Abstract
Metastatic liver tumors (MLTs) from colorectal cancer (CRC) are often treated with stereotactic body radiation therapy (SBRT). The present study aimed to examine the predictive factors for response of MLTs to SBRT. A total of 39 MLTs from 24 patients with CRC were retrospectively analyzed. Radiotherapy for MLT was typically performed with a prescribed dose equivalent to a biologically effective dose (BED)10 of 100 Gy. The median follow-up period was 16 months (range, 5–64 months). The median prescribed dose and total BED10 were 56 Gy (range, 45–72 Gy) and 97.5 Gy (range, 71.7–115.5 Gy), respectively, in a median of 8 fractions (range, 4–33 fractions). The 1- and 2-year local control rates were 67.2 and 35.9%, respectively. For patients with MLT treated with ablative SBRT (BED10 ≥100 Gy in ≤5 fractions), the 1- and 2-year local control rates were 83.3 and 62.5%, respectively. Univariate analysis showed that primary tumor location (left-sided colon), maximum tumor diameter (≤30 mm) and ablative SBRT (BED10 ≥100 Gy in ≤5 fractions) were significantly associated with improved local control (P=0.0058, P=0.0059 and P=0.0268, respectively). Multivariate analysis showed that tumor diameter was significantly associated with improved local control (P=0.0314). In addition, patients who received ablative SBRT had significantly prolonged overall survival times compared with those treated with non-ablative SBRT (P=0.0261). To conclude, tumors ≤30 mm that can be treated with ablative SBRT are associated with good local control rates. The primary tumor location may affect the radiosensitivity of MLTs.
Keywords: stereotactic body radiation therapy, intensity modulated radiotherapy, stereotactic ablative radiotherapy, liver metastasis, colorectal cancer, rectal cancer
Introduction
Patients with colorectal cancer (CRC) often have metastases at initial presentation or during follow-up (1,2). The liver is one of the most frequent sites of metastasis (1,2). Newly developed chemotherapeutic agents, including targeted therapies, have improved the progression-free and overall survival times of patients with metastatic CRC (3,4). There is also evidence supporting the benefit of surgery to treat metastases from CRCs, with surgery improving overall survival time (5–7). The prolonged survival times of patients have highlighted the importance of local therapy for CRC and limited metastatic disease. In patients who are unfit for surgery, alternative local therapeutic approaches, including radiofrequency ablation (RFA), are available to treat liver metastases; these approaches are minimally invasive and can achieve good local control (8,9). However, their indication is currently limited to relatively small tumors that are located far away from critical structures (8,9).
Modern radiotherapeutic techniques, including intensity-modulated radiation therapy (IMRT) and stereotactic body radiation therapy (SBRT), have recently become more frequently utilized to curatively treat limited metastatic tumors (10–14). SBRT, also termed stereotactic ablative radiation therapy, is a proven curative treatment for medically inoperable small tumors in the lung and liver; it delivers highly conformal radiation in a limited number of high-dose fractions, providing excellent primary tumor control with minimal toxicity (12–21). Radiotherapy in alternatively fractionated regimens with a greater number of fractions may be required to reduce the biologically effective doses (BEDs) to normal tissues when treating liver tumors located in the hepatic portal regions. In those cases, IMRT may be considered a more conventional schedule (22). However, there are limited data that compare SBRT with conventionally fractionated radiotherapy of curative intent.
The present study aimed to evaluate the optimal radiotherapy regimen for liver metastases from CRC.
Materials and methods
Patients
The present study was conducted according to the principles of the Declaration of Helsinki. The institutional review board of Miyakojima IGRT Clinic (Osaka, Japan) approved data collection and analysis (approval no. 9). A total of 39 liver tumors in 24 patients who received definitive radiotherapy at Miyakojima IGRT Clinic between November 2007 and December 2014 were analyzed. All eligible patients were histologically diagnosed with primary colorectal adenocarcinoma and radiographically diagnosed with liver metastases using computed tomography (CT) or magnetic resonance imaging (MRI). Tumors from patients who were followed up for <6 months without any failures were excluded. Written informed consent for radiotherapy was obtained from all patients. Patient characteristics are shown in Table I.
Table I.
Characteristics of the patients (n=24) and tumors (n=39).
| Characteristics | n (%) | Median (range) |
|---|---|---|
| Gender | ||
| Male | 14 (58.3) | – |
| Female | 10 (41.7) | – |
| Age, years | – | 64 (43–84) |
| Performance status | ||
| 0 | 14 (58.3) | – |
| 1 | 10 (41.7) | – |
| Primary tumor location | ||
| Right-sided colon | 4 (16.7) | – |
| Left-sided colon | 14 (58.3) | – |
| Rectum | 6 (25.0) | – |
| Interval between the initial treatment and radiotherapy, months | – | 34 (5–145) |
| Number of liver metastasis at the initial radiotherapy | ||
| 1 | 18 (75.0) | – |
| 2 | 4 (16.7) | – |
| 3 | 2 (8.3) | – |
| Usage of chemotherapy after initial surgery to radiotherapy | ||
| Yes | 21 (87.5) | – |
| No | 3 (12.5) | – |
| Presence of metastasis at the initial diagnosis | ||
| Yes | 8 (33.3) | – |
| No | 16 (66.7) | – |
| History of local therapya for metastasis | ||
| Yes | 16 (66.7) | – |
| No | 8 (33.3) | – |
| History of chemotherapy | ||
| Yes | 21 (87.5) | – |
| No | 3 (12.5) | – |
| History of radiotherapy | ||
| Yes | 4 (16.7) | – |
| No | 20 (83.3) | – |
| Presence of extra diseases outside the field of radiotherapy | ||
| Yes | 5 (20.8) | – |
| No | 19 (79.2) | – |
| Primary tumor location | ||
| Right-sided colon | 7 (17.9) | – |
| Left-sided colon | 22 (56.4) | – |
| Rectum | 10 (25.6) | – |
| GTV, cc | – | 8.7 (0.4–134.7) |
| Size of maximum diameter, mm | 35.6 (7.0–116.9) | |
| ≤30 | 18 (46.2) | – |
| 30–50 | 13 (33.3) | – |
| >50 | 8 (20.5) | – |
| PTV, cc | – | 52.8 (12.3–243.0) |
| Total dose, Gy | – | 56.0 (45.0–72.0) |
| Number of fractions | – | 8 (4–33) |
| Fraction size, Gy | – | 7.0 (2.0–12.0) |
| Total prescription BED10, Gy | – | 97.5 (71.7–115.5) |
Local therapy included surgery, radiofrequency ablation, and radiotherapy. GTV, gross tumor volume; PTV, planning target volume; BED, biologically effective dose.
SBRT technique
Radiotherapy for liver tumors was performed as previously described (23). Briefly, CT and MRI scans for treatment planning were obtained using a 4-slice BrightSpeed ExcelÔ (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) between November 2007 and June 2014, and a 64-slice SOMATOM Definition AS Open RT Pro edition (Siemens AG, Munich, Germany) from July 2014 onward, as well as the SIGNA EXCITE HDx 1.5T (GE Healthcare Bio-Sciences), respectively. Planning contrast-enhanced 4-dimensional CT scans and gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MRI images were used to determine gross tumor volume. Planning target volume (PTV) was created by adding a 4–8-mm margin in all directions to the internal target volume (ITV). The prescribed radiation doses were documented at the reference point using conformal beams in 17 tumors, or designed to deliver the prescribed dose to cover 95% of the PTV using IMRT in 22 tumors. A prescribed dose equivalent to a BED10 of ~100 Gy was administered to the liver metastases from CRC. Radiotherapy was performed using a 6-MV linear accelerator (Novalis BrainLAB AG, Feldkirchen, Germany).
Follow-up
Local control was defined as the absence of local failure. Local control and survival times were defined as the intervals between the start of radiotherapy and the date of diagnosis of local failure or the date of mortality, respectively. Local failures were identified by experienced physicians using CT and MRI, and defined by any regrowth of the target tumor or the appearance of tumor staining in the target tumor on contrast-enhanced images. Toxicity was evaluated for the 29 treatments for 39 tumors in 24 patients. No hematological toxicities were considered to exist in patients who exhibited hematological abnormalities prior to radiotherapy and developed no apparent changes from the baseline. Toxicity was evaluated using the Common Terminology Criteria for Adverse Events version 4.0 (24).
Statistical analysis
The data are expressed as the medians, with the ranges in parentheses, unless otherwise indicated. Cumulative local control and survival estimates were calculated using the Kaplan-Meier method, and statistical differences were evaluated by the log-rank test. The Cox proportional hazards model was performed to evaluate factors affecting local control and survival. Results are reported as hazard ratios (HR) and corresponding 95% confidence intervals (CI). Variables with P<0.2 by univariate analysis were entered into the multivariate model. Multivariate analyses were performed with a Cox regression analysis. All statistical analyses were performed using JMP software version 12.2.0 (SAS Institute, Cary, NC, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Eligible patients and tumors
Of the 24 eligible patients, 14 patients received radiotherapy for a single liver metastasis, 3 patients received radiotherapy for two different lesions simultaneously, 3 patients received radiotherapy for two different lesions that occurred sequentially, and 2 patients received radiotherapy for three different lesions simultaneously. In addition, 1 patient received radiotherapy for one lesion and then for three lesions simultaneously 4 months later, and 1 patient received radiotherapy for two lesions simultaneously and then for one lesion 1 month later. The median follow-up times were 11 months (range, 5–52 months) and 16.5 months (range, 6–64 months) for local control and survival, respectively. Ablative SBRT, defined as BED10 ≥100 Gy in ≤5 fractions, was performed for 16 tumors in 8 patients.
Grade 1 nausea and fatigue were observed in 2 patients including 1 patient who developed grade 1 alkaline phosphatase (ALP) elevation and grade 2 γ-glutamyl transpeptidase (GGT) elevation following radiotherapy. Furthermore, 1 patient developed grade 1 aspartate aminotransferase (AST), alanine aminotransferase (ALT) and ALP elevation, and grade 2 GGT elevation following two different treatments. One patient developed grade 1 ALP elevation alone. In addition, 1 patient developed grade 1 AST, ALT and ALP elevation and grade 3 GGT elevation due to chemotherapy. One patient developed grade 2 AST and ALP elevation, and grade 3 GGT and blood bilirubin elevation due to cholangitis caused by a recurrent tumor. Furthermore, 1 patient developed a hemorrhagic duodenal ulcer; however, there was no evident causal association between that event and radiotherapy.
Local control
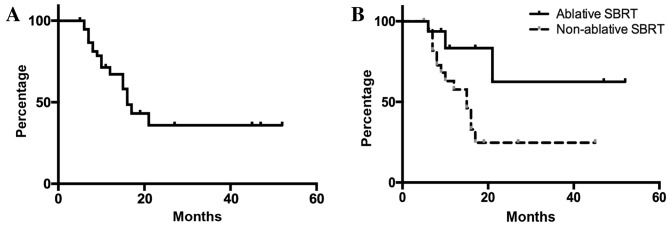
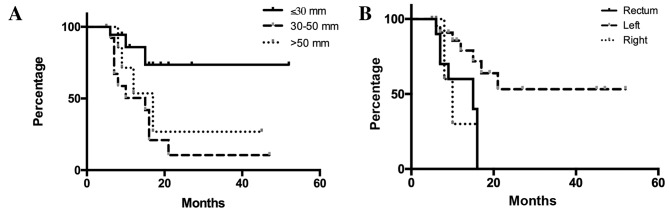
In all 39 liver metastases, the local control rates were 67.2 and 35.9% after 1 and 2 years, respectively (Fig. 1). When patients were divided into three groups based on tumor size (maximum diameter of GTV, ≤30, 30–50 and >50 mm), patients with tumors ≤30 mm in maximum diameter had significantly improved local control compared with the other two groups (Fig. 2A). When patients were stratified according to the primary tumor, defined as right-sided colon (cecum, ascending and transverse colon), left-sided colon (descending, sigmoid and rectosigmoidal) or rectal cancers, liver metastases from rectal cancer were shown to be associated with a significantly poorer local control rate compared with those from the left-sided colon (Fig. 2B).
Figure 1.
Local control in all eligible patients. (A) Outcomes following radiotherapy for metastatic liver tumors. For 39 metastatic tumors, the median local control time among the patients was 16 months. (B) The 1- and 2-year local control rates were 83.3 and 62.5% in the ablative SBRT group and 57.7 and 24.7% in the non-ablative SBRT group, respectively (P=0.0320).
Figure 2.
Differences in local control according to tumor sizes and the primary tumor location. (A) The 1- and 2-year local control rates were 85.9 and 73.6% for patients with tumors ≤30 mm, 50.4 and 10.5% for patients with tumors measuring 30–50 mm and 71.4 and 26.8% for patient with tumors >50 mm, respectively. A significant difference in local control rates was observed among the three groups (P=0.0174). In the two-group analyses, the P-values were P=0.0053 (≤30 vs. 30–50 mm), P=0.0869 (≤30 vs. >50 mm) and P=0.4148 (30–50 vs. >50 mm). (B) The 1-year local control rates were 30.0, 85.6 and 60.0% in liver metastases from the right-sided colon, left-sided colon and rectum, respectively. A significant difference was observed among the three groups (P=0.0115). In the two-group analyses, the P-values were P=0.0043 (rectum vs. left-sided colon), P=0.7001 (rectum vs. right-sided colon) and P=0.0608 (right-sided vs. left-sided colon).
Univariate analyses showed that colon cancer, left-sided location of primary tumor, the use of ablative SBRT and tumor size ≤30 mm were significantly associated with improved local control rates. In addition, multivariate analysis showed that tumor size <30 mm was a significant independent predictor of local control (Table II).
Table II.
Factors associated with local control in 39 tumors.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Factor | n (%) | MST, months | P-value | HR (95% CI) | P-value | HR (95% CI) |
| Age, years | ||||||
| <65 | 19 (48.7) | 16 | 0.9486 | 1.000 | – | – |
| ≥65 | 20 (51.3) | 17 | 0.969 (0.368–2.600) | – | ||
| Gender | ||||||
| Male | 25 (64.1) | 16 | 0.7442 | 1.000 | – | – |
| Female | 14 (35.9) | 21 | 0.839 (0.261–2.309) | – | ||
| Performance Status | ||||||
| 0 | 25 (64.1) | 17 | 0.8508 | 1.000 | – | – |
| 1 | 14 (35.9) | 12 | 0.908 (0.344–2.649) | – | ||
| Primary tumor location | ||||||
| Left-sided colon | 22 (56.4) | N/A | 0.0058 | 1.000 | 0.1068 | 1.000 |
| Other | 17 (43.6) | 10 | 4.370 (1.529–14.199) | 3.728 (0.766–23.295) | ||
| Ablative SBRT | ||||||
| Yes | 16 (41.0) | N/A | 0.0268 | 1.000 | 0.4817 | 1.000 |
| No | 23 (59.0) | 15 | 3.558 (1.144–15.594) | 2.292 (0.205–24.041) | ||
| BED10, Gy | ||||||
| <100 | 20 (51.3) | 15 | 0.0751 | 1.000 | 0.7181 | 1.000 |
| ≥100 | 19 (48.7) | N/A | 0.401 (0.126–1.094) | 1.464 (0.148–9.748) | ||
| Maximum diameter of the GTV, mm | ||||||
| ≤30 | 18 (46.2) | N/A | 0.0059 | 1.000 | 0.0314 | 1.000 |
| >30 | 21 (53.8) | 15 | 4.625 (1.506–20.084) | 3.940 (1.121–18.692) | ||
| PTV margin, mm | ||||||
| <8 | 17 (43.6) | 16 | 0.1350 | 1.000 | 0.6641 | 1.000 |
| 8 | 22 (56.4) | 21 | 0.478 (0.177–1.265) | 1.441 (0.296–9.156) | ||
| Interval between the initial treatment and radiotherapy, months | ||||||
| ≥35 | 19 (48.7) | 16 | 0.5991 | 1.000 | – | – |
| <35 | 20 (51.3) | 21 | 0.771 (0.284–2.051) | – | ||
MST, median survival time; HR, hazard ratio; CI, confidence interval; SBRT, stereotactic body radiation therapy; BED, biologically effective dose; GTV, gross tumor volume; PTV, planning target volume; N/A, not applicable.
Survival times
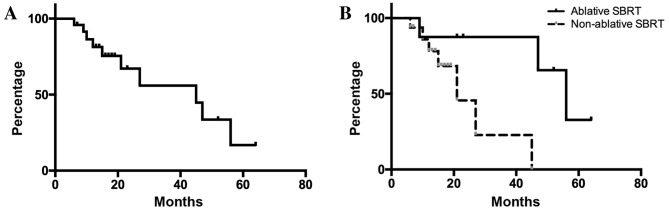
For all 24 patients, the overall survival rates were 81.3 and 67.1% at 12 and 24 months, respectively (Fig. 3). In univariate analyses, ablative SBRT, BED10 ≥100 Gy, an interval between the initial surgical treatment for the primary tumor and radiotherapy ≥35 months, and presence of a single liver metastasis were significantly associated with improved survival outcomes (Table III). No significant independent factors were detected by multivariate analysis.
Figure 3.
Overall survival of all eligible patients. (A) Outcomes following radiotherapy for patients with metastatic liver tumors. For the 24 patients, the median survival time was 45 months. (B) The 2- and 3-year overall survival rates were 87.5 and 87.5% in the ablative SBRT group, and 45.6 and 22.8% in the non-ablative SBRT group, respectively. Patients who received ablative SBRT showed significantly prolonged survival compared with those who received non-ablative SBRT (P=0.0261).
Table III.
Factors associated with overall survival in 24 patients.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Factor | n (%) | MST, months | P-value | HR (95% CI) | P-value | HR (95% CI) |
| Age, years | ||||||
| <65 | 13 (54.2) | 47 | 0.3912 | 1.000 | – | – |
| ≥65 | 11 (45.8) | 27 | 1.964 (0.412–10.545) | – | ||
| Gender | ||||||
| Male | 14 (58.3) | 27 | 0.7355 | 1.000 | – | – |
| Female | 10 (41.7) | 47 | 0.801 (0.212–3.001) | – | ||
| Performance Status | ||||||
| 0 | 14 (58.3) | 27 | 0.5065 | 1.000 | – | – |
| 1 | 10 (41.7) | 47 | 0.636 (0.151–2.398) | – | ||
| Primary tumor location | ||||||
| Colon | 18 (75.0) | 45 | 0.6331 | 1.000 | – | – |
| Rectum | 6 (25.0) | 21 | 1.414 (0.295–5.256) | – | ||
| Left-sided colon | 11 (45.8) | 45 | 0.5105 | 1.000 | – | – |
| Other | 13 (54.2) | 21 | 1.562 (0.385–5.691) | – | ||
| Ablative SBRT | ||||||
| Yes | 8 (33.3) | 56 | 0.0182 | 1.000 | 0.4688 | 1.000 |
| No | 16 (66.7) | 12 | 8.673 (1.387–169.233) | 1.121×10−8 (0–25.088) | ||
| BED10, Gy | ||||||
| <100 | 13 (54.2) | 21 | 0.0060 | 1.000 | 0.1802 | 1.000 |
| ≥100 | 11 (45.8) | 56 | 0.092 (0.005–0.544) | 3.133×10−9 (0–9.697×10−39) | ||
| Maximum diameter of the GTV, mm | ||||||
| ≤30 | 9 (37.5) | 27 | 0.4843 | 1.000 | – | – |
| >30 | 15 (62.5) | 45 | 1.715 (0.409–11.580) | – | ||
| ≤50 | 17 (70.8) | 47 | 0.3458 | 1.000 | – | – |
| >50 | 7 (29.2) | 45 | 2.066 (0.416–8.634) | – | ||
| PTV margin, mm | ||||||
| <8 | 11 (45.8) | 21 | 0.2071 | 1.000 | – | – |
| 8 | 13 (54.2) | 45 | 0.445 (0.113–1.573) | – | ||
| Interval between the initial treatment and radiotherapy, months | ||||||
| ≥35 | 11 (45.8) | 21 | 0.0028 | 1.000 | 0.4246 | 1.000 |
| <35 | 13 (54.2) | 56 | 0.079 (0.004–0.456) | 0.353 (0.010- 3.807) | ||
| Presence of metastasis at the initial diagnosis | ||||||
| Yes | 8 (33.3) | 45 | 0.3683 | 1.000 | – | – |
| No | 16 (66.7) | 27 | 1.984 (0.475–13.384) | – | ||
| History of local therapya for metastasis | ||||||
| Yes | 16 (66.7) | 45 | 0.4530 | 1.000 | – | – |
| No | 8 (33.3) | N/A | 0.564 (0.084–2.313) | – | ||
| History of chemotherapy | ||||||
| Yes | 21 (87.5) | 45 | 0.3970 | 1.000 | – | – |
| No | 3 (12.5) | N/A | 2.903 (0.147–20.092) | – | ||
| History of radiotherapy | ||||||
| Yes | 4 (16.7) | 15 | 0.1113 | 1.000 | 0.1131 | 1.000 |
| No | 20 (83.3) | 45 | 0.201 (0.033–1.545) | 0.154 (0.013–1.629) | ||
| Presence of extra diseases outside the field of radiotherapy | ||||||
| Yes | 5 (20.8) | 56 | 0.6227 | 1.000 | – | – |
| No | 19 (79.2) | 27 | 1.438 (0.354–7.363) | – | ||
| Number of liver metastases | ||||||
| 1 | 18 (75.0) | 47 | 0.0323 | 1.000 | 0.1246 | 1.000 |
| >1 | 6 (25.0) | 21 | 5.294 (1.160–27.017) | 3.845 (0.689–30.348) | ||
Local therapy included surgery, radiofrequency ablation, and radiotherapy. MST, median survival time; SBRT, stereotactic body radiation therapy; HR, hazard ratio; CI, confidence interval; BED, biologically effective dose; GTV, gross tumor volume; PTV, planning target volume; N/A, not applicable.
Discussion
The recommended treatment for patients with oligometastases in the liver is surgical resection (10,11). However, the resection rate of hepatic metastases has been reported to be only 0.8–22% (25). Liver metastases are known to have different sensitivities to radiation therapy based on the location of the primary tumor; it has been reported that liver metastases derived from CRCs were significantly more resistant to radiation compared with liver metastases derived from non-colorectal cancers (15–17). Thus, patients with CRC metastases may benefit from dose escalation. In addition, a hypoxic cell radiosensitizer may potentially improve local control (26).
The present data indicated that liver metastases from rectal cancer were more resistant to radiotherapy compared with those from colon cancer. Conversely, patients with metastases from left-sided colon cancer showed favorable local control rates following radiotherapy. CRC biological behavior was previously shown to depend on tumor location, as it varies for tumors originating from the right-sided colon, left-sided colon and rectum (27–33). Significant overexpression of p53 that can affect the response to radiotherapy in rectal cancer in comparison with colon cancer has been reported (33,34). Ayiomamitis et al (33) reported significant overexpression of p53 in rectal cancer compared with colon cancer tissue samples. In addition, Spitz et al (34) reported that p53 immunohistochemical staining of pretreatment biopsy specimens correlated with the extent of residual disease following chemoradiation in patients with rectal cancer. To the best of our knowledge, this is the first study to investigate the association between the radiosensitivity of liver metastases and primary tumor location.
Treatment with ablative SBRT, which was defined as a prescribed BED10 ≥100 Gy in ≤5 fractions, was found to lead to improved local control and survival rates compared with a conventional fractionated schedule. High-dose regimens have been described for metastatic liver tumors (16,18,19). Dose escalation of SBRT for liver metastasis can improve local control (18,19). However, the optimal regimen of SBRT remains unclear. The linear-quadratic (LQ) model has been widely used to compare differentially fractionated radiotherapeutic regimens, despite several limitations (35). Our previous studies have described a prescription using IMRT based on the LQ model (23,36,37). Hypofractionated radiotherapy at higher doses per fraction may increase radiation-induced damages due to direct cytotoxicity, and may lead to microvascular disruption (38–41). It was suggested that liver metastases should be treated by ablative SBRT as often as possible, and the present data support the findings reported in previous studies (15,16).
In the present study, adding margins >8 mm to the ITV led to improved outcomes, although the differences were not significant. When performing SBRT for liver tumors, no margins are usually added to the ITV (12–14,16–21,23). However, only a few studies have demonstrated how many GTV-to-CTV expansions are required in order to reach the gross and microscopic diseases in liver metastasis (41,42). Beginning in July 2012, the PTV margin was reduced from 8 to 4–6 mm in order to minimize focal liver damage. A possible CTV margin of 2–4 mm could therefore exist in tumors with a PTV margin of 8 mm in the present study.
The present study had several limitations. It was a retrospective study with a small sample size and a relatively short follow-up period. In addition, selection bias could exist in the present study, since tumors in the hepatic portal region were often treated with a more conventional regimen. Although a maximum tumor diameter ≤30 mm was the only prognostic factor for local control by multivariate analysis, the present results were compatible with those in previous studies (20,21). Chemotherapy was less thoroughly described and heterogeneous in the present study. Chemotherapy and other supportive care may differentially affect results, since no significant differences were observed in survival time despite primary tumor location significantly affecting local control in the present study. In addition, molecular biological analysis using immunohistochemistry and gene evaluation in isolated tissues may reveal differences in biological behavior of metastatic CRCs in future clinical studies.
To conclude, patients with liver metastases ≤30 mm in size from colorectal adenocarcinoma that received ablative SBRT exhibited favorable local control. Location of the primary tumor may affect the radiosensitivity of liver metastases.
Acknowledgements
The authors would like to thank Editage (www.editage.jp) for English language editing.
References
- 1.Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: Is there a limit? J Clin Oncol. 2005;23:8490–8499. doi: 10.1200/JCO.2004.00.6155. [DOI] [PubMed] [Google Scholar]
- 2.Aranda E, Abad A, Carrato A, Cervantes A, García-Foncillas J, García Alfonso P, García Carbonero R, Gómez España A, Tabernero JM, Díaz-Rubio E. Treatment recommendations for metastatic colorectal cancer. Clin Transl Oncol. 2011;13:162–178. doi: 10.1007/s12094-011-0636-7. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 4.Giantonio BJ, Levy DE, O'dwyer PJ, Meropol NJ, Catalano PJ, Benson AB, III, Eastern Cooperative Oncology Group A phase II study of high-dose bevacizumab in combination with irinotecan, 5-fluorouracil, leucovorin, as initial therapy for advanced colorectal cancer: Results from the eastern cooperative oncology group study E2200. Ann Oncol. 2006;17:1399–1403. doi: 10.1093/annonc/mdl161. [DOI] [PubMed] [Google Scholar]
- 5.Rees M, Tekkis PP, Welsh FK, O'Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann Surg. 2008;247:125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 6.Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, Jaeck D. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer. 1996;77:1254–1262. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1254::AID-CNCR5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Cirocchi R, Trastulli S, Boselli C, Montedori A, Cavaliere D, Parisi A, Noya G, Abraha I. Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Database Syst Rev. 2012;13:CD006317. doi: 10.1002/14651858.CD006317.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minami Y, Kudo M. Radiofrequency ablation of liver metastases from colorectal cancer: A literature review. Gut Liver. 2013;7:1–6. doi: 10.5009/gnl.2013.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network, corp-author. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. [Jun 12;2016 ];Guidelines version 2.2016 updates colon cancer. [PubMed]
- 11.https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. [Jun 12;2016 ];National Comprehensive Cancer Network: Guidelines version 2.2016 rectal cancer.
- 12.Rieber J, Streblow J, Uhlmann L, Flentje M, Duma M, Ernst I, Blanck O, Wittig A, Boda-Heggemann J, Krempien R, et al. Stereotactic body radiotherapy (SBRT) for medically inoperable lung metastases-A pooled analysis of the German working group ‘stereotactic radiotherapy’. Lung Cancer. 2016;97:51–58. doi: 10.1016/j.lungcan.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Hanna GG, Landau D. Stereotactic body radiotherapy for oligometastatic disease. Clin Oncol (R Coll Radiol) 2015;27:290–297. doi: 10.1016/j.clon.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Takeda A, Sanuki N, Kunieda E. Role of stereotactic body radiotherapy for oligometastasis from colorectal cancer. World J Gastroenterol. 2014;20:4220–4229. doi: 10.3748/wjg.v20.i15.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binkley MS, Trakul N, Jacobs LR, von Eyben R, Le QT, Maxim PG, Loo BW, Jr, Shultz DB, Diehn M. Colorectal histology is associated with an increased risk of local failure in lung metastases treated with stereotactic ablative radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:1044–1052. doi: 10.1016/j.ijrobp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Takeda A, Sanuki N, Tsurugai Y, Oku Y, Aoki Y. Stereotactic body radiotherapy for patients with oligometastases from colorectal cancer: Risk-adapted dose prescription with a maximum dose of 83–100 Gy in five fractions. J Radiat Res. 2016;57:400–405. doi: 10.1093/jrr/rrw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmed KA, Caudell JJ, El-Haddad G, Berglund AE, Welsh EA, Yue B, Hoffe SE, Naghavi AO, Abuodeh YA, Frakes JM, et al. Radiosensitivity differences between liver metastases based on primary histology suggest implications for clinical outcomes after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2016;95:1399–1404. doi: 10.1016/j.ijrobp.2016.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vautravers-Dewas C, Dewas S, Bonodeau F, Adenis A, Lacornerie T, Penel N, Lartigau E, Mirabel X. Image-guided robotic stereotactic body radiation therapy for liver metastases: Is there a dose response relationship? Int J Radiat Oncol Biol Phys. 2011;81:e39–e47. doi: 10.1016/j.ijrobp.2010.12.047. [DOI] [PubMed] [Google Scholar]
- 19.Wulf J, Guckenberger M, Haedinger U, Oppitz U, Mueller G, Baier K, Flentje M. Stereotactic radiotherapy of primary liver cancer and hepatic metastases. Acta Oncol. 2006;45:838–847. doi: 10.1080/02841860600904821. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita H, Onishi H, Matsumoto Y, Murakami N, Matsuo Y, Nomiya T, Nakagawa K, Japanese Radiological Society multi-institutional SBRT study group (JRS-SBRTSG) Local effect of stereotactic body radiotherapy for primary and metastatic liver tumors in 130 Japanese patients. Radiat Oncol. 2014;9:112. doi: 10.1186/1748-717X-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, Chidel MA, Pugh TJ, Franklin W, Kane M, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 22.Engels B, Gevaert T, Everaert H, De Coninck P, Sermeus A, Christian N, Storme G, Verellen D, De Ridder M. Phase II study of helical tomotherapy in the multidisciplinary treatment of oligometastatic colorectal cancer. Radiat Oncol. 2012;7:34. doi: 10.1186/1748-717X-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doi H, Shiomi H, Masai N, Tatsumi D, Igura T, Imai Y, Oh RJ. Threshold doses and prediction of visually apparent liver dysfunction after stereotactic body radiation therapy in cirrhotic and normal livers using magnetic resonance imaging. J Radiat Res. 2016;57:294–300. doi: 10.1093/jrr/rrw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Apr 3;2017 ];Common terminology criteria for adverse events version 4.0. doi: 10.1016/j.jaad.2012.02.010. [DOI] [PubMed]
- 25.Folprecht G, Grothey A, Alberts S, Raab HR, Köhne CH. Neoadjuvant treatment of unresectable colorectal liver metastases: Correlation between tumour response and resection rates. Ann Oncol. 2005;16:1311–1319. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
- 26.Brown JM, Diehn M, Loo BW., Jr Stereotactic ablative radiotherapy should be combined with a hypoxic cell radiosensitizer. Int J Radiat Oncol Biol Phys. 2010;78:323–327. doi: 10.1016/j.ijrobp.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loupakis F, Yang D, Yau L, Feng S, Cremolini C, Zhang W, Maus MK, Antoniotti C, Langer C, Scherer SJ, et al. Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst. 2015;107:pii:dju427. doi: 10.1093/jnci/dju427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, Agarwal A, Maru DM, Sieber O, Desai J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623–4632. doi: 10.1002/cncr.26086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Distler P, Holt PR. Are right- and left-sided colon neoplasms distinct tumors? Dig Dis. 1997;15:302–311. doi: 10.1159/000171605. [DOI] [PubMed] [Google Scholar]
- 30.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 31.Hutchins G, Southward K, Handley K, Magill L, Beaumont C, Stahlschmidt J, Richman S, Chambers P, Seymour M, Kerr D, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–1270. doi: 10.1200/JCO.2010.30.1366. [DOI] [PubMed] [Google Scholar]
- 32.Loupakis F, Ruzzo A, Cremolini C, Vincenzi B, Salvatore L, Santini D, Masi G, Stasi I, Canestrari E, Rulli E, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101:715–721. doi: 10.1038/sj.bjc.6605177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayiomamitis GD, Notas G, Zaravinos A, Zizi-Sermpetzoglou A, Georgiadou M, Sfakianaki O, Kouroumallis E. Differences in telomerase activity between colon and rectal cancer. Can J Surg. 2014;57:199–208. doi: 10.1503/cjs.031312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitz FR, Giacco GG, Hess K, Larry L, Rich TA, Janjan N, Cleary KR, Skibber JM. p53 immunohistochemical staining predicts residual disease after chemoradiation in patients with high-risk rectal cancer. Clin Cancer Res. 1997;3:1685–1690. [PubMed] [Google Scholar]
- 35.Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 7th. Philadelphia: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 36.Doi H, Oh RJ, Miura H, Masai N, Shiomi H, Inoue T. Outcomes and toxicity of radiotherapy for refractory bone and soft tissue sarcomas. Mol Clin Oncol. 2016;4:83–88. doi: 10.3892/mco.2015.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miura H, Masai N, Oh RJ, Shiomi H, Sasaki J, Inoue T. Approach to dose definition to the gross tumor volume for lung cancer with respiratory tumor motion. J Radiat Res. 2013;54:140–145. doi: 10.1093/jrr/rrs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thames HD, Suit HD. Tumor radioresponsiveness versus fractionation sensitivity. Int J Radiat Oncol Biol Phys. 1986;12:687–691. doi: 10.1016/0360-3016(86)90081-7. [DOI] [PubMed] [Google Scholar]
- 39.Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: Implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS) Radiat Res. 2012;177:311–327. doi: 10.1667/RR2773.1. [DOI] [PubMed] [Google Scholar]
- 40.Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: Are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88:254–262. doi: 10.1016/j.ijrobp.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian Y, Zeng ZC, Ji Y, Xiao YP. Microinvasion of liver metastases from colorectal cancer: Predictive factors and application for determining clinical target volume. Radiat Oncol. 2015;10:125. doi: 10.1186/s13014-015-0428-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welter S, Theeqarten D, Trarbach T, Maletzki F, Stamatis G, Tötsch M. Safety distance in the resection of colorectal lung metastases: A prospective evaluation of satellite tumor cells with immunohistochemistry. J Thorac Cardiovasc Surg. 2011;141:1218–1222. doi: 10.1016/j.jtcvs.2010.08.089. [DOI] [PubMed] [Google Scholar]