Abstract
Survivin is a bifunctional protein that suppresses apoptosis and regulates cell division, and is highly expressed in various cancer types. Mucins are high-molecular-weight, heavily glycosylated proteins. In the present study, the association between survivin, mucin 2 (MUC2) and MUC5 expression, and the clinicopathological features of colorectal cancer (CRC) were investigated. The immunohistochemistry and western blotting results demonstrated that survivin was highly expressed in CRC tissues and rarely expressed in normal colon tissues. Moreover, the overexpression of survivin and MUC5 was strongly associated with lymph node metastasis, poor cellular differentiation, advanced tumor stage and a poor prognosis in CRC. By contrast, low expression of MUC2 was significantly associated with lymph node metastasis, poor cellular differentiation and an advanced tumor stage in CRC. The results of the present study suggest that survivin, MUC2 and MUC5 levels may be associated with tumor progression and could be used to aid the early diagnosis and clinical characterization of CRC.
Keywords: survivin, mucin 2, mucin 5, colorectal cancer
Introduction
Despite recent advances in detection and treatment, colorectal cancer (CRC) remains the third most common type of cancer and a major cause of cancer-related mortality worldwide (1,2). There have been a number of recent advances in CRC screening. Probing a combination of sensitive and specific molecular markers could be particularly promising for early diagnosis, prediction of drug response and other clinical applications (3). Survivin is a member of the inhibitor of apoptosis protein family. High survivin expression levels are associated with poor outcomes in the majority of cancer types (4–9). There is also evidence of survivin expression in specific adult tissues, including healthy oral epithelium, colonic epithelium, placenta and healthy endometrium (4,10,11). A recent study reported that survivin can also act as a subunit of the chromosomal passenger complex (CPC), and direct the other subunits of CPC such as Aurora-B, Borealin and the inner centromere protein to regulate chromosome separation and cell division (4,12).
Mucins are high-molecular-weight, heavily glycosylated proteins (13). At present, >20 mucin types have been identified and classified into two separate classes according to their structure and function (14). The two structurally and functionally distinct classes are: i) Secreted gel-forming mucins (MUC2, MUC5AC, MUC5B and MUC6) and ii) transmembrane mucins (MUC1, MUC3A, MUC3B, MUC4, MUC12 and MUC17). The MUC2 glycoprotein is a secreted mucin that consists of two distinct regions with a high degree of internal homology (15). MUC2 is commonly expressed in the healthy colonic epithelium and expression is decreased in non-mucinous colon adenocarcinomas (16–18). MUC2 and MUC5AC are clustered at the same chromosomal locus (11p15.5), and their expression and function may be regulated by a common mechanism (19). The MUC5AC gene is primarily expressed in the gastric and tracheobronchial mucosa; however, MUC5AC is not expressed in the healthy colonic epithelium (20). Although the expression of MUC5AC increases in differentiated CRC, the absence of MUC5AC expression in tumors can be a prognostic factor for more aggressive colon adenocarcinomas (21). Moreover, the expression of MUC2 and MUC5 is regulated by an extracellular signal-regulated kinase pathway in epithelial growth factor (EGF)/RAS proto-oncogene, GTPase (Ras)/Raf proto-oncogene, serine/threonine kinase (Raf)-positive cells (22). A study have indicated that EGF-mutant cancer cell lines express high levels of survivin (23). At present, however, to the best of our knowledge, no studies have investigated the link between survivin expression and MUC2/MUC5 expression in CRC. In the present study, the expression of survivin and its association with MUC2, MUC5 and the clinicopathological features of CRC were examined.
Materials and methods
Patients and tissue samples
CRC and normal tissue samples were obtained from 6 patients who underwent surgery at the Affiliated Hospital of Guilin Medical University (Guilin, China). A total of 20 normal colon mucosa samples and 139 advanced carcinomas (76 men and 63 women) were obtained from the Affiliated Hospital of Guilin Medical University and the archive of Hiroshima University Hospital (Hiroshima, Japan). All samples were obtained following approval by the Ethics Committees of Guilin Medical University and Hiroshima University. All patient records were complete, and each diagnosis was obtained by attending clinicians. Histologically, 117 carcinoma cases were classified as well/moderately differentiated and 22 as poorly differentiated according to the criteria of the Japanese Society for colorectal cancer (10,11). Tissues from each patient were fixed in formalin, cut into parallel 4–5-mm sections and embedded in paraffin. Tissue sections 4-µm thick were stained with hematoxylin and eosin for immunohistochemical examination. Informed consent was obtained from all subjects.
Immunohistochemistry
For immunohistochemical examination, tissue sections (4 µm) were incubated with the following primary antibodies: MUC2 (catalog no. NCL-MUC2; mouse monoclonal antibody, dilution, 1:100; Novocastra; Leica Microsystems GmbH, Wetzlar, Germany), MUC5 (catalog no. NCL-MUC5; mouse monoclonal antibody, dilution 1:100; Novocastra; Leica Microsystems GmbH), survivin (cat no. NB500-201, dilution, 1:1,000; Novus Biologicals, LLC, Littleton, CO, USA) and Ki-67 (cat no. M7240, MIB-1, mouse monoclonal antibody, dilution, 1:100; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA). All were incubated at 4°C overnight following antigen retrieval by microwave treatment in citrate buffer (pH 6.0; ZSGB-Bio, Beijing, China) and detection by the avidin-biotin peroxidase complex system using a labeled streptavidin-biotin kit (Dako; Agilent Technologies, Inc.) according to manufacturers's protocol. For MUC2, MUC5, survivin and Ki-67, immunoreactivity was graded according to the percentage of positive tumor cells as follows: Strong, >60% of tumor cells intensely stained; moderate, >20% intensely stained; mild, 5–20% intensely stained; or negative, <5% intensely stained. The expression levels of Survivin MUC2, MUC5 and Ki-67 were also graded as high (>20% of positive cells) or low (<20% of positive cells).
Western blot analysis
Colorectal tissues were lysed in radioimmunoprecipitation lysis buffer (cat no. R0020; Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) according to manufacturer's protocol. Protein concentrations were detected by the Bradford method, using bovine serum albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) as the standard. Equal amounts of tissue extract (40 µg) underwent 10% SDS-PAGE separation and were then transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA) for antibody blotting. The membrane was then blocked with 5% nonfat dried milk (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at room temperature for 1 h, and incubated with primary antibodies at 4°C overnight and secondary antibodies (cat no. sc-2004, sc-2005, dilution, 1:2,000; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature and then an enhanced chemiluminescence kit (cat no. 170-5061; Clarity™ Western ECL Substrate; Bio-Rad Laboratories, Inc.) was used for visualization of specific protein antigens. Then, images of the membrane were captured in a darkroom, and the results were analyzed. The primary antibodies were anti-MUC2 (cat no. NCL-MUC2; mouse monoclonal antibody; dilution 1:1,000; Novocastra; Leica Microsystems GmbH), anti-MUC5 (cat no. NCL-MUC5; mouse monoclonal antibody, dilution, 1:1,000; Novocastra; Leica Microsystems GmbH), anti-survivin (cat no. NB500-201, dilution, 1:2,000; Novus Biologicals, LLC) and anti-β-actin (cat no. TA-09, dilution, 1:5,000; ZSGB-Bio).
Statistical analysis
The SPSS software package v.17.0 (SPSS, Inc., Chicago, IL, USA) was used for analysis. A χ2 test was used for comparison of data between groups. Survival analyses were conducted using the Kaplan-Meier method and survival characteristics were compared using log-rank tests. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of survivin, MUC2 and MUC5 in healthy and cancerous colon mucosa
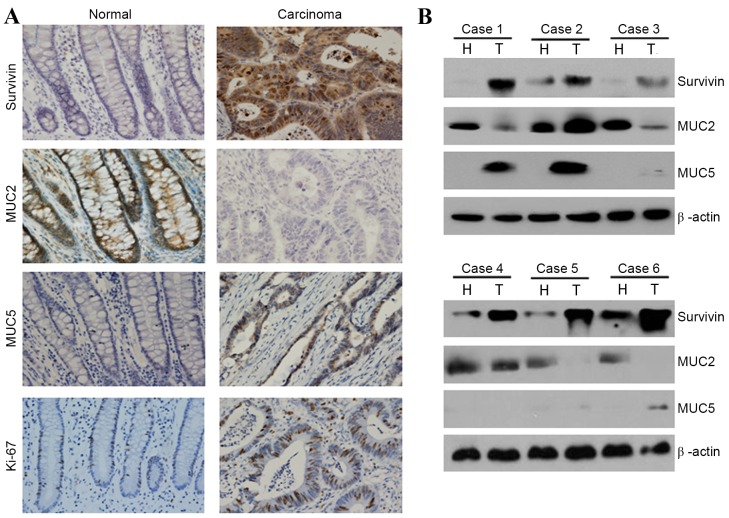
Expression levels of survivin, MUC2 and MUC5 were measured by immunohistochemical methods in 20 normal colon mucosa samples and 139 carcinoma cases. High survivin expression rates (nucleus, cytoplasmic staining) in healthy colon mucosa and CRC cases were 0% (0/20) and 39.57% (55/139), respectively (Table I and Fig. 1A). Rates of high MUC2 expression (cytoplasmic staining) in the healthy colon mucosa and in CRC tissue were 100% (20/20) and 48.20% (67/139), respectively (Table I and Fig. 1A). Rates of high MUC5 expression (cytoplasmic staining) in the healthy colon mucosa and CRC tissue were 0% (0/20) and 28.06% (39/139), respectively (Table I and Fig. 1A). In conclusion, the expression and staining intensity of survivin and MUC5 were significantly increased in CRC tissues (P<0.01), whereas those of MUC2 were significantly decreased in CRC tissues (P<0.01).
Table I.
Survivin, MUC2 and MUC5 expression in normal colon mucosa and cancer.
| Survivin expression, n | MUC2 expression, n | MUC5 expression, n | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tissue type | Total | Low | High | P-value | Low | High | P-value | Low | High | P-value |
| Normal | 20 | 20 | 0 | <0.01a | 0 | 20 | <0.01a | 20 | 0 | <0.01a |
| Cancer | 139 | 84 | 55 | 72 | 67 | 100 | 39 | |||
Normal tissue vs. cancer tissue. MUC, mucin.
Figure 1.
Examples and quantification of survivin and mucin expression in healthy and CRC cells. (A) Immunohistochemical staining for MUC2, MUC5 and survivin in healthy colorectal tissues and adjacent CRC tissues. Representative case of MUC2, MUC5 and survivin expression in normal adjacent tissues and CRC tissues are shown (magnification, ×200). (B) Expression of MUC2, MUC5 and survivin in healthy and cancerous colorectal tissues was examined by western blot analysis. β-actin expression was used as a control. MUC, mucin; CRC, colorectal cancer; H, healthy tissue; T, tumor tissue.
Western blot analysis revealed that the expression levels of survivin and MUC5 in healthy colon mucosa tissues were lower than levels in CRC tissues (Fig. 1B). However, expression levels of MUC2 in the healthy colon mucosa were higher than levels in CRC tissues (Fig. 1B). These findings support the immunohistochemical data, as high expression of MUC2 was observed in healthy colon mucosa, whereas high expression of survivin and MUC5 was observed in CRC cells (Fig. 1B).
Survivin expression and correlation with MUC2, MUC5, Ki-67 and clinicopathological features in CRC
The present study examined the correlation between survivin, MUC2, MUC5 and Ki-67 in CRC cases. A total of 55/139 patients with CRC exhibited high survivin expression levels (Table II). Moreover, of the 55 patients with high survivin expression, 28 exhibited high expression of MUC5 and 41 exhibited high expression of Ki-67, whereas 40 patients exhibited low expression of MUC2. This demonstrated that survivin expression was directly associated with MUC5 and Ki-67 expression, and inversely correlated with MUC2 expression in CRC (P<0.01).
Table II.
Survivin expression and its correlation with MUC2 and MUC5 expression in colorectal cancer.
| Survivin expression | |||
|---|---|---|---|
| Clinicopathological factor | Low (n=84) | High (n=55) | P-value |
| MUC2 expression, n | <0.01 | ||
| Low | 32 | 40 | |
| High | 52 | 15 | |
| MUC5 expression, n | <0.01 | ||
| Low | 73 | 27 | |
| High | 11 | 28 | |
| Ki-67 expression, n | <0.01 | ||
| Low | 57 | 14 | |
| High | 27 | 41 | |
MUC, mucin.
Patients with high survivin expression levels demonstrated significantly increased incidences of lymph node metastasis (P<0.01) and stage-D tumors (P<0.01) (8 Edition, Japanese Classification of Colorectal Carcinoma) (10,11) compared with cases exhibiting low survivin expression levels (Table III). However, no evidence of association between survivin expression level and tumor size, gender or histological differentiation was found.
Table III.
Survivin expression and its correlation with clinicopathological findings in colorectal cancer.
| Survivin expression | |||
|---|---|---|---|
| Clinicopathological factor | Low (n=84) | High (n=55) | P-value |
| Tumor size (mm), n | 0.56 | ||
| ≥50 | 37 | 27 | |
| <50 | 47 | 28 | |
| Histological differentiation, n | <0.01 | ||
| Poor | 10 | 12 | |
| Well/moderate | 74 | 43 | |
| Lymph node metastasis, n | <0.01 | ||
| Negative | 60 | 16 | |
| Positive | 24 | 39 | |
| Sex, n | 0.31 | ||
| Male | 43 | 33 | |
| Female | 41 | 22 | |
| Tumor stage, n | <0.01 | ||
| B/C | 76 | 39 | |
| D | 8 | 16 | |
Patients with low MUC2 expression levels demonstrated significantly lower cell differentiation (P<0.01) and higher incidences of lymph node metastasis (P<0.05) and stage-D tumors (P<0.01) when compared with cases displaying high expression of MUC2 (Table IV). The association between clinicopathological factors and MUC5 expression in CRC was also examined. In comparison to MUC2, patients expressing high levels of MUC5 tended to exhibit poor CRC cell differentiation (P<0.05), higher rates of lymph node metastasis (P<0.01) and higher tumor stage (P<0.01) (Table IV).
Table IV.
MUC2 and MUC5 expression and its correlation with clinicopathological findings in colorectal cancer.
| MUC2 expression | MUC5 expression | |||||
|---|---|---|---|---|---|---|
| Clinicopathological factor | Low (n=72) | High (n=67) | P-value | Low (n=100) | High (n=39) | P-value |
| Tumor size (mm), n | 0.099 | 0.99 | ||||
| ≥50 | 38 | 26 | 46 | 18 | ||
| <50 | 34 | 41 | 54 | 21 | ||
| Histological differentiation, n | <0.01 | <0.05 | ||||
| Poor | 17 | 5 | 12 | 10 | ||
| Well/moderate | 55 | 62 | 88 | 29 | ||
| Lymph node metastasis, n | <0.05 | <0.01 | ||||
| Negative | 34 | 43 | 66 | 11 | ||
| Positive | 38 | 24 | 34 | 28 | ||
| Sex, n | 0.21 | |||||
| Male | 38 | 38 | 58 | 18 | ||
| Female | 34 | 29 | 42 | 21 | ||
| Tumor stage, n | <0.01 | <0.01 | ||||
| B/C | 54 | 61 | 90 | 25 | ||
| D | 18 | 6 | 10 | 14 | ||
MUC, mucin.
Survival analysis
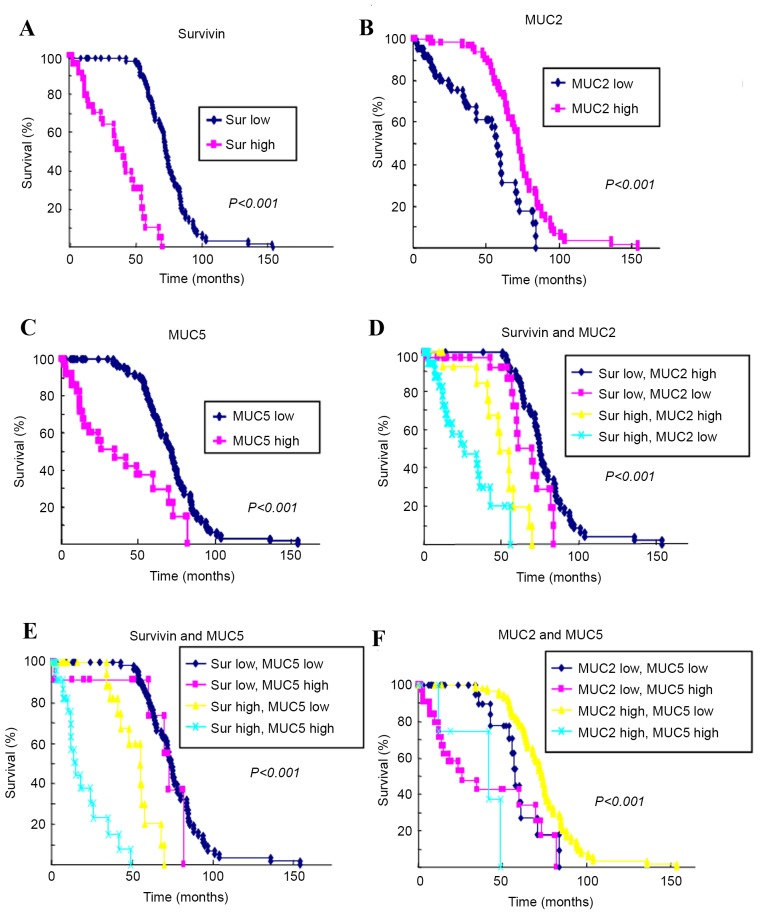
The Kaplan-Meier method was used to assess the survival rate of 139 patients who expressed survivin, MUC2 and MUC5 (Fig. 2). The 5-year survival rate of those whose tumors expressed low levels of survivin and MUC5 was higher than that of patients with high expression levels of survivin and MUC5. By comparison, the 5-year survival rate of those with tumors expressing high levels of MUC2 was higher than that of patients whose tumors expressed low levels of MUC2.
Figure 2.
Kaplan-Meier survival curves of patients with colorectal cancer according to MUC2, MUC5 and survivin expression levels. (A) The 5-year survival rate of patients expressing high survivin levels was 10.3 vs. 80.7% for those expressing low survivin levels. (B) The 5-year survival of patients with high MUC2 expression was 40.2 vs. 73.9% for those expressing low MUC2 levels. (C) The 5-year survival rate of patients expressing high MUC5 levels was 29.8 vs. 69.9% for those expressing low survivin levels. (D) Comparison of the 5-year survival rate of patients with the indicated expression levels of (D) survivin and MUC2, (E) 5 survivin and MUC5, and (F) MUC2 and MUC5. MUC, mucin.
Discussion
Survivin is a bifunctional protein that suppresses apoptosis and regulates cell division, which is highly expressed in various cancer types (12). Additionally, >20 mucins are classified as either secreted mucins or transmembrane mucins according to their structure and function (13). MUC2 and MUC5 are secreted mucins. However, to the best of our knowledge, there have been no studies to date investigating the correlation of survivin expression with MUC2 and MUC5 expression in CRC. The present study focused on the expression levels of survivin, MUC2 and MUC5 in healthy and CRC tissues using immunohistochemical analysis. Additionally, the present study aimed to investigate the potential of these biomarkers to aid in the early diagnosis of CRCs, as well as other clinical applications.
In this study, survivin was revealed to be expressed at high levels in CRC tissues, but at low levels in healthy colon tissues (Fig. 1 and Table I). Moreover, patients with high expression of survivin demonstrated significantly poorer cellular differentiation, higher rates of lymph node metastasis and a higher incidence of stage-D tumors than did cases with low expression of survivin. Furthermore, overexpression of survivin has previously been found to be associated with poor prognosis in CRC, HCC, and head and neck cancer (4,10–12).
The present study also examined the expression levels of MUC2 and MUC5 in healthy colon mucosa and in CRC patients. MUC2 was found to be expressed at high levels in normal colon tissue and at lower levels in CRC tissue (Fig. 1 and Table I). Moreover, cases with low expression of MUC2 demonstrated significantly poorer cell differentiation, higher rates of lymph node metastasis and a higher incidence of stage-D tumors compared with cases with high levels of expressed MUC2. Similarly, several studies have shown that loss of MUC2 expression is correlated with poor prognosis in CRC (24–26). Notably, however, another study found that overexpression of MUC2 is associated with poorer overall survival (27). In a further previous study, decreased expression of MUC2 was found to be associated with colon carcinogenesis, decreased apoptosis and increased migration of intestinal epithelial cells (20).
A number of studies have shown that low expression of MUC2 is associated with poorly differentiated adenocarcinoma of the colon and rectum (28,29). By contrast, in the present study, MUC5 was expressed at high levels in CRC tissues but at low levels in normal colon tissues (Fig. 1 and Table I). Similarly, other studies have shown that MUC5AC is not detected in the normal colon, but is frequently found in adenomas and carcinomas (18,30–32). Other prior studies reported that an increase in expression of MUC5AC was observed in sporadic cancer with high microsatellite instability (33) and that MUC5AC expression in intrahepatic cholangiocarcinoma was found to be an independent prognostic factor by multivariate survival analysis (34). The presence of MUC2 and/or MUC5AC in colorectal mucinous adenocarcinoma has been shown to be associated with proximal (right-sided) CRC location (32,33). There was no statistically significant association between gender and expression of MUC2 and/or MUC5 in the present study. Expression of survivin was also compared with the expression of MUC2, MUC5 and Ki-67 (Fig. 1; Table II). Survivin expression was found to be directly correlated with MUC5 and Ki-67 expression, and inversely correlated with MUC2 expression (Fig. 1 and Table II). These findings led to the hypothesis that survivin and MUC5 are expressed at high levels in CRC, whereas low MUC2 expression levels confer a poor prognosis in CRC.
In conclusion, the present study revealed that the normal-to-carcinoma sequence was significantly associated with the high expression and staining intensity of survivin and MUC5 (P<0.01), and the low expression of MUC2. Additionally, cases with high expression of survivin and MUC5 and/or low expression of MUC2 demonstrated significantly increased rates of lymph node metastasis and incidences of advanced tumor stage. Therefore, increased survivin and MUC5 or decreased MUC2 expression levels are associated with the malignant potential of colon carcinoma. Further investigations using appropriate techniques based on clinical data are required to confirm the present findings.
Acknowledgements
This research was supported in part by grants from The National Natural Science Foundation of China (no. 81460411), Guangxi University of Science and Technology Research Projects (no. ZD20140094), Guangxi Undergraduate Innovation Program (201510601016) The Natural Science Foundation of Guangxi (grant no. 2015GXNSFAA139110).
References
- 1.Klimczak A, Kempińska-Mirosławska B, Mik M, Dziki L, Dziki A. Incidence of colorectal cancer in Poland in 1999–2008. Arch Med Sci. 2011;7:673–678. doi: 10.5114/aoms.2011.24138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JC, Shaw RD. Update on colon cancer screening: Recent advances and observations in colorectal cancer screening. Curr Gastroenterol Rep. 2014;16:403. doi: 10.1007/s11894-014-0403-3. [DOI] [PubMed] [Google Scholar]
- 4.Qi G, Kudo Y, Ando T, Tsunematsu T, Shimizu N, Siriwardena SB, Yoshida M, Keikhaee MR, Ogawa I, Takata T. Nuclear Survivin expression is correlated with malignant behaviors of head and neck cancer together with Aurora-B. Oral Oncol. 2010;46:263–270. doi: 10.1016/j.oraloncology.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Qi G, Ogawa I, Kudo Y, Miyauchi M, Siriwardena BS, Shimamoto F, Tatsuka M, Takata T. Aurora-B expression and its correlation with cell proliferation and metastasis in oral cancer. Virchows Arch. 2007;450:297–302. doi: 10.1007/s00428-006-0360-9. [DOI] [PubMed] [Google Scholar]
- 6.Qi G, Kudo Y, Tang B, Liu T, Jin S, Liu J, Zuo X, Mi S, Shao W, Ma X, et al. PARP6 acts as a tumor suppressor via downregulating Survivin expression in colorectal cancer. Oncotarget. 2016;7:18812–18824. doi: 10.18632/oncotarget.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikeguchi M, Ueta T, Yamane Y, Hirooka Y, Kaibara N. Inducible nitric oxide synthase and survivin messenger RNA expression in hepatocellular carcinoma. Clin Cancer Res. 2002;8:3131–3136. [PubMed] [Google Scholar]
- 8.Wakana Y, Kasuya K, Katayanagi S, Tsuchida A, Aoki T, Koyanagi Y, Ishii H, Ebihara Y. Effect of survivin on cell proliferation and apoptosis in gastric cancer. Oncol Rep. 2002;9:1213–1218. [PubMed] [Google Scholar]
- 9.Sui L, Dong Y, Ohno M, Watanabe Y, Sugimoto K, Tokuda M. Survivin expression and its correlation with cell proliferation and prognosis in epithelial ovarian tumors. Int J Oncol. 2002;21:315–320. [PubMed] [Google Scholar]
- 10.Qi G, Tuncel H, Aoki E, Tanaka S, Oka S, Kaneko I, Okamoto M, Tatsuka M, Nakai S, Shimamoto F. Intracellular localization of survivin determines biological behavior in colorectal cancer. Oncol Rep. 2009;22:557–562. doi: 10.3892/or_00000471. [DOI] [PubMed] [Google Scholar]
- 11.Tuncel H, Shimamoto F, Qi H Kaneko Guangying, Aoki E, Jikihara H, Nakai S, Takata T, Tatsuka M. Nuclear Aurora B and cytoplasmic survivin expression is involved in lymph node metastasis of colorectal cancer. Oncol Lett. 2012;3:1109–1114. doi: 10.3892/ol.2012.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang B, Liang X, Tang F, Zhang J, Zeng S, Jin S, Zhou L, Kudo Y, Qi G. Expression of USP22 and Survivin is an indicator of malignant behavior in hepatocellular carcinoma. Int J Oncol. 2015;47:2208–2216. doi: 10.3892/ijo.2015.3214. [DOI] [PubMed] [Google Scholar]
- 13.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: Mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006;1765:189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Itoh Y, Kamata-Sakurai M, Denda-Nagai K, Nagai S, Tsuiji M, Ishii-Schrade K, Okada K, Goto A, Fukayama M, Irimura T. Identification and expression of human epiglycanin/MUC21: A novel transmembrane mucin. Glycobiology. 2008;18:74–83. doi: 10.1093/glycob/cwm118. [DOI] [PubMed] [Google Scholar]
- 15.Toribara NW, Gum JJ, Culhane PJ, Lagace RE, Hicks JW, Petersen GM, Kim YS. MUC-2 human small intestinal mucin gene structure. Repeated arrays and polymorphism. J Clin Invest. 1991;88:1005–1013. doi: 10.1172/JCI115360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manne U, Weiss HL, Grizzle WE. Racial differences in the prognostic usefulness of MUC1 and MUC2 in colorectal adenocarcinomas. Clin Cancer Res. 2000;6:4017–4025. [PubMed] [Google Scholar]
- 17.Gurevich LE, Kazantseva IA, Korsakova NA, Tsar'Kov PV, Polishchuk LO. Expression of type 1 and type 2 mucins in colonic epithelial tumors. Arkh Patol. 2007;69:12–16. (In Russian) [PubMed] [Google Scholar]
- 18.Ishizu H, Kumagai J, Eishi Y, Takizawa T, Koike M. Mucin core protein expression by colorectal mucinous carcinomas with or without mucus hyperplasia. J Gastroenterol. 2004;39:125–132. doi: 10.1007/s00535-003-1263-z. [DOI] [PubMed] [Google Scholar]
- 19.Kanoh A, Takeuchi H, Kato K, Waki M, Usami K, Irimura T. Interleukin-4 induces specific pp-GalNAc-T expression and alterations in mucin O-glycosylation in colonic epithelial cells. Biochim Biophys Acta. 2008;1780:577–584. doi: 10.1016/j.bbagen.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Bartman AE, Sanderson SJ, Ewing SL, Niehans GA, Wiehr CL, Evans MK, Ho SB. Aberrant expression of MUC5AC and MUC6 gastric mucin genes in colorectal polyps. Int J Cancer. 1999;80:210–218. doi: 10.1002/(SICI)1097-0215(19990118)80:2<210::AID-IJC9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 21.Imai Y, Yamagishi H, Fukuda K, Ono Y, Inoue T, Ueda Y. Differential mucin phenotypes and their significance in a variation of colorectal carcinoma. World J Gastroenterol. 2013;19:3957–3968. doi: 10.3748/wjg.v19.i25.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrais M, Pigny P, Copin MC, Aubert JP, Van Seuningen I. Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J Biol Chem. 2002;30:32258–32267. doi: 10.1074/jbc.M204862200. [DOI] [PubMed] [Google Scholar]
- 23.Okamoto K, Okamoto I, Okamoto W, Tanaka K, Takezawa K, Kuwata K, Yamaguchi H, Nishio K, Nakagawa K. Role of Survivin in EGFR inhibitor-induced apoptosis in non-small cell lung cancers positive for EGFR mutations. Cancer Res. 2010;15:10402–10410. doi: 10.1158/0008-5472.CAN-10-2438. [DOI] [PubMed] [Google Scholar]
- 24.Kang H, Min BS, Lee KY, Kim NK, Kim SN, Choi J, Kim H. Loss of E-cadherin and MUC2 expressions correlated with poor survival in patients with stages II and III colorectal carcinoma. Ann Surg Oncol. 2011;18:711–719. doi: 10.1245/s10434-010-1338-z. [DOI] [PubMed] [Google Scholar]
- 25.Lugli A, Zlobec I, Baker K, Minoo P, Tornillo L, Terracciano L, Jass JR. Prognostic significance of mucins in colorectal cancer with different DNA mismatch-repair status. J Clin Pathol. 2007;60:534–539. doi: 10.1136/jcp.2006.039552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perçinel S, Savaş B, Ensari A, Kuzu I, Kuzu MA, Bektaş M, Cetinkaya H, Kurşun N. Mucins in the colorectal neoplastic spectrum with reference to conventional and serrated adenomas. Turk J Gastroenterol. 2007;18:230–238. [PubMed] [Google Scholar]
- 27.Perez RO, Bresciani BH, Bresciani C, Proscurshim I, Kiss D, Gama-Rodrigues J, Pereira DD, Rawet V, Cecconnello I, Habr-Gama A. Mucinous colorectal adenocarcinoma: Influence of mucin expression (Muc1, 2 and 5) on clinico-pathological features and prognosis. Int J Colorectal Dis. 2008;23:757–765. doi: 10.1007/s00384-008-0486-0. [DOI] [PubMed] [Google Scholar]
- 28.Baas IO, Mulder JW, Offerhaus GJ, Vogelstein B, Hamilton SR. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol. 1994;172:5–12. doi: 10.1002/path.1711720104. [DOI] [PubMed] [Google Scholar]
- 29.Lanza G, Jr, Maestri I, Dubini A, Gafa R, Santini A, Ferretti S, Cavazzini L. p53 expression in colorectal cancer: Relation to tumor type, DNA ploidy pattern and short-term survival. Am J Clin Pathol. 1996;105:604–612. doi: 10.1093/ajcp/105.5.604. [DOI] [PubMed] [Google Scholar]
- 30.Biemer-Hüttmann AE, Walsh MD, McGuckin MA, Ajioka Y, Watanabe H, Leggett BA, Jass JR. Immunohistochemical staining patterns of MUC1, MUC2, MUC4, and MUC5AC mucins in hyperplastic polyps, serrated adenomas, and traditional adenomas of the colorectum. J Histochem Cytochem. 1999;47:1039–1048. doi: 10.1177/002215549904700808. [DOI] [PubMed] [Google Scholar]
- 31.Biemer-Huttmann AE, Walsh MD, McGuckin MA, Simms LA, Young J, Leggett BA, Jass JR. Mucin core protein expression in colorectal cancers with high levels of microsatellite instability indicates a novel pathway of morphogenesis. Clin Cancer Res. 2000;6:1909–1916. [PubMed] [Google Scholar]
- 32.Kocer B, Soran A, Erdogan S, Karabeyoglu M, Yildirim O, Eroglu A, Bozkurt B, Cengiz O. Expression of MUC5AC in colorectal carcinoma and relationship with prognosis. Pathol Int. 2002;52:470–477. doi: 10.1046/j.1440-1827.2002.01369.x. [DOI] [PubMed] [Google Scholar]
- 33.Losi L, Scarselli A, Benatti P, de Leon M Ponz, Roncucci L, Pedroni M, Borghi F, Lamberti I, Rossi G, Marino M, et al. Relationship between MUC5AC and altered expression of MLH1 protein in mucinous and non-mucinous colorectal carcinomas. Pathol Res Pract. 2004;200:371–377. doi: 10.1016/j.prp.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Aishima S, Kuroda Y, Nishihara Y, Taguchi K, Taketomi A, Maehara Y, Tsuneyoshi M. Gastric mucin phenotype defies tumour progression and prognosis of intrahepatic cholangiocarcinoma: Gastric foveolar type is associated with aggressive tumour behaviour. Histopathology. 2006;49:35–44. doi: 10.1111/j.1365-2559.2006.02414.x. [DOI] [PubMed] [Google Scholar]