive cancer cases, indicating that loss of MLH1/MSH2 gene expression is involved in the carcinogenesis of some cases of ESCC.
COX-2 overexpression has been associated with a strong inflammatory response and gastrointestinal carcinoma development (33). However, past studies have identified a low expression of the COX-2 gene in gastric and colorectal cancer cases, associated with gene promoter hypermethylation (34,35). Additionally, it has been reported that COX-2 expression is regulated by promoter methylation in human esophageal cancer cell lines (36). However, in the present study, a low level of COX-2 expression was observed in 63.2% (12/19) of LGIN cases, 65.7% (46/70) of HGIN/CIS cases, and 59.6% (31/52) of invasive cancer cases, therefore indicating no association between the loss of COX-2 expression and tumor progression.
Tobacco smoking and alcohol consumption are two major risk factors for ESCC, and there is increasing evidence for the ability of tobacco smoke-associated carcinogens and polymorphisms of carcinogen-metabolizing genes to modulate DNA methylation in certain types of tobacco-associated cancer, including lung and upper aerodigestive tract cancer (37,38). Cigarette smoke was demonstrated to induce the promoter methylation of genes including FHIT and COX-2 in ESCC (14,23,39). E-cadherin promoter methylation was previously observed to be associated with increasing number of pack-years in HNC (40). There is also evidence to support the association of alcohol use with aberrant DNA methylation patterns in several types of cancer (20). ESCC has been suggested to be associated with promoter methylation of particular genes, including FHIT and MLH1 (39,41,42). In general, chronic inflammation induced by alcohol/tobacco use is considered an inducer of aberrant DNA methylation (43).
In the present study, expression of the p53 gene was also analyzed. p53 is a tumor-suppressor gene, and the p53 protein is crucial in a number of processes, including growth suppression, apoptosis and DNA repair (44). Tobacco and alcohol consumption may cause p53 mutation, one of the most frequent events in esophageal carcinogenesis (45). Point mutations in the p53 gene typically occur at an early stage of ESCC and correlate with tumor progression (46), therefore suggesting an important role of this genetic alteration in ESCC development. However, in the present study as well as a previous study, altered p53 expression was not significantly associated with the early stage of ESCC progression (47). From an epidemiological point of view, HNC and esophageal cancer are associated with the same carcinogens, including alcohol and tobacco (45,48). Alterations in the p53 gene and its expression have been reported in a variety of types of epithelial tumor and premalignant lesion, including HNC and esophageal cancer (45,48). In the present study, the percentage of tumors with p53 overexpression was higher in ESNs with HNC compared with in ESN with other cancer.
In conclusion, methylation-modulated absent or reduced expression of 5 TRGs in the early stages of esophageal tumorigenesis was demonstrated. A loss or reduced protein expression of these TRGs was identified as associated with ESN progression. An improved understanding of the methylation modulation of TRGs will provide new insights into esophageal carcinogenesis, cancer treatment and reveal feasible targets for chemoprevention. Further investigations in this area are required to gain an improved insight into ESCC development and to support the development of novel therapeutic strategies.
References
- 1.Japan Esophageal Society, corp-author. [May 1;2009 ];http://esophagus.jp Comprehensive Registry of Esophageal Cancer in Japan. (3rd). 2002
- 2.Muto M, Horimatsu T, Ezoe Y, Morita S, Miyamoto S. Improving visualization techniques by narrow band imaging and magnification endoscopy. J Gastroenterol Hepatol. 2009;24:1333–1346. doi: 10.1111/j.1440-1746.2009.05925.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang XM, Guo MZ. The value of epigenetic markers in esophageal cancer. Front Med China. 2010;4:378–384. doi: 10.1007/s11684-010-0230-3. [DOI] [PubMed] [Google Scholar]
- 4.Ahrens TD, Werner M, Lassmann S. Epigenetics in esophageal cancers. Cell Tissue Res. 2014;356:643–655. doi: 10.1007/s00441-014-1876-y. [DOI] [PubMed] [Google Scholar]
- 5.Gao H, Wang LD, Zhou Q, Hong JY, Huang TY, Yang CS. p53 Tumor suppressor gene mutation in early esophageal precancerous lesions and carcinoma among high-risk populations in Henan. Cancer Res. 1994;54:4342–4346. [PubMed] [Google Scholar]
- 6.Montesano R, Hollstein M, Hainaut P. Genetic alterations in esophageal cancer and their relevance to etiology and pathogenesis: A review. Int J Cancer. 1996;69:225–235. doi: 10.1002/(SICI)1097-0215(19960621)69:3<225::AID-IJC13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Abedi-Ardekani B, Dar NA, Mir MM, Zargar SA, Lone MM, Martel-Planche G, Villar S, Mounawar M, Saidi F, Malekzadeh R, Hainaut P. Epidermal growth factor receptor (EGFR) mutations and expression in squamous cell carcinoma of the esophagus in central Asia. BMC Cancer. 2012;12:602. doi: 10.1186/1471-2407-12-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori R, Ishiguro H, Kimura M, Mitsui A, Sasaki H, Tomoda K, Mori Y, Ogawa R, Katada T, Kawano O, et al. PIK3CA mutation status in Japanese esophageal squamous cell carcinoma. J Surg Res. 2008;145:320–326. doi: 10.1016/j.jss.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 9.Ushijima T. Detection and interpretation of altered methylation patterns in cancer cells. Nat Rev Cancer. 2005;5:223–231. doi: 10.1038/nrc1571. [DOI] [PubMed] [Google Scholar]
- 10.Kalari S, Pfeifer GP. Identification of driver and passenger DNA methylation in cancer by epigenomic analysis. Adv Genet. 2010;70:277–308. doi: 10.1016/B978-0-12-380866-0.60010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sato F, Meltzer SJ. CpG island hypermethylation in progression of esophageal and gastric cancer. Cancer. 2006;106:483–493. doi: 10.1002/cncr.21657. [DOI] [PubMed] [Google Scholar]
- 12.Mandelker DL, Yamashita K, Tokumaru Y, Mimori K, Howard DL, Tanaka Y, Carvalho AL, Jiang WW, Park HL, Kim MS, et al. PGP9.5 promoter methylation is an independent prognostic factor for esophageal squamous cell carcinoma. Cancer Res. 2005;65:4963–4968. doi: 10.1158/0008-5472.CAN-04-3923. [DOI] [PubMed] [Google Scholar]
- 13.Tzao C, Hsu HS, Sun GH, Lai HL, Wang YC, Tung HJ, Yu CP, Cheng YL, Lee SC. Promoter methylation of the hMLH1 gene and protein expression of human mutL homolog 1 and human mutS homolog 2 in resected esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2005;130:1371. doi: 10.1016/j.jtcvs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Zhang GY, Ma CX, Liu QL, Le XP, Ding Y, Zhang QX. Detection of methylation of hMSH2 gene promoter region of esophageal cancer. Zhonghua Zhong Liu Za Zhi. 2005;27:541–543. (In Chinese) [PubMed] [Google Scholar]
- 15.Lee EJ, Lee BB, Kim JW, Shim YM, Hoseok I, Han J, Cho EY, Park J, Kim DH. Aberrant methylation of fragile histidine triad gene is associated with poor prognosis in early stage esophageal squamous cell carcinoma. Eur J Cancer. 2006;42:972–980. doi: 10.1016/j.ejca.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Lee EJ, Lee BB, Han J, Cho EY, Shim YM, Park J, Kim DH. CpG island hypermethylation of E-cadherin (CDH1) and integrin alpha4 is associated with recurrence of early stage esophageal squamous cell carcinoma. Int J Cancer. 2008;123:2073–2079. doi: 10.1002/ijc.23598. [DOI] [PubMed] [Google Scholar]
- 17.Ling ZQ, Li P, Ge MH, Hu FJ, Fang XH, Dong ZM, Mao WM. Aberrant methylation of different DNA repair genes demonstrates distinct prognostic value for esophageal cancer. Dig Dis Sci. 2011;56:2992–3004. doi: 10.1007/s10620-011-1774-z. [DOI] [PubMed] [Google Scholar]
- 18.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 19.Oze I, Matsuo K, Wakai K, Nagata C, Mizoue T, Tanaka K, Tsuji I, Sasazuki S, Inoue M, Tsugane S, Research Group for the Development and Evaluation of Cancer Prevention Strategies in Japan Alcohol drinking and esophageal cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2011;41:677–992. doi: 10.1093/jjco/hyr026. [DOI] [PubMed] [Google Scholar]
- 20.Varela-Rey M, Woodhoo A, Martinez-Chantar ML, Mato JM, Lu SC. Alcohol, DNA methylation, and cancer. Alcohol Res. 2013;35:25–35. [PMC free article] [PubMed] [Google Scholar]
- 21.Talukdar FR, Ghosh SK, Laskar RS, Mondal R. Epigenetic, genetic and environmental interactions in esophageal squamous cell carcinoma from northeast India. PLoS One. 2013;8:e60996. doi: 10.1371/journal.pone.0060996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 23.Meng XY, Zhu ST, Zhou QZ, Li P, Wang YJ, Zhang ST. Promoter methylation regulates cigarette smoke-stimulated cyclooxygenase-2 expression in esophageal squamous cell carcinoma. J Dig Dis. 2012;13:208–213. doi: 10.1111/j.1751-2980.2012.00578.x. [DOI] [PubMed] [Google Scholar]
- 24.Gabbert HE, Shimoda T, Hainaut P, Nakamura Y, Field JK, Inoue H. Squamous cell carcinoma of the oesophagus. World Health Organization Classification of Tumours. Pathology and Genetics. In: Hamilton SR, Aaltonen LA, editors. Tumours of the Digestive System. Lyon: IARC Press; 2000. pp. 8–19. [Google Scholar]
- 25.Kitamura A, Yashima K, Okamoto E, Andachi H, Hosoda A, Kishimoto Y, Shiota G, Ito H, Kaibara N, Kawasaki H. Reduced Fhit expression occurs in the early stage of esophageal tumorigenesis: No correlation with p53 expression and apoptosis. Oncology. 2001;61:205–211. doi: 10.1159/000055376. [DOI] [PubMed] [Google Scholar]
- 26.Baba Y, Watanabe M, Baba H. Review of the alterations in DNA methylation in esophageal squamous cell carcinoma. Surg Today. 2013;43:1355–64. doi: 10.1007/s00595-012-0451-y. [DOI] [PubMed] [Google Scholar]
- 27.Esteller M. CpG island hypermethylation and tumor suppressor genes: A booming present, a brighter future. Oncogene. 2002;21:5427–5440. doi: 10.1038/sj.onc.1205600. [DOI] [PubMed] [Google Scholar]
- 28.Uehara H, Miyamoto M, Kato K, Cho Y, Kurokawa T, Murakami S, Fukunaga A, Ebihara Y, Kaneko H, Hashimoto H, et al. Deficiency of hMLH1 and hMSH2 expression is a poor prognostic factor in esophageal squamous cell carcinoma. J Surg Oncol. 2005;92:109–115. doi: 10.1002/jso.20332. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi A, Yashima K., Takeda Y, Sasaki S, Kawaguchi K, Harada K, Murawaki Y, Ito H. Fhit, E-cadherin, p53, and activation-induced cytidine deaminase expression in endoscopically resected early stage esophageal squamous neoplasia. J Gastroenterol Hepatol. 2012;27:1752–1758. doi: 10.1111/j.1440-1746.2012.07216.x. [DOI] [PubMed] [Google Scholar]
- 30.Ishii T, Murakami J, Notohara K, Cullings HM, Sasamoto H, Kambara T, Shirakawa Y, Naomoto Y, Ouchida M, Shimizu K, et al. Oesophageal squamous cell carcinoma may develop within a background of accumulating DNA methylation in normal and dysplastic mucosa. Gut. 2007;56:13–19. doi: 10.1136/gut.2005.089813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiricny J. Replication errors: Challenging the genome. EMBO J. 1998;17:6427–6436. doi: 10.1093/emboj/17.22.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang J, Sasco AJ, Fu C, Xue H, Guo G, Hua Z, Zhou Q, Jiang Q, Xu B. Aberrant DNA methylation of P16, MGMT, and hMLH1 genes in combination with MTHFR C677T genetic polymorphism in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:118–125. doi: 10.1158/1055-9965.EPI-07-0733. [DOI] [PubMed] [Google Scholar]
- 33.Konturek PC, Kania J, Burnat G, Hahn EG, Konturek SJ. Prostaglandins as mediators of COX-2 derived carcinogenesis in gastrointestinal tract. J Physiol Pharmacol. 2005;56(Suppl 5):S57–S73. [PubMed] [Google Scholar]
- 34.Toyota M, Shen L, Ohe-Toyota M, Hamilton SR, Sinicrope FA, Issa JP. Aberrant methylation of the cyclooxygenase 2 CpG island in colorectal tumors. Cancer Res. 2000;60:4044–4048. [PubMed] [Google Scholar]
- 35.Huang L, Zhang KL, Li H, Chen XY, Kong QY, Sun Y, Gao X, Guan HW, Liu J. Infrequent COX-2 expression due to promoter hypermethylation in gastric cancers in Dalian, China. Hum Pathol. 2006;37:1557–1567. doi: 10.1016/j.humpath.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 36.Meng XY, Zhu ST, Zong Y, Wang YJ, Li P, Zhang ST. Promoter hypermethylation of cyclooxy-genase-2 gene in esophageal squamous cell carcinoma. Dis Esophagus. 2011;24:444–449. doi: 10.1111/j.1442-2050.2010.01159.x. [DOI] [PubMed] [Google Scholar]
- 37.Mani S, Szymańska K, Cuenin C, Zaridze D, Balassiano K, Lima SC, Matos E, Daudt A, Koifman S, Filho VW, et al. DNA methylation changes associated with risk factors in tumors of the upper aerodigestive tract. Epigenetics. 2012;7:270–277. doi: 10.4161/epi.7.3.19306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin RK, Hsieh YS, Lin P, Hsu HS, Chen CY, Tang YA, Lee CF, Wang YC. The tobacco-specific carcinogen NNK induces DNA methyltransferase 1 accumulation and tumor suppressor gene hypermethylation in mice and lung cancer patients. J Clin Invest. 2010;120:521–532. doi: 10.1172/JCI40706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mori M, Mimori K, Shiraishi T, Alder H, Inoue H, Tanaka Y, Sugimachi K, Huebner K, Croce CM. Altered expression of Fhit in carcinoma precarcinomatous lesions of the esophagus. Cancer Res. 2000;60:1177–1182. [PubMed] [Google Scholar]
- 40.Hasegawa M, Nelson HH, Peters E, Ringstrom E, Posner M, Kelsey KT. Patterns of gene promoter methylation in squamous cell cancer of the head and neck. Oncogene. 2002;21:4231–4236. doi: 10.1038/sj.onc.1205528. [DOI] [PubMed] [Google Scholar]
- 41.Morita M, Oyama T, Nakata S, Ono K, Sugaya M, Uramoto H, Yoshimatsu T, Hanagiri T, Sugio K, Yasumoto K. Expression of fhit in esophageal epithelium and Carcinoma: Reference to drinking, smoking and multicentric carcinogenesis. Anticancer Res. 2006;26:2243–2248. [PubMed] [Google Scholar]
- 42.van Engeland M, Weijenberg MP, Roemen GM, Brink M, De Bruïne AP, Goldbohm RA, van den Brandt PA, Baylin SB, de Goeij AF, Herman JG. Effects of dietary folate and alcohol intake on promoter methylation in sporadic colorectal cancer: The Netherlands cohort study on diet and cancer. Cancer Res. 2003;63:3133–3137. [PubMed] [Google Scholar]
- 43.Ushijima T, Okochi-Takada E. Aberrant methylations in cancer cells: Where do they come from? Cancer Sci. 2005;96:206–211. doi: 10.1111/j.1349-7006.2005.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollestein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- 45.Toh Y, Oki E, Ohgaki K, Sakamoto Y, Ito S, Egashira A, Saeki H, Kakeji Y, Morita M, Sakaguchi Y, et al. Alcohol drinking, cigarette smoking, and development of squamous cell carcinoma of the esophagus: Molecular mechanisms of carcinogenesis. Int J Clin Oncol. 2010;15:135–144. doi: 10.1007/s10147-010-0057-6. [DOI] [PubMed] [Google Scholar]
- 46.Kaneko K, Katagiri A, Konishi K, Kurahashi T, Ito H, Kumekawa Y, Yamamoto T, Muramoto T, Kubota Y, Nozawa H, et al. Study of p53 gene alteration as a biomarker to evaluate the malignant risk of Lugol-unstained lesion with non-dysplasia in the oesophagus. Br J Cancer. 2007;96:492–498. doi: 10.1038/sj.bjc.6603582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chino O, Kijima H, Shimada H, Nishi T, Tanaka H, Kise Y, Kenmochi T, Himeno S, Machimura T, Tanaka M, et al. Accumulation of p53 in esophageal squamous cell carcinoma. Int J Mol Med. 2001;8:359–163. [PubMed] [Google Scholar]
- 48.Perez-Ordoñez B, Beauchemin M, Jordan RC. Molecular biology of squamous cell carcinoma of the head and neck. J Clin Pathol. 2006;59:445–453. doi: 10.1136/jcp.2003.007641. [DOI] [PMC free article] [PubMed] [Google Scholar]