Abstract
The immunobiology of breast cancer (BC) subtypes, including luminal cancer, remains unclear. Cluster of differentiation (CD)8+ tumor-infiltrating lymphocytes (TIL) are essential components of tumor-specific cellular adaptive immunity. However, only few studies have addressed the significance of cluster of differentiation 8+(CD8+) TIL in patients with luminal BC. The present study aimed to evaluate the predictive and prognostic significance of CD8+ TIL in patients with luminal B/human epidermal growth factor receptor 2 (HER 2)-negative BC treated with anthracycline-based neoadjuvant chemotherapy (NC). A total of 31 patients who underwent breast-conserving surgery or mastectomy post-NC were enrolled. Immunostaining for CD8+ TIL was performed using rabbit monoclonal antibodies against human CD8+. Intra- and peritumoral CD8+ TIL expression levels were classified into high and low, based on the median value of each. CD8+ TIL expression data were demonstrated to be correlated with disease-free survival (DFS) and overall survival (OS), using Kaplan-Meier and Cox's proportional hazards regression tests. The results revealed that, among all clinicopathological characteristics, only pathological complete response (pCR) was significantly correlated with intratumoral CD8+ TIL expression (P=0.016). A total of 9/16 patients (56%) with high intratumoral CD8+ TIL expression achieved pCR, in contrast with 2 out of 15 patients (13.3%) with low expression (P=0.016). High expression of intratumoral CD8+ TIL was significantly associated with OS (log-rank test, P=0.023). Multivariate Cox regression analysis revealed that intratumoral expression of CD8+ TIL was an independent prognostic factor for OS [hazard ratio (HR)=2.82; 95% confidence interval (CI)=0.911–4.833, P=0.007], but not for DFS (HR=1.11; 95% CI=0.282–2.078; P=0.508). In conclusion, the results of the present study suggested that high intratumoral CD8+ TIL expression was significantly predictive of pCR post-NC, and represented an independent prognostic factor for improved OS. In contrast, low intratumoral CD8+ TIL expression was a strong predictor of lack of pCR to NC, as well as an independent prognostic factor for poor OS. Assessment of the immune response in conjunction with the usual parameters may aid in the further stratification of patients with luminal B/HER 2-negative BC regarding the prediction of pCR post-NC and overall prognosis.
Keywords: prediction, prognosis, cluster of differentiation 8+, lymphocytes, breast cancer, luminal B, human epidermal growth factor receptor 2-negative, neoadjuvant chemotherapy
Introduction
Breast cancer (BC) is the most common type of female malignancy in Saudi Arabia with evidence of an increased annual incidence from 23.5 cases/100,000 people in 2000 to 34.5 cases/100,000 people in 2010 (1,2). BC remains a heterogeneous disease and includes a range of clinical patterns, pathological characteristics, prognostic factors and responses to different types of treatment. An intrinsic molecular classification has defined four main BC subtypes: Human epidermal growth factor receptor 2 (HER 2), triple-negative, and luminal A and B (3).
Although the luminal types of cancer share similarities, previously conducted studies using next-generation sequencing technology have revealed that luminal A and B BC should be perceived as distinct subtypes, with specific oncogenic drivers, rather than more proliferative varieties of the luminal tumor subtype (4). It has been reported that luminal B BC exhibits a lower expression of hormone receptors, higher expression of proliferation markers and higher histologic grade compared with luminal A cancer (3,5). Furthermore, patients with luminal B cancer exhibit worse prognosis and have a distinct profile of response to hormonal and chemotherapy (4), and numerous efforts have been made to improve survival rates through early diagnosis and multiple therapies (6). However, the limitations of the current therapeutic modalities and advances in molecular diagnostics have resulted in increasing requirements for defining novel prognostic and predictive tools (7).
Neoadjuvant chemotherapy (NC) or primary systemic therapy is considered the standard treatment for locally advanced BC. However, its utilization has increased to treat patients with operable BC (8). One of the main advantages of NC is the reduction in tumor size, allowing for an increased incidence of conservative surgery with improved cosmetic outcomes (9). In addition, beyond initiating an early systemic treatment for clinically undetectable micrometastases, NC provides an opportunity to evaluate the tumor sensitivity to different chemotherapeutic regimens (10). Furthermore, the pathological response to NC may possess prognostic value, as pathological complete response (pCR) may be correlated with improved disease-free survival (DFS) and overall survival (OS), particularly in patients with triple-negative and enriched-HER 2 (11). However, for patients with a luminal B tumor, factors predicting pCR post-NC appear unclear, and no significant correlation between pCR and outcomes, including DFS and OS, has been reported (12).
There is growing interest among researchers towards ‘tumor-specific adaptive immune response’. Infiltrating inflammatory cells, particularly lymphocytes and macrophages, often surround tumor cells. Previous studies have demonstrated that cells of the adaptive immune system perform surveillance and eliminate nascent tumors, in a process termed immunosurveillance (13). Tumor antigens drive the development of tumor-specific adaptive immune responses (14).
Cluster of differentiation 8+ (CD8+) T lymphocytes are essential components of tumor-specific cellular adaptive immunity that attack tumor cells presenting tumor-associated antigen peptide with major histocompatibility complex class I on their surface. CD8+ T cells produce interferon-γ following interactions with their tumor targets, which subsequently lead to cell cycle inhibition, apoptosis, angiostasis and induction of macrophage tumoricidal activity (13–15). When the immune system fails to eliminate all tumor cells, tumors with reduced immunogenicity may emerge with the capability to escape immune attacks. This combination of host-protective and tumor-promoting functions of inflammatory/immune cells has led to the concept of ‘cancer immuno-editing’ (13,16).
Despite the fact that high levels of tumor infiltrating lymphocytes (TILs) have been suggested to be predictive of pCR post-NC in certain BC, the predictive value of TIL subtypes for pCR in different molecular subtypes, particularly in luminal B BC remains unclear (17–21). Additionally, studies on the association between CD8+ T-cell infiltration and OS in BC have presented conflicting results (22–28).
In contrast, CD8+ TILs were extensively studied for their prognostic significance in different types of cancer. Immunohistochemistry (IHC) studies have demonstrated that CD8+ TILs have a favorable effect on OS in different types of solid tumors, including ovarian (29), renal (30), lung (31) and pancreatic (32) cancer. Taking into consideration the heterogeneity of patients with BC, it is necessary to address the subtype-specific immunobiology of distinct molecular and histological subtypes. The interaction between the immune response, intrinsic tumor subtype, and treatment strategy are all potential contributors to the outcome of the disease (33,34).
Notably, the immunobiology of patients with certain BC subtypes, including those with luminal cancers, remains to be elucidated. Few studies have addressed the predictive and/or prognostic significance of CD8+ TIL in patients with the luminal subtypes of BC (3,4,19,20).
In the present study, the predictive and prognostic significance of CD8+ TIL expression was retrospectively evaluated in a cohort of patients with luminal B/HER 2-negative BC treated with anthracycline-based NC.
Patients and methods
Study population
The present study included 31 female patients with stage II (n=12) and stage III (n=19) luminal B tumors treated with NC followed by breast-conserving surgery or mastectomy. The median age of the patients was 53 years (range, 31–68) Follow-ups were performed at the King Khalid University Hospital (Riyadh, Saudi Arabia) between December 2009 and December 2014.
All patients underwent a true-cut core needle biopsy prior to NC for diagnosis of invasive breast carcinoma according to the World Health Organization histopathological diagnostic criteria and confirmation of the luminal B subtype (35). NC consisted, for 14 patients (45.2 %), of a sequential dose dense AC-T chemotherapy regimen [doxorubicin intravenous (IV) 60 mg/m2/cyclophosphamide IV 600 mg/m2 every 2 weeks for 4 cycles followed by docetaxel 75 mg/m2 q. 2 weeks for 4 cycles, with granulocyte colony stimulating factor support]. The remaining 17 patients (54.8%) received a fluorouracil epirubicin cyclophosphamide 100-T chemotherapy regimen [5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, cyclophosphamide 500 mg/m2 q. 3 weeks for 3 cycles followed by docetaxel 100 mg/m2 q. 3 weeks for 3 cycles]. All patients underwent breast surgery within 4 weeks following the last course of NC.
Medical records were collected for all patients in order to acquire, and review the clinical information including age, sex, and initial clinical T and N stage as recommended by the 7th American Joint Committee on Cancer (36). A pair of formalin-fixed paraffin-embedded tumor samples, consisting of a pre-chemotherapy biopsy and post-chemotherapy resection specimens were collected from each patient. Pathology reports, hematoxylin and eosin (H&E)-stained sections, and IHC slides for basic biomarkers were reviewed to acquire pathological information including pathological T and N stage following NC, histologic tumor type (37), histologic grade according to Scarff-Bloom-Richardson (38), Ki-67 score, presence of carcinoma in situ, lymphatic invasion, tumor margins, estrogen receptor (ER) and progesterone receptor (PR), and HER 2 status. BC was identified as luminal B subtype based on positive hormonal expression ER and/or PR >1%, HER 2-negative (score 0 or 1 through IHC or fluorescence in situ hybridization-negative if IHC was score 2), and a Ki-67 score >14%. (5,35).
pCR was defined as the complete disappearance of all invasive tumor cells from breast tissue and regional lymph nodes regardless of the presence of residual ductal carcinoma in situ (17). The present study was approved by the Institutional Review Board of King Khalid University Hospital (Riyadh, Saudi Arabia) and written informed consent was obtained from all participants.
IHC and CD8+ TIL quantification
The paraffin blocks from all 31 patients were retrieved from the archives of the histopathology unit at the King Khalid University Hospital (Riyadh, Saudi Arabia). Then, 4-µm thick sections were cut from the pre-chemotherapy biopsies. The sections obtained were processed and stained with H&E in order to identify the areas with dense lymphocytic infiltration. Furthermore, unstained sections were subsequently obtained and stained using an IHC method for CD8+ T cells. First the cells were incubated in hot air oven for 25 to 30 min at 60 to 65°C. The slides were loaded on automated IHC stainer (Ventana Medical system BenchMark XT; Roche Applied Science, Penzberg, Germany), Ultra View Universal DAB Detection system according to manufacturers protocol, (#760-500; Ventana Benchmark XT; Roche Applied Science). The slides were deparaffinized and the endogenous peroxidase activity was blocked within the closed detection system according to the manufacturers protocol. Antigen epitope retrieval was performed by unmasking with standard CC1 (cell conditioning) ready to use solution for 60 min at 95°C. Incubation was performed with primary antibody, CD8 rabbit monoclonal antibody, RTU (#REF.790-4460; Ventana Benchmark XT; Roche Applied Science) for 24 min at 37°C. The secondary antibody from the kit was the used for the second incubation for ~20 min at 36°C. The staining was visualized by using the light microscopy (Nikon-Eclipse-80 i) and only cells expressing strong membranous and cytoplasmic staining for CD8+ were counted as positive.
CD8+ lymphocytic infiltration was interpreted as intratumoral if they were encountered within the tumor cell nests, whereas it was interpreted as peritumoral if they were encountered within the adjacent stroma; defined as CD8+ cells within one tumor cell diameter of the tumor (26).
A semi-quantitative analysis of the intratumoral and peritumoral CD8+ positive lymphocytes was performed using a Nikon Eclipse 80i microscope (Nikon Corporation, Tokyo, Japan) by two experienced histopathologists (A.R and S.H) who were blind to the patients' clinical background and survival data. The numbers of both intratumoral and peritumoral lymphocytes were manually counted with the help of a numbered grid and eyepiece graticules.
The number of intratumoral and peritumoral lymphocytes was counted in three high power fields (magnification, ×400). The counts were performed in areas of maximum lymphocytic infiltration. Foci demonstrating hemorrhage and/or necrosis were excluded. The mean of the three counts was calculated and recorded for intratumoral and peritumoral zones in each patient. Intratumoral and peritumoral CD8+ lymphocytes expression levels were classified as either high and low based on values ≤ or > the median value of each, respectively.
Statistical analysis
To achieve the aims of the present study, the effect of CD8+ TIL expression was evaluated using two main end points: pCR post-NC and the patients' outcome as indicated by the DFS and OS. The associations between clinicopathological characteristics and pCR were analyzed using the chi-squared test for categorical variables. Mean differences were analyzed using a paired t-test. DFS was measured as the time between the date of diagnosis and the date of the last follow-up or disease relapse. OS was measured as the time between the date of diagnosis and the date of the last follow-up or mortality. OS and DFS rates were analyzed using the Kaplan-Meier estimator method and differences in DFS or OS among subgroups were evaluated for significance using the log-rank test. Cox's proportional hazards regression models were used for multivariate survival analyses to estimate the hazard ratio (HR) of CD8+ TILs adjusted by potential confounding factors, including age at diagnosis, tumor histological type, tumor grade, disease stage, pCR and lymphovascular invasion. The Wald test was used to evaluate the significance of individual coefficients in the model. All statistical analyses were performed using SPSS software (version 16; SPSS, Inc., Chicago, IL, USA). P<0.05 (two-tailed) was considered to indicate a statistically significant difference.
Results
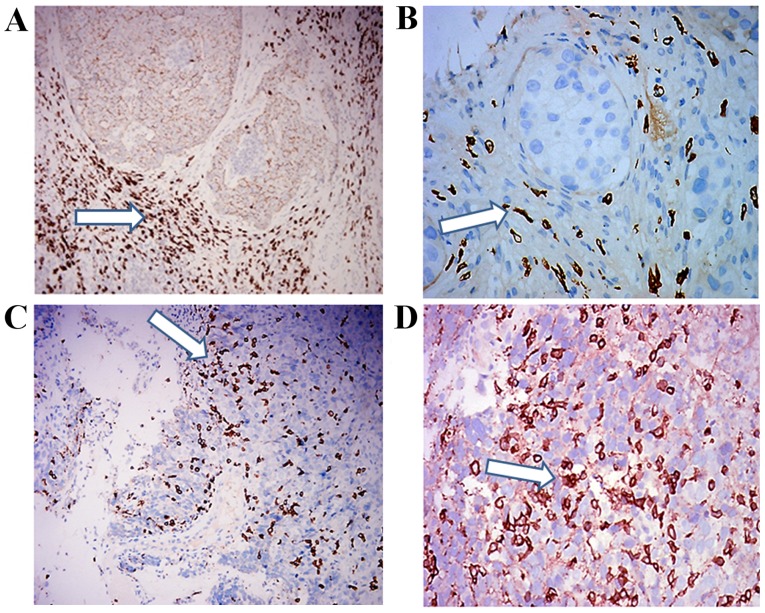
A total of 31 patients with luminal B/HER 2-negative invasive BC were enrolled into the present study. The demographic and clinicopathological data of patients are presented in Table I. Characteristically, 11/31 (35.5%) patients achieved pCR post-NC, while 20/31 (64.5%) did not. The median value was 9 (range, 3–53) for intratumoral CD8+ TIL expression and 50 (range, 28–116) for peritumoral expression. Intratumoral CD8+ TIL expression was high in 16 patients (58.1%) and low in 15 patients (41.9%). High and low peritumoral CD8+ TIL expression levels were recorded in 20 (64.5%) and 11 (35.5%) patients, respectively (Table I). Intra- and peri-tumoral CD8+ TIL infiltration of luminal B BC samples were illustrated in Fig. 1.
Table I.
Clinicopathological characteristics of patients with luminal B breast cancer, including expression of CD8+ TIL.
| Clinicopathological characteristic | Value (%) |
|---|---|
| Age, years | |
| Mean ± standard deviation | 50.58±1.58 |
| >50 | 13 (42) |
| ≤50 | 18 (58) |
| Histological type | |
| Infiltrating ductal | 26 (83.9) |
| Lobular | 5 (16.1) |
| Tumor grade at initial biopsy | |
| Grade II | 16 (51.6) |
| Grade III | 15 (48.4) |
| Lymphovascular invasion | |
| Present | 9 (29) |
| Absent | 22 (71) |
| Disease stage | |
| Stage II | 12 (27.5) |
| Stage III | 19 (72.5) |
| pCR | |
| Present | 11 (35.5) |
| Absent | 20 (64.5) |
| Peritumoral CD8+TIL | |
| High | 20 (64.5) |
| Low | 11 (35.5) |
| Intratumoral CD8+TIL | |
| High | 16 (58.1) |
| Low | 15 (41.9) |
pCR, pathological complete response; CD8, cluster of differentiation 8; TIL, tumor-infiltrating lymphocytes.
Figure 1.
Representative image of a luminal B breast cancer tissue sample with CD8+ TIL immunostaining. Photomicrographs of breast carcinoma with a predominantly peritumoral CD8+ TIL infiltrate as indicated by the arrows at (A) magnification, ×200 and (B) magnification, ×400. Photomicrographs of breast carcinoma with a predominantly intratumoral CD8+ TIL infiltrate as indicated by the arrows at (C) magnification, ×200 and (D) magnification, ×400. CD8, cluster of differentiation 8; TIL, tumor-infiltrating lymphocytes.
Correlations between clinicopathological features and pCR were only identified to be significant for intratumoral expression of CD8+ TIL (Table II). A total of 9/16 patients (56%) with high intratumoral CD8+ TIL expression achieved a pCR, in contrast with only 2/15 patients (13.3%) with low intratumoral CD8+ TIL expression (P=0.016). No significant correlation was identified between peritumoral CD8+ TIL expression and pCR (P=0.135).
Table II.
Prediction of pCR according to the clinicopathological characteristics of patients with luminal B breast cancer.
| Pathological response | ||||
|---|---|---|---|---|
| Clinicopathological characteristic | Non pCR No. (%) | pCR No. (%) | P-value | |
| Age, years | ||||
| >50 | 13 | 9 (29) | 4 (13) | 0.654 |
| <50 | 18 | 11 (35) | 7 (23) | |
| Histological type | ||||
| Infiltrating ductal | 26 | 17 (55) | 9 (29) | 0.595 |
| Lobular | 5 | 3 (10) | 2 (6) | |
| Tumor grade | ||||
| Grade II | 16 | 12 (39) | 4 (13) | 0.189 |
| Grade III | 15 | 8 (26) | 7 (22) | |
| Lymphovascular invasion | ||||
| Present | 9 | 5 (16) | 4 (13) | 0.581 |
| Absent | 22 | 15 (48) | 7 (23) | |
| Disease stage | ||||
| Stage II | 12 | 10 (32) | 2 (7) | 0.086 |
| Stage III | 19 | 10 (32) | 9 (29) | |
| Peritumoral CD8+TIL | ||||
| High | 20 | 11 (35) | 9 (29) | 0.135 |
| Low | 11 | 9 (29) | 2 (7) | |
| Intratumoral CD8+TIL | ||||
| High | 16 | 7 (23) | 9 (29) | 0.016 |
| Low | 15 | 13 (42) | 2 (6) | |
pCR, pathological complete response; CD8, cluster of differentiation 8; TIL, tumor-infiltrating lymphocytes.
Correlations were studied between peri- and intra-tumoral expression of CD8+ TIL, and clinicopathological characteristics, in addition to pCR. The results confirmed that only intratumoral CD8+ TIL expression was significantly correlated with pCR (Table III).
Table III.
Correlations between clinicopathological characteristics of patients with luminal B breast cancer and the expression of CD8+ TIL.
| Intratumoral CD8+ No. (%) | Peritumoral CD8+ No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| Clinicopathological characteristic | High | Low | P-value | High | Low | P-value | |
| Age, years | 0.677 | 0.2 | |||||
| >50 | 13 | 5 (16) | 8 (26) | 10 (32) | 3 (10) | ||
| <50 | 18 | 11 (35) | 7 (23) | 10 (32) | 8 (26) | ||
| Histological type | 0.186 | 0.405 | |||||
| Infiltrating ductal | 26 | 12 (39) | 14 (45) | 16 (52) | 10 (32) | ||
| Lobular | 5 | 4 (13) | 1 (3) | 4 (13) | 1 (3) | ||
| Tumor grade | 0.838 | 0. 553 | |||||
| Grade II | 16 | 9 (29) | 7 (23) | 10 (32) | 6 (19) | ||
| Grade III | 15 | 7 (23) | 8 (25) | 10 (32) | 5 (16) | ||
| Disease stage | 0.862 | 0.423 | |||||
| Stage II | 12 | 5 (16) | 7 (23) | 7 (23) | 5 (16) | ||
| Stage III | 19 | 11 (35) | 8 (26) | 13 (42) | 6 (19) | ||
| Lymphovascular invasion | 0.204 | 0.287 | |||||
| Present | 9 | 6 (19) | 3 (10) | 7 (23) | 2 (6) | ||
| Absent | 22 | 10 (32) | 12 (39) | 13 (42) | 9 (29) | ||
| pCR | 0.016 | 0.135 | |||||
| Present | 11 | 9 (29) | 2 (6) | 9 (29) | 2 (7) | ||
| Absent | 20 | 7 (23) | 13 (42) | 11 (35) | 9 (29) | ||
CD8, cluster of differentiation 8; TIL, tumor-infiltrating lymphocytes; pCR, pathological complete response.
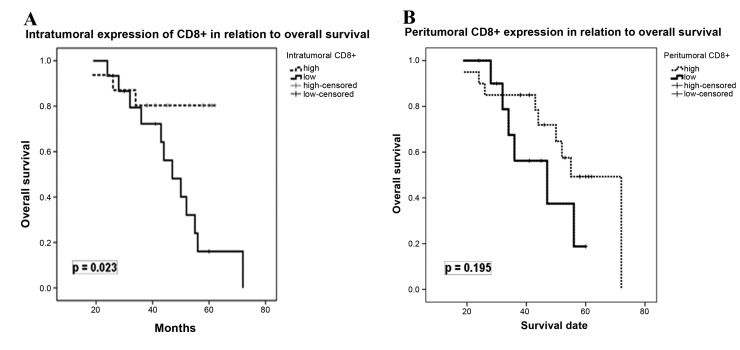
Univariate analysis revealed that high expression of intratumoral CD8+ TILs was significantly associated with OS (P=0.023), but not with DFS (P=0.869). In contrast, peritumoral CD8+ TIL expression exhibited no significant effect on OS (P=0.195) and DFS (P=0.651). Fig. 2 depicts the correlations between intra-and peri-tumoral CD8+ TIL expression, and OS. A multivariate Cox's regression model was performed to assess the correlation between the expression of CD8+ TIL, and DFS and OS, including the following covariates: Age at diagnosis, tumor histological type, tumor grade, disease stage, pCR and lymphovascular invasion. As Table IV demonstrates, intratumoral expression of CD8+ TIL was identified as an independent prognostic factor for OS [HR=2.82; 95% confidence interval (CI)=0.911–4.833; P=0.007], but not for DFS (HR=1.11; 95% CI=0.282–2.078; P=0.508). No statistically significant effect of any of other covariates was observed for DFS and OS.
Figure 2.
Kaplan-Meier survival curves demonstrating the association between CD8+ tumor-infiltrating lymphocyte expression and disease outcome of patients with luminal B breast cancer. Survival curves for (A) Intratumoral CD8+ expression and (B) Peritumoral CD8+ expression. CD8, cluster of differentiation 8.
Table IV.
Disease-free and overall survival according to clinicopathological characteristics of patients with luminal B breast cancer and pCR.
| Disease-free survival | Overall survival | |||||
|---|---|---|---|---|---|---|
| Clinicopathological characteristic | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age: <50 vs. >50 years | 1.67 | 0.187–1.591 | 0.268 | 1.55 | 0.078–2.248 | 0.309 |
| Histological type: | 1.1 | 0.238–1.984 | 0.688 | 1.62 | 0.376–3.867 | 0.211 |
| Ductal vs. lobular | ||||||
| Tumor grade: II vs. III | 0.88 | 0.369–2.376 | 0.891 | 1.76 | 0.051–1.466 | 0.131 |
| Disease stage: II vs. III | 0.96 | 0.338–2.251 | 0.778 | 1.98 | 0.920–4.689 | 0.064 |
| Lymphovascular invasion: | 1.02 | 0.330–2.196 | 0.74 | 1.32 | 0.455–2.974 | 0.245 |
| Absent vs. present | ||||||
| pCR: Absent vs. present | 1.12 | 0.211–1.434 | 0.69 | 1.66 | 0.011–1.408 | 0.072 |
| Peritumoral CD8+ TIL: | 0.89 | 0.455–3.974 | 0.607 | 0.78 | 0.193–1.738 | 0.956 |
| Low vs. high | ||||||
| Intratumoral CD8+ TIL: | 1.11 | 0.228–2.078 | 0.508 | 2.82 | 0.911–4.883 | 0.007 |
| Low vs. high | ||||||
pCR, pathological complete response; CD8, cluster of differentiation 8; TIL, tumor-infiltrating lymphocytes.
Discussion
The traditional prognostic markers for BC include clinical stage, lymph node involvement, tumor grade, ER and PR status, and the presence of HER 2 abnormalities (39). However, the heterogeneity of the disease, together with limitations of current therapeutic modalities and advances in molecular diagnostics have led to increasing interest in identifying novel prognostic and predictive tools (7). Therefore, in the present study, the expression of CD8+ TILs was evaluated in patients with luminal B/HER 2-negative BC, in order to identify its potential predictive and/or prognostic value. The present study involved a cohort of 31 patients treated with anthracycline-taxane-based NC, which is considered the standard treatment for early BC, particularly for this molecular subtype.
The contribution of the different TIL subpopulations to the clinical and biological characteristics of BC remains unclear, particularly in terms of prediction of chemotherapy efficacy. The results of the present study revealed that the intratumoral expression of CD8+ TIL may be predictive of pCR in this patient population. Similar to previous studies (17,21,22,40–42), 56% of patients with high intratumoral expression of CD8+ TIL achieved a pCR, in contrast with 13.3% for patients with low intratumoral CD8+ TIL.
Denkert et al (17) investigated intratumoral and stromal lymphocytes in a total of 1,058 pre-therapeutic BC core biopsies from two neoadjuvant anthracycline/taxane-based studies. It was concluded that the presence of tumor-associated lymphocytes in BC is an independent predictor of response to anthracycline/taxane-based chemotherapy, and provides useful information for oncologists to identify the subgroup of patients that are most likely to benefit from this type of chemotherapy. A multicentric neoadjuvant pilot study by Nabholtz et al (21) investigated the value of CD8+ expression as a predictor of pCR in a series of 60 patients with operable stage II–III triple-negative BC (TNBC), treated with anthracycline-taxane-based chemotherapy plus the anti-HER 1 monoclonal antibody panitumumab. It was reported that high CD8+ TILs counts (≥118) predicted an 84% probability of pCR, as opposed to low counts (<118) yielding a 10% probability. In addition, West et al (40) demonstrated that higher TIL counts detected by eight-gene expression profiling were correlated with the pCR rate in TNBC- and HER 2-positive tumors. In a previous systematic review and meta-analysis, Mao et al (41) observed that a high number of TILs, either stromal or intra-tumoral or both, is a significant predictor of pCR for patients treated with NC. TILs detected in the pre-treatment biopsy indicated a 2.5 and 5 times increased probability of pCR in patients that were TNBC- and HER 2-positive, respectively. Notably, no reported effects were observed in ER-positive patients (41).
It was also observed that chemotherapy induced a high rate of pCR in TIL-positive patients and was able to convert a TIL-negative tumor into a TIL-positive one. It has previously been reported that taxane-based chemotherapy converted 7/21 breast tumors from TIL-negative to TIL-positive (42). Post-chemotherapy TIL status has been identified to be associated with an improvement in clinical response (42). Furthermore, a comprehensive analysis of the immune characteristics in a small group of patients with breast carcinoma demonstrated an increase in CD8 and a decrease in CD4 and CD20 lymphocytes following chemotherapy (43). Notably, the results of the present study revealed that 86.7% of patients with low intratumoral CD8+ TIL counts did not achieve a pCR. This finding may have an important clinical impact regarding neoadjuvant management of patients with luminal B BC. The potential capability to predict a low probability of pCR may allow for the consideration of alternative therapeutic strategies, including endocrine therapy or the potential addition of immunotherapy.
Cytotoxic T cells, identifiable by CD8+ expression, form a major component of the adaptive immune system. Cells that present foreign antigens in association with the major histocompatibility complex class I molecule are recognized by cytotoxic T lymphocytes through a specific interaction between the presented antigen and the T-cell receptor (44). This interaction causes the activated T cell to release proteins, including perforin and granzyme, enabling cytotoxic activity through membranolysis (44). These mechanisms act on tumor cells which, unlike normal cells, present atypical antigens (45). However, regulatory T cells, which express forkhead box P3, act by decreasing the immune response to self-antigens. The hypothesis that regulatory T cells may be recruited by tumors to evade immune destruction is supported by the observation that T cell ablation in mice enables an effective anti-tumor response (46). Thus, it is not surprising that the prognostic importance of lymphocytic infiltration has been demonstrated in different types of solid tumor.
Previous IHC studies have suggested that tumor infiltrating CD8+ TILs may exhibit antitumor activity, as indicated by their favorable effect on patient survival in colorectal (47), ovarian (29), renal (30), lung (31) and pancreatic (32) cancer. Furthermore, in colorectal cancer, the density and location of CD8+ TILs possess prognostic value superior to and independent of the International Union against Cancer tumor node metastasis classification (47). However, such findings have not been confirmed in patients with BC, and in contrast to the unique predictive function of CD8+ TILs for pCR, reports on the association between CD8+ T-cell infiltration and BC survival have presented conflicting results (22–28). Nevertheless, two previous studies have investigated a larger series of cases. Mahmoud et al (23) used a retrospective cohort of 1,334 patients with primary BC to demonstrate that total CD8+ TILs were independently associated with an improved survival rate in BC. Baker et al (48), investigated 1,953 BC cases and demonstrated that the independent favorable prognostic effect of total CD8+ TILs was observed only in tumors that were ER-negative.
These controversial results with regards to the contribution of TIL to tumor progression and clinical outcome in BC may be partially due to the fact that this effect may be restricted to certain tumor subtypes. Another potential explanation may be associated with the method of TIL analysis. Numerous studies have used H&E-stained sections in their evaluation (22,27,28). Evidently, the use of IHC to detect TILs is advantageous for several reasons, including the ability to directly quantify cells that express a given marker, in addition to accurately determining their specific localization within a tissue (23). It is not possible to acquire this information using conventional methods of gene-expression analysis (23,25). Thus, this is why IHC was used in the present study to address the potential value of CD8+ TILs in a well-characterized group of patients with luminal B BC. The results revealed that the intratumoral expression of CD8+ TIL was an independent prognostic factor for improved OS, as patients with high intratumoral CD8+ TIL counts had a 2.82 times higher probability of OS, compared with those with low intratumoral CD8+ TIL counts.
Overall, the results of the present study may have important clinical implications. Considering the prognosis of luminal B/HER 2-negative cancer and the various profiles of hormonal and chemotherapy sensitivity, immunobiological stratification according to the expression of CD8+ TILs may improve the management of these patients (4,6). In addition, this type of immunological profiling may represent the first step towards a more improved understanding of the potential function of antitumor immune responses in mediating the clinical outcome of NC. Furthermore, it may direct novel immunotherapeutic approaches, including the use of programmed death-1 pathway inhibitors for the treatment of patients with BC (49).
In conclusion, the results of the present study suggested that high intratumoral CD8+ TILs expression is significantly predictive of pCR post-NC (56%) and represents an independent prognostic factor for improved OS in patients with luminal B/HER 2-negative BC. In contrast, low intratumoral CD8+ TIL expression was identified as a strong predictor of lack of pCR to NC (13.3%), in addition to an independent prognostic factor for poor OS. Assessment of the immune response, in conjunction with the usual parameters, may aid in the stratification of patients with luminal B/HER 2-negative BC regarding the prediction of pCR post-NC and overall prognosis. Further studies to develop TIL-based predictive and therapeutic strategies in patients with luminal B BC are warranted.
Acknowledgements
The authors would like to thank and acknowledge the support they received from the college of Medicine Research Center, Deanship of Scientific Research, King Saud University and the laboratory technicians for their help with the IHC analysis.
Glossary
Abbreviations
- AC-T
doxorubicin cyclophosphamide docetaxel
- BC
breast cancer
- CD8+
cluster of differentiation 8+
- DFS
disease-free survival
- ER
estrogen receptor
- H&E
hematoxylin and eosin
- HER 2
human epidermal growth factor receptor 2
- NC
neoadjuvant chemotherapy
- OS
overall survival
- pCR
pathologic complete response
- PR
progesterone receptor
- TIL
tumor-infiltrating lymphocytes
- TNBC
triple-negative breast cancer
References
- 1.Alghamdi IG, Hussain II, Alghamdi MS, El-Sheemy MA. The incidence rate of female breast cancer in Saudi Arabia: An observational descriptive epidemiological analysis of data from Saudi Cancer Registry 2001–2008. Breast Cancer (Dove Med Press) 2013;5:103–109. doi: 10.2147/BCTT.S50750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Rikabi A, Husain S. Increasing prevalence of breast cancer among Saudi patients attending a tertiary referral hospital: A retrospective epidemiologic study. Croat Med J. 2012;53:239–243. doi: 10.3325/cmj.2012.53.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijin M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications; Proc Natl Acad Sci USA; 2001; pp. 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ades F, Zardavas D, Bozovic-Spasojevic I, Pugliano L, Fumagalli D, De Azambuja E, Viale G, Sotiriou C, Piccart M. Luminal B breast cancer: Molecular characterization, clinical management, and future perspectives. J Clin Oncol. 2014;32:2794–2803. doi: 10.1200/JCO.2013.54.1870. [DOI] [PubMed] [Google Scholar]
- 5.Prat A, Cheang MC, Martin M, Parker JS, Carrasco E, Caballero R, Tyldesley S, Gelmon K, Bernard PS, Nielsen TO, Perou CM. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal a breast cancer. J Clin Oncol. 2013;31:203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ladoire S, Mignot G, Dabakuyo S, Amould L, Apetoh L, Rébé C, Coudert B, Martin F, Bizollon MH, Vanoli A, et al. In situ immune response after neoadjuvant chemotherapy for breast cancer predicts survival. J Pathol. 2011;224:389–400. doi: 10.1002/path.2866. [DOI] [PubMed] [Google Scholar]
- 7.Cleator S, Ashworth A. Molecular profiling of breast cancer: Clinical implications. Br J Cancer. 2004;90:1120–1124. doi: 10.1038/sj.bjc.6601667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bear HD, Anderson S, Brown A, Smith R, Mamounas EP, Fisher B, Margolese R, Theoret H, Soran A, Wickerham DL, Wolmark N. National Surgical Adjuvant Breast and Bowel Project Protocol B-27. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: Preliminary results from national surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol. 2003;21:4165–4174. doi: 10.1200/JCO.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J Natl Cancer Inst. 2005;97:188–194. doi: 10.1093/jnci/dji021. [DOI] [PubMed] [Google Scholar]
- 10.Chollet P, Amat S, Cure H, De Latour M, Le Bouedec G, Mouret-Reynier MA, Ferriere JP, Achard JL, Dauplat J, Penault-Llorca F. Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer. 2002;86:1041–1046. doi: 10.1038/sj.bjc.6600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich J, Huober J, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 12.Seo AN, Lee HJ, Kim EJ, Kim HJ, Jang MH, Lee HE, Kim YJ, Kim JH, Park SY. Tumour-infiltrating CD8 lymphocytes as an independent predictive factor for pathological complete response to primary systemic therapy in breast cancer. Br J Cancer. 2013;109:2705–2713. doi: 10.1038/bjc.2013.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: The roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv Immunol. 2006;90:1–50. doi: 10.1016/S0065-2776(06)90001-7. [DOI] [PubMed] [Google Scholar]
- 14.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 15.Melichar B, Študentova H, Kalábová H, Vitásková D, Čermáková P, Hornychová H, Ryška A. Predictive and Prognostic significance of tumor-infiltrating lymphocytes in patients with breast cancer treated with neoadjuvant systemic therapy. Anticancer Res. 2014;34:1115–1125. [PubMed] [Google Scholar]
- 16.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: From immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 17.Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Seo JY, Ahn JH, Ahn SH, Gong G. Tumor-associated lymphocytes predict response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013;16:32–39. doi: 10.4048/jbc.2013.16.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ladoire S, Arnould L, Apetoh L, Coudert B, Martin F, Chauffert B, Fumoleau P, Ghiringhelli F. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin Cancer Res. 2008;14:2413–2420. doi: 10.1158/1078-0432.CCR-07-4491. [DOI] [PubMed] [Google Scholar]
- 20.Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48:3342–3354. doi: 10.1016/j.ejca.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 21.Nabholtz JM, Abrial C, Mouret-Reynier MA, Dauplat MM, Weber B, Gligorov J, Forest AM, Tredan O, Vanlemmens L, Petit T, et al. Multicentric neoadjuvant phase II study of panitumumab combined with an anthracycline/taxane based chemotherapy in operable triple negative breast cancer: Identification of biologically-defined signatures predicting treatment impact. Ann Oncol. 2014;25:1570–1577. doi: 10.1093/annonc/mdu183. [DOI] [PubMed] [Google Scholar]
- 22.Lee AH, Gillett CE, Ryder K, Fentiman IS, Miles DW, Millis RR. Different patterns of inflammation and prognosis in invasive carcinoma of the breast. Histopathology. 2006;48:692–701. doi: 10.1111/j.1365-2559.2006.02410.x. [DOI] [PubMed] [Google Scholar]
- 23.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-Infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949–1955. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 24.Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14:R48. doi: 10.1186/bcr3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, Earl HM, Poole CJ, Hiller L, Dunn JA, et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25:1536–1543. doi: 10.1093/annonc/mdu191. [DOI] [PubMed] [Google Scholar]
- 26.Matkowski R, Gisterek I, Halon A, Lacko A, Szewczyk K, Staszek U, Pudelko M, Szynglarewicz B, Szelachowska J, Zolnierek A, Kornafel J. The prognostic role of tumor-infiltrating CD4 and CD8 T lymphocytes in breast cancer. Anticancer Res. 2009;29:2445–2451. [PubMed] [Google Scholar]
- 27.Carlomagno C, Perrone F, Lauria R, De Laurentiis M, Gallo C, Morabito A, Pettinato G, Panico L, Bellelli T, Apicella A, et al. Prognostic significance of necrosis, elastosis, fibrosis and inflammatory cell reaction in operable breast cancer. Oncology. 1995;52:272–277. doi: 10.1159/000227472. [DOI] [PubMed] [Google Scholar]
- 28.Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, Syrjänen K. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28A:859–864. doi: 10.1016/0959-8049(92)90134-N. [DOI] [PubMed] [Google Scholar]
- 29.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer; Proc Natl Acad Sci USA; 2005; pp. 18538–18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: Clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- 31.Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, et al. Predominant infiltration of macrophages and CD8(+) T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008;113:1387–1395. doi: 10.1002/cncr.23712. [DOI] [PubMed] [Google Scholar]
- 32.Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28:e26–e31. doi: 10.1097/00006676-200401000-00023. [DOI] [PubMed] [Google Scholar]
- 33.Camp BJ, Dyhrman ST, Memoli VA, Mott LA, Barth RJ., Jr In situ cytokine production by breast cancer tumor-infiltrating lymphocytes. Ann Surg Oncol. 1996;3:176–184. doi: 10.1007/BF02305798. [DOI] [PubMed] [Google Scholar]
- 34.Muraro E, Martorelli D, Turchet E, Miolo G, Scalone S, Comaro E, Talamini R, Mastorci K, Lombardi D, Perin T, et al. A different immunologic profile characterizes patients with HER 2-overexpressing and HER 2-negative locally advanced breast cancer: Implications for immune-based therapies. Breast Cancer Res. 2011;13:R117. doi: 10.1186/bcr3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumors of the breast. 4th. Vol. 4. Lyon: International Agency for Research on Cancer; 2012. [Google Scholar]
- 36.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging Manual. 7th. New York: Springer; 2010. [Google Scholar]
- 37.Ellis IO, Galea M, Broughton N, Locker A, Blamey RW, Elston CW. Pathological prognostic factors in breast cancer: II. Histological type. relationship with survival in a large study with long-term follow-up. Histopathology. 1992;20:479–489. doi: 10.1111/j.1365-2559.1992.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 38.Elston CW, Elli IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 2002;41:151–153. doi: 10.1046/j.1365-2559.2002.14691.x. [DOI] [PubMed] [Google Scholar]
- 39.Schnitt SJ. Classification and prognosis of invasive breast cancer: From morphology to molecular taxonomy. Mod Pathol. 2010;23(Suppl 2):S60–S64. doi: 10.1038/modpathol.2010.33. [DOI] [PubMed] [Google Scholar]
- 40.West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13:R126. doi: 10.1186/bcr3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao Y, Qu Q, Zhang Y, Liu J, Chen X, Shen K. The value of tumor infiltrating lymphocytes (TILS) for predicting response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. PLoS One. 2014;9:e115103. doi: 10.1371/journal.pone.0115103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demaria S, Volm MD, Shapiro RL, Yee HT, Oratz R, Formenti SC, Muggia F, Symmans WF. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin Cancer Res. 2001;7:3025–3030. [PubMed] [Google Scholar]
- 43.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer; Proc Natl Acad Sci USA; 2012; pp. 2796–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berke G. The binding and lysis of target cells by cytotoxic lymphocytes: Molecular and cellular aspects. Annu Rev Immunol. 1994;12:735–773. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- 45.Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: A common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–5218. [PubMed] [Google Scholar]
- 47.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 48.Baker K, Lachapelle J, Zlobec I, Bismar TA, Terracciano L, Foulkes WD. Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology. 2011;58:1107–1116. doi: 10.1111/j.1365-2559.2011.03846.x. [DOI] [PubMed] [Google Scholar]
- 49.Lee SM, Chow LQ. A new addition to the PD-1 checkpoint inhibitors for non-small cell lung cancer-the anti-PDL1 antibody-MED14736. Transl Lung Cancer Res. 2014;3:408–410. doi: 10.3978/j.issn.2218-6751.2014.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]