We have a love–hate relationship with cholesterol. On one hand, cholesterol is an essential component of cell membranes and serves as the precursor to steroid hormones and bile acids. On the other hand, cholesterol can clog blood vessels and give rise to cardiovascular disease. Moreover, oxidized metabolites of cholesterol, termed oxysterols, are cytotoxic in a variety of different cell types and contribute to atherosclerosis (1). Thus, it is crucial that excess cholesterol be removed from the body. In a recent issue of PNAS, Sonada et al. (2) described an unexpected role for the pregnane X receptor (PXR) in protecting against cholesterol toxicity.
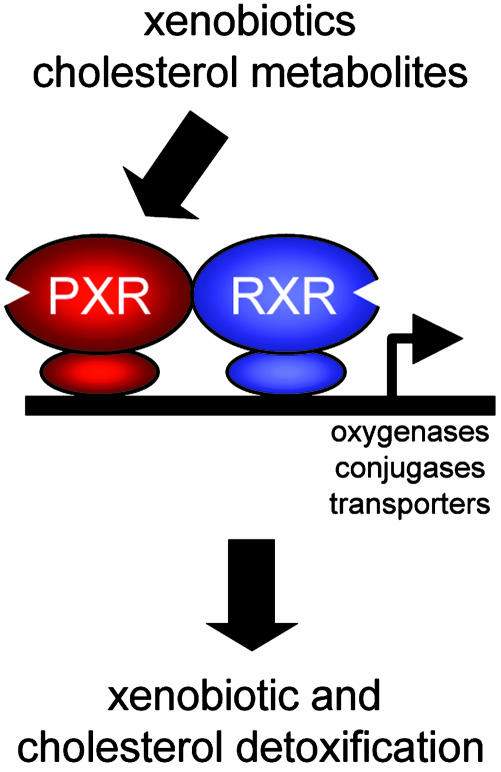
PXR is a member of the steroid/thyroid hormone receptor family of ligand-activated transcription factors that is expressed in liver, intestine, and kidney (3–5). Unlike the classical steroid hormone receptors, which are selectively activated by their cognate hormones at nanomolar or even picomolar concentrations, PXR is activated by micromolar concentrations of a structurally diverse collection of foreign chemicals, or xenobiotics, including the antibiotic rifampicin, the cancer drug taxol, and the herb Saint John's wort. This remarkable promiscuity is facilitated by the unusual ligand-binding pocket of PXR, which is both very large and smooth, allowing it to bind to many different chemicals (6). Once activated, PXR binds to DNA as a heterodimer with the retinoid X receptor and stimulates the transcription of genes encoding cytochrome P450 enzymes, conjugation enzymes, and transporters, which collectively promote xenobiotic metabolism. These findings have led to a “xenosensor” model (Fig. 1), in which PXR detects potentially harmful xenobiotics and induces genes that flush the offending chemicals from the body (3–5). An undesirable side effect of this xenobiotic detoxification system is that drugs that activate PXR can stimulate the metabolism of other drugs, sometimes with life-threatening consequences.
Fig. 1.
Model for PXR-induced detoxification of xenobiotics and cholesterol. Activation of PXR by xenobiotics or cholesterol metabolites stimulates the transcription of genes encoding oxygenases, conjugases, and transporters, which detoxify xenobiotics and cholesterol. PXR binds to DNA as a heterodimer with the retinoid X receptor (RXR).
Although PXR is proposed to function as a xenosensor, there have been tantalizing hints that it might also detect and coordinate the detoxification of metabolic intermediates produced by the body itself. First, PXR is activated in cell-based assays by many different chemicals with steroid backbones, including bile acid and oxysterol metabolites of cholesterol (7–9). In fact, PXR was named based on its activation by a number of different C21 steroids, including the hormone progesterone (10). However, activation of PXR requires higher concentrations of these chemicals than are typically measured in vivo, which draws into question the physiological relevance of these observations. Second, Shenoy et al. (7) showed that treatment of hepatocytes in vitro with 2,3-oxidosqualene:lanosterol cyclase inhibitors, which block the conversion of squalene 2,3-oxide into the cholesterol precursor lanosterol, activates PXR, presumably through the accumulation of squalene intermediates that serve as PXR ligands (11). Third, there is evidence that PXR can be activated by cholesterol metabolites in vivo: Two groups showed that mice lacking the enzyme sterol 27-hydroxylase, which is required for the conversion of cholesterol to bile acids, have increased PXR activity due to elevated concentrations of bile acid precursors such as 5β-cholestane-3α, 7α, 12α-triol (12, 13). By activating PXR, these bile acid precursors induce a shunt pathway of sterol side chain shortening, which results in the formation of bile acids. Although this finding demonstrates the existence of a feed-forward regulatory pathway by which potentially toxic bile acid intermediates activate PXR and induce their own metabolism, it is not known whether this pathway operates under normal physiologic conditions. Finally, activation of PXR protects against the hepatotoxic effects of high concentrations of bile acids administered in the diet (8, 9). Together, these studies raise the possibility that PXR evolved to detect and detoxify not only xenobiotics but also cholesterol precursors and metabolites (Fig. 1).
To address whether PXR has a physiological role in cholesterol metabolism, Sonoda et al. (2) fed wild-type mice and mice lacking PXR (Pxr–/–) a diet enriched in cholesterol and cholic acid, a bile acid that blocks cholesterol catabolism in the liver and enhances cholesterol absorption in the intestine. The Pxr–/– mice have no overt phenotype under normal laboratory conditions (8, 14). Remarkably, the cholesterol/cholic acid diet killed ≈40% of the Pxr–/– mice by day 10 and 100% of the mice by day 60 while causing no lethality in wild-type animals. Death in the Pxr–/– mice was preceded by several days of lethargy, hypothermia, and weight loss and by dramatic increases in the serum concentrations of bilirubin and bile acids, two markers of hepatobiliary defects. Histologic examination showed that the livers from the cholesterol/cholic-acidfed Pxr–/– mice were normal with respect to their overall morphology and the number of bile ducts. However, these studies revealed hepatitis in the Pxr–/– mice manifested by infiltration of mononuclear cells in the periportal and midzonal areas of the hepatic lobes. The Pxr–/– mice also had markedly elevated concentrations of plasma aspartate transaminase and alanine transaminase, two markers of hepatocellular injury, and increased expression of genes induced by inflammation. In addition to causing hepatotoxicity, the cholesterol/ cholic acid diet caused kidney damage in the Pxr–/– mice, as evidenced by increased concentrations of blood urea nitrogen and creatine and induction of proinflammatory genes. Taken together, these data demonstrate an essential role for PXR in protecting against acute toxicity caused by a high-cholesterol diet.
What is the molecular mechanism underlying the protective actions of PXR? Chemicals that activate PXR such as pregnenolone 16α-carbonitrile and spironolactone increase both the production of bile and the concentration of cholesterol in the bile, suggesting a role for this receptor in regulating cholesterol homeostasis (15, 16). Sonoda et al. (2) show that two genes regulated by PXR, cytochrome P450 3A11 (Cyp3a11) and organic anion transporting peptide 2 (Oatp2), are induced in liver by the cholesterol/cholic acid diet in wild-type mice but not in Pxr–/– mice, which suggests that this diet produces a PXR ligand. CYP3A isozymes are best known for oxidizing drugs but also hydroxylate various endogenous steroids, including cholesterol (17). OATP2 is localized on the sinusoidal membrane of hepatocytes, where it transports a variety of endogenous and exogenous compounds, including conjugated and unconjugated bilirubin and conjugated steroids and bile acids into the liver, where they are further metabolized and excreted from the body. Notably, activation of the nuclear receptor CAR, a xenobiotic receptor closely related to PXR that induces Cyp3a11 but not Oatp2, did not prevent the lethality of the cholesterol/cholic acid diet in the Pxr–/– mice. These data point to OATP2 as a candidate for protecting against cholesterol toxicity. How this might occur is not clear. Although OATP2 deficiency has not been described, mutations in a number of other transporters cause liver disease (18).
Pregnane X receptors protect against acute toxicity caused by a high-cholesterol diet.
Cholesterol toxicity is usually thought of as occurring over long periods of time as in cardiovascular disease. The work of Sonoda et al. (2) reveals an unexpected acute toxicity caused by excess cholesterol, which raises a number of interesting questions. What chemicals kill the Pxr–/– mice, and how do they cause death? What are the ligands generated by the cholesterol/cholic acid diet that activate PXR? Finally, and perhaps most importantly, do these studies translate to humans? Do polymorphisms and mutations in PXR influence cholesterol metabolism and the onset and progression of liver disease in people? Notably, PXR agonists such as rifampicin and phenobarbital have long been used clinically to treat pruritis associated with liver disease (19). The study by Sonoda et al. (2) raises the intriguing possibility that PXR agonists may be useful for treating other aspects of liver disease, including hepatitis and renal failure associated with liver pathology.
See companion article on page 2198 in issue 6 of volume 102.
References
- 1.Leonarduzzi, G., Sottero, B. & Poli, G. (2002) J. Nutr. Biochem. 13, 700–710. [DOI] [PubMed] [Google Scholar]
- 2.Sonoda, J., Chong, L. W., Downes, M., Barish, G. D., Coulter, S., Liddle, C., Lee, C.-H. & Evans, R. M. (2005) Proc. Natl. Acad. Sci. USA 102, 2198–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie, W. & Evans, R. M. (2001) J. Biol. Chem. 276, 37739–37742. [DOI] [PubMed] [Google Scholar]
- 4.Kliewer, S. A., Goodwin, B. & Willson, T. M. (2002) Endocr. Rev. 23, 687–702. [DOI] [PubMed] [Google Scholar]
- 5.Dussault, I. & Forman, B. M. (2002) Crit. Rev. Eukaryot. Gene Expr. 12, 53–64. [DOI] [PubMed] [Google Scholar]
- 6.Watkins, R. E., Wisely, G. B., Moore, L. B., Collins, J. L., Lambert, M. H., Williams, S. P., Willson, T. M., Kliewer, S. A. & Redinbo, M. R. (2001) Science 292, 2329–2333. [DOI] [PubMed] [Google Scholar]
- 7.Shenoy, S. D., Spencer, T. A., Mercer-Haines, N. A., Alipour, M., Gargano, M. D., Runge-Morris, M. & Kocarek, T. A. (2004) Drug Metab. Dispos. 32, 66–71. [DOI] [PubMed] [Google Scholar]
- 8.Staudinger, J. L., Goodwin, B., Jones, S. A., Hawkins-Brown, D., MacKenzie, K. I., LaTour, A., Liu, Y., Klaassen, C. D., Brown, K. K., Reinhard, J., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie, W., Radominska-Pandya, A., Shi, Y., Simon, C. M., Nelson, M. C., Ong, E. S., Waxman, D. J. & Evans, R. M. (2001) Proc. Natl. Acad. Sci. USA 98, 3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kliewer, S. A., Moore, J. T., Wade, L., Staudinger, J. L., Watson, M. A., Jones, S. A., McKee, D. D., Oliver, B. B., Willson, T. M., Zetterstrom, R. H., et al. (1998) Cell 92, 73–82. [DOI] [PubMed] [Google Scholar]
- 11.Shenoy, S. D., Spencer, T. A., Mercer-Haines, N. A., Abdolalipour, M., Wurster, W. L., Runge-Morris, M. & Kocarek, T. A. (2004) Mol. Pharmacol. 65, 1302–1312. [DOI] [PubMed] [Google Scholar]
- 12.Goodwin, B., Gauthier, K. C., Umetani, M., Watson, M. A., Lochansky, M. I., Collins, J. L., Leitersdorf, E., Mangelsdorf, D. J., Kliewer, S. A. & Repa, J. J. (2003) Proc. Natl. Acad. Sci. USA 100, 223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dussault, I., Yoo, H. D., Lin, M., Wang, E., Fan, M., Batta, A. K., Salen, G., Erickson, S. K. & Forman, B. M. (2003) Proc. Natl. Acad. Sci. USA 100, 833–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie, W., Barwick, J. L., Downes, M., Blumberg, B., Simon, C. M., Nelson, M. C., Neuschwander-Tetri, B. A., Brunt, E. M., Guzelian, P. S. & Evans, R. M. (2000) Nature 406, 435–439. [DOI] [PubMed] [Google Scholar]
- 15.Klaassen, C. D. (1969) J. Pharmacol. Exp. Ther. 168, 218–223. [PubMed] [Google Scholar]
- 16.Turley, S. D., Spady, D. K. & Dietschy, J. M. (1983) Gastroenterology 84, 253–264. [PubMed] [Google Scholar]
- 17.Bodin, K., Andersson, U., Rystedt, E., Ellis, E., Norlin, M., Pikuleva, I., Eggertsen, G., Bjorkhem, I. & Diczfalusy, U. (2002) J. Biol. Chem. 277, 31534–31540. [DOI] [PubMed] [Google Scholar]
- 18.Kullak-Ublick, G. A. & Meier, P. J. (2000) Clin. Liver Dis. 4, 357–385. [DOI] [PubMed] [Google Scholar]
- 19.Bachs, L., Pares, A., Elena, M., Piera, C. & Rodes, J. (1989) Lancet 1, 574–576. [DOI] [PubMed] [Google Scholar]