Abstract
We have previously reported a hyperlipidemic state in two strains of Apc-deficient mice, Min and Apc1309, associated with low expression levels of lipoprotein lipase (LPL) in the liver and small intestine, and enforced induction of LPL mRNA by peroxisome proliferator-activated receptor (PPAR)α and PPARγ agonists clearly suppressed hyperlipidemia and intestinal polyp formation in these mice. Meanwhile, a compound, NO-1886, has been shown to increase LPL mRNA and protein levels but not to possess PPARα and PPARγ agonistic activity. In this study, therefore, the effects of NO-1886 on hyperlipidemia and intestinal polyp formation were investigated in Min mice. Administration of 400 and 800 ppm NO-1886 in the diet for 13 weeks from 7 weeks of age caused a reduction of serum triglycerides to 39% and 31% of the untreated value, respectively, and the values for very low-density lipoprotein cholesterol, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were improved almost to the wild-type level with a corresponding elevation of the LPL mRNA. Moreover, total numbers of intestinal polyps in the groups receiving NO-1886 at 400 and 800 ppm were decreased to 48% and 42% of the control value, respectively. We also found that NO-1886 suppressed cyclooxygenase-2 transcriptional promoter activity in a reporter gene assay and reduced cyclooxygenase-2 mRNA levels in the small intestine of Min mice. These results indicate that suppression of serum lipid levels by increasing LPL activity may contribute to a reduction of intestinal polyp formation with Apc-deficiency, and NO-1886 and its derivatives could be useful as chemopreventive agents for colon cancer.
Colon cancer is one of the most frequent cancers in developed countries, and many epidemiological studies have suggested a correlation with obesity and hyperlipidemia (1, 2). Recently, we reported an age-dependent hyperlipidemic state in Apc-deficient Min and Apc1309 mice, animal models of human familial adenomatous polyposis (3, 4). The mRNA levels for lipoprotein lipase (LPL), which catalyzes hydrolysis of triglycerides, were shown to be down-regulated in the livers and small intestines of these Apc-deficient animals compared with their wild-type counterparts. We also demonstrated that treatment with a peroxisome proliferator-activated receptor α (PPARα) agonist, bezafibrate, and a PPARγ agonist, pioglitazone, concomitantly suppressed hyperlipidemia and intestinal polyp formation in the mice with induction of LPL mRNA (3, 4). Thus, LPL expression levels may correlate with intestinal polyp development in Apc-deficient mice.
LPL is the major enzyme responsible for the hydrolysis of triglyceride-rich lipoproteins such as chylomicrons and very low-density lipoprotein (VLDL). LPL mRNA is expressed ubiquitously in the body but especially in adipose tissue and skeletal muscle, where it is synthesized then transferred to the surface of endothelial cells, to become bound to membrane-anchored heparan sulfate proteoglycans (5, 6). There are reports on association of hyperlipidemia with lowered or lack of LPL (7, 8). However, there have been no reports directly addressing links between LPL and colon carcinogenesis.
There has been great interest in development of an LPL-selective inducer for effective control of hypertriglyceridemia and low levels of high-density lipoprotein cholesterol (HDL-C) in serum. Several diethyl benzyl phosphonate derivatives were examined and one example, NO-1886, has been reported to increase LPL mRNA and protein levels, resulting in a reduction of plasma triglycerides and an increase in HDL-C levels in rats (9). NO-1886 also improves obesity in rats through induction of fatty acid oxidation-related enzymes, such as long-chain acyl-CoA dehydrogenase and acetyl-CoA acyltransferase (10). Moreover, NO-1886 reduces high-cholesterol diet-induced atherosclerotic lesions in rat coronary arteries (9).
It is well known that PPARα and PPARγ agonists improve hypertriglyceridemia and hypercholesterolemia through induction of lipid metabolism-related genes such as LPL (11). Moreover, these agonists have been documented to show antiproliferative and proapoptotic effects in various types of cancer cells, including colon cancer cells (12). Using a reporter gene assay, NO-1886 was revealed not to possess PPARα and PPARγ agonistic activity, unlike bezafibrate and pioglitazone (10). Thus, the LPL selective activator, NO-1886, may be a very essential agent for determining the relationship between hyperlipidemia due to LPL depression and colon carcinogenesis. In this study, we therefore examined the effects of 400 and 800 ppm NO-1886 in the diet on both hyperlipidemia and intestinal polyp formation in Min mice and demonstrated concomitant suppression of both. Taking account of the fact that NO-1886 also suppressed cyclooxygenase-2 (COX-2) mRNA expression, possible mechanisms of its action in Apc-deficient mice and the usage of NO-1886 or its derivatives as possible candidates of colon cancer prevention are also discussed.
Materials and Methods
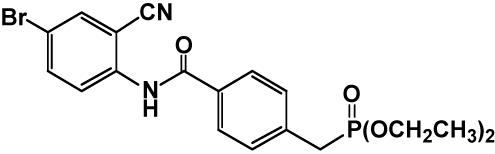
Animals and Chemicals. Female C57BL/6-ApcMin/+ mice (Min mice) were purchased from The Jackson Laboratory at 5 weeks of age and genotyped by the method reported in ref. 13. We used female animals for experimental convenience because there are no significant differences in the numbers of intestinal polyps and serum lipid levels between males and females (3, 4, 14). Heterozygotes of the Min strain and wild-type (C57BL/6J) mice were acclimated to laboratory conditions for 2 weeks. Three to five mice were housed per plastic cage with sterilized softwood chips as bedding in a barrier-sustained animal room airconditioned at 24 ± 2°C and 55% humidity on a 12-h light/dark cycle. The LPL selective inducer NO-1886, 4-[(4-bromo-2-cyanophenyl)carbamoyl]benzylphosphonate, was chemically synthesized at Otsuka Pharmaceutical Factory (9). Its structure is shown in Fig. 1. NO-1886 was well mixed at concentrations of 400 and 800 ppm with AIN-76A powdered basal diet (CLEA Japan, Tokyo). The chemical was confirmed to be stable under the experimental conditions used in this study.
Fig. 1.
Structure of NO-1886.
Animal Experiments. To investigate the effects of NO-1886 on both hyperlipidemia and intestinal polyp formation, 10–13 female Min mice at 7 weeks of age were given 0 (control), 400, or 800 ppm NO-1886 in the diet for 13 weeks. Food and water were available ad libitum. The animals were observed daily for clinical signs and mortality. Body weights and food consumption were measured weekly. At the kill time points, animals were anesthetized with ether, and blood samples were collected from the abdominal aorta. Serum levels of triglycerides and total cholesterol were measured as reported in ref. 3. In addition, the levels of the major lipoprotein classes for cholesterol, VLDL, low-density lipoprotein (LDL), and high-density lipoprotein (HDL), were measured by HPLC (15). The experiments were conducted according to the “Guidelines for Animal Experiments in National Cancer Center” of the Committee for Ethics of Animal Experimentation of the National Cancer Center.
The intestinal tract was removed, filled with 10% buffered formalin, and separated into the small intestine, cecum, and colon. The small intestine was divided into the proximal segment (≈4 cm in length), and then the proximal (middle) and distal halves of the remainder. All segments were opened longitudinally and fixed flat between sheets of filter paper in 10% buffered formalin. The numbers and sizes of polyps and their distributions in the intestine were assessed with a stereoscopic microscope (3).
RT-PCR Analysis. Tissue samples from the normal parts of liver and small intestine of mice (n = 3 each) were rapidly deep-frozen in liquid nitrogen and stored at -80°C. Cells of a human colon adenocarcinoma cell line, DLD-1, were purchased from the Health Science Research Resources Bank (Osaka) and cultured according to the supplier's instructions. Total RNA was isolated from tissues by using Isogen (Nippon Gene, Tokyo), treated with DNase (Invitrogen) and applied at 3-μg aliquots in a final volume of 20 μl for synthesis of cDNA by using an Omniscript RT Kit (Qiagen, Hilden, Germany) and an oligo(dT) primer. The mixture was incubated for 10 min at 32°C, then 50 min at 42°C, and immediately cooled on ice. As an internal control to confirm the integrity of the isolated mRNA, β-actin was used (3). PCR was performed with specific primers for mouse LPL (5′-primer-GGATCCGTGGCCGCAGCAGACGCAGGAAGA, 3′-primer-GAATTCCATCCAGTTGATGAATCTGGCCAC) (16), COX-1 (5′-GTCATCAAGGAGTCCCGAG, 3′-CCAGTTTCTTCAGTGAGGC), COX-2 (5′-CACACTCTATCACTGGCACC, 3′-CTCTCTGCTCTGGTCAATGG), inducible nitric oxide (iNOS) (5′-CTTGGAGCGAGTTGTGGATTG, 3′-CAGGAAGTAGGTGAGGGC), PPARγ (5′-TGAGACCAACAGCCTGACG, 3′-GATGTCAAAGGAATGCGAGTGG), PPARα (5′-TCTTACCTGTGAACACGACCTG, 3′-AGCAGTGGAAGAATCGGACC). PCR amplification of 1 μl of cDNA was carried out in a final volume of 10 μl with an PTC-200 DNA Engine (MJ Research, Waltham, MA), by using a HotStarTaq (Qiagen). Cycling conditions were as follows: 94°C for 20 sec, annealing temperature (60–64°C) for 30 sec, 72°C for 80 sec, and 25–35 cycles after an initial step of 95°C for 15 min. A final elongation step of 72°C for 10 min completed the PCR. The products were then electrophoresed in 2% agarose gels.
Reporter Gene Assay for COX-2 Promoter-Dependent Transcriptional Activity. Stable transfectants containing pB2-Gal-BSD and pCOX-2/B2-Gal-BSD in the genome DNA of DLD-1 cells were prepared as described in ref. 17. Cells were seeded at a density of 2 × 104 cells per well in 96-well plates and precultured for 24 h. They were then cotreated with type α TGF (TGFα) (100 ng/ml) and/or NO-1886 (2.5, 5, and 10 μM), and the total β-gal activities of cells in each well were determined by colorimetric assay with o-nitrophenyl-β-d-gal as described in ref. 17. The background β-gal activity of DLD-1 cells was determined with a control nontreated culture of DLD-1/B2-Gal-BSD cells, and the value was set as 0. The basal β-gal activity of nontreated DLD-1/COX2-B2-Gal-BSD was set as 100%. The percentage of β-gal activity with each treatment was then calculated by using data from triplicate wells. The values for β-gal activity were normalized for total protein amount. All assays were carried out in triplicate, and the experiment was repeated at least twice.
Statistical Analysis. The results were expressed as mean ± SE values, and statistical analysis was performed with Student's t test. Differences were considered to be statistically significant with P < 0.05.
Results
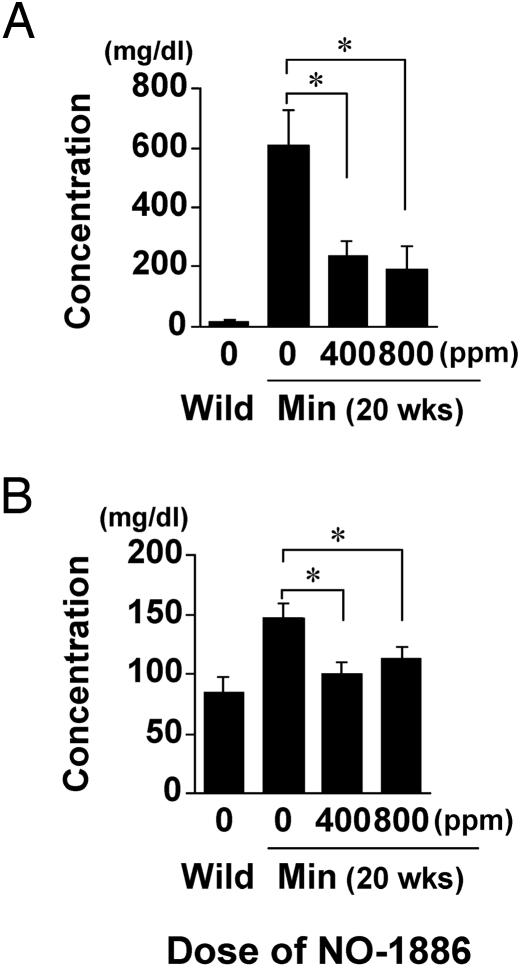
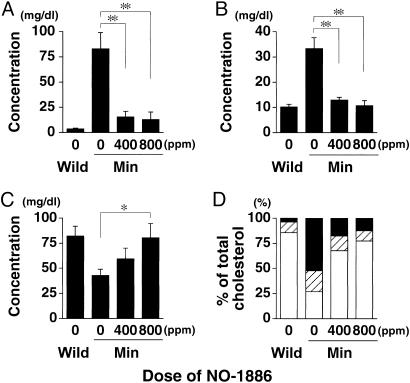
Improvement of Serum Lipid Levels in Min Mice by NO-1886. Consistent with our previous reports (3, 4), a hyperlipidemic state was observed in the Min mice fed the basal diet at 20 weeks of age. Namely, serum levels of triglycerides at 20 weeks of age were dramatically increased to almost 30 times the wild-type value (Fig. 2A). Total cholesterol levels in Min mice were also increased 1.7-fold (Fig. 2B). Moreover, VLDL cholesterol (VLDL-C) levels in the basal diet group of Min mice were 24-fold higher than in their wild-type counterparts (Fig. 3A). LDL cholesterol (LDL-C) levels were increased 3.3-fold, whereas HDL-C was decreased to 50% of the wild-type value (Fig. 3 B and C). The proportions of HDL-C, LDL-C, and VLDL-C in the total cholesterol in Min mice (27%, 21%, and 52%, respectively) were almost opposite to those in wild-type mice (86%, 10%, and 4%, respectively) (Fig. 3D).
Fig. 2.
Suppression of serum lipid levels in Min mice by NO-1886. Values for serum levels of triglyceride (A) and total cholesterol (B) in female Min mice given diet containing NO-1886 at doses of 0 (n = 7), 400 (n = 8), and 800 ppm (n = 10) for 13 weeks and wild-type mice (n = 6) are shown. Data are means; bars are SE. *, P < 0.05; **, P < 0.01.
Fig. 3.
Serum levels of cholesterol lipoproteins in female Min mice treated with NO-1886. Shown are lipoprotein classes in female Min mice given diets containing NO-1886 at doses of 0, 400, and 800 ppm for 13 weeks and wild-type mice. (A) VLDL cholesterol. (B) LDL cholesterol. (C) HDL cholesterol. (D) The proportions of cholesterol lipoproteins. Open box, HDL cholesterol; crosshatched box, LDL cholesterol; filled box, VLDL cholesterol). Data are means; bars are SE (n = 5). *, P < 0.05; **, P < 0.01.
Administration of 400 and 800 ppm NO-1886 did not affect body weights or clinical signs of Min mice throughout the experimental period. Amounts of daily food intake were not different among groups, and daily intakes of NO-1886 in the 400- and 800-ppm groups of Min mice were 1.2–1.5 mg per mouse per day and 2.8–3.1 mg per mouse per day, respectively. In addition, there were no changes observed in any organ weights that could be attributable to toxicity. Administration of 400 and 800 ppm NO-1886 clearly decreased serum levels of triglycerides to 39% and 31% of the untreated control value, respectively (Fig. 2 A). The levels of total cholesterol were also decreased to 69% and 77% of the untreated control value (Fig. 2B). Furthermore, levels of both triglyceride-rich lipoproteins, VLDL-C and LDL-C, were dramatically suppressed by NO-1886 treatment. The levels of VLDL-C in the groups treated with NO-1886 at 400 and 800 ppm were reduced to 19% and 15% of the untreated control value, and the levels of LDL-C were to 39% and 32% of the untreated control value, respectively (Fig. 3 A and B). In contrast, HDL-C levels were increased to the wild-type value at 800 ppm (Fig. 3C). Overall, administration of NO-1886 improved the balance of HDL-C, LDL-C, and VLDL-C in the total cholesterol of Min mice (Fig. 3D).
Suppression of Intestinal Polyp Formation in Min Mice by NO-1886. Table 1 summarizes data for the number and distribution of intestinal polyps in the basal diet and NO-1886-treated groups. Almost all polyps developed in the small intestine, with only a few in the colon (Table 1). The total number of polyps were significantly decreased by administration of 400- and 800-ppm NO-1886 to 48% and 42% of the untreated control value, respectively, with reduction in the proximal, middle, and distal parts by 63%, 57%, and 45% with 400 ppm, and by 74%, 63%, and 49% with 800 ppm. Treatment with NO-1886 also significantly decreased the numbers of colon polyps.
Table 1. Suppression of intestinal polyp development in Min mice by NO-1886.
| Small intestine
|
||||||
|---|---|---|---|---|---|---|
| Dose, ppm | No. of mice | Proximal | Middle | Distal | Colon | Total |
| 0 | 7 | 23.1 ± 4.2 | 37.1 ± 11.1 | 60.4 ± 12.2 | 1.0 ± 0.2 | 121.7 ± 26.0 |
| 400 | 8 | 8.5 ± 1.4 (37)* | 16.1 ± 3.8 (43) | 33.0 ± 6.5 (55) | 0.4 ± 0.2 (38)† | 58.0 ± 10.8 (48)† |
| 800 | 10 | 5.9 ± 1.0 (26)* | 13.8 ± 2.6 (37)† | 30.5 ± 5.0 (51)† | 0.3 ± 0.2 (30)† | 50.5 ± 7.8 (42)* |
Data are means ± SE of the number of polyps per mouse. Numbers in parentheses are percentages of the control value.
Significantly different from the basal diet group at P < 0.01
Significantly different from the basal diet group at P < 0.05
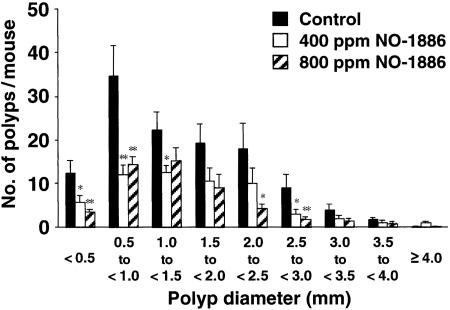
Fig. 4 shows the size distributions of intestinal polyps in the basal diet and NO-1886-treated groups. The main polyp sizes observed in the basal diet groups were 0.5–3.0 mm in diameter. Administration of NO-1886 reduced the numbers of polyps of all sizes.
Fig. 4.
Effects of NO-1886 on the size distribution of intestinal polyps in Min mice. Min mice were fed a basal diet (filled box) or a diet containing 400 ppm (open box) or 800 ppm (hatched box) NO-1886 for 13 weeks. The number of polyps per mouse in each size class is given as a mean value; bars are SE. *, P < 0.05; **, P < 0.01.
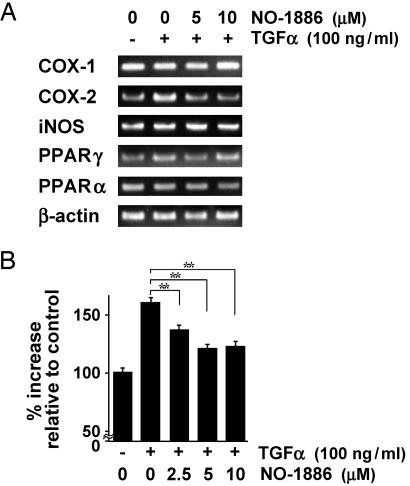
Down-Regulation of COX-2 Transcriptional Activity by NO-1886. Expression of enzymes associated with inflammation has been reported to be increased in colon carcinogenesis (18). To cast light on mechanisms of the effect of NO-1886 on colon carcinogenesis, we investigated expression levels of mRNAs for COX-1, COX-2, and iNOS in DLD-1 human colon cancer cells by RT-PCR. As shown in Fig. 5A, the TGFα-stimulated mRNA levels for COX-2 were reduced to nonstimulated mRNA levels by NO-1886 in DLD-1 cells. On the other hand, there was no obvious variation in the mRNA levels for COX-1 and iNOS (Fig. 5A). In addition, we confirmed that NO-1886 did not change the mRNA levels for PPARγ and PPARα in DLD-1 cells (Fig. 5A). Fig. 5B shows the results for β-gal reporter gene assay in DLD-1 cells. Treatment of cells with 100 ng/ml TGFα for 48 h increased COX-2 transcriptional levels to 1.6-fold of the control value, whereas NO-1886 at 5 and 10 μM suppressed TGFα-stimulated COX-2 transcriptional activity to 1.2-fold of the control value. No significant decrease of cell viability was observed after 48 h culture with NO-1886 at these concentrations.
Fig. 5.
Suppression of COX-2 mRNA level and COX-2 transcriptional activity in a human colon cancer cell line by NO-1886. (A) RT-PCR analysis of mRNA expression levels for COX-1, COX-2, iNOS, PPARγ, and PPARα in DLD-1 cells treated with the indicated dose of TGFα and/or NO-1886. (B) Reporter gene assay for COX-2 promoter-dependent transcriptional activity in DLD-1 cells. All assays were carried out in triplicate, and data are representative of at least two separate experiments. Data are means; bars are SD. **, P < 0.01.
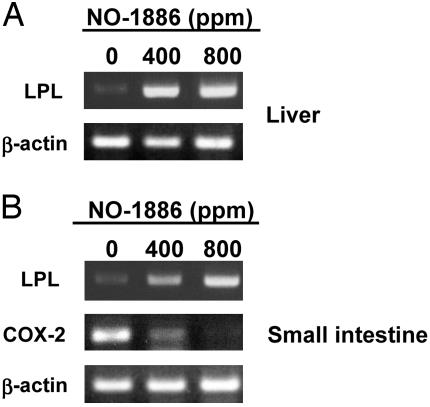
Expression of LPL and COX-2 mRNAs in Liver and Small Intestine in Min Mice Assessed by RT-PCR. LPL mRNA levels in the liver and the small intestine in the Min mice at 20 weeks of age were very low but were markedly increased by the treatment with NO-1886 dose-dependently (Fig. 6). Consistent with the in vitro data in Fig. 5, administration of NO-1886 reduced mRNA levels of COX-2 in normal parts of small intestine of Min mice at 20 weeks of age (Fig. 6B).
Fig. 6.
Changes of mRNA levels for LPL and COX-2 in the liver and small intestine of female Min mice. (A) RT-PCR analysis of LPL mRNA expression in the livers of female Min mice given diets containing NO-1886 at doses of 0, 400, and 800 ppm for 13 weeks. (B) RT-PCR analysis of LPL and COX-2 mRNA expression in normal parts of the small intestine of female Min mice given diets containing NO-1886 at doses of 0, 400, and 800 ppm for 13 weeks. Data are representative of three mice of each group.
Discussion
This study provided clear evidence that administration of the LPL selective inducer NO-1886, which increases LPL mRNA and protein levels, suppresses both hyperlipidemia and intestinal polyp formation in Min mice. Decrease in serum triglycerides, VLDL-C and LDL-C, and an increase in HDL-C were demonstrated, with elevation of LPL mRNA level and suppression of COX-2 expression. NO-1886 is shown not to have a potential of PPARα and PPARγ agonists (10). It is therefore speculated that LPL activity itself may play an important role in the intestinal polyp formation in Apc-deficient mice.
We earlier reported dramatically increased serum levels of triglycerides and markedly low levels of liver and small intestine LPL mRNA in Min mice compared with their wild-type counterparts (3, 4). This data provided concrete evidence that the expression levels of LPL, which catalyzes hydrolysis of triglycerides, correlate with hypertriglyceridemia in Min mice. At present, it still cannot be stated with certainty whether hyperlipidemia is a leading cause of intestinal polyp formation. Colon tumors induced by 1,2-dimethylhydrazine in rats, however, are not linked to serum lipid levels (19). As hyperlipidemia and polyp formation could be related to Apc-deficiency independently, we now address whether low LPL activity and high serum lipid levels could promote intestinal polyp formation in these mice.
It has been reported that inflammation-associated enzymes such as COX-2 and iNOS are overexpressed in colon carcinogenesis (18). Treatment with NO-1886 reduced COX-2 expression levels in a reporter gene assay as well as normal parts of the small intestine of Min mice. Immunohistochemically, expression of COX-2 is reported to be observed in normal parts of the small intestine in Min mice (20). Moreover, it is well known that expression of COX-2 is markedly elevated in colon cancers of humans and AOM-treated rats and in intestinal polyps of Apc-deficient mice (21–23), playing an important role in cancer cell proliferation and angiogenesis (24). Therefore, down-regulation of COX-2 by NO-1886 is clearly one possible mechanism underlying suppression of intestinal polyp development.
There is evidence that prostaglandin (PG)E2, produced by COX-1 and COX-2, is a potent inhibitor of LPL expression in macrophages (25). PGE2 levels are also known to be elevated in both human and rodent colon tumors (26, 27). These findings support the speculation that the role of LPL in intestinal polyp development may be associated with the arachidonic cascade.
In conclusion, this study indicates that the LPL selective inducer, NO-1886, has potential benefits for treatment of both hyperlipidemia and intestinal polyp development. LPL may be a good target for chemoprevention. Thus, NO-1886 and its derivatives are suggested to be promising candidate chemopreventive agents for colon cancer. It is very important to now clarify LPL functions and their significance for cancer development.
Acknowledgments
This work was supported by Grants-in-Aid for Cancer Research, the Third-Term Comprehensive 10-Year Strategy for Cancer Control, and Research on Advanced Medical Technology from the Ministry of Health, Labour, and Welfare of Japan; a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (J.S.P.S.); and a Grant from the Public Trust Nishi Cancer Research Fund.
Abbreviations: COX, cyclooxygenase; HDL, high-density lipoprotein; HDL-C, HDL cholesterol; iNOS, inducible NO; LDL, low-density lipoprotein; LDL-C, LDL cholesterol; LPL, lipoprotein lipase; PPAR, peroxisome proliferator-activated receptor; VLDL, very low-density lipoprotein; VLDL-C, VLDL cholesterol.
References
- 1.Le Marchand, L., Wilkens, L. R., Kolonel, L. N., Hankin, J. H. & Lyu, L. C. (1997) Cancer Res. 57, 4787-4794. [PubMed] [Google Scholar]
- 2.Bruce, W. R., Wolever, T. M. & Giacca, A. (2000) Nutr. Cancer 37, 19-26. [DOI] [PubMed] [Google Scholar]
- 3.Niho, N., Takahashi, M., Kitamura, T., Shoji, Y., Itoh, M., Noda, T., Sugimura, T. & Wakabayashi, K. (2003) Cancer Res. 63, 6090-6095. [PubMed] [Google Scholar]
- 4.Niho, N., Takahashi, M., Shoji, Y., Takeuchi, Y., Matsubara, S., Sugimura, T. & Wakabayashi, K. (2003) Cancer Sci. 94, 960-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenkovich, C. F., Chen, S. H., Wims, M., Luo, C. C., Li, W. H. & Chan, L. (1989) J. Lipid Res. 30, 423-431. [PubMed] [Google Scholar]
- 6.Goldberg, I. J. (1996) J. Lipid Res. 37, 693-707. [PubMed] [Google Scholar]
- 7.Gehrisch, S. (1999) Curr. Atheroscler. Rep. 1, 70-78. [DOI] [PubMed] [Google Scholar]
- 8.Mead, J. R., Irvine, S. A. & Ramji, D. P. (2002) J. Mol. Med. 80, 753-769. [DOI] [PubMed] [Google Scholar]
- 9.Tsutsumi, K., Inoue, Y., Shima, A., Iwasaki, K., Kawamura, M. & Murase, T. (1993) J. Clin. Invest. 92, 411-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doi, M., Kondo, Y. & Tsutsumi, K. (2003) Metabolism 52, 1547-1550. [DOI] [PubMed] [Google Scholar]
- 11.Schoonjans, K., Staels, B. & Auwerx, J. (1996) Biochim. Biophys. Acta 1302, 93-109. [DOI] [PubMed] [Google Scholar]
- 12.Rosen, E. D. & Spiegelman, B. M. (2001) J. Biol. Chem. 276, 37731-37734. [DOI] [PubMed] [Google Scholar]
- 13.Moser, A. R., Pitot, H. C. & Dove, W. F. (1990) Science 247, 322-324. [DOI] [PubMed] [Google Scholar]
- 14.Mutoh, M., Watanabe, K., Kitamura, T., Shoji, Y., Takahashi, M., Kawamori, T., Tani, K., Kobayashi, M., Maruyama, T., Kobayashi, K., et al. (2002) Cancer Res. 62, 28-32. [PubMed] [Google Scholar]
- 15.Usui, S., Hara, Y., Hosaki, S. & Okazaki, M. (2002) J. Lipid Res. 43, 805-814. [PubMed] [Google Scholar]
- 16.Schoonjans, K., Peinado-Onsurbe, J., Lefebvre, A. M., Heyman, R. A., Briggs, M., Deeb, S., Staels, B. & Auwerx, J. (1996) EMBO J. 15, 5336-5348. [PMC free article] [PubMed] [Google Scholar]
- 17.Mutoh, M., Takahashi, M., Fukuda, K., Matsushima-Hibiya, Y., Mutoh, H., Sugimura, T. & Wakabayashi, K. (2000) Carcinogenesis 21, 959-963. [DOI] [PubMed] [Google Scholar]
- 18.Bing, R. J., Miyataka, M., Rich, K. A., Hanson, N., Wang, X., Slosser, H. D. & Shi, S. R. (2001) Clin. Cancer Res. 7, 3385-3392. [PubMed] [Google Scholar]
- 19.Barton, T. P., Cruse, J. P. & Lewin, M. R. (1987) Br. J. Cancer 56, 451-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hull, M. A., Booth, J. K., Tisbury, A., Scott, N., Bonifer, C., Markham, A. F. & Coletta, P. L. (1999) Br. J. Cancer 79, 1399-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sano, H., Kawahito, Y., Wilder, R. L., Hashiramoto, A., Mukai, S., Asai, K., Kimura, S., Kato, H., Kondo, M. & Hla, T. (1995) Cancer Res. 55, 3785-3789. [PubMed] [Google Scholar]
- 22.DuBois, R. N., Radhika, A., Reddy, B. S. & Entingh, A. J. (1996) Gastroenterology 110, 1259-1262. [DOI] [PubMed] [Google Scholar]
- 23.Williams, C. S., Luongo, C., Radhika, A., Zhang, T., Lamps, L. W., Nanney, L. B., Beauchamp, R. D. & DuBois, R. N. (1996) Gastroenterology 111, 1134-1140. [DOI] [PubMed] [Google Scholar]
- 24.Tsujii, M., Kawano, S., Tsuji, S., Sawaoka, H., Hori, M. & DuBois, R. N. (1998) Cell 93, 705-716. [DOI] [PubMed] [Google Scholar]
- 25.Desanctis, J. B., Varesio, L. & Radzioch, D. (1994) Immunology 81, 605-610. [PMC free article] [PubMed] [Google Scholar]
- 26.Pugh, S. & Thomas, G. A. (1994) Gut 35, 675-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chulada, P. C., Thompson, M. B., Mahler, J. F., Doyle, C. M., Gaul, B. W., Lee, C., Tiano, H. F., Morham, S. G., Smithies, O. & Langenbach, R. (2000) Cancer Res. 60, 4705-4708. [PubMed] [Google Scholar]