Abstract
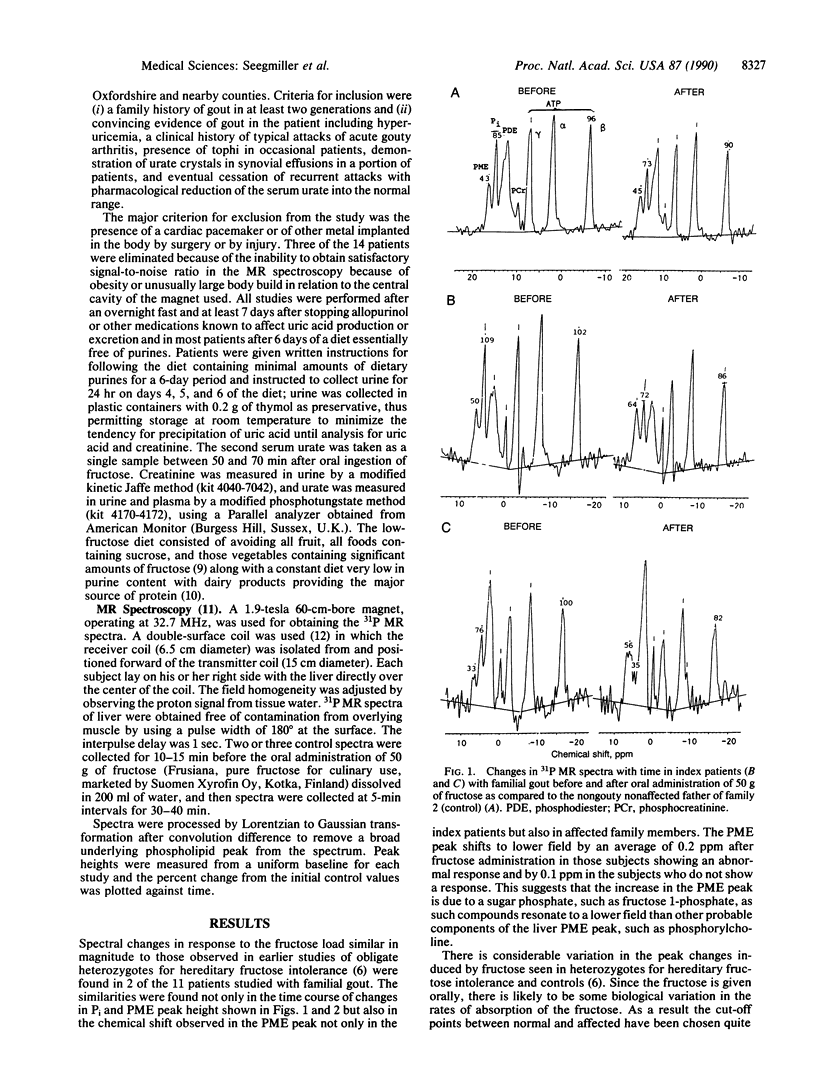
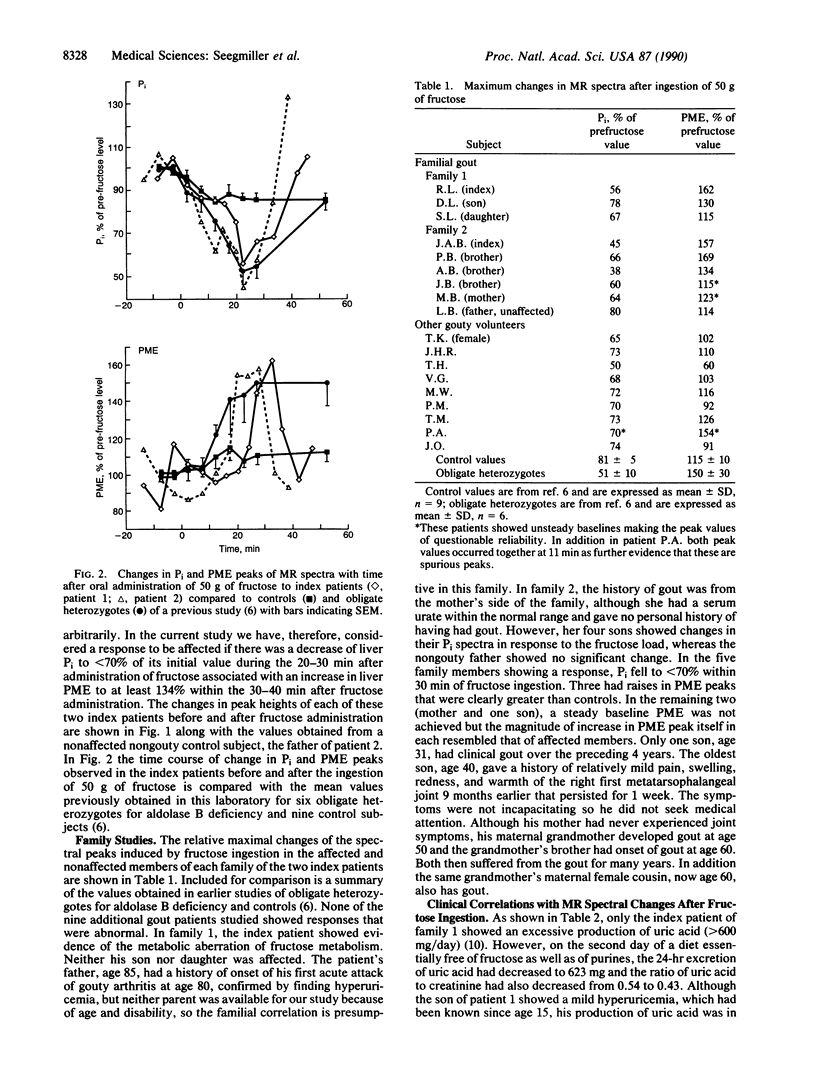
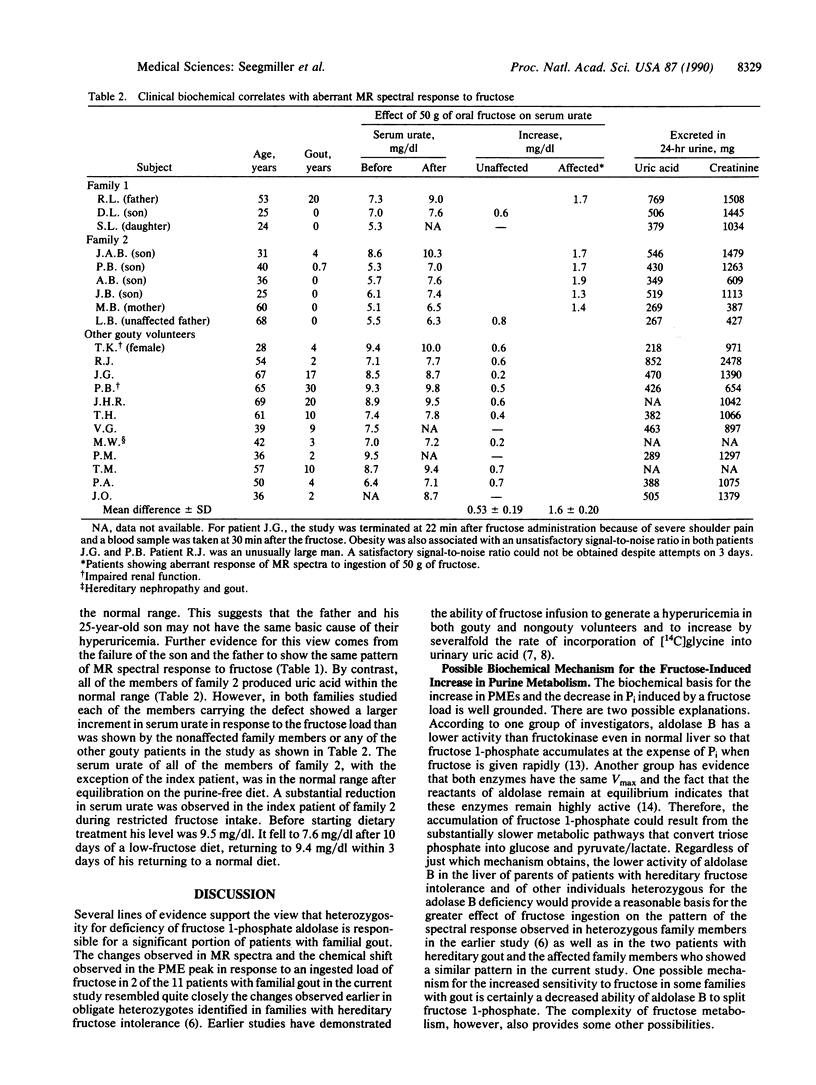
The hyperuricemia responsible for the development of gouty arthritis results from a wide range of environmental factors and underlying genetically determined aberrations of metabolism. 31P magnetic resonance spectroscopy studies of children with hereditary fructose intolerance revealed a readily detectable rise in phosphomonoesters with a marked fall in inorganic phosphate in their liver in vivo and a rise in serum urate in response to very low doses of oral fructose. Parents and some family members heterozygous for this enzyme deficiency showed a similar pattern when given a substantially larger dose of fructose. Three of the nine heterozygotes thus identified also had clinical gout, suggesting the possibility of this defect being a fairly common cause of gout. In the present study this same noninvasive technology was used to identify the same spectral pattern in 2 of the 11 families studied with hereditary gout. In one family, the index patient's three brothers and his mother all showed the fructose-induced abnormality of metabolism, in agreement with the maternal inheritance of the gout in this family group. The test dose of fructose used produced a significantly larger increment in the concentration of serum urate in the patients showing the changes in 31P magnetic resonance spectra than in the other patients with familial gout or in nonaffected members, thus suggesting a simpler method for initial screening for the defect.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode J. C., Zelder O., Rumpelt H. J., Wittkamp U. Depletion of liver adenosine phosphates and metabolic effects of intravenous infusion of fructose or sorbitol in man and in the rat. Eur J Clin Invest. 1973 Sep;3(5):436–441. doi: 10.1111/j.1365-2362.1973.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Cox T. M., Camilleri M., O'Donnell M. W., Chadwick V. S. Pseudodominant transmission of fructose intolerance in an adult and three offspring: Heterozygote detection by intestinal biopsy. N Engl J Med. 1982 Aug 26;307(9):537–540. doi: 10.1056/NEJM198208263070906. [DOI] [PubMed] [Google Scholar]
- Cross N. C., Cox T. M. Molecular analysis of aldolase B genes in the diagnosis of hereditary fructose intolerance in the United Kingdom. Q J Med. 1989 Nov;73(271):1015–1020. [PubMed] [Google Scholar]
- Hultman E., Nilsson L. H., Sahlin K. Adenine nucleotide content of human liver. Normal values and fructose-induced depletion. Scand J Clin Lab Invest. 1975 May;35(3):245–251. doi: 10.1080/00365517509095736. [DOI] [PubMed] [Google Scholar]
- Itakura M., Sabina R. L., Heald P. W., Holmes E. W. Basis for the control of purine biosynthesis by purine ribonucleotides. J Clin Invest. 1981 Apr;67(4):994–1002. doi: 10.1172/JCI110150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäenpä P. H., Raivio K. O., Kekomäki M. P. Liver adenine nucleotides: fructose-induced depletion and its effect on protein synthesis. Science. 1968 Sep 20;161(3847):1253–1254. doi: 10.1126/science.161.3847.1253. [DOI] [PubMed] [Google Scholar]
- Oberhaensli R. D., Rajagopalan B., Taylor D. J., Radda G. K., Collins J. E., Leonard J. V., Schwarz H., Herschkowitz N. Study of hereditary fructose intolerance by use of 31P magnetic resonance spectroscopy. Lancet. 1987 Oct 24;2(8565):931–934. doi: 10.1016/s0140-6736(87)91419-x. [DOI] [PubMed] [Google Scholar]
- Perheentupa J., Raivio K. Fructose-induced hyperuricaemia. Lancet. 1967 Sep 9;2(7515):528–531. doi: 10.1016/s0140-6736(67)90494-1. [DOI] [PubMed] [Google Scholar]
- Radda G. K., Rajagopalan B., Taylor D. J. Biochemistry in vivo: an appraisal of clinical magnetic resonance spectroscopy. Magn Reson Q. 1989 Apr;5(2):122–151. [PubMed] [Google Scholar]
- Raivio K. O., Becker 7. A., Meyer L. J., Greene M. L., Nuki G., Seegmiller J. E. Stimulation of human purine synthesis de novo by fructose infusion. Metabolism. 1975 Jul;24(7):861–869. doi: 10.1016/0026-0495(75)90133-x. [DOI] [PubMed] [Google Scholar]
- SEEGMILLER J. E., GRAYZEL A. I., LASTER L., LIDDLE L. Uric acid production in gout. J Clin Invest. 1961 Jul;40:1304–1314. doi: 10.1172/JCI104360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe F., Della Corte E., Bonetti E., Abbondanza A., Abbati A., De Stefano F. Fructose-induced hyperuricaemia. Lancet. 1970 Dec 19;2(7686):1310–1311. doi: 10.1016/s0140-6736(70)92269-5. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G., Bontemps F., Vincent M. F. Cytosolic purine 5'-nucleotidases of rat liver and human red blood cells: regulatory properties and role in AMP dephosphorylation. Adv Enzyme Regul. 1988;27:297–311. doi: 10.1016/0065-2571(88)90023-4. [DOI] [PubMed] [Google Scholar]
- Vincent M. F., Van den Berghe G., Hers H. G. Effect of fructose on the concentration of phosphoribosylpyrophosphate in isolated hepatocytes. Adv Exp Med Biol. 1986;195(Pt B):615–621. doi: 10.1007/978-1-4684-1248-2_96. [DOI] [PubMed] [Google Scholar]
- Vincent M. F., Van den Berghe G., Hers H. G. Increase in phosphoribosyl pyrophosphate induced by ATP and Pi depletion in hepatocytes. FASEB J. 1989 May;3(7):1862–1867. doi: 10.1096/fasebj.3.7.2469615. [DOI] [PubMed] [Google Scholar]
- Woods H. F., Eggleston L. V., Krebs H. A. The cause of hepatic accumulation of fructose 1-phosphate on fructose loading. Biochem J. 1970 Sep;119(3):501–510. doi: 10.1042/bj1190501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berghe G., Bronfman M., Vanneste R., Hers H. G. The mechanism of adenosine triphosphate depletion in the liver after a load of fructose. A kinetic study of liver adenylate deaminase. Biochem J. 1977 Mar 15;162(3):601–609. doi: 10.1042/bj1620601. [DOI] [PMC free article] [PubMed] [Google Scholar]



