Abstract
Secretion of granular glands from the skin of amphibians is a fascinating resource of active substances, particularly for cancer therapy in clinical practice of Traditional Chinese Medicine. A variety of anti-tumor peptides have been isolated from different toads and frogs; however, no anti-tumor peptides are reported in toad venom of Bufo gargarizans. Firstly, soluble fraction from fresh toad venom (FTV) was compared with that from dried toad venom (DTV), using HPLC analysis. It was revealed that FTV has a different HPLC chromatography compared with DTV. Soluble fraction of FTV is more toxic to SH-SY5Y cells than that of DTV, as evaluated by MTT assay. Secondly, it was identified that protein components from soluble fractions of FTV and DTV possess different patterns by SDS-PAGE analysis, and proteins from FTV are also more toxic than that from DTV. Thirdly, an immobilized basic fibroblast growth factor (bFGF) affinity column was used to isolate bFGF-binding components from soluble fraction of FTV, and it was identified that bFGF-binding components prohibited bFGF-dependent neurite growth of SH-SY5Y cells. Finally, it was identified that bFGF-binding components activated apoptosis via upregulation of caspase-3 and caspase-8 expression in SH-SY5Y cells. These data suggest that FTV contains active components that interact with bFGF and activate apoptosis in SH-SY5Y cells.
Keywords: toad venom, bFGF-binding component, cytotoxicity, apoptosis, SH-SY5Y cells
Introduction
As a traditional Chinese medicine (TCM), the dried secretion from skin glands of Bufo gargarizans (toad venom) has been used in the treatment of various types of inflammation in China for thousands of years (1). Previous studies reported that toad venom has an activity in tumor inhibition. For instance, extraction of toad venom inhibited the growth of non-small cell lung cancer (2,3). Active components of toad venom, including telocinobufagin, marinobufagin, bufalin, bufotalin and resibufogenin, were reported to exhibit a tumor-inhibiting activity (4–6). Bufalin and cardiotonic steroid, isolated from toad venom, were identified to cause apoptosis in human prostate and breast cancer cells by upregulating the expression of caspase family genes in tumor cells (7,8). Bufalin can also suppress cell proliferation and inhibit the migration and invasion of tumor cells, although the molecular mechanisms remain unknown (6,9,10). By contrast, arenobufagin, another major component of toad venom, was reported as a specific inhibitor of VEGF-mediated angiogenesis (6).
Compared with the anti-tumor activity of the aforementioned small molecules from toad venom of B. gargarizans, studies on the anti-tumor activity of skin secretion from other amphibians are focused on peptides that are considered to be more sensitive and/or specific than small molecules (11–14). In previous studies, a large number of anti-tumor peptides were reported from different toads and frogs (15–17). Peptides from the magainin family were isolated from African clawed frog (Xenopus laevis) skin (18,19). Maganin 1 and maganin 2, two peptides with 23 amino acid residues, were reported to inhibit cell proliferation in NCI-H82 and NCI-H526 cells at a low dose (19,20). Since maganin 2 has a high specific cytotoxicity, its derivative via amino acid modification has been approved as a novel medicine in cancer treatment (21,22). In addition, maximin and bombinin families, identified in Bombina maxima and Bombina orientalis, were reported to inhibit the proliferation of tumor cells (23).
However, the active peptides of toad venom from B. gargarizans are unknown since toad venom is always prepared into the dried form from secretion of toad, a procedure that possibly denatures or damages active proteins and peptides. The identification and detection of possible anti-tumor peptides from skin gland secretions of B. gargarizans remains a challenge, and has not been studied. In the present study, protein components and their anti-tumor activity of fresh toad venom (FTV) were compared with those of dried toad venom (DTV). Furthermore, basic fibroblast growth factor (bFGF), one of the growth factors involved in angiogenesis in cancer metastasis (24–26), was selected as a biomarker for cancers to investigate protein components from toad venom. A bFGF-immobilized affinity column was used to capture active components that can interact with bFGF. The bFGF can inhibit the apoptosis induced by oridonin in L929 tumor cells (26), and cause tumor migration by regulating several pathways (27,28). The mechanism of anti-tumor activity of active components was preliminarily studied.
Materials and methods
Collection and treatment of toad venom
FTV was collected from the Laboratory Animal Center at Zhejiang University (Hangzhou, China) according to the method recommended in Pharmacopoeia of People's Republic of China (1). To prepare soluble fraction of toad venom, 0.6 g of FTV was extracted with 10 ml PBS (0.2 M, pH 7.2) in a 15 ml tube. The tube was incubated at 16°C for 4 h on an agitator at 210 rpm and followed by centrifugation of 12,000 × g at 4°C for 15 min. Supernatant was collected as soluble fraction of FTV. Another 0.6 g of FTV was baked at 37°C for 24 h to prepare DTV. The soluble fraction of DTV was prepared using the method described for the preparation of the soluble fraction of FTV.
Cell culture
Human SH-SY5Y-EGFP cells were purchased from Hangzhou Neuropeptide Biological Science and Technology, Inc., Ltd. (NUPTEC; Hangzhou, China) and maintained as described previously (29) with a little modification. Human SH-SY5Y, Hep G2 and HUVEC cells were purchased from Type Cell Culture Collection of the Chinese Academy of Sciences (Beijing, China). HUVEC cells were cultured in endothelial cell medium (ECM) medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) while other cells were cultured in DMEM (HyClone; GE Healthcare Life Sciences). The two mediums were supplemented with 10% fetal bovine serum (FBS) (FBS; HyClone; GE Healthcare Life Sciences) and 0.3% penicillin-streptomycin solution (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany), at 37°C under a 5% CO2 and 95% air humidified atmosphere in a CO2 cell incubator (Sanyo, Moniguchi, Osaka, Japan). The medium was replaced every other day.
High-pressure liquid chromatography (HPLC) analysis
HPLC analysis of samples was performed on an Agilent 1260 Liquid Chromatographer (Agilent, Santa Clara, CA, USA). Soluble fractions from FTV and DTV were filtrated by a 0.2 µm filter (Axygen; Corning, Inc., Corning, NY, USA) and 30 µl of each filtrated sample was loaded onto an Agilent reverse phase (RP) C8 column (Agilent) and eluted at a flow rate of 1 ml/min in a gradient of 5% buffer B (100% acetonitrile) (Scharlab, Barcelona, Spain) vs. 90% buffer A (0.1% H3PO4 in water) for 10 min. Components in eluates were detected at 214 nm.
Isolation of total proteins from soluble fraction of toad venom
Soluble fractions of FTV and DTV stored at −80°C freezer were incubated on ice until they were completely thawed. In total, 24 ml of pre-cold acetone at −80°C was mixed with 6 ml of thawed soluble fractions of FTV or DTV. The mixtures were centrifuged at 4°C, 13,400 × g for 10 min. The supernatant was discarded, and subsequently, pelleted proteins were re-dissolved in 6 ml of double distilled water.
Determination of cytotoxicity
Human SH-SY5Y-EGFP cells were seeded in 96-well plates (Corning, Inc.) at an initial density of 1.0×104 cells per well and incubated at 37°C for 24 h. Cells were treated with soluble fraction of FTV and DTV, and their isolated proteins were prepared from 0, 0.1625, 0.325, 0.75, 1.5, 3 and 6 mg toad venom wt/ml at 37°C for 24 h. The MTT Cell Proliferation and Cytotoxicity Assay kit (Sangon; Biotech, Co., Ltd., Shanghai, China) was used to determine cytotoxicity. MTT, whose reducing capacity indicates cellular activity, was added to each well and incubated at 37°C for 4 h, according to the manufacturer's protocol. Formazan solution was added to each well to resolve MTT formazan crystals. Absorbance at 490 nm, indicating cellular activity, was determined on a Mustiskan FC scanning multi-well spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Protein analysis
For SDS-PAGE analysis, protein samples were prepared as aforementioned. The soluble fraction of FTV or DTV (10 µl) were mixed with 5 µl 5X SDS PAGE sample loading buffers (Sangon, Biotech, Co., Ltd.) and mixtures were boiled for 5 min. The samples were then loaded onto a 12% SDS-PAGE gel and electrophoresed for 40 min at 110V. Proteins on the gel were detected by Coomassie brilliant blue R250 staining (Ourchem, Shanghai, China).
Identification of active components
A bFGF-immobilized affinity column (NUPTEC) was used to identify active peptides from the soluble fraction of toad venom. In total, 20 ml soluble fraction of toad venom was loaded to a bFGF affinity column and the same volume of sample was loaded to a human serum albumin (HSA) affinity column (NUPTEC) as a control. Subsequent to loading, the column was eluted by 5 column volumes of 50 mM Tris-HCl buffer (pH 7.2) and 50 mM PBS (pH 6.0) in order, followed by elution of 3 column volumes of 100 mM glycine buffer (pH 3.0) for active components. To identify active components, an Agilent 1260 HPLC system was used to detect absorbance at 214 nm. Collected eluates from affinity columns were loaded onto an Agilent C-8 column and eluted at the flow rate of 1 ml/min using a 3% buffer B (100% acetonitrile) against a 97% buffer A (0.1% H3PO4 in water) for 10 min.
bFGF-inhibiting assay of bFGF-binding components
SH-SY5Y cells (1×104) were plated into 96-well plates in triplicate and incubated at 37°C in a 5% CO2 and 95% air humidified atmosphere in a CO2 cell incubator for 24 h, followed by treatment with a final concentration of 1 µM bFGF (NUPTEC). The bFGF-binding component was added to medium at various doses of 0, 5, 25, 50 and 100 µg/ml (final concentration) along with bFGF. Morphology of the cells was visualized using a Leica DM3000B microscope (Leica Microsystems GmbH, Wetzlar, Germany; magnification, ×200) and images were captured using LAS software (version 4.2; Leica Microsystems GmbH) and the length of 150 neurites was measured randomly for each sample at day 1, 2, 3, 4 and 5, respectively.
Cytotoxicity assay of bFGF-binding components
SH-SY5Y, Hep G2 and HUVEC cells (1×104) were plated into 96-well plates in triplicate and incubated at 37°C for 24 h as aforementioned. SH-SY5Y and Hep G2 cells were cultured in DMEM while HUVEC cells were cultured in ECM. Cells were then treated with bFGF-binding component at various doses of 0, 5, 25, 50 and 100 µg/ml (final concentration), respectively. Cell viability was determined using the MTT Cell Proliferation and Cytotoxicity Assay kit after 24 h treatment.
Reverse transcription-polymerase chain reaction (RT-PCR) assay
Gene expression of caspase-3 and caspase-8 was detected by RT-PCR analysis. SH-SY5Y cells were plated into a 6-well plate (Corning, Inc.) at an initial density of 1.0×105 cells/well. After 24 h, cells were treated with bFGF-binding component at various doses of 0, 5, 25 and 50 µg/ml (final concentration). Total RNAs were extracted by TRIzol reagent (Thermo Fisher Scientific, Inc.) and reverse-transcribed into cDNAs by FastQuant RT kit (Tiangen Biotech, Beijing, China), according to the manufacturer's protocol. Primers for caspase-3 (accession no. BC016926; www.ncbi.nlm.nih.gov), caspase-8 (accession no. AH007578; www.ncbi.nlm.nih.gov) and β-actin (accession no. DQ407611.1; www.ncbi.nlm.nih.gov) were designed and synthesized by Sangon (Biotech, Co., Ltd.) for RT PCR analysis (Table I). The PCRs were performed at 95°C for 2 min, followed by 25 cycles of denaturing at 95°C for 15 sec, annealing at 61°C for 15 sec and extending at 72°C for 45 sec. The PCR products were analyzed on 1% agarose gels and signal intensities were quantified by Quantity One (version 4.5; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Table I.
Primers used in reverse transcription-polymerase chain reaction.
| Primers | Sequences |
|---|---|
| Caspase-3 | |
| Forward | CTGGACTGCGGTATTGAGAC |
| Reverse | CCGGGTGCGGTAGAGTAAGC |
| Caspase-8 | |
| Forward | CTGCAGAGGGAACCTGGTACATCC |
| Reverse | CATCAATCAGAAGGGAAGA |
| β-actin | |
| Forward | GATCATTGCTCCTCCTGAG |
| Reverse | ACTCCTGCTTGCTGATCCA |
Western blot analysis
SH-SY5Y cells were cultured and treated as aforementioned. After 24 h, cells were collected with ice-cold PBS to 1.5 ml centrifuge tubes, and centrifuged at 4°C and 200 × g for 5 min. The supernatant was discarded and collected cells were immediately lysed with lysis buffer (20 mM sucrose, 1 mM EDTA, 20 µM Tris-HCl, pH 7.2, 1 mM DTT, 10 mM KCl, 1.5 mM MgCl2, containing 1 mM phenylmethanesulfonyl fluoride, PMSF). Lysed cells were then centrifuged at 4°C, 13,400 × g for 10 min and total protein was collected. Protein concentrations were determined to normalize different samples using Pierce BCA Protein kit (Thermo Fisher Scientific, Inc.), following the instructions. Total proteins were then loaded to 12% SDS-PAGE gel and electrophoresed for 40 min at 110V and then transferred to a polyvinylidene fluoride (PVDF) membrane (Bio-Rad Laboratories, Inc.). The membrane was then blocked with 5% skim milk, and incubated with the anti-caspase-3 (cat. no. ER30804; 1:2,000) or anti-caspase-8 (cat. no. ET1603-16; 1:2,000) primary antibodies (Hua'an Biotech, Hangzhou, China), and goat anti-rabbit IgG secondary antibodies (HRP-conjugated; cat. no. HA1001-100; 1:2,000; Hua'an Biotech), respectively. The target proteins were visualized using an enhanced chemiluminescence detection system (Tiangen Biotech).
Statistical analysis
Significance of differences between treatments and control were analyzed by analysis of variance using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) and considered significant at P<0.05.
Results
Different pattern of components from FTV and DTV
By HPLC analysis, no significant differences in soluble fractions were identified between FTV and DTV on chromatography beyond 15 min retention time, although there were a number of chromatographic peaks showing difference in peak area (Fig. 1). However, there was an apparent difference between FTV and DTV within the first 15 min retention time. Particularly, FTV had three much larger peaks than DTV, and one large peak that was not observed in DTV within the first 5 min retention time (Fig. 1), indicating that FTV had more polar molecules than DTV.
Figure 1.
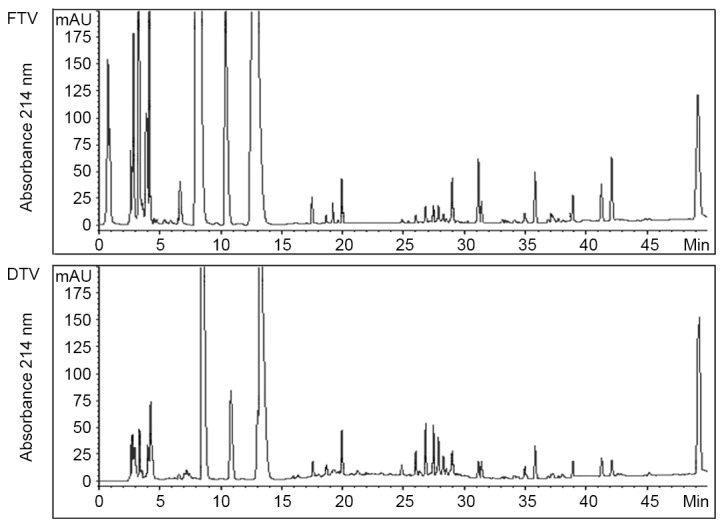
High performance liquid chromatography analysis of soluble fractions from FTV and DTV. Data show one of similar results from two independent experiments. FTV, fresh toad venom; DTV, dried toad venom.
Different cytotoxicity of components from FTV and DTV
Based on different patterns of components from FTV and DTV (Fig. 1), cytotoxicity of soluble fractions from FTV and DTV was compared in human SH-SY5Y cells to establish a link between HPLC chromatography and cytotoxicity. MTT assay demonstrated that cell survival decreased in SH-SY5Y cells treated by soluble fractions from FTV and DTV in a dose-dependent manner, indicating the cytotoxicity of FTV and DTV. However, FTV had a more significant cytotoxicity compared with DTV (P=0.15, 0.0089, 0.0024, 0.0019, 0.0056, 0.0027 and 0.0014 at concentrations 0, 0.1625, 0.325, 0.75, 1.5, 3 and 6 mg/ml, respectively; Fig. 2A). Alternatively, the cytotoxicity was also compared in EGFP-expressing SH-SY5Y cells. As fluorescence-imaged in Fig. 2B, FTV and DTV demonstrated cytotoxicity as compared with controls, however, FTV was more cytotoxic compared with DTV.
Figure 2.
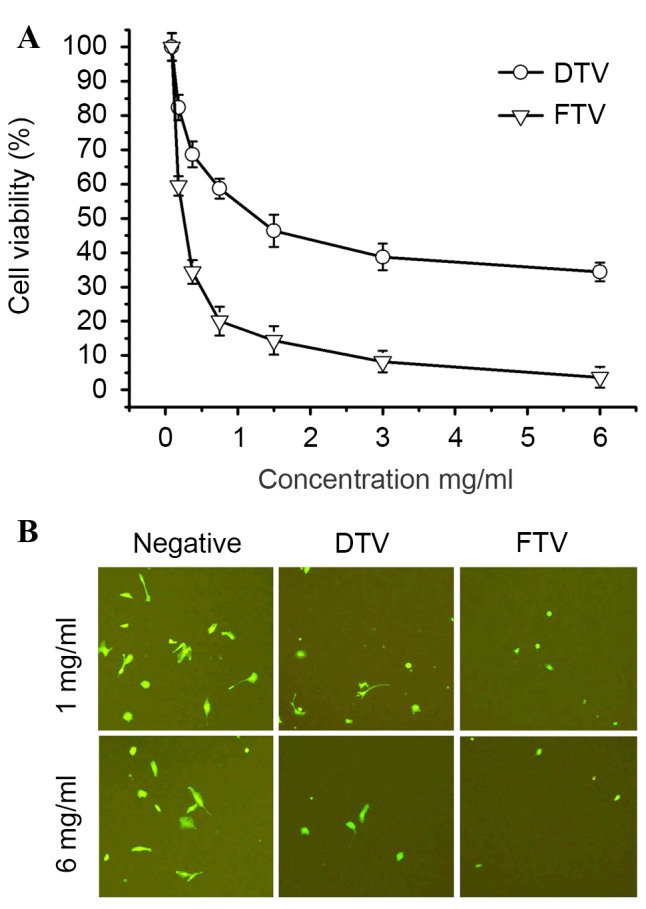
Cytotoxicity of soluble fractions from FTV and DTV. (A) Cytotoxicity of FTV and DTV in human SH-SY5Y cells determined by MTT assay. Data show the mean ± standard error from three independent experiments. (B) Cytotoxicity of FTV and DTV in EGFP-expressing SH-SY5Y cells imaged by an inverted fluorescence microscope. Data show one of similar results from three independent experiments. Scale bar, 200 µm. FTV, fresh toad venom; DTV, dried toad venom.
Protein components of toad venom and their cytotoxicity
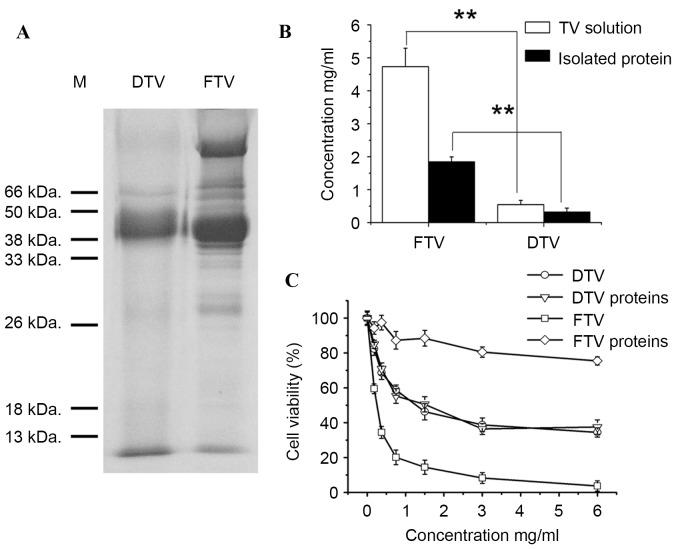
According to HPLC analysis of soluble fractions of toad venom, the main difference between FTV and DTV was polar molecular components (Fig. 1). Water-soluble protein/peptide is one type of highly polar biomolecules. Thus, water-soluble protein patterns were compared between FTV and DTV by SDS-PAGE analysis. As shown in Fig. 3A, FTV had more water-soluble protein bands than DTV, consistent with the evidence that FTV had more and larger peaks on HPLC chromatography compared with DTV within the first 5 min of retention time (Fig. 1). Subsequent protein analysis demonstrated that soluble fraction of FTV has a significantly higher protein level than DTV (P=0.0025 and P=0.0064, respectively; Fig. 3B).
Figure 3.
Cytotoxicity of water-soluble proteins isolated from FTV and DTV. (A) SDS-PAGE analysis of water-soluble proteins from FTV and DTV. Data show one of similar results from two independent experiments. (B) Content of total water-soluble proteins from FTV and DTV. (C) Cytotoxicity of water-soluble proteins isolated from FTV and DTV. Data show mean ± standard error from three independent experiments. **P<0.01. M, marker; TV, toad venom; FTV, fresh toad venom; DTV, dried toad venom.
Furthermore, to determine whether difference in water-soluble protein component of toad venom contributed to the cytotoxicity, protein fractions were isolated from soluble fractions of FTV and DTV, and the cytotoxicity was investigated in human SH-SY5Y cells. Consistent with the aforementioned results that FTV demonstrated a higher cytotoxicity than DTV (Fig. 2), FTV was more toxic to SH-SY5Y cells than DTV (P=0.21, 0.0072, 0.0055, 0.0031, 0.0021, 0.0051 and 0.0060 and concentrations 0, 0.1625, 0.325, 0.75, 1.5, 3 and 6 mg/ml, respectively; Fig. 3C). In parallel to this observation, the protein fraction of FTV (FTV proteins) was also more toxic to cells than the protein fraction of DTV (DTV proteins) (P=0.21, 0.0072, 0.0055, 0.0031, 0.0021, 0.0051 and 0.0060 and 0, 0.1625, 0.325, 0.75, 1.5, 3 and 6 mg/ml, respectively), and notably, the latter revealed only slight toxicity (Fig. 3C), suggesting that protein fraction is involved in the cytotoxicity of toad venom and toxic proteins only retained in FTV.
Identification of bFGF-binding components from FTV
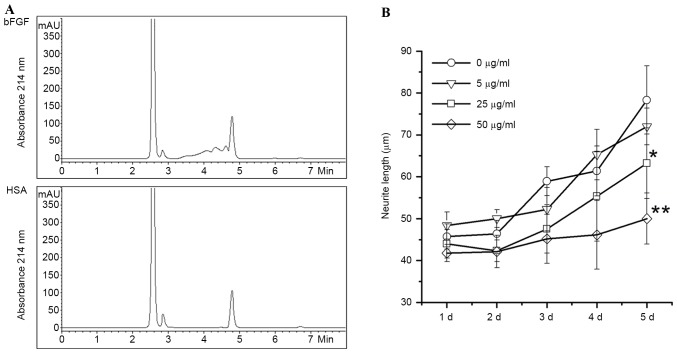
bFGF is one of the biomarkers in angiogenesis for cancer metastasis. In order to identify anti-tumor components from FTV, a bFGF-immobilized affinity column was used to capture bFGF-binding components from FTV proteins, followed by identification of HPLC analysis. As shown in Fig. 4A, the bFGF-affinity column captured three specific peaks identified by HPLC chromatography, but the HSA-affinity control column did not.
Figure 4.
High performance liquid chromatography analysis and activity of bFGF-binding peptides from FTV. (A) Three chromatographic peaks were identified from eluted components of bFGF-affinity column loaded with the soluble fraction from FTV. Elute from HSA-affinity column loaded with soluble fraction from FTV was used as a negative control. Data show one of similar results from two independent experiments. (B) bFGF-induced neurite outgrowth of SH-SY5Y was inhibited by bFGF-binding components extracted from FTV. *P<0.05 vs. 0 µg/ml (P=0.028), **P<0.01 vs. 0 µg/ml (P=0.0081). FTV, fresh toad venom; HAS, human serum albumin; bFGF, basic fibroblast growth factor.
To verify the activity of bFGF-binding components of FTV, eluates from the bFGF-affinity column were used to prohibit bFGF-induced neurite outgrowth of SH-SY5Y cells. Data revealed that the neurite outgrowth of human SH-SY5Y cells induced by bFGF is significantly prohibited by bFGF-binding components from FTV in a dose-dependent manner compared with the control (P=0.028 and 0.0081 at concentrations 25 and 50 µg/ml, respectively; Fig. 4B).
Apoptosis induced by bFGF-binding components
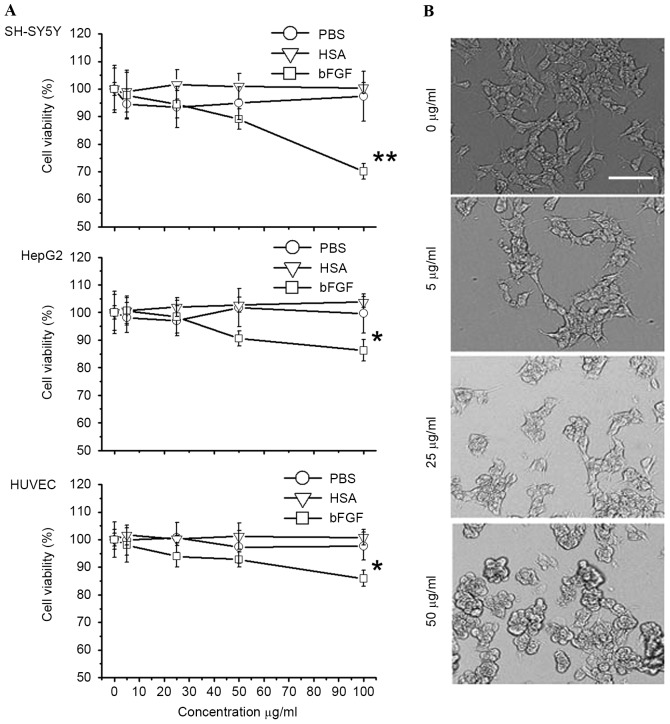
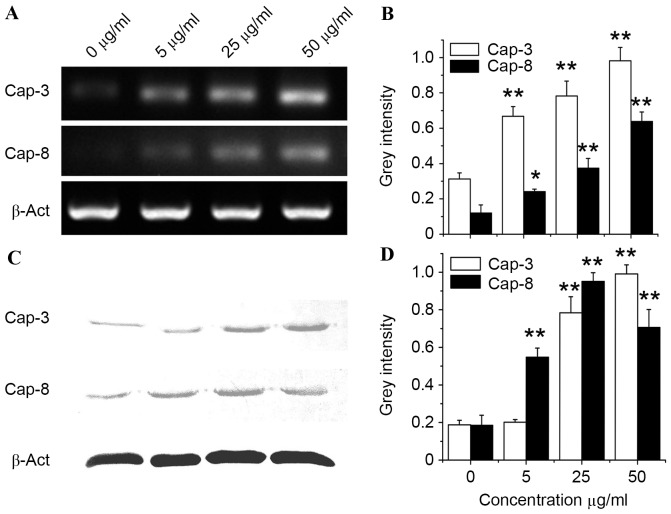
In the present study, the cytotoxicity of bFGF-binding components to human SH-SY5Y, Hep G2 and HUVEC cells was shown to be specific (P=0.0014, 0.031 and 0.035 for SH-SY5Y, HepG2 and HUVEC cells, respectively), since PBS and HSA-binding components did not show cytotoxicity (Fig. 5A). Particularly in the SH-SY5Y cells, the morphology of cells was greatly affected following treatment with bFGF-binding components (Fig. 5B). To further investigate the mechanism of cytotoxicity induced by bFGF-binding components, gene and protein expression of caspase-3 and caspase-8 (two biomarkers for apoptosis) was studied by analysis of mRNA and protein level in SH-SY5Y cells treated with bFGF-binding components. Data from RT-PCR, as well as western blot analysis, revealed that gene (P=0.0075, 0.0043 and 0.0031 at concentrations 5, 25 and 50 mg/ml, respectively, vs. the Cap-3 control; P=0.022, 0.0077 and 0.0059 at concentrations 5, 25 and 50 mg/ml, respectively, vs. the Cap-8 control) and protein (P=0.13, 0.0037 and 0.0023 at concentrations 5, 25 and 50 mg/ml, respectively, vs. the Cap-3 control; P=0.0090, 0.0041 and 0.0055 at concentrations 5, 25 and 50 mg/ml, respectively, vs. the Cap-8 control) expression of caspase-3 and caspase-8 were significantly upregulated by bFGF-binding components in a dose-dependent manner (Fig. 6), suggesting that bFGF-binding components can induce cytotoxicity, possibly via activation of apoptosis in human SH-SY5Y cells.
Figure 5.
Viability of human SH-SY5Y cells reduced by bFGF-binding peptides. (A) Death of SH-SY5Y, Hep G2 and HUVEC cells was induced by bFGF-binding peptides extracted from FTV. (B) Morphology of SH-SY5Y cells following treatment by bFGF-binding peptides. Data show one of similar results from three independent experiments. **P<0.01 (P=0.0014), *P<0.05 vs. 0 µg/ml bFGF-binding peptide (P=0.031 in HepG2 and P=0.035 in HUVEC cells). Data show mean ± standard error from three independent experiments.
Figure 6.
Apoptosis related molecules in SH-SY5Y treated by bFGF-binding peptides. (A) Expression of caspase-3 and caspase-8 genes was upregulated by bFGF-binding peptides extracted from FTV, as detected by RT-PCR analysis. (B) Quantification of mRNA levels of caspase-3 and caspase-8 following normalization to β-actin. (C) Expression of caspase-3 and caspase-8 proteins was upregulated by bFGF-binding peptides extracted from FTV, as detected by western blot analysis. (D) Quantification of protein levels of caspase-3 and caspase-8 following normalization to β-actin. **P<0.01 vs. control. *P<0.05 vs. control. Data show mean ± standard error from three independent experiments. Cap-3, caspase-3; Cap-8, caspase-8; β-Act, β-actin.
Discussion
Anti-tumor peptides secreted from the skin of frogs and toads have been extensively reported and they give a promising progress in cancer research and clinical practice (14,15,19,30). However, these peptides exhibit a high diversity in different species, including Pipidae and Discoglossidae. B. gargarizans is one of the most important species of Bufonidae in China, and the majority of studies are focused on the anti-tumor activity of small molecular components (4,6,7,13). It has not been reported that this species has anti-tumor peptides secreted from skin glands. In the present study, components from B. gargarizans that contributed to the inhibition of proliferation of SH-SY5Y neuroblastoma cells were identified.
DTV has been used as a traditional medicine in China for thousands of years (1). In the present study, DTV was identified to possess fewer peptides than FTV, since treatment may be harmful for peptides when FTV is prepared for DTV. This is supported by the observation in the present study that FTV had more protein bands and components than DTV, as evidenced by HPLC and SDS-PAGE analysis. Along with the difference in protein components, FTV had more significant cytotoxicity in SH-SY5Y neuroblastoma cells than DTV and isolated proteins/peptides from FTV were more cytotoxic to tumor cells than those from DTV.
To identify active peptides from FTV, a bFGF-immobilized affinity column was used to capture bFGF-binding components from FTV protein fractions, and three potential peptides that could inhibit the proliferation of bFGF-dependent cells including HUVEC and SH-SY5Y, were identified (31,32). In parallel to the inhibition of cell proliferation, these bFGF-binding components could also upregulate the gene and protein expression of caspase-3 and caspase-8 in tumor cells, suggesting that the cytotoxicity induced by bFGF-binding components possibly came from the activation of apoptosis, and that the anti-tumor activity of bFGF-binding components isolated from FTV was contributed by inhibition of bFGF activity and down-regulation of bFGF gene expression. This is consistent with bFGF being markedly involved in tumor growth (31–33).
Acknowledgements
The present study was supported by the startup fund (grant no. 2012FR017) from Zhejiang A&F University in China, Zhejiang Provincial Natural Science Foundation (grant no. LQ14H280007) in China, and Prior project (grant no. T12B13292014) from Hangzhou Hygiene Bureau Zhejiang, China.
References
- 1.The State Pharmacopoeia Commission of PR China, corp-author. Pharmacopoeia of the People's Republic of China. 2010. Vol. 1. Chemical Industry Publishing House; Beijing: 2010. pp. 360–360. [Google Scholar]
- 2.Meng Z, Yang P, Shen Y, Bei W, Zhang Y, Ge Y, Newman RA, Cohen L, Liu L, Thornton B, et al. Pilot study of huachansu in patients with hepatocellular carcinoma, nonsmall-cell lung cancer, or pancreatic cancer. Cancer. 2009;115:5309–5318. doi: 10.1002/cncr.24602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang SJ, Zhang YT, Zhao JH, Shen LN, Shi F, Feng NP. Preparation and in vitro anti-tumor properties of toad venom extract-loaded solid lipid nanoparticles. Pharmazie. 2013;68:653–660. [PubMed] [Google Scholar]
- 4.Qiao L, Huang YF, Cao JQ, Zhou YZ, Qi XL, Pei YH. One new bufadienolide from Chinese drug ‘Chan'Su’. J Asian Nat Prod Res. 2008;10:233–237. doi: 10.1080/10286020701603146. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Ma X, Li F, Wang J, Chen H, Wang G, Lv X, Sun C, Jia J. Preparative separation and purification of bufadienolides from Chinese traditional medicine of ChanSu using high-speed counter-current chromatography. J Sep Sci. 2010;33:1325–1330. doi: 10.1002/jssc.200900782. [DOI] [PubMed] [Google Scholar]
- 6.Zhang DM, Liu JS, Deng LJ, Chen MF, Yiu A, Cao HH, Tian HY, Fung KP, Kurihara H, Pan JX, Ye WC. Arenobufagin, a natural bufadienolide from toad venom, induces apoptosis and autophagy in human hepatocellular carcinoma cells through inhibition of PI3K/Akt/mTOR pathway. Carcinogenesis. 2013;34:1331–1342. doi: 10.1093/carcin/bgt060. [DOI] [PubMed] [Google Scholar]
- 7.Zhu Z, Li E, Liu Y, Gao Y, Sun H, Wang Y, Wang Z, Liu X, Wang Q, Liu Y. Bufalin induces the apoptosis of acute promyelocytic leukemia cells via the downregulation of survivin expression. Acta Haematol. 2012;128:144–150. doi: 10.1159/000339424. [DOI] [PubMed] [Google Scholar]
- 8.Hong SH, Kim GY, Chang YC, Moon SK, Kim WJ, Choi YH. Bufalin prevents the migration and invasion of T24 bladder carcinoma cells through the inactivation of matrix metalloproteinases and modulation of tight junctions. Int J Oncol. 2013;42:277–286. doi: 10.3892/ijo.2012.1683. [DOI] [PubMed] [Google Scholar]
- 9.Chen YY, Lu HF, Hsu SC, Kuo CL, Chang SJ, Lin JJ, Wu PP, Liu JY, Lee CH, Chung JG, Chang JB. Bufalin inhibits migration and invasion in human hepatocellular carcinoma SK-Hep1 cells through the inhibitions of NF-κB and matrix metalloproteinase-2/−9-signaling pathways. Environ Toxicol. 2015;30:74–82. doi: 10.1002/tox.21896. [DOI] [PubMed] [Google Scholar]
- 10.Zhai XF, Fang FF, Liu Q, Meng YB, Guo YY, Chen Z. MiR-181a contributes to bufalin-induced apoptosis in PC-3 prostate cancer cells. BMC Complement Altern Med. 2013;13:325. doi: 10.1186/1472-6882-13-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller YE. Bombesin-like peptides: From frog skin to human lung. Am J Respir Cell Mol Biol. 1990;3:189–190. doi: 10.1165/ajrcmb/3.3.189. [DOI] [PubMed] [Google Scholar]
- 12.Simmaco M, Severini C, De Biase D, Barra D, Bossa F, Roberts JD, Melchiorri P, Erspamer V. Six novel tachykinin- and bombesin-related peptides from the skin of the Australian frog Pseudophryne guntheri. Peptides. 1990;11:299–304. doi: 10.1016/0196-9781(90)90086-K. [DOI] [PubMed] [Google Scholar]
- 13.Lee WH, Li Y, Lai R, Li S, Zhang Y, Wang W. Variety of antimicrobial peptides in the Bombina maxima toad and evidence of their rapid diversification. Eur J Immunol. 2005;35:1220–1229. doi: 10.1002/eji.200425615. [DOI] [PubMed] [Google Scholar]
- 14.King JD, Leprince J, Vaudry H, Coquet L, Jouenne T, Conlon JM. Purification and characterization of antimicrobial peptides from the Caribbean frog, Leptodactylus validus (Anura: Leptodactylidae) Peptides. 2008;29:1287–1292. doi: 10.1016/j.peptides.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Bevins CL, Zasloff M. Peptides from frog skin. Annu Rev Biochem. 1990;59:395–414. doi: 10.1146/annurev.bi.59.070190.002143. [DOI] [PubMed] [Google Scholar]
- 16.Bechinger B, Zasloff M, Opella SJ. Structure and orientation of the antibiotic peptide magainin in membranes by solid-state nuclear magnetic resonance spectroscopy. Protein Sci. 1993;2:2077–2084. doi: 10.1002/pro.5560021208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westerhoff HV, Zasloff M, Rosner JL, Hendler RW, De Waal A, Vaz Gomes A, Jongsma PM, Riethorst A, Juretić D. Functional synergism of the magainins PGLa and magainin-2 in Escherichia coli, tumor cells and liposomes. Eur J Biochem. 1995;228:257–264. doi: 10.1111/j.1432-1033.1995.00257.x. [DOI] [PubMed] [Google Scholar]
- 18.Ohsaki Y, Gazdar AF, Chen HC, Johnson BE. Antitumor activity of magainin analogues against human lung cancer cell lines. Cancer Res. 1992;52:3534–3538. [PubMed] [Google Scholar]
- 19.Baker MA, Maloy WL, Zasloff M, Jacob LS. Anticancer efficacy of Magainin2 and analogue peptides. Cancer Res. 1993;53:3052–3057. [PubMed] [Google Scholar]
- 20.Giacometti A, Ghiselli R, Cirioni O, Mocchegiani F, D'Amato G, Orlando F, Sisti V, Kamysz W, Silvestri C, Naldoski P, et al. Therapeutic efficacy of the magainin analogue MSI-78 in different intra-abdominal sepsis rat models. J Antimicrob Chemother. 2004;54:654–660. doi: 10.1093/jac/dkh390. [DOI] [PubMed] [Google Scholar]
- 21.Shin SY, Kang JH, Jang SY, Kim Y, Kim KL, Hahm KS. Effects of the hinge region of cecropin A(1–8)-magainin 2(1–12), a synthetic antimicrobial peptide, on liposomes, bacterial and tumor cells. Biochim Biophys Acta. 2000;1463:209–218. doi: 10.1016/S0005-2736(99)00210-2. [DOI] [PubMed] [Google Scholar]
- 22.Takeshima K, Chikushi A, Lee KK, Yonehara S, Matsuzaki K. Translocation of analogues of the antimicrobial peptides magainin and buforin across human cell membranes. J Biol Chem. 2003;278:1310–1315. doi: 10.1074/jbc.M208762200. [DOI] [PubMed] [Google Scholar]
- 23.Gibson BW, Tang DZ, Mandrell R, Kelly M, Spindel ER. Bombinin-like peptides with antimicrobial activity from skin secretions of the Asian toad, Bombina orientalis. J Biol Chem. 1991;266:23103–23111. [PubMed] [Google Scholar]
- 24.Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea; Proc Natl Acad Sci USA; 2002; pp. 8868–8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demirkesen C, Büyükpinarbasili N, Ramazanoğlu R, Oğuz O, Mandel NM, Kaner G. The correlation of angiogenesis with metastasis in primary cutaneous melanoma: A comparative analysis of microvessel density, expression of vascular endothelial growth factor and basic fibroblastic growth factor. Pathology. 2006;38:132–137. doi: 10.1080/00313020600557565. [DOI] [PubMed] [Google Scholar]
- 26.Huang J, Wu L, Tashiro S, Onodera S, Ikejima T. Fibroblast growth factor-2 suppresses oridonin-induced L929 apoptosis through extracellular signal-regulated kinase-dependent and phosphatidylinositol 3-kinase-independent pathway. J Pharmacol Sci. 2006;102:305–313. doi: 10.1254/jphs.FPJ06004X. [DOI] [PubMed] [Google Scholar]
- 27.Liu JF, Crepin M, Liu JM, Barritault D, Ledoux D. FGF-2 and TPA induce matrix metalloproteinase-9 secretion in MCF-7 cells through PKC activation of the Ras/ERK pathway. Biochem Biophys Res Commun. 2002;293:1174–1182. doi: 10.1016/S0006-291X(02)00350-9. [DOI] [PubMed] [Google Scholar]
- 28.Suyama K, Shapiro I, Guttman M, Hazan RB. A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell. 2002;2:301–314. doi: 10.1016/S1535-6108(02)00150-2. [DOI] [PubMed] [Google Scholar]
- 29.Qian Y, Zheng Y, Tiffany-Castiglioni E. Valproate reversibly reduces neurite outgrowth by human SY5Y neuroblastoma cells. Brain Res. 2009;1302:21–33. doi: 10.1016/j.brainres.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 30.Ferreira PM, Lima DJ, Debiasi BW, Soares BM, Kda C Machado, Jda C Noronha, Dde J Rodrigues, Sinhorin AP, Pessoa C, Vieira GM., Jr Antiproliferative activity of Rhinella marina and Rhaebo guttatus venom extracts from Southern Amazon. Toxicon. 2013;72:43–51. doi: 10.1016/j.toxicon.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Li D, Wang H, Xiang JJ, Deng N, Wang PP, Kang YL, Tao J, Xu M. Monoclonal antibodies targeting basic fibroblast growth factor inhibit the growth of B16 melanoma in vivo and in vitro. Oncol Rep. 2010;24:457–463. doi: 10.3892/or_00000879. [DOI] [PubMed] [Google Scholar]
- 32.Polec A, Fedorcsak P, Eskild A, Tanbo TG. The interplay of human chorionic gonadotropin (hCG) with basic fibroblast growth factor and adipokines on angiogenesis in vitro. Placenta. 2014;35:249–253. doi: 10.1016/j.placenta.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Xu Z, Shi H, Mei Q, Shen Y, Xu J. Effects of macrophage metalloelastase on the basic fibroblast growth factor expression and tumor angiogenesis in murine colon cancer. Dig Dis Sci. 2012;57:85–91. doi: 10.1007/s10620-011-1838-0. [DOI] [PubMed] [Google Scholar]