Abstract
X-linked ribosomal protein S4 (RPS4X) has previously been reported to be associated with cisplatin resistance and clinical outcome in bladder and ovarian cancer. However, the value of RPS4X as a diagnostic and prognostic marker in intrahepatic cholangiocarcinoma (ICC) has not yet been investigated. The present study evaluated the expression pattern, and diagnostic and prognostic value of RPS4X in patients with ICC. Retrospective analysis was performed for a total of 201 patients with intrahepatic cholangiocarcinoma, and 8 patients with inflammation of the bile duct. Immunohistochemistry was performed using tissue microarrays to characterize the expression profile of RPS4X. Receiver operating characteristic (ROC) curves, the Kaplan-Meier estimator and Cox regression analysis were applied to evaluate the potential diagnostic and prognostic value of RPS4X in ICC. RPS4X was significantly upregulated in ICC tissues compared with the inflamed bile duct tissues. When differentiating ICC from normal controls, ROC analysis of RPS4X gave an area under the curve value of 0.9030 (sensitivity, 82.59%; specificity, 100%). RPS4X expression was significantly positively correlated with serum alkaline phosphatase levels. Survival analysis demonstrated that RPS4X expression levels were an independent prognostic factor for overall survival. Therefore, RPS4X expression levels may serve as a novel diagnostic and prognostic marker in ICC.
Keywords: X-linked ribosomal protein S4, intrahepatic cholangio-carcinoma, receiver operating characteristic curve, prognosis, immunohistochemistry
Introduction
Intrahepatic cholangiocarcinoma (ICC) constitutes the second most prevalent primary hepatic malignancy following hepatocellular carcinoma (HCC) (1,2). Whilst more uncommon in the United States and Europe, the incidence rates of this malignancy are high in China (2,3). Although the diagnostic and surgical approaches for the treatment of ICC have been improved to a certain extent, the survival rates for patients with ICC remain unfavorable (4). As adjuvant therapy is frequently ineffective for patients with ICC, complete surgical resection is currently the only curative treatment (5). However, the majority of patients with ICC are diagnosed at an advanced stage with intrahepatic and lymph node metastases, when curative surgery is not a viable option (6–8). Despite numerous advances in ICC research, the mechanisms underlying ICC progression remain poorly understood, and further studies to identify diagnostic and prognostic factors are required.
X-linked human ribosomal protein S4 (RPS4X) encodes a component of the 40S subunit of the ribosomal complex (9) and is not subject to X-inactivation (10). It has also been reported that RPS4X haploinsufficiency serves a role in Turner syndrome (11). Previous studies have suggested that RPS4X may be important in tumor progression, and demonstrated that RPS4X physically interacts with Y-box binding protein-1 (YB-1) in breast and ovarian cancer cell lines (12,13). The RPS4X/YB-1 complex is critical in counteracting cisplatin resistance in MCF7 and MDA-MB-231 breast cancer cells (12,13). Furthermore, RPS4X has been identified as an independent prognostic factor for ovarian and bladder cancer (13,14). However, the potential diagnostic and prognostic function of RPS4X in patients with ICC remains to be elucidated. In the present study, the expression profile of RPS4X, and its diagnostic and prognostic significance in patients with ICC was evaluated. This may aid in the future treatment and management of patients with ICC.
Patients and methods
Patients
A total of 201 patients (146 male, 55 female; age range, 27–81) with ICC, who underwent surgical resection at the Eastern Hepatobiliary Surgery Hospital (Shanghai, China) between July 2000 and December 2006, were included in the current study. Inflamed bile duct (IBD) samples were collected as normal control tissues from 8 patients with ICC who underwent a hepatectomy at the same hospital between March 2008 and September 2008. ICC tissue samples were pathologically diagnosed at the time of surgery and independently examined by two pathologists. No patients in the present study received chemotherapy or radiotherapy prior to surgery. Tumor stage was defined according to the American Joint Committee on Cancer Tumor-Node-Metastasis (TNM) staging system (15).
Follow-up
Patient follow-up examinations were performed monthly for the first 2–3 months following surgery, and every 2–6 months thereafter. Where tumor recurrence or metastasis was suspected, magnetic resonance imaging, positron emission tomography-computed tomography and biopsies were performed. Overall survival (OS) was defined as the time from the date of hepatectomy to the final follow-up or mortality. The time to recurrence (TTR) was defined as the time from the date of hepatectomy to the first relapse, distant metastasis. The median follow-up time was 22.5 months (range, 0.2–92.3 months). Of the 201 patients with ICC, the TTR information of 73 patients was not accessible for the follow-up period or until the date of mortality.
For the use of clinical materials in the current study, written informed consent was obtained from patients, in addition to approval from The Ethics Committee of the Eastern Hepatobiliary Surgery Hospital. All experiments were performed in accordance with the approved guidelines of the Eastern Hepatobiliary Surgery Hospital.
Tissue microarray construction (TMA), immunohistochemistry (IHC), signal evaluation and integrated optical density (IOD) analysis
TMA construction was performed as described previously (16,17). Representative formalin-fixed paraffin-embedded tumor tissues, fixed in 10% neutral formaldehyde at room temperature for 12–24 h and embedded in paraffin, were collected and used to construct a long-distance peritumoral TMA chip. For IHC, 4-µm-thick sections were used and IOD analysis were performed for evaluating the expression of RPS4X as described previously (18). An anti-RPS4X polyclonal antibody was purchased from Abmart (Shanghai, China; dilution, 1:200; cat. no. P30129S) and an EnVision Detection kit (cat. no. GK500705: Gene Tech, Shanghai, China), which included a horseradish peroxidase secondary antibody, was used with ChemMate™ diaminobenzidine Chromogen reagent (Gene Tech Biotechnology Co., Ltd. Shanghai, China), to visualize tissue antigens. Slides omitting the primary antibodies were produced as the negative control for the IHC assay. Images were captured under high-power magnification (×200, light microscopy). Mean IOD values were calculated and analyzed using Image-Pro Plus software (version 6.0; Media Cybernetics, Inc., Rockville, MD, USA). For the determination of high or low RPS4X expression levels, optimal cutoff IOD values were estimated using X-tile software (version 3.6.1; Yale University, New Haven, CT, USA).
Statistical analysis
All statistical analyses were performed with SPSS software (version 13.0; SPSS, Inc., Chicago, IL, USA). Differences between variables were assessed using the χ2 (chi-square) test or Mann-Whitney U test (scatter dot plot). Data are presented as the mean ± standard error of the mean. The Kaplan-Meier estimator was used to assess survival and the log-rank test was applied to compare survival rates between patient subgroups. Univariate and multivariate analyses were performed using the Cox's proportional hazards regression model. The clinicopathological variables that were determined to be significant in univariate analysis were further evaluated using Cox's multivariate proportional hazards regression analysis. Receiver operating characteristic (ROC) curve analysis was used to determine the predictive significance of parameters. P<0.05 was considered to indicate a statistically significant difference.
Results
RPS4X is significantly upregulated in ICC tissue samples
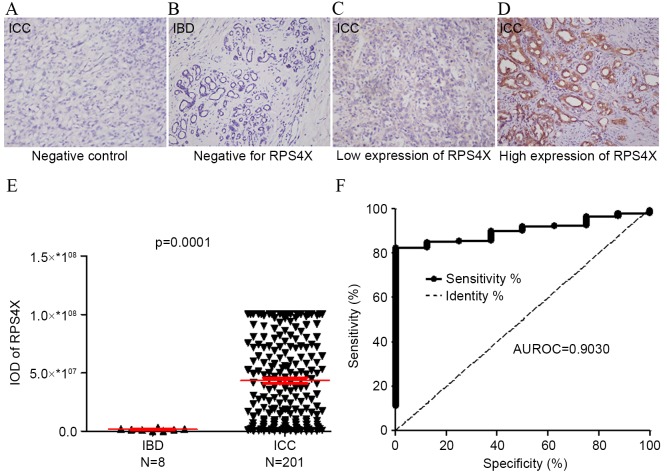
A total of 201 ICC tissue samples and 8 IBD tissue samples used to construct a TMA, and RPS4X expression levels were detected using IHC analysis (Fig. 1). Staining for RPS4X in the ICC tissues (Fig. 1C and D) was observed to be significantly more intense compared with that observed in the IBD control tissues (Fig. 1B). The IOD of each tissue sample was quantitatively analyzed, and the results demonstrated that the staining intensity of RPS4X in the ICC group was significantly higher compared with that of the IBD control group (P=0.0001; Fig. 1E). In addition, ROC analysis revealed that the area under the curve (AUC) value of RPS4X was 0.9030, where the optimal cutoff IOD value was 29993910, providing a sensitivity of 82.59% and a specificity of 100% for detecting ICC (Fig. 1F).
Figure 1.
RPS4X is significantly upregulated in ICC tissue samples. RPS4X expression in 8 IBD cases and 201 ICC cases was analyzed using immunohistochemistry. Representative images (magnification, ×200) taken from the tissue microarray of the (A) ICC negative control, (B) IBD negative for RPS4X, (C) ICC with low expression of RPS4X and (D) ICC with high expression of RPS4X. (E) The IOD for RPS4X was obtained and differences between the ICC and IBD tissues were analyzed via the Mann-Whitney U test. (F) ROC curve analysis of RPS4X for discriminating between ICC and IBD lesions. At a cut-off IOD level of 29993910, RPS4X exhibited 82.59% sensitivity and 100% specificity for detecting ICC. AUROC, 0.9030; 95% confidence interval, 0.8533–0.9527. RPS4X, X-linked ribosomal protein S4; IBD, inflamed bile duct; ICC intrahepatic cholangiocarcinoma; IOD, integrated optical density; ROC, receiver operator characteristic; AUROC, area under the ROC curve.
High RPS4X expression levels are associated with the clinicopathological features of patients with ICC
The association between the clinicopathological features of ICC and RPS4X expression levels was retrospectively investigated (Table I). The cohort included 91 cases of TNM stage I (45.3%), 79 cases of stage II (39.3%), 5 cases of stage III (2.5%) and 26 cases of stage IV (12.9%) ICC. RPS4X expression levels in the ICC tissue samples were determined to be high in 111/201 cases (55.2%) and low in 90/201 cases (44.8%). χ2 test indicated a correlation between RPS4X expression levels and serum alkaline phosphatase levels (P=0.031). However, no significant association was observed between RPS4X expression levels and other clinicopathological parameters, including age, gender, liver cirrhosis, serum carcinoembryonic antigen (CEA), serum carbohydrate antigen (CA) 19–9, serum alanine transaminase, serum γ-glutamyl transpeptidase (GGT), tumor size, tumor number, microvascular invasion and TNM stage.
Table I.
Association between RPS4X expression and the clinicopathological characteristics of patients with ICC.
| RPS4X | |||
|---|---|---|---|
| Clinicopathological characteristic | Low | High | P-value |
| Age, years | 0.138 | ||
| <52 | 40 | 61 | |
| >52 | 50 | 50 | |
| Gender | 0.403 | ||
| Male | 68 | 78 | |
| Female | 22 | 33 | |
| Liver cirrhosis status | 0.138 | ||
| Absent | 56 | 80 | |
| Present | 34 | 31 | |
| Serum CEA, µg/l | 0.721 | ||
| <5 | 72 | 91 | |
| >5 | 18 | 20 | |
| Serum CA19-9, U/ml | 0.573 | ||
| ≤37 | 41 | 55 | |
| >37 | 49 | 56 | |
| Serum ALT, U/l | 0.172 | ||
| ≤75 | 80 | 91 | |
| >75 | 10 | 20 | |
| Serum GGT, U/l | 0.180 | ||
| ≤50 | 36 | 34 | |
| >50 | 54 | 76 | |
| Serum ALP, U/l | 0.031a | ||
| <119 | 29 | 52 | |
| >119 | 61 | 58 | |
| Tumor size, cm | 0.944 | ||
| ≤5 | 32 | 40 | |
| >5 | 58 | 71 | |
| Tumor number | 0.487 | ||
| Single | 72 | 93 | |
| Multiple | 18 | 18 | |
| Microvascular invasion status | 0.662 | ||
| Absent | 59 | 76 | |
| Present | 31 | 35 | |
| TNM stage | 0.899 | ||
| I | 41 | 50 | |
| II | 34 | 45 | |
| III | 3 | 2 | |
| IV | 12 | 14 | |
P<0.05. ICC, intrahepatic cholangiocarcinoma; RPS4X, X-linked ribosomal protein S4; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19–9; ALT, alanine transaminase; GGT, γ-glutamyl transpeptidase; ALP, alkaline phosphatase; TNM, tumor node metastasis.
High RPS4X expression levels indicate poor survival in patients with ICC
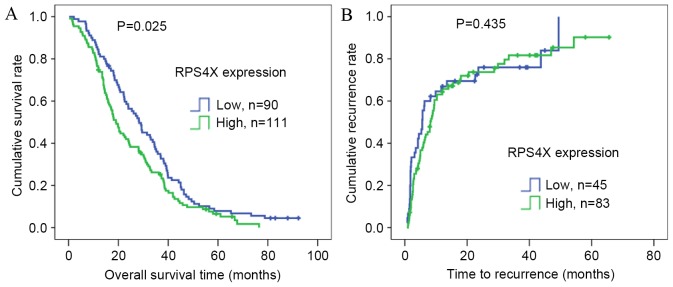
To determine the prognostic value of RPS4X in postsurgical patients with ICC, Kaplan-Meier OS analysis was conducted in 201 patients, and TTR analysis was performed in 128 patients according to the collected prognostic information. Univariate and multivariate analyses of the risk factors influencing OS and TTR are listed in Table II. Univariate analysis demonstrated that serum GGT levels (P=0.029), tumor number (P=0.043), TNM stage (P=0.003) and RPS4X expression (P=0.026) were significantly associated with OS. Only tumor size (P=0.031) was significantly associated with TTR, as demonstrated by univariate analysis. OS and TTR curves according to the IOD values from RPS4X staining are presented in Fig. 2, respectively. In addition, Kaplan-Meier analyses revealed that a high RPS4X expression may indicate poor survival rate of patients with ICC following surgery (P=0.025; Fig. 2A), but not TTR.
Table II.
Univariate and multivariate analysis of the prognostic value of the clinicopathological characteristics of patients with ICC.
| OS | TTR | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| Clinicopathological characteristic | P-value | HR | 95% Cl | P-value | P-value | HR | 95% Cl | P-value |
| Age, years: ≤52 vs. >52 | 0.971 | 0.117 | ||||||
| Gender: Male vs. female | 0.497 | 0.319 | ||||||
| Liver cirrhosis: Absent vs. present | 0.970 | 0.453 | ||||||
| Serum CEA, µg/l: ≤10 vs. >10 | 0.065 | 0.957 | ||||||
| Serum CA19-9, U/ml: ≤75 vs. >75 | 0.099 | 0.552 | ||||||
| Serum ALT, U/l: ≤119 vs. >119 | 0.741 | 0.625 | ||||||
| Serum GGT, U/l: ≤50 vs. >50 | 0.029a | 0.134 | 0.373 | |||||
| Serum ALP, U/l: ≤119 vs. >119 | 0.094 | 0.573 | ||||||
| Tumor size, cm: ≤5 vs. >5 | 0.085 | 0.031a | 1.608 | 1.045–2.472 | 0.031a | |||
| Tumor number: Single vs. multiple | 0.043a | 0.144 | 0.188 | |||||
| Microvascular invasion: Absent vs. present | 0.078 | 0.847 | ||||||
| TNM stage: I vs. II vs. III vs. IV | 0.003a | 1.258 | 1.086–1.457 | 0.002a | 0.135 | |||
| RPS4X expression: Low vs. high | 0.026a | 1.424 | 1.065–1.904 | 0.017a | 0.438 | |||
P<0.05. ICC, intrahepatic cholangiocarcinoma; RPS4X, X-linked ribosomal protein S4; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19–9; ALT, alanine transaminase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; TNM, tumor node metastasis; NS, non significant; OS, overall survival; TTR, time to recurrence; CI, confidence interval; HR, hazard ratio.
Figure 2.
Kaplan-Meier estimator survival analysis of RPS4X expression in patients with ICC. (A) Probability analysis of the post-operative overall survival demonstrated that patients with ICC expressing high levels of RPS4X had a poorer prognosis compared with those expressing low levels. (B) The time to recurrence for patients with ICC expressing high and low levels of RPS4X did not significantly differ. RPS4X, X-Linked ribosomal protein S4; ICC, intrahepatic cholangiocarcinoma.
As presented in Table II, TNM stage [(hazard ratio (HR) 1.258; 95% confidence interval (CI), 1.086–1.457; P=0.002)] and RPS4X staining intensity (HR 1.424; 95% CI, 1.065–1.904; P=0.017) were the independent risk factors identified for OS. Tumor size (HR 1.608; 95% CI 1.045–2.472; P=0.031) was identified as an independent risk factor for TTR.
Discussion
ICC is a rare liver malignancy, originating from the epithelium of the intrahepatic biliary duct (19). Due to its early-stage invasion, widespread metastasis and ineffective therapeutic options, ICC has a high mortality rate and a poor prognosis (20). Molecular profiling of the tumor is a necessary part of treatment selection, and the immunohistochemical assessment of ICC biomarkers can provide predictive and/or prognostic information for patients with this disease. According to numerous previous studies, the number of tumors (single vs. multiple), completeness of resection (R0) and the presence of vascular invasion and lymph node metastases are identified as the most important prognostic factors in patients with ICC (21–24). However, other potential prognostic biomarkers for ICC remain to be elucidated.
Previous studies have demonstrated that low expression levels of RPS4X were associated with an increased risk of disease recurrence and mortality in patients with bladder and ovarian cancer (13,14). The present study aimed to determine the association between RPS4X expression levels and the clinical outcome of patients with ICC. Tissue samples from a population of 201 patients with ICC and 8 patients with IBD were analyzed using IHC. The results indicated that RPS4X expression was abnormally increased in the ICC tissue specimens compared with the normal IBD tissues. In this cohort of 201 patients with ICC, Kaplan-Meier OS analysis demonstrated that high levels of RPS4X expression were associated with a shorter survival time and poor prognosis following surgical resection of the tumor. Multivariate Cox regression analysis also revealed that RPS4X expression levels were an independent prognostic marker in patients with ICC. However, Kaplan-Meier analyses indicated no significant association between RPS4X expression levels and TTR in the present study. Concordantly, multivariate Cox regression analysis also excluded RPS4X expression levels as an independent prognostic marker for TTR. However, the missing TTR data (73/201) from the follow-up period potentially impacted the TTR analysis. To the best of our knowledge, the present study is the first to demonstrate that the overexpression of RPS4X is associated with the poor prognosis of patients with ICC.
The poor prognosis of patients with ICC following tumor resection has not improved over the last decade, which is primarily due to late stage diagnosis leading to high rates of metastasis and recurrence (25,26). Plasma serum markers for ICC, including CA19-9 and CEA, usually possess high specificity, but low sensitivity; CA19-9 is increased in ~50% of ICC cases, whereas CEA is elevated in 15–20% of ICC cases (27,28). Therefore, these serum markers are insufficiently sensitive for a definitive diagnosis. In the present study, ROC analysis of RPS4X expression determined an AUC value of 0.9030 with a sensitivity of 82.59% and a specificity of 100%. This result indicates that immunohistochemical staining of RPS4X in tissues enables the differentiation between ICC tissues and IBD. Tissue biopsy is not routinely recommended for patients with ICC that are going to undergo curative resection (29). However, a pathological diagnosis is required prior to systemic chemotherapy or radiotherapy (29), thus tissue markers, such as RPS4X should be further assessed in biopsy tissues. A previous study demonstrated that the knockdown of RPS4X expression was able to decrease DNA synthesis and induce cisplatin resistance in MCF7 and MDA-MB-231 breast cancer cell lines (11). However, whether RPS4X serves a role in the response of patients with ICC to systemic adjuvant therapy, including 5-FU (fluorouracil) -based radiation and gemcitabine/5-FU, requires further clarification.
In conclusion, the findings of the present study indicate that increased RPS4X expression levels are a diagnostic and prognostic biomarker for ICC, which is able to independently identify patients with a poor clinical prognosis.
Acknowledgements
The present study was supported by the Medical Guide Fund of the Shanghai Science and Technology Committee (grant no. 134119a0202).
References
- 1.Zhang GW, Lin JH, Qian JP, Zhou J. Identification of risk and prognostic factors for patients with clonorchiasis-associated intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2014;21:3628–3637. doi: 10.1245/s10434-014-3710-x. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 4.Brown KM, Parmar AD, Geller DA. Intrahepatic cholangiocarcinoma. Surg Oncol Clin N Am. 2014;23:231–246. doi: 10.1016/j.soc.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawarada Y, Yamagiwa K, Das BC. Analysis of the relationships between clinicopathologic factors and survival time in intrahepatic cholangiocarcinoma. Am J Surg. 2002;183:679–685. doi: 10.1016/S0002-9610(02)00853-X. [DOI] [PubMed] [Google Scholar]
- 6.Dhanasekaran R, Hemming AW, Zendejas I, George T, Nelson DR, Soldevila-Pico C, Firpi RJ, Morelli G, Clark V, Cabrera R. Treatment outcomes and prognostic factors of intrahepatic cholangiocarcinoma. Oncol Rep. 2013;29:1259–1267. doi: 10.3892/or.2013.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roayaie S, Guarrera JV, Ye MQ, Thung SN, Emre S, Fishbein TM, Guy SR, Sheiner PA, Miller CM, Schwartz ME. Aggressive surgical treatment of intrahepatic cholangiocarcinoma: Predictors of outcomes. J Am Coll Surg. 1998;187:365–372. doi: 10.1016/S1072-7515(98)00203-8. [DOI] [PubMed] [Google Scholar]
- 8.Zhou XD, Tang ZY, Fan J, Zhou J, Wu ZQ, Qin LX, Ma ZC, Sun HC, Qiu SJ, Yu Y, et al. Intrahepatic cholangiocarcinoma: Report of 272 patients compared with 5,829 patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2009;135:1073–1080. doi: 10.1007/s00432-009-0547-y. [DOI] [PubMed] [Google Scholar]
- 9.Watanabe M, Furuno N, Goebl M, Go M, Miyauchi K, Sekiguchi T, Basilico C, Nishimito T. Molecular cloning of the human gene, CCG2, that complements the BHK-derived temperature-sensitive cell cycle mutant tsBN63: Identity of CCG2 with the human X chromosomal SCAR/RPS4X gene. J Cell Sci. 1991;100:35–43. doi: 10.1242/jcs.100.1.35. [DOI] [PubMed] [Google Scholar]
- 10.Zinn AR, Alagappan RK, Brown LG, Wool I, Page DC. Structure and function of ribosomal protein S4 genes on the human and mouse sex chromosomes. Mol Cell Biol. 1994;14:2485–2492. doi: 10.1128/MCB.14.4.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe M, Zinn AR, Page DC, Nishimoto T. Functional equivalence of human X- and Y-encoded isoforms of ribosomal protein S4 consistent with a role in Turner syndrome. Nat Genet. 1993;4:268–271. doi: 10.1038/ng0793-268. [DOI] [PubMed] [Google Scholar]
- 12.Garand C, Guay D, Sereduk C, Chow D, Tsofack SP, Langlois M, Perreault E, Yin HH, Lebel M. An integrative approach to identify YB-1-interacting proteins required for cisplatin resistance in MCF7 and MDA-MB-231 breast cancer cells. Cancer Sci. 2011;102:1410–1417. doi: 10.1111/j.1349-7006.2011.01948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsofack SP, Meunier L, Sanchez L, Madore J, Provencher D, Mes-Masson AM, Lebel M. Low expression of the X-linked ribosomal protein S4 in human serous epithelial ovarian cancer is associated with a poor prognosis. BMC Cancer. 2013;13:303. doi: 10.1186/1471-2407-13-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paquet ÉR, Hovington H, Brisson H, Lacombe C, Larue H, Têtu B, Lacombe L, Fradet Y, Lebel M. Low level of the X-linked ribosomal protein S4 in human urothelial carcinomas is associated with a poor prognosis. Biomark Med. 2015;9:187–197. doi: 10.2217/bmm.14.115. [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Compton CC. The American joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 16.Albanghali M, Green A, Rakha E, Aleskandarany M, Nolan C, Ellis I, Cheung KL. Construction of tissue microarrays from core needle biopsy-a systematic literature review. Histopathology. 2016;68:323–332. doi: 10.1111/his.12802. [DOI] [PubMed] [Google Scholar]
- 17.Tan D, Li Q, Deeb G, Ramnath N, Slocum HK, Brooks J, Cheney R, Wiseman S, Anderson T, Loewen G. Thyroid transcription factor-1 expression prevalence and its clinical implications in non-small cell lung cancer: A high-throughput tissue microarray and immunohistochemistry study. Hum Pathol. 2003;34:597–604. doi: 10.1016/S0046-8177(03)00180-1. [DOI] [PubMed] [Google Scholar]
- 18.Jin GZ, Li Y, Cong WM, Yu H, Dong H, Shu H, Liu XH, Yan GQ, Zhang L, Zhang Y, et al. iTRAQ-2DLC-ESI-MS/MS based identification of a new set of immunohistochemical biomarkers for classification of dysplastic nodules and small hepatocellular carcinoma. J Proteome Res. 2011;10:3418–3428. doi: 10.1021/pr200482t. [DOI] [PubMed] [Google Scholar]
- 19.Sang H, Li T, Li H, Liu J. Gab1 regulates proliferation and migration through the PI3K/Akt signaling pathway in intrahepatic cholangiocarcinoma. Tumour Biol. 2015;36:8367–8377. doi: 10.1007/s13277-015-3590-0. [DOI] [PubMed] [Google Scholar]
- 20.Hyder O, Hatzaras I, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, et al. Recurrence after operative management of intrahepatic cholangiocarcinoma. Surgery. 2013;153:811–818. doi: 10.1016/j.surg.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali SM, Clark CJ, Zaydfudim VM, Que FG, Nagorney DM. Role of major vascular resection in patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2013;20:2023–2028. doi: 10.1245/s10434-012-2808-2. [DOI] [PubMed] [Google Scholar]
- 22.de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, Pulitano C, Barroso E, Clary BM, Aldrighetti L, et al. Intrahepatic cholangiocarcinoma: An international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29:3140–3145. doi: 10.1200/JCO.2011.35.6519. [DOI] [PubMed] [Google Scholar]
- 23.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, D'Angelica M, DeMatteo RP, Fong Y, Schwartz L, et al. Intrahepatic cholangiocarcinoma: Rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 24.Ribero D, Pinna AD, Guglielmi A, Ponti A, Nuzzo G, Giulini SM, Aldrighetti L, Calise F, Gerunda GE, Tomatis M, et al. Surgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: A multi-institutional analysis of 434 patients. Arch Surg. 2012;147:1107–1113. doi: 10.1001/archsurg.2012.1962. [DOI] [PubMed] [Google Scholar]
- 25.Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013;145:1215–1229. doi: 10.1053/j.gastro.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo X, Yuan L, Wang Y, Ge R, Sun Y, Wei G. Survival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: A large single-center cohort study. J Gastrointest Surg. 2014;18:562–572. doi: 10.1007/s11605-013-2447-3. [DOI] [PubMed] [Google Scholar]
- 28.Maithel SK, Gamblin TC, Kamel I, Corona-Villalobos CP, Thomas M, Pawlik TM. Multidisciplinary approaches to intrahepatic cholangiocarcinoma. Cancer. 2013;119:3929–3942. doi: 10.1002/cncr.28312. [DOI] [PubMed] [Google Scholar]
- 29.Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic Cholangiocarcinoma: Expert consensus statement. HPB (Oxford) 2015;17:669–680. doi: 10.1111/hpb.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]