Abstract
Magnetic resonance (MR) techniques provide opportunities to non-invasively characterize neurobiological milestones of adolescent brain development. Juxtaposed to the critical finalization of brain development is initiation of alcohol and substance use, and increased frequency and quantity of use, patterns that can lead to abuse and addiction. This review provides a comprehensive overview of existing MR studies of adolescent alcohol and drug users. The most common alteration reported across substance used and MR modalities is in the frontal lobe (63% of published studies). This is not surprising, given that this is the last region to reach neurobiological adulthood. Comparatively, evidence is less consistent regarding alterations in regions that mature earlier (e.g., amygdala, hippocampus), however newer techniques now permit investigations beyond regional approaches that are uncovering network-level vulnerabilities. Regardless of whether neurobiological signatures exist prior to the initiation of use, this body of work provides important direction for ongoing prospective investigations of adolescent brain development, and the significant impact of alcohol and substance use on the brain during the second decade of life.
Keywords: adolescence, adolescent, MRI, DTI, fMRI, MRS, resting state, task, alcohol, marijuana, nicotine, drug use, poly-drug use, substance abuse, addiction, vulnerabilities, brain development, frontal lobe, hippocampus, reward sensitivity, impulsivity
1. Introduction
Adolescence is an age period, typically spanning the second decade of life, characterized by physiological and social maturation that occurs along with significant structural, functional and neurochemical brain changes (Paus, 2005; Spear, 2000). This special issue provides a comprehensive set of reviews of preclinical and clinical studies detailing adolescent brain maturation across multiple domains: neurobiological development, cognition, affect, motivation, reward sensitivity, puberty and sex differences, stress and adversity, sleep physiology, the social brain, genetic influences and the emergence of psychopathology. The purpose of this review is to provide an evaluation of the existing human data, obtained using non-invasive magnetic resonance (MR) neuroimaging methods that document alcohol- and drug-related neurobiological consequences in currently using adolescents.
Over the past decade, MR techniques have dramatically improved the ability to characterize adolescent-related brain changes, due in part to the evolution and availability of higher field-strength scanners, hardware and software innovations, and advanced MR sequences. As a result, MR studies have been able to significantly advance the field in our understanding of neuromaturational changes from childhood through adolescence (Casey et al., 2008; Dahl, 2004; Ernst and Mueller, 2008; Giedd and Rapoport, 2010; Gogtay et al., 2004; Luciana, 2013; Pfefferbaum et al., 1994; Sowell et al., 2004; Steinberg, 2010), and into late adolescence/emerging adulthood (Bennett and Baird, 2006; Sullivan et al., 2011). Adolescent brain reorganization, refinements and functional improvements map onto enhanced cognitive abilities (Casey et al., 2005; Paus, 2005), with the most prominent changes occurring in frontally-based regions and functionally connected subcortical structures (Anderson, 2001; Casey et al., 2000; Giedd et al., 1999; Klenberg et al., 2001; Pfefferbaum et al., 1994; Rosso et al., 2004; Sowell et al., 2001; Sowell et al., 2004; Williams et al., 1999), including age-related increases in frontal lobe gamma-Amino butyric acid (GABA) levels (Silveri et al., 2013). While such changes make adolescence a time of neurobiological opportunity, increased propensity to seek out novel stimulation and engage in risk-taking behaviors, such as using alcohol and drugs, enhance the potential vulnerabilities of this age period. Characterizing the neurobiology underlying immature cognitive and behavioral responses, which result in suboptimal self-regulatory control, have been areas of extensive investigation (Casey et al., 2000; Casey and Jones, 2010; Dempster, 1992; Durston et al., 2006; Schweinsburg et al., 2004; Silveri et al., 2011). Accordingly, the frontal lobes, and limbic/hippocampal circuitries, have been identified as regions particularly vulnerable to early and escalating alcohol and substance use. Due to ethical considerations prohibiting administration of alcohol and drugs to human youth, animal studies have proven invaluable in initiatives to identify consequences of alcohol and drug use on the brain and behavior in adolescents, under controlled laboratory conditions. The companion preclinical review on alcohol and drug effects in this issue (c.f., Spear, this issue), as well as a wealth of past studies, demonstrate strong evidence for age-specific alcohol and drug effects, which in humans could influence the initiation, escalation of use, and risk for abuse during the adolescent period.
Age of initiation and escalation of alcohol and drug use, as well as a high prevalence of substance use disorders (SUDs), overlap with the critical period of adolescent brain reorganization. The Monitoring the Future (MTF) survey has been an essential resource for understanding changing rates of and attitudes towards alcohol and drug use since 1975. In addition to alcohol, drugs assessed include tobacco/nicotine (cigarette smoking, hookah use and e-cigarettes), marijuana, synthetic cannabinoids (K2/Spice), prescription and over-the-counter drugs (including non-medical opioid and stimulant use, cough/cold medicines), ecstasy (“Molly”), other amphetamine-type stimulants (methamphetamine), synthetic stimulants (bath salts), hallucinogens (e.g., salvia), and inhalants. A summary of lifetime prevalence of alcohol and selected drug use, from 44,900 students in grades 8, 10 and 12, from the 2015 MTF survey (Johnston et al., 2016) are presented in Table 1.
Table 1.
2015 MTF Survey Results: Adolescent Lifetime Use Prevalence of Alcohol, Marijuana and Other Drugs
| 8th Graders | 10th Graders | 12th Graders | 4-year change | ||
|---|---|---|---|---|---|
| Alcohol | Any | 26.1% | 47.1% | 64.0% | ↑ 37.9% |
| Drunk | 10.9% | 28.6% | 46.7% | ↑ 35.8% | |
| Marijuana | 15.5% | 31.1% | 44.7% | ↑ 29.2% | |
| Nicotine | Cigarettes | 13.3% | 19.9% | 31.1% | ↑ 17.8% |
| Smokeless | 8.6% | 12.3% | 13.2% | ↑ 4.6% | |
| E-cigarettes | 9.5% | 14.0% | 16.2% | ↑ 6.7% | |
| Amphetamines | 6.8% | 9.7% | 10.8% | ↑ 4.0% | |
| Methamphetamine | 0.8% | 1.3% | 1.0% | ↑ 0.2% | |
| MDMA | 2.3% | 3.8% | 5.9% | ↑ 3.6% | |
| Inhalants | 9.4% | 7.2% | 5.7% | ↓ 3.7% | |
| Any Illicit Drug | 20.5% | 34.7% | 48.9% | ↑ 28.4% | |
| Any Illicit Drug (not marijuana) | 10.3% | 14.6% | 21.1% | ↑ 10.8% |
Percent prevalence for each substance is taken directly from Table 5 of the 2015 MTF survey results (Johnston et al., 2016). Four-year change was calculated to reflect, within this particular survey year, the average increase (or decrease for inhalants) in lifetime prevalence of use from 8th grade to 12th grade.
Alcohol continues to be the most commonly used substance among adolescents; however, marijuana use has been increasing over time. Recent surveys have also considered new drugs and modes of drug use, including use of e-cigarettes and synthetic cannabinoids. This review focuses specifically on adolescent MR studies of the most frequently used substances, alcohol and marijuana, and selected other drugs when available (nicotine, stimulants, ecstasy, inhalants, and poly-drug use). Studies included were limited to those conducted in current alcohol- and/or drug-using adolescents that were age 10 and older, excluding studies that began at age 18, or that were specific to emerging adulthood (age 18–25+ years). Although limited to adolescents, this review notably highlights the rapidly growing data available, obtained using multiple imaging modalities that permit comparison of regions and networks affected across multiple substances. While the collection of results presented in this review may reflect drug use in general, regardless of age, longitudinal studies currently in progress will be instrumental in characterizing adolescence as a unique period of neurobiological vulnerability to alcohol and drugs. Fulfilling the inclusion criteria were, to our knowledge, 103 published structural, functional and spectroscopy studies, together comprising data from thousands of alcohol- and substance-using adolescents and healthy, non-using comparison subjects.
2. Magnetic Resonance Technologies
Structural magnetic resonance imaging (MRI) provides quantitative information regarding brain tissue and volume, by using techniques such as voxel-based morphometry (VBM) to segment images into gray matter (GM) volume, white matter (WM) volume, and cerebrospinal fluid (CSF) volume (Ashburner and Friston, 2000). Parcellation methods also are used to obtain measures of cortical thickness (Fischl and Dale, 2000). Diffusion tensor imaging (DTI) is a structural technique that enables measurement of the restricted diffusion of water in tissue to examine white matter architecture (directional diffusion, fractional anisotropy (FA), with higher levels typically reflecting better white matter integrity), and average water diffusion (mean diffusivity (MD)) (Mukherjee et al., 2008a; Mukherjee et al., 2008b). Functional magnetic resonance imaging (fMRI) provides information regarding brain responses to passive activity in a resting state (rsfMRI) or to external stimuli (task fMRI), by acquiring blood oxygenation level dependent (BOLD) signals, which reflect hemodynamic responses to transient neural activity that result from changes in the ratio of oxyhemoglobin and deoxyhemoglobin (Khanna et al., 2015). More recent advances in fMRI-based connectivity analyses permit assessment of network-level properties of brain function and intrinsic/resting state functional connectivity (rsFC) (Ernst et al., 2015). Arterial spin labeling (ASL) also is a functional technique, used to quantify cerebral perfusion (Petersen et al., 2006). Magnetic resonance spectroscopy (MRS) is used to acquire signals from nuclei capable of producing a resonance signal, such as hydrogen protons (1H), to determine relative metabolite concentrations (Cohen-Gilbert et al., 2014). Metabolites assessed in 1H MRS are N-acetyl Aspartate (NAA, marker of neuronal integrity), choline (Cho, cellular synthesis and degradation), creatine + phosphocreatine (Cr + PCr, markers of cellular energetic state), myo-Inositol (ml, phospholipid metabolism and maintenance of osmotic equilibrium), glutathione (GSH, oxidative stress), and glutamate (Glu), glutamine (Gln) and GABA (amino acid neurotransmitters, involved in cellular metabolism). Metabolites are typically normalized to internal standards, the unsuppressed peak arising from water or the creatine peak.
3. Neurobiological Findings in Current Alcohol and Drug Using Adolescents
To date, of the existing empirical publications reflecting MR neuroimaging data from adolescent current users of alcohol and drugs, the majority of studies are on marijuana (46 unique publications from 2004 to 2016), followed by alcohol (38 unique publications from 2000 to 2016), and considerably fewer publications focusing on other drugs (Figure 1). When broken down by imaging modality, roughly one third of marijuana and one third of alcohol studies were functional, whereas 45% of alcohol and 33% of marijuana studies were structural (MRI).
Figure 1.
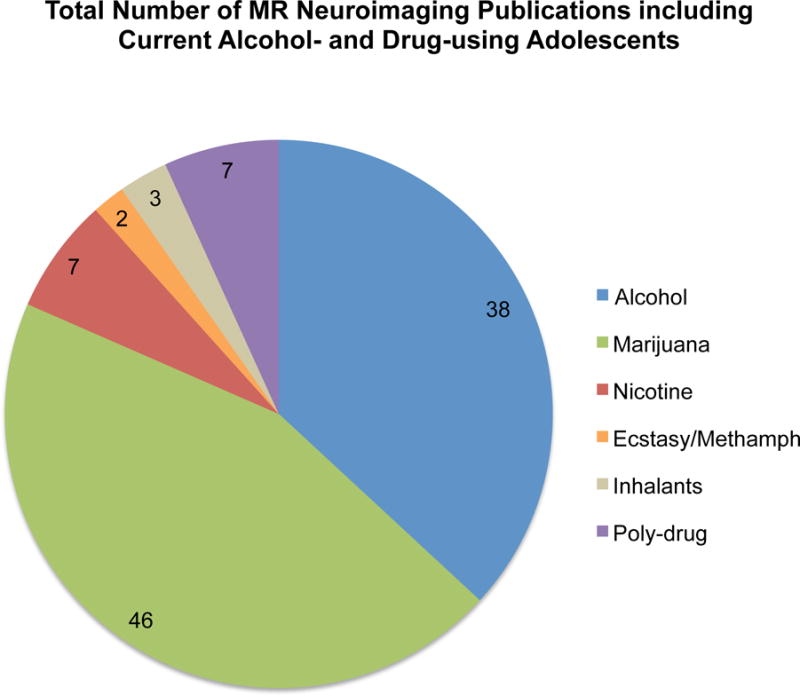
Total number of publications including current alcohol- and drug-using adolescents: 103 MR Studies. Numbers within each section reflect the total number of publications per alcohol/substance.
For other drugs, fewer published studies exist (19 unique publications, Figure 1) that have exclusively examined adolescent current substance users: nicotine (7 studies), amphetamine-type stimulants (ecstasy and methamphetamine, 2 studies), inhalants (3 studies), and poly-drug use (7 studies).
3.1. Alcohol
Given the near-ubiquity of alcohol use and misuse among adolescents, a significant body of neuroimaging literature examining the impact of substance use in this age group has focused on alcohol. Although cognitive studies in adolescent heavy drinkers have most consistently identified deficits in memory, attention, visuospatial skills, and executive functions (Lisdahl et al., 2013a), the existing research on the impact of alcohol on adolescent brain development has yet to yield consistent, replicated findings.
For alcohol, the most consistently published regional alterations reported were in the frontal lobe (61% of studies), followed by alterations in the temporal lobe (45% of studies) and then parietal lobe (32% of studies), in current adolescent alcohol users. Consistent alterations reported in MR studies of adolescent alcohol users, plotted as a function of brain region and imaging modality, are presented in Figures 2. Findings from these studies, as well as those reporting no significant regional differences, are described in great detail below. Notably, only 8 out of 31 studies that had specific frontal-related hypotheses failed to document significant frontal alterations (effects were non-significant) in current adolescent alcohol users relative to a comparison group.
Figure 2.
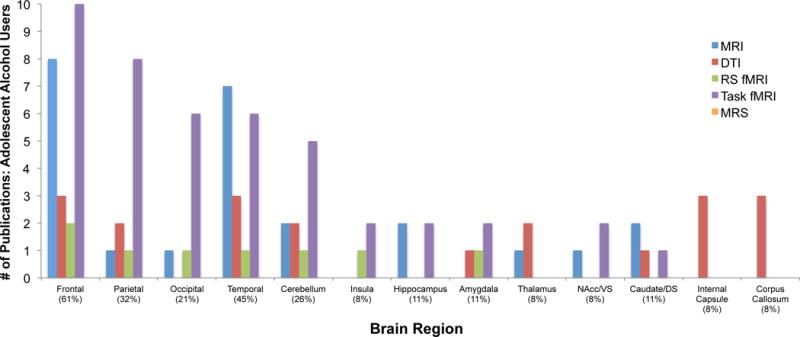
Studies reporting significant brain alterations by region in adolescent alcohol users: 38 MR studies. Percentages in parentheses refer to percentage of published studies (y-axis) documenting significant alterations within a given region of interest (x-axis). Abbreviations – MRI: magnetic resonance imaging; DTI: diffusion tensor imaging; RS fMRI: resting state/task functional magnetic resonance imaging; MRS: magnetic resonance spectroscopy; VS: ventral striatum; DS: dorsal striatum
MRI: Structural Brain Changes
The majority of MRI studies in adolescent alcohol users have focused on regions where damage has been observed in adult alcohol dependent or heavy-drinking populations: prefrontal cortex (PFC) and hippocampus (Oscar-Berman and Marinkovic, 2007). Smaller PFC volumes have been reported in adolescent binge drinkers and those with alcohol use disorders (AUDs) (De Bellis et al., 2005; Fein et al., 2013; Medina et al., 2008; Whelan et al., 2014), with volume inversely correlated with amount of consumption (De Bellis et al., 2005). Recent cross-sectional data from a large adolescent cohort (674 adolescents meeting no/low alcohol or drug use criteria and 134 adolescents exceeding criteria) demonstrate smaller frontal and temporal volumes and thinner frontal, temporal and cingulate cortices in the high alcohol exposure group (Pfefferbaum et al., 2015). Others have reported smaller temporal volumes in AUD adolescents, independent of childhood trauma status (Brooks et al., 2014). Longitudinal evidence suggests declining cortical thickness in individuals who initiate alcohol involvement during adolescence in PFC (Luciana et al., 2013; Squeglia et al., 2015) and temporal (Squeglia et al., 2014; Squeglia et al., 2015) regions, and attenuated white matter growth (Luciana et al., 2013). Importantly, a co-twin control study reported that smaller orbitofrontal cortex (OFC) volumes were associated with alcohol use, perhaps reflecting pre-existing risk factors rather than alcohol-related toxicity (Malone et al., 2014). To this end, prospective studies indicate pre-existing decrements in prefrontal (Squeglia et al., 2014) and ACC (Cheetham et al., 2014) volumes are associated with subsequent heavy drinking. Conversely, the IMAGEN study of 14 year olds from England, France, Ireland and Germany identified structural risk factors, with smaller parahippocampal volumes, but not aberrant frontal volumes, predicting later drinking (Whelan et al., 2014), and others reporting no pre-existing frontal abnormalities in those who later transitioned to drinking (Luciana et al., 2013). Together, structural imaging data indicate that frontal lobe volume abnormalities are both a pre-existing risk factor, and a consequence of adolescent alcohol use.
Evidence is more mixed for hippocampal alterations. For instance, adolescents with AUD were reported to have smaller hippocampal volumes (De Bellis et al., 2000; Nagel et al., 2005), and altered hippocampal asymmetry (Medina et al., 2007b), with smaller volumes associated with earlier age of drinking onset (De Bellis et al., 2000). In contrast, others have reported no significant differences in hippocampal volumes and adolescent alcohol use (Fein et al., 2013; Malone et al., 2014; Whelan et al., 2014; Wilson et al., 2015), although alcohol use histories were relatively limited. A longitudinal investigation also found that initiating heavy drinking was not linked to a change in hippocampal volume (Squeglia et al., 2014). It is possible that inconsistent hippocampal findings may be attributable to the extent and duration of alcohol involvement, as well as other methodological factors, such as manual vs. automated parcellation analyses for determining tissue volumes.
Reports of structural changes in other brain regions are more limited. In one study, ventral striatal volumes were positively associated and OFC volumes were negatively associated with frequency of alcohol use in a juvenile justice-involved sample (Thayer et al., 2012). A twin study identified amygdala deficits associated with drinking, with co-twin analyses indicating that alterations were likely pre-existing (Wilson et al., 2015), which is consistent with other studies (Dager et al., 2015; Hill et al., 2013b). Finally, recent binge drinking was associated with lower cerebellar gray and white matter volumes, even in healthy adolescents who didn’t meet criteria for AUD (Lisdahl et al., 2013b). However, one study suggested that lower cerebellar white matter might also be pre-existing (Squeglia et al., 2014). Overall, structural changes in limbic circuitry and the cerebellum associated with adolescent alcohol use merit further inquiry.
DTI: White Matter Architecture
While a number of studies have employed DTI to characterize the impact of alcohol drinking on white matter in adolescence, only three studies to date have examined the impact of alcohol use in isolation from effects of other substances (Elofson et al., 2013). First, FA in the rostral body and isthmus region of the corpus callosum were higher in the AUD group (De Bellis et al., 2008), which contradicts evidence for reduced FA in the corpus collosum of adults with AUDs (Pfefferbaum et al., 2006). This reversed pattern observed in adolescents is interpreted as reflecting early white matter development (higher FA) that may predispose individuals toward substance use, followed by gradual degeneration of tracts (e.g., lower FA). To this end, the adolescent AUD group demonstrated a negative association between rostrum FA and age that was not observed in healthy controls (De Bellis et al., 2008). Others reported higher FA in adolescents with AUD in a limbic pathway and the stria terminalis, and no regions of lower FA (Cardenas et al., 2013). However, studies of older adolescents (age 16–19, without AUD) documented lower FA associated with alcohol use (Jacobus et al., 2013a; McQueeny et al., 2009). In sub-clinical samples of adolescent binge versus non-binge drinkers, lower FA was observed in corpus callosum, superior longitudinal fasciculus, corona radiata, internal and external capsules, and commissural, limbic, brainstem, and cortical projection fibers in the heavier-drinking group, with no regions of increased FA (Jacobus et al., 2009; McQueeny et al., 2009). Results from the same participants re-examined at 3-year follow-up indicated that binge drinkers maintained lower FA in superior longitudinal fasciculus, corpus callosum, internal capsule, and thalamic fibers, with an additional decline in FA in some regions (Jacobus et al., 2013a). In a prospective study, adolescents who initiated drinking after a baseline assessment showed either no change or increased FA; however, a non-using control group was not included in study results, making it difficult to interpret whether trajectories deviate from normative development (Jacobus et al., 2013b). In a separate sample of adolescents, initiating drinking during a 2-year inter-scan interval was associated with attenuated increases in FA in temporal and caudate white matter compared to continuous nondrinkers (Luciana et al., 2013). Development of DTI techniques and ongoing collection of large longitudinal samples should help clarify trajectories and answer important questions regarding relationships between white matter changes and adolescent alcohol use.
FMRI: Resting State and Task-Based
Only one rsfMRI alcohol study has been published to date, documenting that low amygdala-OFC connectivity was associated with greater alcohol use in 12–25-year-old boys (Peters et al., 2015). This effect was most robust in 14–16 year olds, and was mediated by testosterone levels. Given the increasing application of rsfMRI and fMRI-based connectivity analyses integrating task and resting state data (Ernst et al., 2015), more studies reporting on network-level FC in alcohol-using youth are likely on the horizon. Data from one ASL study also are available, showing that current adolescent alcohol users had higher rCBF frontal (bilateral superior frontal gyrus), right temporal pole, left posterior cingulate, and left insula, but lower rCBF in right superior temporal gyrus and left parieto-occipital cortex relative to non-users. Interestingly, adolescents that would go on to use alcohol after the baseline assessment (future users) had higher rCBF in frontal (left mid orbital gyrus) and left lingual areas and lower rCBF in bilateral temporal and parietal areas relative to non-users. Currently using adolescents, however, only differed significantly from future users in left middle temporal gyrus and left cerebellum and (higher rCBF in current users) (Ramage et al., 2015).
In contrast, there are ample data from studies employing task fMRI to probe spatial and visual working memory, memory encoding, inhibitory control, reward processing and alcohol cue reactivity in alcohol-using adolescents. Most published studies have focused on working memory, which is known to be impaired following prolonged heavy alcohol use (Lisdahl et al., 2013a). Adolescents (15–17 years), with and without AUDs abstinent for five days, performed equally well on spatial working memory (2-back spatial location task), however several significant group differences in brain activation were evident (Tapert et al., 2004). In the AUD group, greater BOLD activation was observed in bilateral parietal regions, while less BOLD activation was observed in left precentral gyrus, mid-occipital regions and cerebellum. Activation differences were of a greater magnitude in individuals endorsing more withdrawal and hangover symptoms. Activation differences may reflect reliance on compensatory mechanisms, recruitment of additional neuronal resources, in AUD to permit successful task performance.
In 15- to 19-year-olds classified as heavy drinkers or light/non drinkers, heavy drinkers showed greater BOLD signal during a visual working memory task, in frontal and parietal regions, and less occipital activation relative to the lighter drinking group (Squeglia et al., 2012). These regions were then used as regions of interest (ROIs) in a longitudinal study that showed reduced frontal and parietal BOLD signal at baseline, more specifically, in left medial frontal gyrus and right inferior parietal lobule ROIs in non-drinking adolescents who would go on to transition to heavy alcohol use compared to those who would not (Squeglia et al., 2012). Existing studies collectively suggest a shift towards greater reliance on fronto-parietal networks, and possibly more effortful processing, associated with working memory in adolescents who consume large amounts of alcohol.
In a study of verbal memory encoding, 16–18 year old binge drinkers showed more BOLD activation in right superior frontal cortex and bilateral posterior parietal cortex, and less occipital activation during novel encoding, relative to non-binge drinking controls. This increased recruitment of working memory networks corresponded with a subtle impairment in verbal encoding, with the binge group showing a trend towards poorer recall(Schweinsburg et al., 2010a). In addition, although binge and non-binge groups did not differ significantly in the magnitude of hippocampal BOLD activation, controls demonstrated significant activation of the left hippocampus during novel encoding, whereas binge drinkers did not. Together these findings suggest altered processing of novel verbal information and suboptimal performance that are associated with binge drinking during adolescence. Binge drinking also was associated with increased prefrontal and parietal activation drinkers during performance of a paired associates task, regardless of co-occurring marijuana use(Schweinsburg et al., 2011).
Inhibitory control has also been assessed in conjunction with fMRI in adolescent alcohol users. In a series of longitudinal studies, 12- to 14-year olds who transitioned to heavy drinking by follow-up exhibited less activation at baseline in right and left frontal areas, motor cortex, cingulate gyrus, middle temporal gyri, and the inferior parietal lobules (Norman et al., 2011), and increased activation at follow-up in areas that mediate response inhibition, including bilateral middle frontal gyri, right inferior parietal lobule, and left cerebellar tonsil (Wetherill et al., 2013b). Similarly, adolescents who transitioned to heavy drinking and experienced alcohol-induced blackouts exhibited more baseline frontal response during response inhibition processing (Wetherill et al., 2013a). Furthermore, fMRI response during inhibitory processing was poor at correctly classifying binge drinkers at age 14 (Whelan et al., 2014), however increased premotor activation was a significant predictor of subsequent drinking at age 16. Together, these studies support the role of inhibition as a risk factor rather than consequence of drinking in this age range.
Brain activation during probability-based decision-making, reward anticipation and receipt, has been examined using Wheel of Fortune (WOF), monetary incentive delay (MID), and gambling tasks. Adolescent onset binge drinkers exhibited reduced response to reward in the WOF task in the left cerebellum, although there were no differences in ventral striatum, which was speculated to reflect reduced salience of task rewards, and/or neurotoxic effects of alcohol on cerebellum (Cservenka et al., 2015b). In the IMAGEN study, in which participants performing the MID task can win or avoid losing money, decreased ventromedial PFC and increased inferior frontal gyrus responses were evident during reward anticipation and receipt, and were robust classifiers of current binge drinking, whereas superior frontal activation during reward receipt predicted future drinking (Whelan et al., 2014). Binge drinkers also displayed greater hippocampal and putamen activation during reward anticipation, and less hippocampal response during reward receipt. More recently, nucleus accumbens (NAcc) activation was examined in adolescents that used alcohol only, marijuana only, tobacco only, marijuana and tobacco, or all three substances, relative to non-users. No relationship was observed between alcohol use and NAcc response during MID reward anticipation or receipt (Karoly et al., 2015). In another example of reward sensitivity, 12–26-year-olds were scanned while performing a gambling task at baseline and again 2 years later (Braams et al., 2016). Baseline testosterone levels, but not baseline NAcc response to reward, predicted subsequent alcohol involvement, whereas greater NAcc activation during reward at follow-up was associated with more drinking. Taken together, while an enhanced reward response may not be pre-existing, there is some evidence that sensitivity of the reward system is enhanced with ongoing alcohol use.
In an fMRI study employing the Iowa Gambling task, binge drinkers made significantly more risky choices and displayed less learning, which was associated with greater recruitment of left amygdala and bilateral insula compared to the non-drinking group (Xiao et al., 2013). Negative urgency (tendency to act impulsively during negative emotions), measured via UPPS (urgency, premeditation, perseverance, and sensation seeking) scale (Whiteside and Lynam, 2001), was positively correlated with insula activation and negatively correlated with OFC activation in binge and non-drinkers, suggesting a role for limbic circuitry, and insula in particular, in risk-taking behaviors that promote heavy episodic alcohol use in adolescence.
During an emotional processing, passive viewing, fMRI task, greater ventromedial PFC and lesser inferior frontal responses classified binge drinkers, who also had less temporal and cuneus activation to angry faces. A lesser frontal response to angry faces also predicted subsequent drinking (Whelan et al., 2014). An fMRI study of brain activation during the passive viewing of alcohol images showed that 14–17 year old adolescents with AUDs had increased left PFC, orbital gyrus, anterior and posterior cingulate gyri, amygdala and parahippocampus responses relative to light-drinker (Tapert et al., 2003). Further, while light drinkers exhibited greater activation in two right frontal regions relative to the AUD group, overall, elevated brain responding to alcohol cues was evident in the adolescent AUD group in regions associated with reward and craving, and areas implicated in self-regulation.
MRS: Neurochemistry
To date, no current MRS data exist for current adolescent alcohol users, despite compelling evidence of at least proton metabolite abnormalities reported in adult alcohol dependent patients (for review see (Meyerhoff et al., 2013)), and emerging adult binge drinkers (Silveri et al., 2014). A recent study of alcohol naïve adolescents and light drinking emerging adults suggest that a positive family history of alcoholism may influence glutamatergic metabolites and impulse control, which together could confer greater genetic risk of problem drinking later in life (Cohen-Gilbert et al., 2015). Clearly, more studies utilizing MRS could help advance what is known about the effects of adolescent alcohol consumption on brain chemistry.
3.2. Marijuana
In the past decade, an increasing number of studies have characterized neurocognitive decrements associated with adolescent marijuana use. Most consistently, deficits have been observed in learning and memory, executive function, processing speed, and attention (Lisdahl et al., 2014), which persist even after several weeks of abstinence (Hanson et al., 2010; Schweinsburg et al., 2008a). Brain regions subserving these abilities, hippocampus, basal ganglia, cerebellum, and frontal cortex, are particularly dense in cannabinoid receptors, and thus are primary target sites for the psychoactive actions of marijuana (Glass et al., 1997; Herkenham et al., 1990). Accordingly, neuroimaging has provided valuable insight into the impact of marijuana use during this age span, particularly given the ongoing maturation, and vulnerabilities of these brain regions to marijuana, throughout adolescence.
Like alcohol, the most consistently published regional alteration in adolescent marijuana users is in the frontal lobe (63% of studies), followed by the parietal lobe (33% of studies) and the temporal lobe (22% of studies). Regional alterations observed in adolescent marijuana users are presented in Figure 3, as well as described below. Again like alcohol studies, only 9 out of 38 studies that had specific frontal-related hypotheses failed to document significant frontal alterations (effects were non-significant) in current adolescent marijuana users relative to a comparison group.
Figure 3.
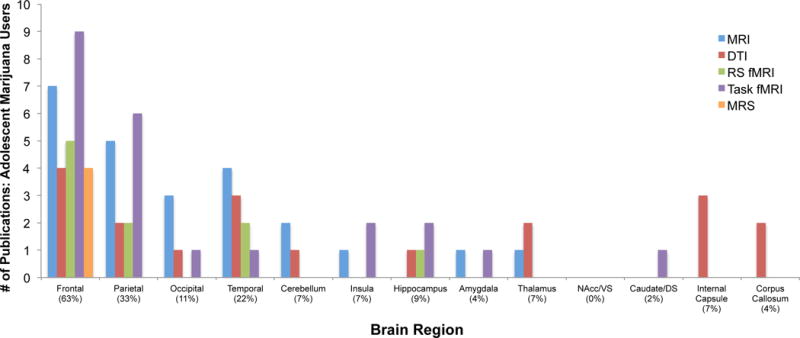
Studies reporting significant brain alterations by region in adolescent marijuana users: 46 MR Studies. Percentages in parentheses refer to percentage of published studies (y-axis) documenting significant alterations within a given region of interest (x-axis). Abbreviations – MRI: magnetic resonance imaging; DTI: diffusion tensor imaging; RS fMRI: resting state/task functional magnetic resonance imaging; MRS: magnetic resonance spectroscopy; VS: ventral striatum; DS: dorsal striatum
A critical caveat of this body of work is that the majority of adolescent marijuana studies include users with relatively heavy concomitant alcohol use. Although some studies include comparison alcohol only groups, or have attempted to statistically control for alcohol use, it is difficult to parse independent from interactive effects of these substances. On the other hand, data from studies including heavy marijuana-using adolescents that report little alcohol use are not only less prevalent, but results may not generalize to the broader population of marijuana-using adolescents. It is similarly difficult to differentiate the influence of comorbid psychiatric disorders and nicotine use, which also are prevalent in adolescent marijuana users.
MRI: Structural Brain Changes
Adolescent marijuana users, abstinent for 28 days, had thicker frontal ACC, medial temporal and occipital cortices than nonusers at baseline (Jacobus et al., 2014). At 1.5 and 3 years after baseline scanning (ages 18–21), marijuana users continued to exhibit thicker cortices than controls in widespread brain regions (Jacobus et al., 2015), with cumulative marijuana exposure throughout adolescence associated with thicker temporal cortices. Notably, many of the cortical differences may have been driven by alcohol use, as lifetime drinking also was associated with thicker cortices. In a separate cross-sectional study of 16–19-year-old adolescents, marijuana users likewise had thicker temporal and parietal cortices, but less cortical thickness in frontal and insula regions (Lopez-Larson et al., 2011). In a third study, abnormalities in cortical thickness were not observed in 10–23-year-olds in a residential treatment program for marijuana use (Kumra et al., 2012). However, alternate structural methods revealed different patterns in the same subjects: marijuana users demonstrated reduced volumes and surface area of frontal and parietal, and increased thalamic volume (Kumra et al., 2012), but no differences in anterior cingulate surface area (Epstein and Kumra, 2014).
In disagreement with previous evidence of structural abnormalities among adolescent marijuana users, some studies have failed to replicate earlier findings. In a study examining 14–18 year old adolescents recruited from the juvenile justice system, current daily marijuana users and nonusers (matched on alcohol use), no group differences were found for any region using a variety of analysis methods, including voxel-based morphometry, surface-based morphometry, and shape analyses of the NAcc, amygdala, hippocampus, and cerebellum (Weiland et al., 2015). It is possible that other characteristics of this sample may have contributed to the lack of observed effects, highlighting the importance for future studies to better delineate personal risk factors contributing to volumetric abnormalities among marijuana users.
Despite evidence of poorer memory function associated with adolescent marijuana use (Schweinsburg et al., 2008a), and that smaller hippocampal volumes have been consistently identified among adults (Lorenzetti et al., 2014; Yucel et al., 2016), very few of the studies investigating the hippocampus have yielded significant hippocampal results. Although no significant overall hippocampal volume differences were evident in a number of studies (Kumra et al., 2012; Medina et al., 2007a; Medina et al., 2007b; Weiland et al., 2015), one study did show that marijuana users did not exhibit the same pattern of greater right>left hippocampal volume asymmetry, which was associated with better verbal learning scores in nonusers (Medina et al., 2007b). It is possible that hippocampal abnormalities manifest in older users with more extensive use histories, particularly given a prospective study indicating no premorbid hippocampal differences in adolescents that later initiated marijuana use (Cheetham et al., 2012). Marijuana users also have been reported to have larger inferior posterior vermis, in which greater abnormalities were associated with poorer executive functioning (Medina et al., 2010), and smaller amygdala volumes (McQueeny et al., 2011), which were later reported to be associated with greater craving in marijuana users across the first 28 days of abstinence (Padula et al., 2015).
Finally, a prospective study of 10–23 year old adolescents revealed less cortical thinning in several higher order association regions between baseline and 18-month follow-up in treatment-seeking marijuana using adolescents (age 16.6) compared to controls, but no effect of having early-onset schizophrenia (Epstein and Kumra, 2015a). Adolescent neuromaturation typically includes cortical thinning (Gogtay et al., 2004); therefore, marijuana use during this age range may interfere with this developmental process, resulting in thicker cortices among heavy users.
DTI: White Matter Architecture
Adolescent marijuana users, who also drank heavily, showed lower FA throughout several widespread brain regions, including left superior longitudinal fasciculus, and inferior frontal and temporal white matter, compared to nonusers (Bava et al., 2009). However, marijuana users also had higher FA in right superior longitudinal fasciculus, internal capsule, and right occipital white matter. In the same set of subjects, poorer neurocognitive function was predictive of abnormalities in temporal and frontal white matter integrity, whereas higher occipital FA was related to better neurocognitive function, which could indicate a compensatory process among marijuana users (Bava et al., 2010). Marijuana users also had lower FA than nonusers in corona radiata and superior longitudinal fasciculus (Jacobus et al., 2009).
In a larger sample of adolescent marijuana users, lower FA at baseline assessment was associated with higher degrees of subsequent aggressive and delinquent risk taking, as well as more substance use, at 18-month follow-up (Jacobus et al., 2013c). Marijuana-related reductions in FA were found to persist at 18-month follow-up, after controlling for baseline DTI indices. Compared to controls, marijuana users also had higher MD, which was correlated with alcohol, but not marijuana use (Bava et al., 2013). Participants were followed an additional 18 months, with a final scan 3 years following baseline assessment, at age 19–22. Marijuana users continued to have lower FA than non-using controls in numerous fiber tracts, as well as a significant decline in FA between baseline and follow-up (Jacobus et al., 2013a). Moreover, a subset of adolescents that were nonusers at baseline initiated heavy alcohol or marijuana + alcohol use by 3-year follow-up; those who initiated marijuana use showed declining FA in several white matter pathways, whereas those who initiated only alcohol use generally showed no change (Jacobus et al., 2013b). It should be noted that there was no control group of continuous non-users included in the study. Another longitudinal investigation identified similar patterns of results among adolescent marijuana users in treatment (Epstein and Kumra, 2015b). Although groups of marijuana users and controls were similar at baseline, marijuana users demonstrated significant decreases, whereas nonusers demonstrated significant increases in FA over time in the inferior longitudinal fasciculus. Taken together, these results provide important evidence that poorer white matter integrity reflects neurotoxicity associated with initiating marijuana use, rather than pre-existing differences.
FMRI: Resting State and Task-Based
To date, three studies have assessed resting state fMRI in adolescent marijuana users. Marijuana users in treatment had significantly increased resting state activity in right parietal and right prefrontal cortices, relative to nonusers. In addition, marijuana users exhibited reduced inter-hemispheric connectivity in cerebellum and superior frontal gyrus, and increased inter-hemispheric connectivity in supramarginal gyrus (Orr et al., 2013). Using the OFC as a seed region, marijuana users showed greater functional connectivity from the OFC seed region to cingulate and prefrontal regions, with a younger age of onset being associated with more aberrant connections (Lopez-Larson et al., 2015).
In a study of adolescents in a juvenile justice day program, divided into high and low marijuana, independent component analysis revealed that high marijuana users had higher resting state frontal lobe activity within the frontotemporal executive control network (Houck et al., 2013). More recently, adolescent marijuana users recruited from a treatment program exhibited lower connectivity between ACC and frontal regions at 18 month follow-up, as well as a decline in connectivity between ACC and dorsolateral prefrontal cortex between baseline and follow-up, compared to community controls (Camchong et al., 2016). Interestingly, lower connectivity between ACC and OFC at baseline was associated with more marijuana use within the interscan period. Finally, an ASL study indicated that at baseline, marijuana users have reduced rCBF in right medial frontal and bilateral temporal regions, and increased blood flow in the precuneus (Jacobus et al., 2012). However, no group differences were observed at 28-day follow-up, which could indicate attenuation of blood flow abnormalities with abstinence.
Given that adolescent marijuana users show deficits on behavioral tasks of working memory, long-term memory, and inhibitory processing (Lisdahl et al., 2014; Schweinsburg et al., 2008a), several fMRI studies have focused on these task domains. In a verbal working memory fMRI task, marijuana users demonstrated poorer working memory performance and failed to deactivate the hippocampus (Jacobsen et al., 2004). In an additional sample of adolescents that used both marijuana and tobacco, during nicotine withdrawal, marijuana users exhibited more posterior brain activation under high working memory load than comparison teens, and demonstrated different patterns of frontoparietal functional connectivity between smoking and withdrawal conditions (Jacobsen et al., 2007b). Together, these results indicate abnormities among marijuana using adolescents that may be masked by nicotine use, yet it is unclear whether these patterns would also be observed in comparison to non-smoking controls.
Marijuana users often have concomitant alcohol use, thus adolescents who used marijuana + alcohol were compared to heavy drinkers and non-using controls after ~8 days of marijuana abstinence (Schweinsburg et al., 2005). During spatial working memory, marijuana users exhibited increased dorsolateral (DLPFC) activation and more inferior and middle frontal deactivation compared to nonusers, despite task performance. These alterations were not observed in heavy drinkers, suggesting compensatory working memory and attention processing associated with heavy marijuana use. After a longer period of abstinence (monitored for 28 days), less DLPFC activation and greater posterior parietal responses were observed relative to controls (Schweinsburg et al., 2008b). It was concluded that marijuana users relied more on spatial rehearsal and attention strategies, and less on general executive processing, demonstrating disrupted response patterns even after 28 days of abstinence. Subsequently, a positive relationship was reported between task performance and brain response in temporal regions, but controls displayed a negative relationship, which could indicate the use of more verbal processing among better performing marijuana users (Padula et al., 2007). Finally, in a cross-sectional study of recent (abstinent 3–7 days) and abstinent users (28–60 days), recent users had more frontal and insular responses than abstinent users (Schweinsburg et al., 2010b). These regions were not activated among controls, and may be involved in working memory updating and inhibitory control. Although these findings have yet to be replicated in a longitudinal sample, these data suggest that performance strategies and brain responses change as abstinence progresses.
Marijuana-using males, recruited from the Netherlands and United States, had a heightened response in prefrontal and posterior parietal regions during a verbal working memory task, but no response differences during a pictorial associative learning task (Jager et al., 2010). In another study, adolescents performed a verbal learning task following ~22 days of marijuana abstinence; despite similar task performance between groups, marijuana users showed bilateral prefrontal hyperactivation, while users of marijuana + alcohol showed response levels intermediate between marijuana-only users and controls. Within the hippocampus, marijuana users failed to show significant task-related activation, whereas marijuana + alcohol users activated similarly as controls in adolescent marijuana users (Schweinsburg et al., 2011). This study offered unique insight by including a marijuana-only group with no nicotine use or psychopathology. In sum, during working memory, recent users show increased prefrontal responses, which may decrease with abstinence, yet longitudinal investigations need to clarify these trajectories. In addition, regardless of abstinence duration, marijuana users show increased response in posterior brain regions. These findings support a model of increased neural effort to maintain performance.
Two investigations have used a go/nogo paradigm to characterize inhibitory processing in adolescent marijuana users. During inhibition trials, marijuana users exhibited increased activation throughout widespread prefrontal, parietal, and occipital regions (Tapert et al., 2007). In another study utilizing a go/nogo task, no differences between groups were found with voxel-wise whole-brain analyses (Behan et al., 2014). However, using a region of interest approach, correlations were observed between bilateral posterior parietal and cerebellar regions in marijuana users, suggesting altered connectivity within inhibition networks. Neural response during a decision-making paradigm, examined among marijuana users from a recent outpatient treatment program, youth with similar psychiatric histories, and healthy controls demonstrated that when making risky decisions, marijuana users had more posterior parietal responses than both comparison groups (De Bellis et al., 2013). In addition, when receiving feedback on decisions, marijuana users showed reduced OFC response compared to both control groups. Taken together, data from executive function tasks indicate recruitment of greater neuronal resources in MJ users compared to non-users, as well as less neuronal responsiveness to feedback. This suggests enhanced brain effort to perform more-frontally mediated tasks, as well as altered connectivity and a blunted response during rewards.
Reward processing also has been examined in adolescent marijuana users using a MID task. In a study of marijuana-using boys and controls, striatal responses to reward anticipation or receipt did not differ between users and controls. Similarly, adolescent marijuana users showed no differences in the NAcc during reward anticipation or receipt, using the same task. Reward response was further examined with a simulated gambling task, in which marijuana users had hyperactivated networks involved in reward processing both during winning and losing conditions, suggesting increased sensitivity to both reward and punishment (Acheson et al., 2015). There is less convincing evidence of altered neuronal responses to reward in adolescent marijuana users on a monetary incentive delay task. However, during a gambling task, marijuana users do exhibit heightened activation regardless of winning or losing, perhaps due to greater risk taking associated with gambling.
In an emotional processing task, in which participants viewed angry and neutral faces during fMRI scanning, marijuana users showed greater bilateral amygdala activation to angry faces compared to neutral faces. In contrast, controls showed more response to neutral faces than angry faces in temporo-parietal and inferior frontal regions, and no difference in amygdalae (Spechler et al., 2015). Finally, during a simple finger-tapping task, greater cingulate responses were observed among controls but not marijuana users (Lopez-Larson et al., 2012). Akin to the altered activation patterns observed in executive and reward tasks, marijuana using adolescents appear to be more responsive to emotion, particularly angry faces, and less responsive to neutral faces or during a simple motor task. Clearly, more fMRI studies utilizing tasks that tap a variety of functional domains are needed to better understand how adolescent marijuana use impacts brain activation, and whether or not differences are antecedent to and/or consequences of use.
MRS: Neurochemistry
Few studies have utilized MRS to characterize neurochemical profiles in adolescent marijuana users (Sneider et al., 2013). An investigation of ACC metabolites identified lower NAA, Glu, Cr, and myo-I in marijuana users (Prescot et al., 2011), as well as low ACC GABA, and replicated findings of lower reduced NAA, Cr, and myo-I published in a subsequent study (Prescot et al., 2013). Adolescents with combined use of methamphetamine and marijuana also demonstrated lower ACC NAA, beyond effects observed in methamphetamine users alone, with no differences in other metabolites (Sung et al., 2013). Cumulative lifetime marijuana exposure and earlier age of onset were associated with lower NAA levels. In contrast, adolescent marijuana users had higher ventrolateral PFC NAA levels than marijuana-using adolescents with bipolar disorder and than adolescents with bipolar disorder alone (Bitter et al., 2014). This inconsistent finding may be related, in part, to a wider age range of the participants, who were 12–21-years-old.
3.3. Other Drugs: Nicotine, Amphetamine-type Stimulants, Inhalants, Poly-drug
While imaging studies of all other drugs used by adolescents are quite sparse, data are described from nicotine, ecstasy and methamphetamine, inhalants, and poly-drug use MR studies (see also, Table 2), most of which employed task fMRI. Notably, no data are available from adolescent opioid-using cohorts. Non-medical use of prescription drugs, in particular, is a growing health concern in a number of countries, including the United States (UNODC, 2011). Early onset of substance use also is not only a robust predictor of increased risk for future substance use disorders (Ramage et al., 2015), adolescents and young adults with stimulant use are at particular risk (Leland et al., 2006). The use of stimulants and other drugs are concerning due to the high risk of dependence, with individuals demonstrating increased risk taking, poor decision making, and high levels of impulsivity, relative to stimulant-naïve individuals, however the neural contributions are less known. Further, not only is co-use common among those with the most substance use, substance use disorders and conduct disorder, and other psychiatric disorders, are typically comorbid in adolescents, reflecting risky decision making behavior among other complexities.
Table 2.
Adolescent MR Studies of Nicotine, Ecstasy and Methamphetamine, Inhalants, and Poly-drug Use
| Authors & Year | Participants | Age | Field Strength | Modality/Task | ROI | Results | Clinical or Cognitive Correlates |
|---|---|---|---|---|---|---|---|
| Nicotine | |||||||
| Jacobsen et al., 2007a | 14 SM (7F) 20 HC (12F) |
17.0 ± 0.7 16.3 ± 1.2 |
3T | DTI | CC FRO Genu ILF SLF int capsule ext capsule |
SM ↑ FA FRO SM ↑ FA int capsule |
SM ↑ FA L int capsule: ↑ reaction time during auditory attention task performance |
| Jacobsen et al., 2007c | 14 SM (8F) 16 HC (7F) |
17.0 ± 0.9 16.2 ± 1.5 |
3T | fMRI (attention) |
HIPP Insula MFG MOG lingual STG |
SM ↑ R STG | SM ↓performance accuracy |
| Jacobsen et al., 2007b | 55 SM (36F) 38 HC (22F) |
16.9 ± 1.2 16.6 ± 1.4 |
1.5T | fMRI (VWM) |
vlPFC inf PAR |
SM ↑ left vlPFC ↑ inf PAR | smoking abstinence: ↓ working memory efficiency |
| Karoly et al., 2015b | 14 MJ (3F) 34 SM (13F) 12 ALC (4F) 17 MJ + SM (4F) 17 PDU (7F) 38 HC (14F) |
15.8 ± 1.4 16.3 ± 1.2 16.0 ± 1.2 15.8 ± 1.2 15.9 ± 1.0 15.8 ± 1.2 |
3T | fMRI (MID) |
NAcc | SM ↓ NAcc during reward anticipation | no behavioral group differences |
| Peter et al., 2011 | 43 SM (19F) 43 HC (19F) |
14.4 ± 0.4 | 3T | fMRI (MID) |
VS | SM ↓ VS activation | SM ↑ reward delay discounting ↑ novelty seeking ↓ VS: ↑ smoking frequency ↓ VS: ↑ reaction time |
| Rubinstein et al., 2011b | 12 LS (5F) 12 HC (5F) |
16.3 ± 1.0 15.7 ± 1.6 |
3T | fMRI (cues) |
ACC HIPP PHP MOG |
LS ↑ activation left ACC, rHIPP, rPHP, bilat MOG to smoking cues | none reported |
| Rubinstein et al., 2011a | 12 LS (5F) 12 HC (5F) |
16.3 ± 1.0 15.7 ± 1.6 |
3T | fMRI (cues) |
insula putamen inf FRO Rolandic operculum mid OCC calcarine |
HC ↑insula, putamen, inf FRO, Rolandic operculum to food/craving cues; not in LS | none reported |
| Amphetamine-Type Stimulants: Ecstasy | |||||||
| Jacobsen et al., 2004a | 6 MDMA (4F) 6 HC (4F) |
17.3 ± 1.1 17.1 ± 1.1 |
1.5T | fMRI (VWM) |
HIPP | MDMA failed to ↓ L HIPP | MDMA ↓ L HIPP: ↑ time since last use |
| Amphetamine-Type Stimulants: Methamphetamine | |||||||
| Churchwell et al., 2012 | 9 Meth (6F) 8 Meth + MJ (5F) 10 HC (7F) |
15.6 ± 1.4 16.2 ± 1.2 15.7 ± 1.5 |
3T | MRI | striatum | Meth + MJ ↑ striatal | Meth + MJ ↑ novelty seeking ↑ meth exposure: ↑ volume & ↑ novelty seeking |
| Inhalants | |||||||
| Hong et al., 2014 | 22 IU (2F) 22 HC (4F) |
17.2 ± 1.4 16.5 ± 2.4 |
3T | MRI | caudate pallidum putamen thalamus AMYG HIPP |
IU ↓ R thalamus | ↓ R thalamus: ↑ inhalant use |
| Takagi et al., 2013 | 14 IU (8F) 11 MJ (3F) 9 HC (6F) |
17.3 ± 1.7 19.7 ± 1.7 19.5 ± 2.6 |
3T | MRI | CC | IU ↓ CC area ↓ thickness ↓ genu | none reported |
| Yuncu et al., 2015 | 19 IU (3F) 19 HC (4F) |
15.5 ± 1.0 16.1 ± 0.8 |
3T | DTI | OCC PAR TEMP |
IU ↑ AD left OCC, PAR, TEMP | IU ↓ executive functioning |
| Poly-Drug/SUD | |||||||
| Dalwani et al., 2011 | 25 PDU (0F) 19 HC (0F) |
16.6 ± 0.2 16.6 ± 0.4 |
3T | MRI | CB PFC precuneus |
PDU ↓ L DLPFC, R lingual gyrus, CB ↑ R precuneus | PDU ↓L DLPFC: ↑substance dependence severity |
| Chumachenko et al., 2015 | 25 SUD (0F) 19 HC (0F) |
16.6 ± 1.2 16.6 ± 1.6 |
3T | MRI | inf FRO Insula OFC PCC STG |
SUD ↓ PCC cortical thickness | ↓ STG CT: ↑ lifetime conduct disorder symptoms |
| Dalwani et al., 2015 | 22 PDU (all F) 21 HC (all F) |
16.1 ± 0.2 16.7 ± 0.3 |
3T | MRI | ACC DLPFC VLPFC PAR mOFC striatum |
PDU ↓ R DLPFC, L VLPFC, ACC, PAR, mOFC | none reported |
| Thatcher et al., 2010 | 24 SUD (12 F) 12 HC (6F) |
16.7 ± 1.0 16.7 ± 1.1 |
3T | DTI | SLF | SUD ↓ FA sup LF; SUD females ↓ FA than SUD males | |
| Clark et al., 2012 | 35 SUD (16F) 20 HC (11F) |
16.8 ± 1.2 16.2 ± 1.0 |
3T | DTI | PFC PAR |
SUD ↓ FA PFC, PAR | SUD ↓ FA: ↑ psychological dysregulation, ↑ marijuana symptoms |
| Weissman et al., 2015 | 50 PDU (23F) 19 HC (13F) |
16.3 ± 0.5 | 3T | RSFC | NAcc FROPAR PFC |
PDU ↑ FC NAcc and FROPAR | PDU ↑ FC: ↑ substance use onset |
| Schneider et al., 2012 | 31 PDU (16F) 31 HC (16F) |
14.6 ± 0.4 14.4 ± 0.4 |
3T | MRI fMRI (MID) |
VS | PDU ↓ VS volume, ↓ VS reward anticipation | PDU ↓ VS reward anticipation: ↑ risk-taking bias |
ACC: anterior cingulate; AD: axial diffusivity; AMYG: Amygdala; AUD: alcohol use disorder; CB: cerebellum; CC: corpus callosum; CT: cortical thickness; DLPFC: dorsolateral prefrontal cortex; FA: fractional anisotropy; FRO: frontal; GM: gray matter; HC: healthy comparison; HIPP: hippocampus; inf: inferior; ILF: inferior longitudinal fasciculus; IU: inhalant users; VLPFC: ventrolateral prefrontal cortex; LS: light smoker; METH: methamphetamine; MFG: middle frontal gyrus; MID: monetary incentive delay; MJ: marijuana; mOFC: medial orbitofrontal cortex; MOG: middle occipital gyrus; NAcc: nucleus accumbens; OCC: occipital; PAR: parietal; PCC: posterior cingulate cortex; PHP: parahippocampus; PDU: poly-drug users; PFC: prefrontal cortex; sup: superior; SLF: superior longitudinal fasciculus; SM: smoker; STG: superior temporal gyrus; SUD: substance use disorder; TEMP: temporal; VLPFC: ventrolateral prefrontal cortex; VS: ventral striatum; VWM: verbal working memory; WM: white matter;: associated/correlated with
Nicotine
While there are no studies investigating morphology in adolescent smokers per se, a recent study investigated young adult male smokers, with an age range that included 16 to 23 year olds (Li et al., 2015). The findings demonstrated significantly larger right caudate volumes, and cortical thinning in frontal regions, left insula, left middle temporal gyrus, right inferior parietal, and right parahippocampus relative to nonsmokers. Lastly, cortical thickness of the OFC and right DLPFC were associated with pack-years and nicotine dependence severity, respectively, in smokers. Consistent with studies of adolescent alcohol and marijuana use, fronto-striatal structural differences also are evident in adolescent/emerging adult smokers.
In a DTI study of adolescent smokers and nonsmokers (with and without prenatal exposure to maternal smoking), increased FA was observed in the anterior cortical white matter in smokers (without prenatal exposure) (Jacobsen et al., 2007a). Significant positive correlations also were reported between adolescent tobacco exposure and FA in the genu of the corpus callosum, and between reaction time during behavioral performance of an auditory attention task and FA of the posterior limb of the left internal capsule. Although altered white matter integrity is typically seen as decreased rather than increased FA, in this context, it was suggested that nicotine may prematurely elicit events ordinarily triggered by cholinergic projections via stimulation of nicotinic receptors.
In a cohort similar to that included in the DTI study, adolescent smokers performing a selective attention task while undergoing fMRI demonstrated increased activation in the right superior temporal gyrus in the auditory condition relative to nonsmokers. With smokers performing worse than nonsmokers, these data suggest altered attentional processing associated with adolescent exposure to nicotine (Jacobsen et al., 2007c). Similarly elevated brain responses also were observed in adolescent smokers (daily use) performing a verbal working memory fMRI task, only activation was greater in left ventrolateral PFC and inferior parietal lobe relative to non-smokers (Jacobsen et al., 2007b). Even with smoking abstinence, alterations persisted, as evidenced by a continued reduction in efficiency of working memory brain circuitry and alterations in the functional connectivity between ventrolateral PFC and other components of the verbal working memory circuitry.
Adolescent nicotine users (tobacco-only), unlike the lack of differences reported for adolescent alcohol and marijuana users performing the MID task, exhibited less activation in the NAcc during reward anticipation than the other two substance groups (Karoly et al., 2015). This difference in adolescent smokers was observed despite similar behavioral performance across all three groups. In the IMAGEN study, adolescent smokers (at least one cigarette in the last 30 days), displayed less activation in the ventral striatum during fMRI reward anticipation, which correlated with smoking frequency (Peters et al., 2011). Notably, the observed hypoactivation during reward anticipation was evident in these adolescents who had mild smoking habits.
When adolescent smokers (1 to 5 cigarettes per day) viewed smoking cues (pictures of people smoking and smoking-related objects), greater activation was evident than during neutral cues in the left ACC, right hippocampus, and right parahippocampal gyrus, and bilateral middle occipital gyri (Rubinstein et al., 2011b). Thus, even low levels of smoking were attributed to heightened reactivity to smoking cues in adolescents. In contrast, adolescent smokers failed to activate regions during the viewing of pleasurable (sweet, salty, and high fat) food cues in insula, interior frontal region, and Rolandic operculum relative to nonsmokers (Rubinstein et al., 2011a). Together these findings show reduced reward sensitivity to appetitive stimuli in adolescent smokers, as evidenced by smaller brain responses to reward anticipation and pleasurable foods, but enhanced responding to smoking cues.
Amphetamine-type Stimulants: Methamphetamine & Ecstasy
Amphetamine-type stimulants (ATS) include amphetamine (e.g., speed), methamphetamine, and 3,4-Methylenedioxymethamphetamine (MDMA, ecstasy), the latter of which are commonly used together in recreational and entertainment settings (e.g., ‘club scene’) (UNODC, 2011). While there are several existing studies examining the effects of MDMA on brain structure and function in emerging and older adults (Cowan, 2007; Mueller et al., 2016), only one study exists that included adolescents under age 18. Adolescent MDMA users exhibited delayed reaction times and failed to deactivate left hippocampus during verbal working memory (Jacobsen et al., 2004). Adolescent users of methamphetamine and combined methamphetamine + marijuana use had increased regional striatal volumes, which were correlated with increased novelty seeking (Churchwell et al., 2012).
Inhalants
A preliminary study of Korean and Australian adolescent inhalant users, relative to healthy adolescents, demonstrated reduced right thalamic volume, which was negatively correlated with severity of inhalant use among Korean participants (Hong et al., 2014). Distinct white matter abnormalities in inhalant users were revealed using DTI, specifically, globally smaller, thinner and more curved CC and a disproportionally thinner genu relative to healthy controls. No differences were observed in the marijuana group (Takagi et al., 2013). These findings are consistent with a study conducted in emerging adults (Yucel et al., 2010). Furthermore, inhalant abusers also exhibited higher axial diffusivity in left parietal, occipital, and temporal white matter (Yuncu et al., 2015).
Poly-Drug
There are relatively few published studies in adolescent poly-drug users. Adolescent males who were poly-drug dependent had smaller left DLPFC, bilateral cerebellum, and right lingual gyrus and higher right precuneus volumes than healthy comparisons subjects. A negative association also was observed between left DLPFC gray matter volume and substance dependence severity in the substance dependence group (Dalwani et al., 2011). In another all male sample, adolescents with substance use disorder (SUD) and conduct problems had less posterior cingulate cortex cortical thickness, which is critical to response inhibition. A positive association between lifetime conduct disorder symptoms and superior temporal gyrus thickness also was reported (Chumachenko et al., 2015). In an all female cohort, adolescent females with severe substance use and conduct problems were found to have significantly smaller overall whole brain gray matter volume, as well as smaller frontal (right DLPFC, left ventrolateral PFC, medial OFC, ACC) and parietal volumes relative to controls (Dalwani et al., 2015).
Evidence of altered white matter integrity, lower FA in superior longitudinal fasciculus, an area implicated in development of executive function, has been reported in adolescents with SUD (Thatcher et al., 2010). In a separate study, adolescents with SUD also displayed white matter disorganization in prefrontal and parietal areas, areas important for behavioral and affective regulation. Lower FA in PFC and parietal regions of interest was associated with greater psychological dysregulation and marijuana-related symptoms in particular in the poly-drug use group (Clark et al., 2012).
From functional imaging studies, adolescents who initiated alcohol, marijuana, nicotine, or other drug use after a substance naïve baseline assessment, showed a positive linear relationship between age of substance use onset and rsFC of the right frontoparietal network and the NAcc (Weissman et al., 2015). This coupling of reward and cognitive control networks may suggest a mechanism by which earlier onset of substance use influences the transition to substance use and dependence. Furthermore, adolescents with problematic substance use exhibited decreased activation in the bilateral ventral striatum during reward anticipation in the MID task (Schneider et al., 2012). Importantly, lower activation of the ventral striatum was significantly associated with greater risk taking in the problematic substance use group.
5. Summary
Over the past two decades, there has been a dramatic increase in knowledge regarding the adolescent brain – characterization of neurodevelopmental changes and milestones that have been captured non-invasively using a variety of MR techniques. Taken together with data from animal models that demonstrate adolescence to be a period of unique sensitivity to alcohol and drugs, there are increasing translational opportunities that will ultimately further our understanding of the consequences of alcohol and drug use on the adolescent brain.
Collectively, studies included in this review confirm that the most common alteration reported across all substances and MR modalities is in the frontal lobe (63% of all published studies). This is not surprising, given that the frontal lobe is the last region to reach neurobiological adulthood and executive functions continue to improve into adulthood. While the studies described here point towards neurotoxic effects of alcohol and drugs (e.g., neurobiological consequences of use), it is important to acknowledge that aberrant neurobiological signatures may have existed prior to the initiation of use. Observed effects could reflect contributions from antecedents of use such as 1) age of first use (Lisdahl et al., 2013a; Luciana et al., 2013; Weissman et al., 2015); 2) family history of addiction (Cohen-Gilbert et al., 2015; Cservenka, 2016; Cservenka et al., 2015a; Heitzeg et al., 2008; Hill et al., 2013a; Schweinsburg et al., 2004; Silveri et al., 2011; Silveri et al., 2004; Spadoni et al., 2013) (for review see (Cservenka, 2016)); 3) childhood maltreatment (Shin et al., 2013; Teicher and Samson, 2016); and/or 4) comorbid psychiatric conditions (De Bellis et al., 2005; Miguel-Hidalgo, 2013; Roberts et al., 2007). Furthermore, most of the available studies in this area are cross-sectional in nature, comparing users to non-users, which can limit data interpretations. An additional factor, likely influencing neurobiological effects of alcohol and drugs during adolescence, is sex differences. Imbedded within only a small number of studies (Caldwell et al., 2005; De Bellis et al., 2005; Fein et al., 2013; McQueeny et al., 2011; Medina et al., 2009; Squeglia et al., 2015), there is growing evidence supporting sex effects on the alcohol- and drug-related brain alterations reported during adolescence. Given the NIH mandate to include investigation of sex effects in studies on addictions and in psychiatry, as well as a burgeoning literature documenting sex-specific differences in brain maturation that likely predate alcohol and drug initiation, readers are directed to the excellent preclinical (c.f., Schultz and Sisk) and clinical (c.f., Gur and Gur) reviews of adolescence and sex differences included in this special issue. Fortunately, federally funded initiatives are now underway to more thoroughly examine risk factors for and the longitudinal impact of alcohol and drug use in large, multisite studies of adolescents: the National Consortium on Alcohol and NeuroDevelopment in Adolescence (N-CANDA) (Brown et al., 2015; Pfefferbaum et al., 2015; Sullivan et al., 2016) (cross-sequential design, ages 12–21), and the Adolescent Brain Cognitive Development (ABCD) Study (10,000 youth beginning at ages 9–10, examined over ten years). Together these national initiatives are combining state-of-the-art multimodal magnetic resonance techniques with cognitive and clinical assessments to examine factors that may precipitate alcohol and drug use, while providing information regarding consequence of initiating and continuing use.
Due to high rates of alcohol and drug co-use, including marijuana, nicotine, and other substances, it has been difficult to attribute abnormalities to any one substance in particular. Some work suggests greater impairments related to alcohol consumption, while other studies have identified more associations with marijuana use and minimal influence of alcohol use. Paradoxically, a few studies have found that users of both marijuana and alcohol appear more similar to controls than users of either substance alone. While it is possible that marijuana has some properties that attenuate alcohol-related neurotoxicity, this notion is not well supported by the extant literature, which offers substantial evidence of abnormalities in marijuana users who also drink. It is likely that there are important methodological differences leading to these contradictory results. Differences may be related to sample characteristics (age, racial/ethnic background, SES), timing and frequency of use, timing of scanning and inter-scan intervals, and psychiatric and other substance use histories, all of which are critical factors. In addition, it is plausible that use of one imaging methodology alone may be insufficient for fully delineating the complex nature of the independent and interactive influences of substances on various neural processes. On the other hand, it is noteworthy that the effects of alcohol, marijuana, nicotine and other drugs are evident among even relatively moderate users with no SUD, such as those meeting sub diagnostic criteria for binge drinking and light nicotine smokers.
Finally, although there is now plentiful evidence of abnormalities associated with adolescent alcohol, marijuana, and other drug use, relationships between neurocognition and patterns of continued use remain unclear. It remains to be determined to what degree neurobiological consequences may resolve with extended abstinence, or continue to decline with increasing use. To that end, more long-term prospective multimodal studies are needed to help elucidate the impacts of various trajectories of use in later life. Regardless of antecedent or consequence, important next steps should focus on a move towards treating adolescents with unhealthy substance use. Increasing initiatives to close the large gap between neuroscience and practitioners that provide clinical care and treatment for adolescent alcohol and substance use disorders (Feldstein Ewing et al., 2015) is clearly warranted, and should be a major priority over the next decade.
Highlights.
Studies described point towards neurotoxic effects of alcohol and drugs
Most published alteration in substance-using adolescents is in the frontal lobe
Brain effects of substance use evident among relatively moderate users
Difficult to parse substance-specific brain abnormalities given co-use
Priority to close gap between neuroscience and practitioners treating adolescents
Acknowledgments
The authors wish to thank Noa Golan for her contributions to this review. Preparation of this review was supported by R01 AA018153 (MMS), K01 DA038207 (AD), and K01 AA022392 (JCG). The content of this review is solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson A, Ray KL, Hines CS, Li K, Dawes MA, Mathias CW, Dougherty DM, Laird AR. Functional activation and effective connectivity differences in adolescent marijuana users performing a simulated gambling task. J Addict. 2015;2015:783106. doi: 10.1155/2015/783106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson V. Assessing executive functions in children: biological, psychological, and developmental considerationst. Pediatr Rehabil. 2001;4:119–136. doi: 10.1080/13638490110091347. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bava S, Frank LR, McQueeny T, Schweinsburg BC, Schweinsburg AD, Tapert SF. Altered white matter microstructure in adolescent substance users. Psychiatry Res. 2009;173:228–237. doi: 10.1016/j.pscychresns.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Mahmood O, Yang TT, Tapert SF. Neurocognitive correlates of white matter quality in adolescent substance users. Brain Cogn. 2010;72:347–354. doi: 10.1016/j.bandc.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Jacobus J, Thayer RE, Tapert SF. Longitudinal changes in white matter integrity among adolescent substance users. Alcohol Clin Exp Res. 2013;37(Suppl 1):E181–189. doi: 10.1111/j.1530-0277.2012.01920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan B, Connolly CG, Datwani S, Doucet M, Ivanovic J, Morioka R, Stone A, Watts R, Smyth B, Garavan H. Response inhibition and elevated parietal-cerebellar correlations in chronic adolescent cannabis users. Neuropharmacology. 2014;84:131–137. doi: 10.1016/j.neuropharm.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Bennett CM, Baird AA. Anatomical changes in the emerging adult brain: a voxel-based morphometry study. Hum Brain Mapp. 2006;27:766–777. doi: 10.1002/hbm.20218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitter SM, Weber WA, Chu WJ, Adler CM, Eliassen JC, Strakowski SM, DelBello MP. N-acetyl aspartate levels in adolescents with bipolar and/or cannabis use disorders. J Dual Diagn. 2014;10:39–43. doi: 10.1080/15504263.2013.869077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams BR, Peper JS, van der Heide D, Peters S, Crone EA. Nucleus accumbens response to rewards and testosterone levels are related to alcohol use in adolescents and young adults. Dev Cogn Neurosci. 2016;17:83–93. doi: 10.1016/j.dcn.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Dalvie S, Cuzen NL, Cardenas V, Fein G, Stein DJ. Childhood adversity is linked to differential brain volumes in adolescents with alcohol use disorder: a voxel-based morphometry study. Metab Brain Dis. 2014;29:311–321. doi: 10.1007/s11011-014-9489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, De Bellis MD, Hooper SR, Clark DB, Chung T, Hasler BP, Colrain IM, Baker FC, Prouty D, Pfefferbaum A, Sullivan EV, Pohl KM, Rohlfing T, Nichols BN, Chu W, Tapert SF. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. J Stud Alcohol Drugs. 2015;76:895–908. doi: 10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Lim KO, Kumra S. Adverse Effects of Cannabis on Adolescent Brain Development: A Longitudinal Study. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Greenstein D, Fouche JP, Ferrett H, Cuzen N, Stein DJ, Fein G. Not lesser but Greater fractional anisotropy in adolescents with alcohol use disorders. Neuroimage Clin. 2013;2:804–809. doi: 10.1016/j.nicl.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Curr Opin Neurobiol. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM. Neurobiology of the adolescent brain and behavior: implications for substance use disorders. J Am Acad Child Adolesc Psychiatry. 2010;49:1189–1201. doi: 10.1016/j.jaac.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons J, Yucel M, Lubman DI. Volumetric differences in the anterior cingulate cortex prospectively predict alcohol-related problems in adolescence. Psychopharmacology (Berl) 2014;231:1731–1742. doi: 10.1007/s00213-014-3483-8. [DOI] [PubMed] [Google Scholar]
- Cheetham A, Allen NB, Whittle S, Simmons JG, Yucel M, Lubman DI. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol Psychiatry. 2012;71:684–692. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Chumachenko SY, Sakai JT, Dalwani MS, Mikulich-Gilbertson SK, Dunn R, Tanabe J, Young S, McWilliams SK, Banich MT, Crowley TJ. Brain cortical thickness in male adolescents with serious substance use and conduct problems. Am J Drug Alcohol Abuse. 2015;41:414–424. doi: 10.3109/00952990.2015.1058389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Carey PD, Ferrett HL, Stein DJ, Yurgelun-Todd DA. Abnormal striatal circuitry and intensified novelty seeking among adolescents who abuse methamphetamine and cannabis. Dev Neurosci. 2012;34:310–317. doi: 10.1159/000337724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Chung T, Thatcher DL, Pajtek S, Long EC. Psychological dysregulation, white matter disorganization and substance use disorders in adolescence. Addiction. 2012;107:206–214. doi: 10.1111/j.1360-0443.2011.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gilbert JE, Jensen JE, Silveri MM. Contributions of magnetic resonance spectroscopy to understanding development: potential applications in the study of adolescent alcohol use and abuse. Dev Psychopathol. 2014;26:405–423. doi: 10.1017/S0954579414000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Gilbert JE, Sneider JT, Crowley DJ, Rosso IM, Jensen JE, Silveri MM. Impact of family history of alcoholism on glutamine/glutamate ratio in anterior cingulate cortex in substance-naive adolescents. Dev Cogn Neurosci. 2015;16:147–154. doi: 10.1016/j.dcn.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan RL. Neuroimaging research in human MDMA users: a review. Psychopharmacology (Berl) 2007;189:539–556. doi: 10.1007/s00213-006-0467-3. [DOI] [PubMed] [Google Scholar]
- Cservenka A. Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend. 2016;158:8–21. doi: 10.1016/j.drugalcdep.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Gillespie AJ, Michael PG, Nagel BJ. Family history density of alcoholism relates to left nucleus accumbens volume in adolescent girls. J Stud Alcohol Drugs. 2015a;76:47–56. [PMC free article] [PubMed] [Google Scholar]
- Cservenka A, Jones SA, Nagel BJ. Reduced cerebellar brain activity during reward processing in adolescent binge drinkers. Dev Cogn Neurosci. 2015b;16:110–120. doi: 10.1016/j.dcn.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dager AD, McKay DR, Kent JW, Jr, Curran JE, Knowles E, Sprooten E, Goring HH, Dyer TD, Pearlson GD, Olvera RL, Fox PT, Lovallo WR, Duggirala R, Almasy L, Blangero J, Glahn DC. Shared genetic factors influence amygdala volumes and risk for alcoholism. Neuropsychopharmacology. 2015;40:412–420. doi: 10.1038/npp.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE. Adolescent development and the regulation of behavior and emotion: introduction to part VIII. Ann N Y Acad Sci. 2004;1021:294–295. doi: 10.1196/annals.1308.034. [DOI] [PubMed] [Google Scholar]
- Dalwani M, Sakai JT, Mikulich-Gilbertson SK, Tanabe J, Raymond K, McWilliams SK, Thompson LL, Banich MT, Crowley TJ. Reduced cortical gray matter volume in male adolescents with substance and conduct problems. Drug Alcohol Depend. 2011;118:295–305. doi: 10.1016/j.drugalcdep.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalwani MS, McMahon MA, Mikulich-Gilbertson SK, Young SE, Regner MF, Raymond KM, McWilliams SK, Banich MT, Tanabe JL, Crowley TJ, Sakai JT. Female adolescents with severe substance and conduct problems have substantially less brain gray matter volume. PLoS One. 2015;10:e0126368. doi: 10.1371/journal.pone.0126368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent- onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, Payne ME, MacFall J. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol Clin Exp Res. 2008;32:395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Wang L, Bergman SR, Yaxley RH, Hooper SR, Huettel SA. Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug Alcohol Depend. 2013;133:134–145. doi: 10.1016/j.drugalcdep.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism - Toward a unified theory of cognitive-development and aging. Dev Rev. 1992;12:45–75. [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–20. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Elofson J, Gongvatana W, Carey KB. Alcohol use and cerebral white matter compromise in adolescence. Addict Behav. 2013;38:2295–2305. doi: 10.1016/j.addbeh.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein KA, Kumra S. Executive attention impairment in adolescents with schizophrenia who have used cannabis. Schizophr Res. 2014;157:48–54. doi: 10.1016/j.schres.2014.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein KA, Kumra S. Altered cortical maturation in adolescent cannabis users with and without schizophrenia. Schizophr Res. 2015a;162:143–152. doi: 10.1016/j.schres.2014.11.029. [DOI] [PubMed] [Google Scholar]
- Epstein KA, Kumra S. White matter fractional anisotropy over two time points in early onset schizophrenia and adolescent cannabis use disorder: A naturalistic diffusion tensor imaging study. Psychiatry Res. 2015b;232:34–41. doi: 10.1016/j.pscychresns.2014.10.010. [DOI] [PubMed] [Google Scholar]
- Ernst M, Mueller SC. The adolescent brain: insights from functional neuroimaging research. Dev Neurobiol. 2008;68:729–743. doi: 10.1002/dneu.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Torrisi S, Balderston N, Grillon C, Hale EA. fMRI functional connectivity applied to adolescent neurodevelopment. Annu Rev Clin Psychol. 2015;11:361–377. doi: 10.1146/annurev-clinpsy-032814-112753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Greenstein D, Cardenas VA, Cuzen NL, Fouche JP, Ferrett H, Thomas K, Stein DJ. Cortical and subcortical volumes in adolescents with alcohol dependence but without substance or psychiatric comorbidities. Psychiatry Res. 2013;214:1–8. doi: 10.1016/j.pscychresns.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Tapert SF, Molina BS. Uniting adolescent neuroimaging and treatment research: Recommendations in pursuit of improved integration. Neurosci Biobehav Rev. 2015 doi: 10.1016/j.neubiorev.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. Epub 2004 May 8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg MM, Nigg JT, Yau WY, Zubieta JK, Zucker RA. Affective circuitry and risk for alcoholism in late adolescence: differences in frontostriatal responses between vulnerable and resilient children of alcoholic parents. Alcohol Clin Exp Res. 2008;32:414–426. doi: 10.1111/j.1530-0277.2007.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Terwilliger R, McDermott M. White matter microstructure, alcohol exposure, and familial risk for alcohol dependence. Psychiatry Res. 2013a;212:43–53. doi: 10.1016/j.pscychresns.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Carter H, McDermott MD, Zezza N, Stiffler S. Amygdala Volume in Offspring from Multiplex for Alcohol Dependence Families: The Moderating Influence of Childhood Environment and 5-HTTLPR Variation. J Alcohol Drug Depend. 2013b;(Suppl 1) doi: 10.4172/2329-6488.S1-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SB, Choi EJ, Kim HH, Suh JE, Takagi MJ, Lubman DI, Kim JW, Kim CD, Yi SH, Yucel M. Decreased thalamic volumes in adolescent inhalant users from Korea and Australia. World J Biol Psychiatry. 2014;15:636–640. doi: 10.3109/15622975.2014.902540. [DOI] [PubMed] [Google Scholar]
- Houck JM, Bryan AD, Feldstein Ewing SW. Functional connectivity and cannabis use in high-risk adolescents. Am J Drug Alcohol Abuse. 2013;39:414–423. doi: 10.3109/00952990.2013.837914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Westerveld M, Pugh KR. Impact of cannabis use on brain function in adolescents. Ann N Y Acad Sci. 2004;1021:384–390. doi: 10.1196/annals.1308.053. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Picciotto MR, Heath CJ, Frost SJ, Tsou KA, Dwan RA, Jackowski MP, Constable RT, Mencl WE. Prenatal and adolescent exposure to tobacco smoke modulates the development of white matter microstructure. J Neurosci. 2007a;27:13491–13498. doi: 10.1523/JNEUROSCI.2402-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biol Psychiatry. 2007b;61:31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Slotkin TA, Mencl WE, Frost SJ, Pugh KR. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007c;32:2453–2464. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- Jacobus J, Goldenberg D, Wierenga CE, Tolentino NJ, Liu TT, Tapert SF. Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology (Berl) 2012;222:675–684. doi: 10.1007/s00213-012-2674-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LR, Yang TT, Tapert SF. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicol Teratol. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Bava S, Tapert SF. White matter characterization of adolescent binge drinking with and without co-occurring marijuana use: a 3-year investigation. Psychiatry Res. 2013a;214:374–381. doi: 10.1016/j.pscychresns.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Infante MA, Bava S, Tapert SF. White matter integrity pre- and post marijuana and alcohol initiation in adolescence. Brain Sci. 2013b;3:396–414. doi: 10.3390/brainsci3010396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Meruelo AD, Castro N, Brumback T, Giedd JN, Tapert SF. Cortical thickness in adolescent marijuana and alcohol users: A three-year prospective study from adolescence to young adulthood. Dev Cogn Neurosci. 2015;16:101–109. doi: 10.1016/j.dcn.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Squeglia LM, Sorg SF, Nguyen-Louie TT, Tapert SF. Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. J Stud Alcohol Drugs. 2014;75:729–743. doi: 10.15288/jsad.2014.75.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Thayer RE, Trim RS, Bava S, Frank LR, Tapert SF. White matter integrity, substance use, and risk taking in adolescence. Psychol Addict Behav. 2013c;27:431–442. doi: 10.1037/a0028235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry. 2010;49:561–572. 572.e561–563. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Institute for Social Research. The University of Michigan; Ann Arbor: 2016. Monitoring the Future national survey results on drug use, 1975–2015 Overview, key findings on adolescent drug use. [Google Scholar]
- Karoly HC, Bryan AD, Weiland BJ, Mayer A, Dodd A, Feldstein Ewing SW. Does incentive-elicited nucleus accumbens activation differ by substance of abuse? An examination with adolescents. Dev Cogn Neurosci. 2015;16:5–15. doi: 10.1016/j.dcn.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna N, Altmeyer W, Zhuo J, Steven A. Functional Neuroimaging: Fundamental Principles and Clinical Applications. Neuroradiol J. 2015;28:87–96. doi: 10.1177/1971400915576311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenberg L, Korkman M, Lahti-Nuuttila P. Differential development of attention and executive functions in 3- to 12-year-old Finnish children. Dev Neuropsychol. 2001;20:407–428. doi: 10.1207/S15326942DN2001_6. [DOI] [PubMed] [Google Scholar]
- Kumra S, Robinson P, Tambyraja R, Jensen D, Schimunek C, Houri A, Reis T, Lim K. Parietal lobe volume deficits in adolescents with schizophrenia and adolescents with cannabis use disorders. J Am Acad Child Adolesc Psychiatry. 2012;51:171–180. doi: 10.1016/j.jaac.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Leland DS, Arce E, Feinstein JS, Paulus MP. Young adult stimulant users’ increased striatal activation during uncertainty is related to impulsivity. Neuroimage. 2006;33:725–731. doi: 10.1016/j.neuroimage.2006.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Gilbart ER, Wright NE, Shollenbarger S. Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry. 2013a;4:53. doi: 10.3389/fpsyt.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Thayer R, Squeglia LM, McQueeny TM, Tapert SF. Recent binge drinking predicts smaller cerebellar volumes in adolescents. Psychiatry Res. 2013b;211:17–23. doi: 10.1016/j.pscychresns.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Wright NE, Kirchner-Medina C, Maple KE, Shollenbarger S. The effects of regular cannabis use on neurocognition in adolescents and young adults. Curr Addiction Rep. 2014;1:144–156. doi: 10.1007/s40429-014-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behav Brain Res. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Rogowska J, Bogorodzki P, Bueler CE, McGlade EC, Yurgelun-Todd DA. Cortico-cerebellar abnormalities in adolescents with heavy marijuana use. Psychiatry Res. 2012;202:224–232. doi: 10.1016/j.pscychresns.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Rogowska J, Yurgelun-Todd D. Aberrant orbitofrontal connectivity in marijuana smoking adolescents. Dev Cogn Neurosci. 2015;16:54–62. doi: 10.1016/j.dcn.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Fornito A, Lubman DI, Yucel M. The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Curr Pharm Des. 2014;20:2138–2167. doi: 10.2174/13816128113199990435. [DOI] [PubMed] [Google Scholar]
- Luciana M. Adolescent brain development in normality and psychopathology. Dev Psychopathol. 2013;25:1325–1345. doi: 10.1017/S0954579413000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Collins PF, Muetzel RL, Lim KO. Effects of alcohol use initiation on brain structure in typically developing adolescents. Am J Drug Alcohol Abuse. 2013;39:345–355. doi: 10.3109/00952990.2013.837057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone SM, Luciana M, Wilson S, Sparks JC, Hunt RH, Thomas KM, Iacono WG. Adolescent drinking and motivated decision-making: a cotwin-control investigation with monozygotic twins. Behav Genet. 2014;44:407–418. doi: 10.1007/s10519-014-9651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Padula CB, Price J, Medina KL, Logan P, Tapert SF. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res. 2011;224:128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueeny T, Schweinsburg BC, Schweinsburg AD, Jacobus J, Bava S, Frank LR, Tapert SF. Altered white matter integrity in adolescent binge drinkers. Alcohol Clin Exp Res. 2009;33:1278–1285. doi: 10.1111/j.1530-0277.2009.00953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Yang TT, Tapert SF. Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addict Biol. 2009;14:457–468. doi: 10.1111/j.1369-1600.2009.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Park A, McQueeny T, Tapert SF. Depressive symptoms in adolescents: associations with white matter volume and marijuana use. J Child Psychol Psychiatry. 2007a;48:592–600. doi: 10.1111/j.1469-7610.2007.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Res. 2010;182:152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007b;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhoff DJ, Durazzo TC, Ende G. Chronic alcohol consumption, abstinence and relapse: brain proton magnetic resonance spectroscopy studies in animals and humans. Curr Top Behav Neurosci. 2013;13:511–540. doi: 10.1007/7854_2011_131. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Brain structural and functional changes in adolescents with psychiatric disorders. Int J Adolesc Med Health. 2013;25:245–256. doi: 10.1515/ijamh-2013-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller F, Lenz C, Steiner M, Dolder PC, Walter M, Lang UE, Liechti ME, Borgwardt S. Neuroimaging in moderate MDMA use: A systematic review. Neurosci Biobehav Rev. 2016;62:21–34. doi: 10.1016/j.neubiorev.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Mukherjee P, Berman JI, Chung SW, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: theoretic underpinnings. AJNR Am J Neuroradiol. 2008a;29:632–641. doi: 10.3174/ajnr.A1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P, Chung SW, Berman JI, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: technical considerations. AJNR Am J Neuroradiol. 2008b;29:843–852. doi: 10.3174/ajnr.A1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr C, Morioka R, Behan B, Datwani S, Doucet M, Ivanovic J, Kelly C, Weierstall K, Watts R, Smyth B, Garavan H. Altered resting-state connectivity in adolescent cannabis users. Am J Drug Alcohol Abuse. 2013;39:372–381. doi: 10.3109/00952990.2013.848213. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula CB, McQueeny T, Lisdahl KM, Price JS, Tapert SF. Craving is associated with amygdala volumes in adolescent marijuana users during abstinence. Am J Drug Alcohol Abuse. 2015;41:127–132. doi: 10.3109/00952990.2014.966198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padula CB, Schweinsburg AD, Tapert SF. Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychol Addict Behav. 2007;21:478–487. doi: 10.1037/0893-164X.21.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Conrod PJ, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Martinot JL, Paus T, Poline JB, Robbins TW, Rietschel M, Smolka M, Strohle A, Struve M, Loth E, Schumann G, Buchel C, Consortium, I. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Peters S, Jolles DJ, Van Duijvenvoorde AC, Crone EA, Peper JS. The link between testosterone and amygdala-orbitofrontal cortex connectivity in adolescent alcohol use. Psychoneuroendocrinology. 2015;53:117–126. doi: 10.1016/j.psyneuen.2015.01.004. [DOI] [PubMed] [Google Scholar]
- Petersen ET, Zimine I, Ho YC, Golay X. Non-invasive measurement of perfusion: a critical review of arterial spin labelling techniques. Br J Radiol. 2006;79:688–701. doi: 10.1259/bjr/67705974. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiol Aging. 2006;27:994–1009. doi: 10.1016/j.neurobiolaging.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rohlfing T, Pohl KM, Lane B, Chu W, Kwon D, Nolan Nichols B, Brown SA, Tapert SF, Cummins K, Thompson WK, Brumback T, Meloy MJ, Jernigan TL, Dale A, Colrain IM, Baker FC, Prouty D, De Bellis MD, Voyvodic JT, Clark DB, Luna B, Chung T, Nagel BJ, Sullivan EV. Adolescent Development of Cortical and White Matter Structure in the NCANDA Sample: Role of Sex, Ethnicity, Puberty, and Alcohol Drinking. Cereb Cortex. 2015 doi: 10.1093/cercor/bhv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescot AP, Locatelli AE, Renshaw PF, Yurgelun-Todd DA. Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. Neuroimage. 2011;57:69–75. doi: 10.1016/j.neuroimage.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescot AP, Renshaw PF, Yurgelun-Todd DA. gamma-Amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug Alcohol Depend. 2013;129:232–239. doi: 10.1016/j.drugalcdep.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage AE, Lin AL, Olvera RL, Fox PT, Williamson DE. Resting-state regional cerebral blood flow during adolescence: associations with initiation of substance use and prediction of future use disorders. Drug Alcohol Depend. 2015;149:40–48. doi: 10.1016/j.drugalcdep.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RE, Roberts CR, Xing Y. Comorbidity of substance use disorders and other psychiatric disorders among adolescents: evidence from an epidemiologic survey. Drug Alcohol Depend. 2007;88(Suppl 1):S4–13. doi: 10.1016/j.drugalcdep.2006.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Young AD, Femia LA, Yurgelun-Todd DA. Cognitive and emotional components of frontal lobe functioning in childhood and adolescence. Ann N Y Acad Sci. 2004;1021:355–362. doi: 10.1196/annals.1308.045. [DOI] [PubMed] [Google Scholar]
- Rubinstein ML, Luks TL, Dryden WY, Rait MA, Simpson GV. Adolescent smokers show decreased brain responses to pleasurable food images compared with nonsmokers. Nicotine Tob Res. 2011a;13:751–755. doi: 10.1093/ntr/ntr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein ML, Luks TL, Moscicki AB, Dryden W, Rait MA, Simpson GV. Smoking-related cue-induced brain activation in adolescent light smokers. J Adolesc Health. 2011b;48:7–12. doi: 10.1016/j.jadohealth.2010.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, Banaschewski T, Barker GJ, Conrod P, Flor H, Garavan H, Heinz A, Ittermann B, Lathrop M, Loth E, Mann K, Martinot JL, Nees F, Paus T, Rietschel M, Robbins TW, Smolka MN, Spanagel R, Strohle A, Struve M, Schumann G, Buchel C, Consortium, I. Risk taking and the adolescent reward system: a potential common link to substance abuse. Am J Psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Curr Drug Abuse Rev. 2008a;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010a;44:111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res. 2008b;163:40–51. doi: 10.1016/j.pscychresns.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Cheung EH, Brown GG, Brown SA, Tapert SF. fMRI response to spatial working memory in adolescents with comorbid marijuana and alcohol use disorders. Drug Alcohol Depend. 2005;79:201–210. doi: 10.1016/j.drugalcdep.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Medina KL, McQueeny T, Brown SA, Tapert SF. The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. J Psychoactive Drugs. 2010b;42:401–412. doi: 10.1080/02791072.2010.10400703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Schweinsburg BC, Nagel BJ, Eyler LT, Tapert SF. Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction. 2011;106:564–573. doi: 10.1111/j.1360-0443.2010.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin SH, Miller DP, Teicher MH. Exposure to childhood neglect and physical abuse and developmental trajectories of heavy episodic drinking from early adolescence into young adulthood. Drug Alcohol Depend. 2013;127:31–38. doi: 10.1016/j.drugalcdep.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Cohen-Gilbert J, Crowley DJ, Rosso IM, Jensen JE, Sneider JT. Altered anterior cingulate neurochemistry in emerging adult binge drinkers with a history of alcohol-induced blackouts. Alcohol Clin Exp Res. 2014;38:969–979. doi: 10.1111/acer.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Rogowska J, McCaffrey A, Yurgelun-Todd DA. Adolescents at risk for alcohol abuse demonstrate altered frontal lobe activation during Stroop performance. Alcohol Clin Exp Res. 2011;35:218–228. doi: 10.1111/j.1530-0277.2010.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Sneider JT, Crowley DJ, Covell MJ, Acharya D, Rosso IM, Jensen JE. Frontal lobe gamma-aminobutyric acid levels during adolescence: associations with impulsivity and response inhibition. Biol Psychiatry. 2013;74:296–304. doi: 10.1016/j.biopsych.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveri MM, Tzilos GK, Pimentel PJ, Yurgelun-Todd DA. Trajectories of adolescent emotional and cognitive development: effects of sex and risk for drug use. Ann N Y Acad Sci. 2004;1021:363–370. doi: 10.1196/annals.1308.046. [DOI] [PubMed] [Google Scholar]
- Sneider JT, Mashhoon Y, Silveri MM. A Review of Magnetic Resonance Spectroscopy Studies in Marijuana using Adolescents and Adults. J Addict Res Ther. 2013;(Suppl 4) doi: 10.4172/2155-6105.S4-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Delis D, Stiles J, Jernigan TL. Improved memory functioning and frontal lobe maturation between childhood and adolescence: a structural MRI study. J Int Neuropsychol Soc. 2001;7:312–322. doi: 10.1017/s135561770173305x. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neuroscientist. 2004;10:372–392. doi: 10.1177/1073858404263960. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Simmons AN, Yang TT, Tapert SF. Family history of alcohol use disorders and neuromaturation: a functional connectivity study with adolescents. Am J Drug Alcohol Abuse. 2013;39:356–364. doi: 10.3109/00952990.2013.818680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spechler PA, Orr CA, Chaarani B, Kan KJ, Mackey S, Morton A, Snowe MP, Hudson KE, Althoff RR, Higgins ST, Cattrell A, Flor H, Nees F, Banaschewski T, Bokde AL, Whelan R, Buchel C, Bromberg U, Conrod P, Frouin V, Papadopoulos D, Gallinat J, Heinz A, Walter H, Ittermann B, Gowland P, Paus T, Poustka L, Martinot JL, Artiges E, Smolka MN, Schumann G, Garavan H. Cannabis use in early adolescence: Evidence of amygdala hypersensitivity to signals of threat. Dev Cogn Neurosci. 2015;16:63–70. doi: 10.1016/j.dcn.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Pulido C, Wetherill RR, Jacobus J, Brown GG, Tapert SF. Brain response to working memory over three years of adolescence: influence of initiating heavy drinking. J Stud Alcohol Drugs. 2012;73:749–760. doi: 10.15288/jsad.2012.73.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Rinker DA, Bartsch H, Castro N, Chung Y, Dale AM, Jernigan TL, Tapert SF. Brain volume reductions in adolescent heavy drinkers. Dev Cogn Neurosci. 2014;9:117–125. doi: 10.1016/j.dcn.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A. Brain development in heavy-drinking adolescents. Am J Psychiatry. 2015;172:531–542. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A behavioral scientist looks at the science of adolescent brain development. Brain Cogn. 2010;72:160–164. doi: 10.1016/j.bandc.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Brumback T, Tapert SF, Fama R, Prouty D, Brown SA, Cummins K, Thompson WK, Colrain IM, Baker FC, De Bellis MD, Hooper SR, Clark DB, Chung T, Nagel BJ, Nichols BN, Rohlfing T, Chu W, Pohl KM, Pfefferbaum A. Cognitive, Emotion Control, and Motor Performance of Adolescents in the NCANDA Study: Contributions From Alcohol Consumption, Age, Sex, Ethnicity, and Family History of Addiction. Neuropsychology. 2016 doi: 10.1037/neu0000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Rohlfing T, Baker FC, Padilla ML, Colrain IM. Developmental change in regional brain structure over 7 months in early adolescence: comparison of approaches for longitudinal atlas-based parcellation. Neuroimage. 2011;57:214–224. doi: 10.1016/j.neuroimage.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung YH, Carey PD, Stein DJ, Ferrett HL, Spottiswoode BS, Renshaw PF, Yurgelun-Todd DA. Decreased frontal N-acetylaspartate levels in adolescents concurrently using both methamphetamine and marijuana. Behav Brain Res. 2013;246:154–161. doi: 10.1016/j.bbr.2013.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M, Lubman DI, Walterfang M, Barton S, Reutens D, Wood A, Yucel M. Corpus callosum size and shape alterations in adolescent inhalant users. Addict Biol. 2013;18:851–854. doi: 10.1111/j.1369-1600.2011.00364.x. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT, Frank LR. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology (Berl) 2007;194:173–183. doi: 10.1007/s00213-007-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA. Annual Research Review: Enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry. 2016 doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher DL, Pajtek S, Chung T, Terwilliger RA, Clark DB. Gender differences in the relationship between white matter organization and adolescent substance use disorders. Drug Alcohol Depend. 2010;110:55–61. doi: 10.1016/j.drugalcdep.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer RE, Crotwell SM, Callahan TJ, Hutchison KE, Bryan AD. Nucleus accumbens volume is associated with frequency of alcohol use among juvenile justice-involved adolescents. Brain Sci. 2012;2:605–618. doi: 10.3390/brainsci2040605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC. World Drug Report. United Nations Publication; 2011. [Google Scholar]
- Weiland BJ, Thayer RE, Depue BE, Sabbineni A, Bryan AD, Hutchison KE. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. J Neurosci. 2015;35:1505–1512. doi: 10.1523/JNEUROSCI.2946-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DG, Schriber RA, Fassbender C, Atherton O, Krafft C, Robins RW, Hastings PD, Guyer AE. Earlier adolescent substance use onset predicts stronger connectivity between reward and cognitive control brain networks. Dev Cogn Neurosci. 2015;16:121–129. doi: 10.1016/j.dcn.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Castro N, Squeglia LM, Tapert SF. Atypical neural activity during inhibitory processing in substance-naive youth who later experience alcohol-induced blackouts. Drug Alcohol Depend. 2013a;128:243–249. doi: 10.1016/j.drugalcdep.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology (Berl) 2013b;230:663–671. doi: 10.1007/s00213-013-3198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, Barker GJ, Bokde AL, Buchel C, Carvalho FM, Conrod PJ, Flor H, Fauth-Buhler M, Frouin V, Gallinat J, Gan G, Gowland P, Heinz A, Ittermann B, Lawrence C, Mann K, Martinot JL, Nees F, Ortiz N, Paillere-Martinot ML, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Strohle A, Schumann G, Garavan H, Consortium, I. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185–189. doi: 10.1038/nature13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The five factor model and impulsivity: Using a structural model of personality to understand impulsivity. Pers Individ Dif. 2001;30:669–689. [Google Scholar]
- Williams BR, Ponesse JS, Schachar RJ, Logan GD, Tannock R. Development of inhibitory control across the life span. Dev Psychol. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Wilson S, Malone SM, Thomas KM, Iacono WG. Adolescent drinking and brain morphometry: A co-twin control analysis. Dev Cogn Neurosci. 2015;16:130–138. doi: 10.1016/j.dcn.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Bechara A, Gong Q, Huang X, Li X, Xue G, Wong S, Lu ZL, Palmer P, Wei Y, Jia Y, Johnson CA. Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychol Addict Behav. 2013;27:443–454. doi: 10.1037/a0027892. [DOI] [PubMed] [Google Scholar]
- Yucel M, Lorenzetti V, Suo C, Zalesky A, Fornito A, Takagi MJ, Lubman DI, Solowij N. Hippocampal harms, protection and recovery following regular cannabis use. Transl Psychiatry. 2016;6:e710. doi: 10.1038/tp.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Zalesky A, Takagi MJ, Bora E, Fornito A, Ditchfield M, Egan GF, Pantelis C, Lubman DI. White-matter abnormalities in adolescents with long-term inhalant and cannabis use: a diffusion magnetic resonance imaging study. J Psychiatry Neurosci. 2010;35:409–412. doi: 10.1503/jpn.090177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuncu Z, Zorlu N, Saatcioglu H, Basay B, Basay O, Zorlu PK, Kitis O, Gelal F. Abnormal white matter integrity and impairment of cognitive abilities in adolescent inhalant abusers. Neurotoxicol Teratol. 2015;47:89–95. doi: 10.1016/j.ntt.2014.11.009. [DOI] [PubMed] [Google Scholar]


