Abstract
Background
Ten percent of pancreatic ductal adenocarcinoma (PDAC) is due to genetic predisposition, including the breast and ovarian cancer syndrome germline mutations BRCA1 and BRCA2. Knowledge of specific genetic mutations predisposing to PDAC may enable risk stratification, early detection and development of effective screening and surveillance programs. We aimed to determine the diagnostic yield of testing for BRCA1/2 germline mutations in a PDAC screening cohort and a PDAC cohort referred for genetic testing.
Methods
Patients in a high-risk PDAC prevention and genetics program or with a personal history of PDAC referred for genetic evaluation underwent testing for BRCA1/2 germline mutations. Clinical BRCA1/2 genetic testing included testing for the 3 Ashkenazi Jewish founder mutations or BRCA1/2 comprehensive testing.
Results
37 patients without PDAC underwent BRCA1/2 testing at our institution. Genetic testing identified 7 patients who were BRCA1/2 carriers for a yield of 18.9%. Six carried Ashkenazi Jewish founder mutations (3 BRCA1, 3 BRCA2), and 1 had a BRCA2 mutation on comprehensive testing. 32 patients with PDAC underwent BRCA1/2 genetic testing. Five had Ashkenazi Jewish founder mutations (two BRCA1, three BRCA2), and 2 had BRCA2 mutations on comprehensive testing. The diagnostic yield was 7/32 (21.8%).
Conclusions
BRCA1/2 testing is useful in PDAC risk stratification and alters risk assignment and screening recommendations for mutation positive patients and their families. Clinical BRCA1/2 testing should be considered in patients of Ashkenazi Jewish descent, with a personal history or family history of PDAC, even in the absence of a family history of breast and ovarian cancer.
Keywords: Pancreatic Neoplasms, Pancreas Cancer Screening, Pancreas Cancer, Diagnosis, BRCA1 Gene, BRCA2 Gene, Genetic Testing
Background
Pancreatic ductal adenocarcinoma (PDAC) is the fourth leading cause of death from malignancy in the United States, with an estimated 45,220 new cases and 38,460 deaths in 20131. Ten percent of PDAC is hereditary2 and certain patient populations, such as those with hereditary pancreatitis3, Peutz-Jeghers syndrome4, familial atypical multiple mole melanoma (FAMMM) syndrome5-9, Lynch syndrome10-12, and the breast-ovarian cancer syndrome (BRCA1 and BRCA2 mutations)13-18 are at the highest risk of PDAC. The BRCA1 and BRCA2 mutations most typically cause breast and ovarian cancer, but are also associated with prostate cancer and PDAC. The association between BRCA2 mutations and PDAC has been described in the literature, especially in the Ashkenazi Jewish population; but the role of BRCA1 mutations and PDAC is less established13, 14, 17, 19-21.
Several high-volume centers have initiated PDAC screening and surveillance protocols22-26. Current guidelines, based on expert opinion, recommend screening for PDAC in a BRCA2 carrier with a first-degree relative with PDAC or with two non-first degree relatives with PDAC27. No guidelines are available for PDAC screening in BRCA1 carriers. The value of genetic testing prior to the initiation of PDAC screening has not been well documented, and there are no guidelines incorporating genetic testing into PDAC risk stratification. We report a single-center experience of clinical BRCA1 and BRCA2 testing in patients seen in a Pancreas Cancer Prevention and Genetics Program. We aimed to determine the diagnostic yield of genetic testing for BRCA1 and BRCA2 germline mutations in a PDAC screening cohort and in a cohort with PDAC who were referred for genetic risk evaluation and testing.
Methods
Patient Selection and Genetic Testing
All consecutive patients referred to the Pancreas Cancer Prevention and Genetics Program between 2005 and 2011 were included (Figure 1.) Medical and social history, including patient's religion, and previous tobacco and alcohol use, were self-reported. Patients met with a genetic counselor for detailed pedigree analysis, and were offered relevant genetic testing based upon the family history by the genetic counselor and pancreas cancer screening clinical provider28. Nearly all Ashkenazi Jewish patients with a mutation in BRCA1 or BRCA2 harbor 1 of 3 founder mutations (BRCA1 185delAG, BRCA1 5382insC, and BRCA2 6174delT) that are therefore tested as a diagnostic panel. In non-Ashkenazi Jews, as well as in all other religious denominations, founder mutations do not exist with such high prevalence; therefore, the entire genes are tested in a comprehensive manner. Clinical BRCA1/2 testing included testing for the three Ashkenazi Jewish founder mutations, or comprehensive sequencing of both BRCA1 and BRCA2 with the BRCA1 5-site rearrangement panel for non-Ashkenazi Jewish patients (Myriad Genetics, Salt Lake City, Utah). Additional large rearrangement testing was not performed. Genetic testing for the Partner and Localizer of BRCA2 (PALB2) gene was not routinely available throughout the study period. The strategy for genetic testing included testing the youngest available affected family member. For patients of Ashkenazi Jewish descent, BRCA1/2 testing was recommended even in the absence of a family history of breast or ovarian cancer. If an affected family member was not available, genetic testing was recommended for the unaffected patient.
Figure 1. Flow Diagram Describing How Patients Enter Into Our Pancreatic Cancer and Genetics Program.
Abbreviations: pts= patients, GT= genetic testing, PDAC= pancreatic ductal adenocarcinoma, AJ= Ashkenazi Jewish, FAMMM= Familial Atypical Multiple Mole Melanoma Syndrome, APC= Adenomatous Polyposis Coli, FAP= Familial Adenomatous Polyposis, HNPCC= Hereditary Nonpolyposis Colorectal Cancer
An additional 20 patients were referred to the Pancreas Cancer Prevention and Genetics Program for PDAC screening recommendations based on genetic testing results that had been performed at other institutions. These mutation carriers were included only in the analyses of impact on clinical management. Thirty two patients were referred following a diagnosis of PDAC. This cohort was analyzed separately. An additional group of 17 patients referred for screening and genetic risk evaluation had a personal history and family history suggestive of hereditary syndromes not related to BRCA1/2. These included FAMMM, Lynch Syndrome, familial adenomatous polyposis (FAP) and familial pancreatitis. Genetic testing for germline mutations that predispose to PDAC, other than BRCA1/2, was undertaken if there was a family history consistent with an autosomal dominant pattern of disease transmission, and either an affected cancer survivor or a living obligate mutation carrier. Individuals without a living affected cancer patient in their family were also tested for germline mutations if there was a very strong family history in an autosomal pattern characteristic of a specific genetic cancer predisposition syndrome. Clinical genetic testing for these diseases was performed at appropriate clinical laboratories.
PDAC Screening
Patients without a prior history of PDAC were stratified into average, moderate, or high-risk of PDAC based on their personal and family history of cancer, according to our program protocol22. High-risk patients included those with ≥ 2 first-degree relatives with PDAC, ≥ 3 first-, second-, or third-degree relatives with PDAC, 1 first-degree and 1 second-degree relative with PDAC if one PDAC occurred at age <55, and those who had a genetic syndrome that was associated with PDAC, including those with known BRCA1 and BRCA2 mutations. Moderate-risk patients included those with ≥ 2 first-, second-, or third-degree relatives with PDAC, patients with one first-degree relative with PDAC at an age younger than 55, and not otherwise meeting criteria for high-risk. Average-risk patients were defined as those without a family history of PDAC or with 1 family member with PDAC over the age of 55 years. Patients in the high-risk group were offered routine laboratory examinations, including complete blood count, basic metabolic function, hepatic function, amylase, lipase, and carbohydrate antigen 19-9 (CA19-9), endoscopic ultrasound (EUS), and magnetic resonance imaging (MRI) of the abdomen with magnetic resonance cholangiopancreatography (MRCP). Patients at moderate risk of PDAC were offered either EUS or MRI/MRCP along with laboratory examinations as above, and those at average risk were offered complete blood count, basic metabolic function, hepatic function, amylase, lipase, and CA 19-9 alone. Patients identified to have BRCA1 or BRCA2 mutations were referred to breast and ovarian cancer screening providers to discuss their breast and ovarian cancer risks and management. Results of baseline multi-organ cancer screening examinations and cancer prevention events are reported.
Results
BRCA1/2 Testing of PDAC Screening Cohort
Thirty seven patients without PDAC, and without known mutations in the family, underwent BRCA1 and BRCA2 testing in conjunction with PDAC screening evaluation at our center (Table 1). The mean age was 53.4 years, 16 (43.2%) were male, 37(100%) were Caucasian, and 32 (86.5%) were of Ashkenazi Jewish descent. Six of the Ashkenazi Jewish patients were found to carry Ashkenazi Jewish founder mutations (two BRCA1 185delAG, 1 BRCA1 5382insC, and 3 BRCA2 6174delT). One BRCA2 3922G>T (E1308X) mutation was identified on comprehensive testing in a non-Ashkenazi Jewish individual. Overall diagnostic yield was 7/37, or 18.9%. Of the Ashkenazi Jewish PDAC screening cohort, 6/32 (18.8%) were found to have BRCA1 or BRCA2 mutations. Twenty four patients had normal testing for the Ashkenazi Jewish founder mutations, and 6 patients had normal comprehensive BRCA1/2 testing, which was undertaken in Ashkenazi Jewish subjects if the founder mutations were not detected but there was a very strong family history consistent with a genetic predisposition.
Table 1. Demographic Information For Patients Referred To Pancreas Cancer Genetics And Screening Program.
| All | PDAC Screening Cohort, BRCA1/2 Genetic Testing | PDAC Screening Cohort, Prior BRCA1/2 Genetic Testing | PDAC Screening Cohort, predictive testing | PDAC Screening cohort, non-BRCA1/2 genetic testing | PDAC Cohort, BRCA1/2 Genetic Testing | PDAC Cohort, non-BRCA1/2 Genetic Testing | |
|---|---|---|---|---|---|---|---|
| Number | 128 | 37 | 20 | 16 | 17 | 32 | 6 |
| Mean Age (years) | 54 | 53.4 | 53.0 | 40.6 | 53.6 | 57.0 | 57.2 |
| Male | 58 (45.3%) | 16(43.2%) | 2 (10%) | 9 (56.3%) | 5 (29.4%) | 20 (62.5%) | 6 (100%) |
| Caucasian | 123 (96%) | 37 (100%) | 19 (95%) | 16 (100%) | 13 (76.5%) | 32 (100%) | 6 (100%) |
| Ashkenazi Jewish | 88 (68.8%) | 32 (86.5%) | 16 (80%) | 8 (50%) | 6 (35.3%) | 26 (81.3%) | 1 (16.7%) |
| Alcohol Use | 82 (64.1%) | 27 (73%) | 13 (65%) | 13 (81.3%) | 8 (47.1%) | 19 (59%) | 2 (20%) |
| Tobacco Use | 46 (35.9%) | 13 (48%) | 10 (50%) | 2 (12.5%) | 5 (29.4%) | 13 (41%) | 3 (50%) |
PDAC Screening Cohort With Prior BRCA1/2 Testing
Twenty patients were referred to our center with BRCA1/2 testing results that had already been obtained at an outside institution. The mean age was 53.0 years, 2 (10%) were male, 19 (95%) were Caucasian, and 16 (80%) were of AJ descent. Thirteen patients had known BRCA1/2 mutations, while 7 patients were referred with negative BRCA1/2 testing. Ten patients had Ashkenazi Jewish founder mutations (3 BRCA1 185delAG, 7 BRCA2 6174delT), and 3 patients had BRCA2 mutations demonstrated with comprehensive testing (9324insA, 3917delC, 3058A>T [K944X]). These patients underwent PDAC screening according to program protocol but were not included in the analysis for diagnostic yield.
Predictive BRCA1/2 Genetic Testing of PDAC Screening Cohort
Sixteen patients were referred to our center for predictive testing following a positive test for a genetic mutation in a family member. The mean age was 40.6, 9 (56.3%) were male, 8 (50%) were Ashkenazi Jewish descent and 16 (100%) were Caucasian. Genetic testing revealed two BRCA1 185delAG mutations, and four BRCA2 mutations (1074delCA, 6024delTA, 6174delT, 5823delAT). These patients were then offered PDAC screening according to post-genetic testing program protocol but were not included in the analysis for diagnostic yield.
Other Genetic Testing: PDAC Screening Cohort
Seventeen unaffected patients had non-BRCA1/2 genetic testing based on family history consistent with Lynch Syndrome (5 patients), FAMMM (6 patients), hereditary pancreatitis (4 patients) or FAP (2 patients). The mean age was 53.6, 5 (29.4%) were male, 13 (76.5%) were Caucasian, and 6 (35.3%) were Ashkenazi Jewish. Two of five patients tested positive for mismatch repair mutations consistent with Lynch Syndrome. Six patients had normal p16 testing undertaken for FAMMM, 3 patients had normal genetic testing for the cationic tryspinogen gene, or PRSS1 for hereditary pancreatitis, and one patient had normal genetic testing for serine protease inhibitor Kazal-type 1 (SPINK1) mutation for hereditary pancreatitis. Two patients underwent genetic testing for adenomatous polyposis coli (APC) mutations for FAP, even though it is uncertain that this mutation predisposes to PDAC. All patients with germline mutations were screened for PDAC under the high-risk protocol as outlined in the methods.
BRCA1/2 Testing of PDAC Cohort
Thirty two patients were referred for BRCA1 and BRCA2 genetic testing following a diagnosis of PDAC. The mean age was 57 years, 20 (62.5%) male, 26 (81.3%) Ashkenazi Jewish, 100% Caucasian (Table 1). Five patients (all Ashkenazi Jewish white males, mean age 56) were demonstrated to have Ashkenazi Jewish founder mutations (two BRCA1 185delAG and three BRCA2 6174delT mutation carriers). Two patients were found to have a mutation on comprehensive testing (BRCA2 5823delAT and BRCA2 6024delTA). The diagnostic yield was 7/32, or 21.8%. The diagnostic yield for the Ashkenazi Jewish PDAC cohort alone was 5/26, or 19.2%.
The mean age of PDAC diagnosis amongst those with PDAC found to carry a BRCA1/2 mutation was 55.7 years (range 48-67, standard deviation 8.6) compared to 61.5 years (range 35-82, standard deviation 12.0) (p=.25) for those without a mutation (Table 2). None of the PDAC patients who tested positive for BRCA1/2 mutations had a history of tobacco use or heavy alcohol use. Typically, only patients with an early age, or a bilateral diagnosis of breast or ovarian cancer are referred for BRCA1/2 testing. Most of the BRCA1/2 mutation carriers in this cohort would have therefore not been advised to undergo testing. Only two patients had a first-degree relative with a BRCA1/2 related cancer: a 48 year-old patient had a mother diagnosed with ovarian cancer at 69, a sister diagnosed with ovarian cancer at 49, and another sister diagnosed with breast cancer at 37; and a 65 year-old patient had a mother with breast cancer diagnosed at age 40 with a subsequent ovarian cancer at age 65, and a brother with prostate cancer at 49. One subject had a father with prostate cancer at age 83, and another subject had a sister with breast cancer at age 66; it is unknown if these cancers are related to a BRCA1/2 mutation. The remaining BRCA1/2 carriers did not have a BRCA1/2-related cancer in any first degree relatives. None of the 7 PDAC patients found to have BRCA1/2 mutations had any first- or second degree relatives with PDAC, although one 49 year-old patient with a BRCA2 6174delT mutation had 3 third-degree relatives with PDAC.
Table 2. Personal and Family History of Patients with PDAC Who Underwent Genetic Testing.
A total of 7 patients with PDAC were found to have mutations in BRCA1 or BRCA2.
| BRCA1 Mutation Carriers | BRCA2 Mutation Carriers | No BRCA Mutation | |
|---|---|---|---|
| Number of Subjects | 2 | 5 | 25 |
| Ashkenazi Jewish Subjects | 2 (100%) | 3 (60%) | 21 (84%) |
| Mean Age at Diagnosis (Years +/- SD) | 49.0 +/- 1.4 | 58.4 +/- 8.9 | 61.5 +/- 12.0 |
| Tobacco Use | 0 | 0 | 3 current, 10 previous |
| Heavy Alcohol Use | 0 | 0 | 2 |
| Personal History of Non-PDAC Cancer | 0 | Breast Cancer in 2 | Breast Cancer in 3 Prostate Cancer in 2 Basal Cell Carcinoma in 1 Neuroendocrine in 1 |
| Number of Patients With First-Degree Relatives with PDAC | 0 | 0* | 4 |
| Number of Patients with First-Degree Relatives with Breast or Ovarian Cancer | 1 | 2 | 5 |
One individual had 3 third-degree relatives with PDAC.
Other Genetic Testing: PDAC Cohort
An additional 6 patients with PDAC were referred for evaluation of a genetic susceptibility to PDAC and underwent non-BRCA1/2 genetic testing. The mean age was 57.2 years, 6 (100%) male and Caucasian, 1 (16%) Ashkenazi Jewish. Five with a significant family history of melanoma or dysplastic nevi underwent p16 testing for FAMMM, and 2/5 were positive for p16 mutations G101W and D153Y. A PDAC patient with a personal history of colon cancer was found to be negative for mismatch repair mutations for Lynch Syndrome.
Risk Stratification of PDAC Screening Cohort
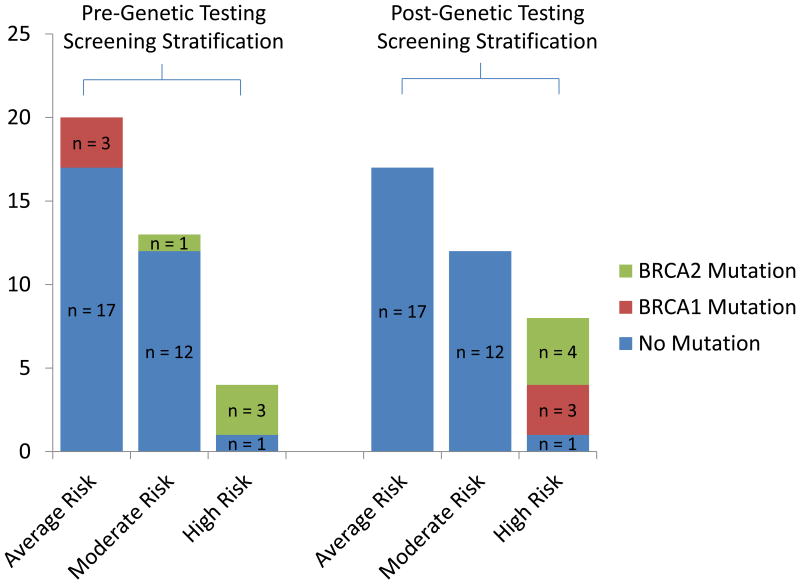
Based on family history prior to genetic testing, risk stratification of this PDAC screening cohort identified 4 patients who were high-risk of PDAC, 13 moderate-risk patients, and 20 average-risk patients (Figure 2). Of the 4 high-risk patients, three (75%) were BRCA2 carriers: two BRCA2 6174delT and one BRCA2 3922G>T mutation. One of the thirteen (7.7%) moderate-risk patients was found to carry a BRCA2 6174delT mutation. A total of 3/20 (15%) average risk patients were found to have BRCA1 mutations: two BRCA1 185delAG mutations and one BRCA1 5382insC mutation. All BRCA1/2 mutation carriers were risk re-classified and transitioned to a high-risk screening protocol. BRCA1/2 mutations were more frequently identified in the high-risk cohort compared to the moderate- and average-risk cohorts (p=.02).
Figure 2. Risk Stratification Of The Unaffected PDAC Screening Cohort Who Underwent Genetic Testing At Our Institution.
Risk stratification prior to genetic testing identified 20 average-risk patients, 13 moderate-risk patients and 4 high-risk patients. Of the 4 high-risk patients, 3 (75%) were BRCA2 carriers. One moderate-risk patient was found to carry a BRCA2 mutation. A total of 3/20 (15%) average risk patients were found to have BRCA1 mutations. All BRCA1/2 mutation carriers were re-classified into a high-risk screening protocol.
Clinical Findings of Cancer Screening and Prevention in BRCA1/2 Carriers
The overall diagnostic yield of multi-organ cancer baseline screening and prevention interventions in BRCA1 and BRCA2 carriers was 7/20, or 35%. Two of 6 (33.3%) BRCA1 mutation carriers had pertinent findings on exploratory surgery undertaken for abnormal pancreas findings on clinical screening, or on prophylactic surgery undertaken as a result of positive genetic testing for BRCA1. One subject was found to have a pancreatic mucinous adenocarcinoma on initial examination, and another subject had an ovarian papillary carcinoma on prophylactic total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH-BSO). Five of 14 (35.7%) BRCA2 mutation carriers had relevant cancer screening findings. These included a 21 mm PDAC in the head of the pancreas, a patient with a 5 mm dilated pancreatic duct in the head of the pancreas, 3 patients with IPMNs including one patient with both a 4 × 6 mm IPMN and a tubal intraepithelial carcinoma found on prophylactic TAH-BSO. One BRCA2 carrier was not recommended to have PDAC screening at the time of analysis due to young age. The remaining BRCA1 and BRCA2 carriers had normal pancreatic, breast and ovarian examinations. In this small sample size, no differences were noted in age of initial screening, tobacco or alcohol history, family history of PDAC or other BRCA-related malignancies between subjects with pancreatic or other findings, and those with normal screening examinations. No adverse events were noted as a result of screening, including adverse reactions to MRI contrast dye, complications of anesthesia during endoscopy, or other complications of the endoscopy itself. Psychosocial impact on patients was not assessed; however, no obvious deleterious effects were noted.
Discussion
Genetic testing for BRCA1 and BRCA2 mutations in both an unaffected population at risk of PDAC and an affected PDAC population demonstrated a high diagnostic yield of 18.9% and 21.8%, respectively. In the Ashkenazi Jewish population, the diagnostic yield for BRCA1 and BRCA2 testing was 18.8% in the unaffected PDAC screening population and 19.2% in the affected PDAC population. In this single-center experience, BRCA2 mutations were more prevalent in the high- and moderate-risk PDAC screening cohort (4/17 or 23.5%), while BRCA1 mutations were demonstrated only in the average-risk cohort (3/20, or 15.0%).
Genetic testing of both our screening and PDAC cohorts revealed 5 BRCA1 and 9 BRCA2 mutations, indicating that genetic testing for both BRCA1 and BRCA2 genes should be performed together in these patients. The screening and cancer prevention strategy was undertaken with the understanding that germline mutation carriers are predisposed to cancer in multiple organs, synchronously and bilaterally, (i.e. breast, ovarian and PDAC) for all patients found to carry BRCA1/2 germline mutations; 3 BRCA1 carriers would not have received any multi-organ cancer screening, and 1 BRCA2 carrier would have received only PDAC screening based on family history alone. Screening and prophylactic surgery in these patients revealed a PDAC and ovarian cancer that would have otherwise been undiagnosed.
The overall diagnostic yield of multi-organ cancer screening and preventive interventions of BRCA1 and BRCA2 carriers was high (35%). It is at present too early to determine the long range clinical consequences of PDAC screening in this patient population. The BRCA2 carrier diagnosed with PDAC on screening is alive and free of disease 7 years after diagnosis. However, the BRCA1 carrier diagnosed with PDAC died shortly after diagnosis, and entrance into our program likely did not alter her overall morbidity and mortality. Positive BRCA1/2 testing allowed family members to make informed decisions regarding their PDAC, breast and ovarian cancer screening and management options. Recent studies have demonstrated that some BRCA1/2 positive PDAC patients may benefit from treatment with poly ADP ribose polymerase (PARP) inhibitors29, 30, and our group has described bi-allelic loss of both BRCA1 and BRCA2 in PDAC patients with germline BRCA1/2 mutations13.
The clinical consequences of identifying PanIN lesions, IPMNs, and a dilated pancreatic duct in other BRCA1/2 mutation carriers remain unknown at this time. All interventions were undertaken only after input and advice from a multi-disciplinary team, and were always based on risk-stratification and clinical circumstance of which genetic testing was only one aspect. It is unknown if PDAC screening will ultimately affect the incidence of PDAC diagnosis, stage at diagnosis, and overall morbidity and mortality from the disease. Interventions undertaken based on abnormal pancreas imaging may ultimately be found to be unnecessary and unadvisable; however, all interventions were performed in a research setting with the ultimate goal of elucidating the efficacy of pancreas cancer screening and prevention. The two ovarian cancer patients remain alive and disease free, suggesting that a comprehensive approach to cancer screening and prevention may favorably impact overall survival. No adverse events were noted as a result of PDAC screening or prophylactic or preventative surgery. Our present investigation is an attempt to determine whether genetic testing can better stratify at-risk individuals and avoid unnecessary screening examinations and interventions in some patients.
PDAC patients were initially referred for genetic testing due to a young age of diagnosis or a family history of breast, ovarian and pancreatic cancers. New York City has a large Ashkenazi Jewish patient population and as the center's genetic testing experience grew, more patients were also referred for genetic testing if they were of Ashkenazi Jewish descent, regardless of age at diagnosis or a family history of breast or ovarian cancers. These referral patterns for BRCA1/2 testing may have introduced selection bias into the cohort, as a large proportion of our PDAC population was young and Ashkenazi Jewish. The utility of genetic testing in a non-Ashkenazi Jewish population requires further study. BRCA1/2 carriers with PDAC were diagnosed at a mean age of 54.1 years, younger, but not significantly different than the mean age of diagnosis for sporadic PDAC. Further study is required to determine if there is a difference in PDAC age of diagnosis between carriers and non-carriers. The absence of first- and second-degree family members with PDAC in the BRCA1/2 cancer cohort is particularly notable, as current PDAC consensus guidelines recommend only screening BRCA2 carriers with either a first degree relative with PDAC, or two non-first degree relatives with PDAC27.
Our study has several important limitations. Many average risk patients in our cohort had a family history of PDAC, but this PDAC was diagnosed at >55 years. Therefore, our average risk cohort may not be representative of a true average risk population, and is likely at a mildly elevated risk of PDAC. The majority of both our screening and PDAC cohorts are Ashkenazi Jewish; therefore these results may largely be applicable to an Ashkenazi Jewish population and may not be broadly generalizable to a non-Ashkenazi Jewish PDAC screening population. We additionally report only the findings on initial screening examination, and cannot fully assess the impact of PDAC screening on disease morbidity and mortality with limited follow-up. We do not have an assessment of the psychosocial impact of PDAC screening and genetic testing in this situation.
In summary, we demonstrate a high diagnostic yield of 18.9% for BRCA1/2 testing in a PDAC prevention and genetics program. While positive BRCA1/2 genetic testing was more frequently demonstrated in the high-risk cohort, BRCA1/2 mutations were identified in average- and moderate-risk individuals suggesting that genetic testing may be a useful adjunct in PDAC risk assessment, even without a strong family history of PDAC, breast cancer or ovarian cancer. Notably, 15% of our average-risk screening population carried a BRCA1 germline mutation. Genetic testing is a useful part of PDAC risk stratification and may alter screening recommendations for mutation positive patients and their families.
A diagnostic yield of 21.9% was demonstrated in a PDAC patient population referred for genetic testing. BRCA1 and BRCA2 mutations were both noted in the PDAC patient cohort referred for genetic testing. Those who tested positive for BRCA1/2 mutations had a trend towards a younger age at PDAC diagnosis. There was a notable absence of PDAC and few BRCA related cancers in first degree relatives of BRCA1/2 carriers. Clinical BRCA1 and BRCA2 testing should be considered in Ashkenazi Jewish patients with a personal or family history of PDAC, even in the absence of a family history of breast and ovarian cancer. Further studies are warranted to understand the role of BRCA1 and BRCA2 mutations in the development of PDAC, and to determine how to implement BRCA1 and BRCA2 testing in a PDAC screening population.
Acknowledgments
SUPPORT: American College of Gastroenterology Clinical Research Award ACG-CR-070-2012, PI: Aimee Lucas
Hirshberg Foundation, PI: Harold Frucht
During the study period, Dr. Lucas received salary and educational support from NIH T32 DK083256 (PI: Timothy C. Wang, Trainee: Aimee L. Lucas) and NIH UL1 TR000040 (PI: Henry Ginsburg, Trainee: Aimee L. Lucas)
Footnotes
Author Contributions: All authors 1) made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; 2) were involved in drafting the article or revising it critically for important intellectual content; and 3) have approved the final version.
The authors indicate no relevant financial disclosures.
References
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Klein AP, Hruban RH, Brune KA, et al. Familial pancreatic cancer. Cancer J. 2001;7:266–73. [PubMed] [Google Scholar]
- 3.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442–6. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 4.Giardiello F, Brensinger J, Tersmette A, et al. Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000;119:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 5.Vasen HF, Wasser M, van Mil A, et al. Magnetic resonance imaging surveillance detects early-stage pancreatic cancer in carriers of a p16-Leiden mutation. Gastroenterology. 2011;140:850–6. doi: 10.1053/j.gastro.2010.11.048. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein AM. Familial melanoma, pancreatic cancer and germline CDKN2A mutations. Hum Mutat. 2004;23:630. doi: 10.1002/humu.9247. [DOI] [PubMed] [Google Scholar]
- 7.Vinarsky V, Fine RL, Assaad A, et al. Head and neck squamous cell carcinoma in FAMMM syndrome. Head & Neck. 2009;31:1524–1527. doi: 10.1002/hed.21050. [DOI] [PubMed] [Google Scholar]
- 8.Lynch HT, Fusaro RM, Lynch JF, et al. Pancreatic cancer and the FAMMM syndrome. Fam Cancer. 2008;7:103–12. doi: 10.1007/s10689-007-9166-4. [DOI] [PubMed] [Google Scholar]
- 9.Vasen HF, Gruis NA, Frants RR, et al. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-Leiden) Int J Cancer. 2000;87:809–11. [PubMed] [Google Scholar]
- 10.Lynch HT, Voorhees GJ, Lanspa SJ, et al. Pancreatic carcinoma and hereditary nonpolyposis colorectal cancer: a family study. Br J Cancer. 1985;52:271–3. doi: 10.1038/bjc.1985.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst. 2004;96:261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kastrinos F, Mukherjee B, Tayob N, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302:1790–5. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lucas AL, Shakya R, Lipsyc MD, et al. High Prevalence of BRCA1 and BRCA2 Germline Mutations With Loss of Heterozygosity In a Series of Resected Pancreatic Adenocarcinoma and Other Neoplastic Lesions. Clin Cancer Res. 2013;19:3396–3403. doi: 10.1158/1078-0432.CCR-12-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hahn SA, Greenhalf B, Ellis I, et al. BRCA2 germline mutations in familial pancreatic carcinoma. Gastroenterology. 2003;124:A548–A548. doi: 10.1093/jnci/95.3.214. [DOI] [PubMed] [Google Scholar]
- 15.Murphy KM, Brune KA, Griffin C, et al. Evaluation of candidate genes MAP2K4, MADH4, ACVR1B, and BRCA2 in familial pancreatic cancer: deleterious BRCA2 mutations in 17% Cancer Res. 2002;62:3789–93. [PubMed] [Google Scholar]
- 16.Couch FJ, Johnson MR, Rabe KG, et al. The prevalence of BRCA2 mutations in familial pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:342–6. doi: 10.1158/1055-9965.EPI-06-0783. [DOI] [PubMed] [Google Scholar]
- 17.Goggins M, Schutte M, Lu J, et al. Germline BRCA2 gene mutations in patients with apparently sporadic pancreatic carcinomas. Cancer Research. 1996;56:5360–5364. [PubMed] [Google Scholar]
- 18.Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. J Natl Cancer Inst. 2002;94:1358–65. doi: 10.1093/jnci/94.18.1358. [DOI] [PubMed] [Google Scholar]
- 19.Ferrone CR, Levine DA, Tang LH, et al. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol. 2009;27:433–8. doi: 10.1200/JCO.2008.18.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Sukhni W, Rothenmund H, Borgida AE, et al. Germline BRCA1 mutations predispose to pancreatic adenocarcinoma. Hum Genet. 2008;124:271–8. doi: 10.1007/s00439-008-0554-0. [DOI] [PubMed] [Google Scholar]
- 21.Axilbund JE, Argani P, Kamiyama M, et al. Absence of germline BRCA1 mutations in familial pancreatic cancer patients. Cancer Biol Ther. 2009;8:131–5. doi: 10.4161/cbt.8.2.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verna EC, Hwang C, Stevens PD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res. 2010;16:5028–37. doi: 10.1158/1078-0432.CCR-09-3209. [DOI] [PubMed] [Google Scholar]
- 23.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol. 2006;4:766–81. doi: 10.1016/j.cgh.2006.02.005. quiz 665. [DOI] [PubMed] [Google Scholar]
- 24.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. quiz e14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175–81. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 26.Brentnall TA, Bronner MP, Byrd DR, et al. Early diagnosis and treatment of pancreatic dysplasia in patients with a family history of pancreatic cancer. Ann Intern Med. 1999;131:247–55. doi: 10.7326/0003-4819-131-4-199908170-00003. [DOI] [PubMed] [Google Scholar]
- 27.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2012 doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol. 2003;21:2397–406. doi: 10.1200/JCO.2003.03.189. [DOI] [PubMed] [Google Scholar]
- 29.Bryant HE, Schultz N, Thomas HD, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 30.Tutt A, Robson M, Garber JE, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–44. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]