Abstract
Increased intestinal permeability has been observed in numerous human autoimmune diseases, including type-1 diabetes (T1D) and its' animal model, the BB-wor diabetic prone rat. We have recently described zonulin, a protein that regulates intercellular tight junctions. The objective of this study was to establish whether zonulin-dependent increased intestinal permeability plays a role in the pathogenesis of T1D. In the BB diabetic-prone rat model of T1D, intestinal intraluminal zonulin levels were elevated 35-fold compared to control BB diabetic-resistant rats. Zonulin up-regulation was coincident with decreased small intestinal transepithelial electrical resistance, and was followed by the production of autoantibodies against pancreatic beta cells, which preceded the onset of clinically evident T1D by ≈25 days. In those diabetic prone rats that did not progress to diabetes, both intraluminal zonulin and transepithelial electrical resistance were similar to those detected in diabetic-resistant animal controls. Blockade of the zonulin receptor reduced the cumulative incidence of T1D by 70%, despite the persistence of intraluminal zonulin up-regulation. Moreover, treatment responders did not seroconvert to islet cell antibodies. Combined together, these findings suggest that the zonulin-induced loss in small intestinal barrier function is involved in the pathogenesis of T1D in the BB diabetic-prone animal model.
Keywords: intestinal permeability, autoimmunity, non-self antigens, ussing chambers
The intestinal epithelium provides the largest mucosal barrier between the internal host and the external environment. Epithelial tight junctions (tj) serve as the principle gate through which intact macromolecules with preserved immunogenicity may cross the intestinal barrier (1). When the integrity of the intestinal epithelial barrier function is compromised (i.e., during prematurity, exposure to radiation, chemotherapy, microorganisms, and their products), intestinal permeability to macromolecules increases (2–4). Consequently, an immune response to environmental antigens may ensue, giving rise to either an autoimmune response in genetically susceptible individuals or tolerance. The specific cells involved in this immune response include antigen-presenting cells, T and natural T killer lymphocytes (NK), B lymphocytes, and plasma cells (5). These cells lie in close proximity to the intestinal epithelial barrier and facilitate immune responsiveness, especially in the presence of elevated intestinal epithelial permeability (6). Recent reports suggest that quantitative and/or qualitative deficiencies in a subset of NK cells occur in several autoimmune diseases, including type 1 diabetes (T1D) (7) and celiac disease (CD) (8). These cells play a pivotal role in the maintenance of immune tolerance by down-regulating the immune response to foreign antigens when they gain access beyond the intestinal mucosal barrier (6). Recent studies in humans and in animal models have linked the presence of increased intestinal permeability to the occurrence of autoimmune diseases (8–13). However, a causative role for the loss of the intestinal barrier function has not been definitively established.
Gastrointestinal (GI) symptoms in T1D have been generally ascribed to altered intestinal motility (14) secondary to autonomic neuropathy (15). However, more recent studies performed in both human subjects affected by T1D (16, 17) and the BB diabetic prone (BBDP) animal model of diabetes (13) suggest that altered intestinal permeability occurs in T1D before the onset of these complications. These observations are compatible with the concept that increased intestinal permeability secondary to alteration of intestinal tj could be involved in the genesis of T1D.
To meet the many diverse physiological challenges to which epithelia are subjected, intercellular tj must be capable of rapid and coordinated responses. This requires the presence of a complex regulatory system that orchestrates the state of tj assembly. Although it is well accepted that tj are dynamic structures, surprisingly little is known about their regulation. The discovery of zonula occludens toxin (Zot), a protein elaborated by Vibrio cholerae (18) and of its receptor (19), has shed some light on the intricate mechanisms involved in the modulation of the intestinal paracellular pathway (20) and led us to the discovery of its eukaryotic counterpart zonulin (21). This protein is involved in the innate immunity of the gut (3) and, when inappropriately up-regulated, appears to play a key role in the increased intestinal permeability and pathogenesis of autoimmune diseases such as CD (22). In this study, we used the combination of the Ussing chamber assay and a recently developed zonulin sandwich ELISA to study whether zonulin was responsible for this early increase in gut permeability typical of BBDP rats (13). Furthermore, we used a synthetic peptide, competitive inhibitor (FZI/0) (23) to confirm the role of zonulin in T1D pathogenesis and to possibly develop therapeutic and/or preventive interventions for autoimmune diseases characterized by leaky gut.
Materials and Methods
Animal Model. White male BB/Wor diabetes-prone (BBDP) and diabetes-resistant (BBDR) rats (age, 20–120 days) were obtained from Biomedical Research Models (Rutland, MA). According to Biomedical Research Models, 80% of BBDP rats present with clinically evident diabetes by age 80 days.
Ex Vivo Experiments. Age-matched male BBDP and BBDR rats (total n = 20) were anesthetized with ketamine and killed at increasing ages (20, 50, 75, and >100 days) by exsanguination following an experimental protocol approved by the University of Maryland Institutional Animal Care and Use Committee.
The abdominal wall was opened, small intestinal loops were isolated, and intraluminal lavage was performed by instillation of 0.5 ml of PBS into the proximal small intestine followed by aspiration. The aspirate was stored at –80°C until analysis of intraluminal zonulin was performed (see below). The small intestine was then opened along the mesenteric border, washed free of intestinal contents, and mounted in Ussing chambers.
Ussing Chamber Assay. Experiments were carried out as we have described (17, 19, 23). Briefly, male BBDP and BBDR rats (age range, 20–110 days) were killed as described above. Five-centimeter segments of intestine (jejunum, ileum, and colon) were removed, rinsed free of the intestinal content, and opened along the mesenteric border. Eight sheets of mucosa were mounted in lucite Ussing chambers, connected to a voltage clamp apparatus (EVC 4000, World Precision Instruments, Saratosa, FL), and bathed with freshly prepared buffer containing 53 mM NaCl, 5 mM KCl, 30.5 mM Na2SO, 30.5 mM mannitol, 1.69 mM Na2HPO4, 0.3 mM NaH2PO4, 1.25 mM CaCl2, 1.1 mM MgCl2, and 25 mM NaHCO3. The bathing solution was maintained at 37°C with water-jacketed reservoirs connected to a constant temperature circulating pump and gassed with 95% O2 and 5% CO2. Potential difference was measured, and short-circuit current and transepithelial electrical resistance (TEER) were calculated (17).
In Vivo Experiments. Twenty-day-old BBDP rats were randomized into two equal groups (n = 15 for each group). The drinking water supply of the treatment group consisted of autoclaved water supplemented with 10 μg/ml zonulin receptor blocker, FZI/0 and HCO–3 1.5 g/dl to buffer gastric acidity. The placebo group received autoclaved water plus HCO–3 1.5 g/dl. The drinking solutions were prepared freshly every day. The rats were housed in HEPA filter cages and fed a standard rat chow diet (Harlan Teklab Diet). In both groups, the total amount of water (including FZI/0 consumed by the treated group) and food intake was recorded daily, and weight gain was monitored weekly. Every 7 days, the rats were housed in metabolic cages, where their drinking water was supplemented with lactulose (900 mg) and mannitol (600 mg), two nonabsorbable sugars. Urine samples were collected for 24 h, lactulose and mannitol were quantified by HPLC, and the lactulose/mannitol (LA/MA) ratio was calculated as described (13). Blood glucose was determined weekly by using the OneTouch glucose monitoring system. Reagent strips for urinalysis were used to monitor urine glucose (Diastix) and ketones (Ketostix) weekly. The onset of diabetes was defined by appearance of urinary ketones, random blood glucose levels ≥250 mg/dl, and confirmed by fasting overnight blood glucose levels ≥200 mg/dl. Upon presentation of diabetes or by the experimental endpoint (age, 80–85 days), the rats were killed, blood was collected for antiislet cell antibodies (anti-ICA) determination, samples of intestinal lavage were collected for zonulin measurements, several organs were harvested and frozen for future analysis, and intestinal tissues were mounted in Ussing chambers to measure TEER as described (24).
Synthesis of the Zonulin Peptide Inhibitor FZI/0. The zonulin synthetic peptide inhibitor FZI/0, capable of competitively blocking the zonulin receptor and the consequent zonulin-dependent opening of the tj, was identified and prepared as described in structure–function analysis studies of the Zot/zonulin receptor binding motif (20, 21) and was obtained from Biopolymer Laboratories, University of Maryland (Baltimore).
Zonulin Sandwich ELISA. Zonulin concentration was determined by using a sandwich ELISA as described (3) with minor modifications. Briefly, plastic microtiter plates (Costar, Cambridge, MA) were coated with polyclonal rabbit, zonulin-specific anti-Zot antibodies (dilution 1:100) overnight at 4°C, and then blocked by incubation with 0.05% PBS–Tween 20 for 15 min at room temperature (RT). A standard curve was obtained by serial dilution of zonulin (0.78–50 ng/ml) in 0.05% PBS-Tween 20. Equal aliquots of each standard and test sample was pipetted into the wells and incubated for 1 h at RT. Unbound zonulin was washed out, and the wells were incubated by agitation with biotinylated anti-Zot antibodies for 1 h at RT. A color reaction was developed by adding 100 μl of Extra-Avidin (Sigma) diluted 1:20,000 in 0.1 M Tris·HCl/1 mM MgCl2/1% BSA, pH 7.3, for 15 min, followed by incubation with 100 μl of a solution containing 1 mg/ml of p-nitrophenyl-phosphate substrate (Sigma). Absorbance was read after 30 min in a spectrophotometer at 405 nm. To define the intra- and interassay precision of the ELISA-sandwich method, the coefficient of variation (CV) was calculated by using three replicates from two samples with different concentrations of zonulin, on three consecutive days. Our past experience generated an interassay ELISA CV of 9.8%. The CV of the intraassay test was 4.2% at day 1, 3.3% at day 2, and 2.9% at day 3. Zonulin concentration was expressed as ng/mg total protein detected in the tested samples (both serum and intestinal lavage).
Detection of Antiislet Antibodies. Serum IgG anti-ICA were measured by means of indirect immunofluorescence assay, using cryosections of rat pancreas derived from both nondiabetic BB and Wistar Hannover rats. The sections were incubated for 30 min with the undiluted rat's serum. After washing, sections were incubated for 30 min with fluorescein-labeled goat anti-rat IgG (Jackson ImmunoResearch catalog no. 112-095-006) diluted 1:100. The slides were washed and examined by using fluorescent microscopy. The immunoassay was considered positive in the presence of three islet cells tested positive per section in both BB- and Wistar Hannover-derived pancreas. All measurements were performed on coded samples that were operator blinded.
Statistical Analysis. All values are exposed as the mean ± SEM. Analysis of difference was determined by Student's t test for paired or unpaired varieties. Analysis of difference for the cumulative incidence of T1D was determined by using the Fisher least significant difference test. P < 0.05 was considered statistically significant.
Results
Role of Zonulin in the Genesis of Intestinal Permeability and Onset of T1D in the ex Vivo BB Rat Model. To determine the temporal relationship between changes in intraluminal secretion of zonulin, intestinal permeability, and serum glucose levels, both BBDP and BBDR rats were studied. There was no difference in intraluminal zonulin concentration between BBDP and BBDR animals at age 20 days (Fig. 1A). At age 50 days, a 4-fold increase in intraluminal zonulin was observed in the BBDP cohort relative to the BBDR rats, which progressed to a 35-fold increase by age 75 days (Fig. 1 A). For all ages studied, intraluminal zonulin was minimally present in the small intestine of the BBDR animals (Fig. 1 A).
Fig. 1.
Zonulin levels and serum glucose levels in both BBDR (A) and BBDP (B) rats at increasing ages. BBDP rats showed an increase in both intraluminal (squares) and serum (circles) zonulin starting from the age 50 days group, whereas differences in serum glucose (triangles, dotted line) were detected only in animals >75 days old. No significant changes were observed in BBDR rats in either zonulin (both serum and luminal) or serum glucose levels at any age group. n = 3–6 for each group. *, P < 0.01 compared to BBDR animals; **, P < 0.005 compared to BBDR animals.
In each animal group, serum zonulin levels correlated with intraluminal zonulin concentration changes (Fig. 1). At age >100 days, BBDP rats without T1D showed low zonulin levels (serum zonulin, 0.1 ± 0.2 ng/mg protein; luminal zonulin, 0.2 ± 0.2 ng/mg protein) and normal serum glucose levels (112 ± 12 mg/dl) compared to BBDP rats that developed T1D. In BBDP rats that developed T1D, increased intraluminal zonulin (age, 50 days) preceded hyperglycemia (age, 75 days) (Fig. 1B) and was coincident with decreased TEER (Fig. 2). No significant changes in TEER were observed between BBDP or BBDR rats at age 20 days (Fig. 2). At age 50 days, a significant decrease in small intestinal TEER was observed in BBDP rats as compared to BBDR animals; this remained significantly decreased in ileal tissues at 75 days (Fig. 2). No changes in TEER were observed in the colon of either BBDP or BBDR rats at any age interval (Fig. 2). These findings are consistent with previous reports (13), confirming that the intestinal permeability changes in these diabetic rats are confined to the small intestine. These changes parallel the known regional distribution of the zonulin intestinal receptor (24).
Fig. 2.
Intestinal resistance (TEER, Ω·cm2) in BBDP (filled bars) and BBDR (open bars) rats. No difference in TEER between BBDR and BBDP rats was observed at age 20 days, irrespective of the intestinal tract examined. By age 50 days, the TEER of the small intestine in BBDP animals was significantly lower in both the jejunum and ileum, whereas the colon showed no differences in TEER between the two groups. Significant differences in ileal TEER were observed also at age 75 days. n = 6 for each group. *, P < 0.05 compared to BBDR animals.
In Vivo Effect of Prolonged Administration of the Zonulin Inhibitor, FZI/0, on the Progression of T1D in BBDP Rats. To confirm the role of zonulin-dependent increased permeability in the pathogenesis of T1D in the BBDP rat model, animals were randomized to two treatment groups, those that received FZI/0 in their drinking water and a control, untreated group. Untreated animals that developed T1D showed an increase in intestinal permeability that was statistically significant starting from age 44 days (Fig. 3A) and that was temporally coincident with increased serum zonulin (Table 1). Conversely, animals treated with FZI/0 that did not develop T1D (average FZI/0 administration: 34.4 ± 6.4 μg per 100 g of body weight, see Table 1) did not show any appreciable increase in intestinal permeability (Fig. 3B), despite serum zonulin levels that were comparable to those detected in the untreated animals (Table 1).
Fig. 3.
In vivo intestinal permeability and serum glucose levels in BBDP rats. (A) Untreated BBDP animals that evolved to T1D showed an increase in intestinal permeability as measure by LA/MA ratio (squares) that became statistically significant at age 44 days (P < 0.05–0.002 age 44–72 days compared to age 30 days). These permeability changes were followed by a significant increase in serum glucose levels (diamonds) starting ≈2 weeks after the increase in intestinal permeability (P < 0.05–0.0001 age 65–72 days compared to age 30 days). (B) Conversely, BBDP rats treated with FZI/0 and that did not develop T1D had no changes in either intestinal permeability or serum glucose levels. The FZI/0-treated animals that developed diabetes (n = 4) and the untreated animals that did not develop diabetes (n = 3) were eliminated from the final analysis. Therefore, n for treated group = 11; n for untreated group = 12.
Table 1. Weight gain, water intake, serum zonulin levels, and presence of ICA in BBDP rats either untreated and with development of T1D (control) or treated with FZI/0 and with no development of T1D.
| Age, days
|
Weight gain, g/week
|
Water intake, ml/day
|
FZI/0 intake, μg/100 g of body weight
|
Serum zonulin, ng/mg protein
|
Serum ICA, % ICA-positive rats
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Control | FZI/0 | Control | FZI/0 | Control | FZI/0 | Control | FZI/0 | ||
| 30 | 11.4 ± 3.96 | 12.3 ± 2.94 | 28.2 ± 13.1 | 27.5 ± 9.9 | 22.0 | 0.2 ± 0.1 | 0.2 ± 0.2 | 0 | 0 |
| 37 | 17.5 ± 3.09 | 17.4 ± 2.86 | 42.6 ± 12.3 | 39.9 ± 11.8 | 31.9 | 0.7 ± 0.2 | 0.9 ± 0.4 | 9 | 0 |
| 44 | 23.9 ± 3.10 | 23.3 ± 3.44 | 46.3 ± 16.5 | 44.5 ± 14.4 | 35.6 | 2.2 ± 0.5 | 2.5 ± 0.7 | 50 | 0 |
| 51 | 29.6 ± 2.78 | 29.5 ± 3.12 | 50.5 ± 15.4 | 41.5 ± 11.7 | 33.2 | 4.8 ± 0.6 | 5.3 ± 0.9 | 83 | 9 |
| 58 | 31.5 ± 1.96 | 30.6 ± 3.61 | 47.0 ± 13.8 | 47.2 ± 14.2 | 37.8 | 4.7 ± 1.1 | 4.4 ± 1.3 | 92 | 9 |
| 65 | 35.8 ± 1.76 | 35.9 ± 2.80 | 56.9 ± 25.0 | 52.4 ± 20.4 | 41.9 | 4.9 ± 0.9 | 5.2 ± 1.6 | 92 | 9 |
| 72 | 37.5 ± 3.11 | 37.0 ± 4.37 | 59.4 ± 25.6 | 47.7 ± 17.9 | 38.2 | 4.7 ± 1.9 | 5.0 ± 1.5 | 92 | 9 |
In the untreated BBDP rats that developed T1D, serum glucose levels sharply increased starting ≈2 weeks after the onset of zonulin-dependent increased permeability (Fig. 3A). No significant changes in serum glucose levels were detected in non-T1D FZI/0-treated animals (Fig. 3B). No differences in weight gain and amount of water intake were observed between treated and untreated groups (Table 1) until the development of diabetes.
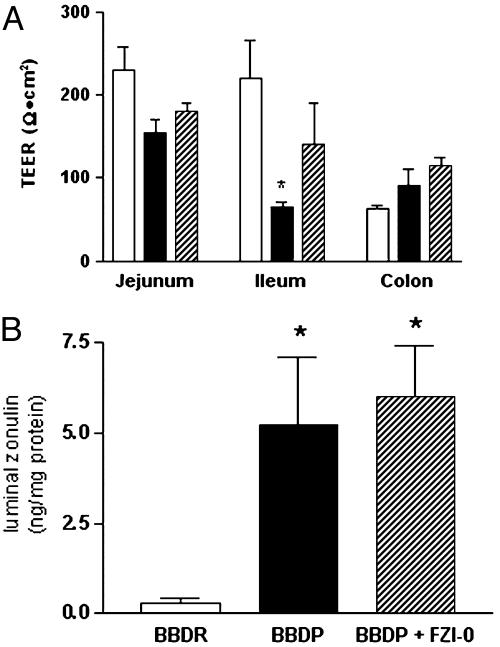
Inhibition of Zonulin-Mediated Increased Intestinal Permeability Reduced the Cumulative Incidence of T1D in BBDP Rats. Eighty percent of the untreated BBDP rats (12 of 15) progressed to the diabetic state at age 69.2 ± 2.9 days (25 days after zonulin-dependent increased intestinal permeability). Conversely, only 27% of the BBDP rats (4 of 15) treated with FZI/0 developed diabetes (P < 0.009). At either the onset of diabetes or the experimental endpoint (age, 80–85 days), animals were killed, and small intestine TEER and intraluminal zonulin were measured. BBDP rats that developed diabetes showed a significant decrement in ileal TEER as compared to BBDR rats, whereas BBDP rats treated with FZI/0 showed a TEER that was not statistically different from that detected in BBDR rats (Fig. 4A). Intraluminal zonulin was markedly elevated in BBDP rats as compared to BBDR rats, irrespective of the FZI/0 treatment (Fig. 4B). These results confirmed the correlation between intraluminal zonulin and TEER (r =–0.91, P < 0.002) and proved that FZI/0 prevented the onset of diabetes by preventing the changes in intestinal permeability (Fig. 3B) through the blockage of the zonulin receptor rather than affecting the zonulin release in the intestinal lumen.
Fig. 4.
In vivo effect of the zonulin inhibitor FZI/0 on TEER (A) and intestinal zonulin release (B) in BBDP rats. Untreated BBDP rats that developed T1D (filled bars) showed a significant decrease in ileal TEER and increase in intestinal luminal zonulin compared to BBDR animals (open bars) (A). FZI/0 treatment (dashed bars) in BBDP animals prevented the ileal TEER decrement (A) without affecting the intraluminal zonulin release (B). n = 7–15 for each group. *, P < 0.02.
Serum Antiislet Antibodies in Control Animals and Animals Treated with FZI/0. In addition to increased intestinal permeability, autoimmune destruction of pancreatic beta cells secondary to the generation of anti-ICA is also a prerequisite for the development of T1D. ICA were detected in the serum of untreated BBDP rats that developed diabetes (Fig. 5B and Table 1) but not in the FZI/0-treated BBDP rats that did not progress to diabetes (Fig. 5A and Table 1). These results suggest that, by blocking the zonulin-mediated increased permeability, the production of autoantibodies against the pancreatic beta cells was also blocked, thus preventing the evolution to T1D.
Fig. 5.

ICA in both FZI/0-treated rats and untreated diabetic animals. No ICA were detected in non-T1D-treated animals (A), whereas untreated rats that developed diabetes showed the presence of the autoantibodies in their serum (B).
Discussion
A normal intestinal mucosal immune response depends on a number of factors, including physical barrier functions, luminal digestion of potential antigens, selective antigen sampling sites, and unique T cell subpopulations that effect suppression. Defects in any individual component may predispose to intestinal inflammation, food allergy, or autoimmunity. Healthy, mature gut mucosa with its intact tj serves as the major organ of defense against foreign antigens, toxins, and macromolecules entering the host via the oral/enteric route. When the integrity of the tj system is compromised, such as during prematurity or exposure to radiation, chemotherapy, and/or toxins an immune response to environmental antigens (including autoimmune diseases and food allergies) may develop (25). The specific cells that are important for this immune response lie in close proximity to the luminal antigens and account for up to 80% of all Ig-producing cells in the body. Another important factor for the intestinal immunological responsiveness is the MHC. HLA class I and II genes are located in the MHC on chromosome 6. These genes code for glycoproteins, which bind peptides, and this HLA–peptide complex is recognized by certain T cell receptors in the intestinal mucosa (reviewed in ref. 25). Susceptibility to at least 50 diseases has been associated with specific HLA class I or II alleles. A common denominator of these diseases is the presence of several preexisting conditions leading to an autoimmune process. The first is a genetic susceptibility for the host immune system to recognize, and potentially misinterpret, an environmental antigen presented within the GI tract. Second, the host must be exposed to the antigen. Finally, the antigen must be presented to the GI mucosal immune system after its paracellular passage (normally prevented by the tj competency) from the intestinal lumen to the gut submucosa. In all cases, increased permeability appears to precede disease and causes an abnormality in antigen delivery that triggers the multiorgan process leading to the autoimmune response.
This temporal relationship has been described in T1D both in BBDP rat animal model (13) and in humans with T1D (15, 16, 26). However, the mediator(s) responsible for the loss of this intestinal barrier function are currently not known. With this study, we have generated evidence suggesting that zonulin, a physiologic modulator of intestinal tj involved in innate immunity (3), is a key step in the pathway leading to the aberrant intestinal permeability observed in the BBDP rats.
The increase in intraluminal zonulin observed in this study was found to: (i) correlate with serum zonulin levels (Fig. 1), (ii) be age-related (Fig. 1), (ii) correlate with an increase in intestinal permeability (Figs. 2 and 3), (iv) precede the onset of diabetes by at least 3 weeks (Figs. 1 and 3), (v) remain high in these BBDP rats (Fig. 1 and Table 1), and (vi) correlate with the progression toward clinically evident diabetes (Fig. 1). These observations suggest a role for zonulin in the pathogenesis of T1D in BBDP rat, prompting us to design in vivo experiments to confirm this hypothesis. Our results using the zonulin inhibitor FZI/0 confirmed a direct link between zonulin up-regulation, loss of the intestinal barrier function, and progression toward T1D (Figs. 3, 4, 5).
Both ex vivo (Fig. 1 and 2) and in vivo (Fig. 3) studies showed significant decreases in small intestinal TEER, starting at age 40–50 days, in rats destined to develop T1D. At this age, two distinct yet interconnected events occurred in the BBDP animals. Intestinal secretion of zonulin increased (4-fold by age 50 days and 35-fold by age 75 days compared to BBDR rats) and a concomitant loss of resistivity of the small intestine was detected. Neither of these pathophysiologic events was observed in the BBDR rats or in BBDP rats that did not progress to develop diabetes. Furthermore, persistently elevated zonulin and decreased small intestinal TEER were followed after 2–3 weeks by seroconversion, hyperglycemia, and onset of clinically evident T1D.
These results suggest that an as yet unidentified trigger(s) is responsible for inappropriate zonulin secretion starting at age 40–50 days in genetically susceptible rats. Among others, dietary proteins are possible triggers stimulating zonulin secretion (27), as also suggested by the observation that BBDP rats fed with hydrolyzed chow have a reduced incidence of T1D (13). In the NOD mouse model of T1D, gluten has been identified as a potential trigger of the autoimmune process (28). In CD, gluten causes increased secretion of zonulin, promoting increased intestinal permeability with continuous exposure of the GI immune system to gluten and other environmental antigens (27). Restriction of gluten reverses small intestinal epithelial damage, restores tj integrity, serum zonulin levels are reduced and intestinal permeability returns to baseline (A.F, unpublished data). CD and T1D are comorbid diseases, as the prevalence of CD among patients with T1D is 6- to 9-fold higher than the general population (29, 30). Two independent studies tracking large cohorts of newborns at high risk for T1D showed that the odds ratio for developing the disease was 4- to 5-fold higher in subjects prematurely exposed (<3 months of age) to gluten (31, 32). One possible explanation for these observations is that gluten, a protein introduced in large quantities in the human diet only after the advent of agriculture, activates the mechanism of zonulin innate immunity (3, 27, 33). In genetically susceptible individuals, this activation would lead to sustained zonulin up-regulation, resulting in the loss of the intestinal barrier function.
Although the link between activation of the GI immune system and pancreatic beta cell destruction is incompletely understood, current knowledge suggests that antigens are presented to the gut-associated lymphoid tissue (GALT) through the paracellular pathway (6). Lymphocytes are known to circulate between GALT, lymph nodes, and other tissues. Migration of lymphocytes into the pancreas appears to be mediated through mucosal vascular addressin (MAdCAM-1) and α4β7 integrin, a gut-specific homing receptor for addressin (34) that is highly expressed in beta cell reactive lymphocytes of T1D patients. MAdCAM-1 is specifically implicated in the homing and recirculation of lymphocytes in the early phase of T1D in NOD mice (35), and its' inhibition before the onset of insulinitis results in reduced incidence of T1D (35). Conversely, inhibition of α4-integrin blocked the spontaneous development of T1D and passive transfer of diabetes from splenic lymphocytes of diabetic mice (36).
Immunomodulation may represent an additional but not mutually exclusive mechanism through which zonulin may influence the autoimmune process in genetically susceptible individuals. We have recently demonstrated that macrophages and, to a lesser extent, lymphocytes are sensitive to zonulin. Specifically, we have shown that activation of the zonulin pathway in human macrophages influences cell-mediated antigen presentation (37). We have also demonstrated that zonulin pathway activation causes an increase in CD3+, CD80+, and HLA-DR expression (M. T. De Magistris, M. Sztein, and A.F., unpublished data), changes that have recently been described in intestinal biopsies obtained from T1D patients (38). Therefore, it is conceivable to hypothesize that the effect of zonulin on both mucosal barrier function and macrophage-mediated antigen presentation and subsequent change in cytokine profile may play in concert in determining the switch from immune tolerance to autoimmunity.
The identification of zonulin pathway activation as a mediator of environmental antigen access to GALT in T1D provides critical information on the first step linking environmental exposures to priming of the GI immune system. Inhibition of the zonulin system may represent an innovative therapeutic tool for the prevention and possibly the treatment of T1D. Clinically, the onset of T1D coincides with the loss of a critical mass of beta cells, leading to dependence on exogenous insulin, and recent studies have suggested that, at time of first presentation, the average patient still possesses as much as 50% of their endogenous insulin production capacity (39). It is tantalizing to hypothesize that early in the diabetic state, the use of the zonulin inhibitor FZI/0 might preserve remaining beta cell function in these patients, thus reducing their insulin requirements and/or restoring euglycemia.
Acknowledgments
This work was supported in part by National Institutes of Health Grants DK-66630 and DK-48373 (to A.F.).
Abbreviations: tj, tight junctions; T1D, type I diabetes; GI, gastrointestinal; Zot, zonula occludens toxin; BBDP, BB/Wor diabetes-prone; BBDR, BB/Wor diabetes-resistant; TEER, transepithelial electrical resistance; ICA, islet cell antibody; CD, celiac disease.
References
- 1.Lee, V. (2000) Eur. J. Pharm. Sci. 11, S41–50. [DOI] [PubMed] [Google Scholar]
- 2.Buell, M. G. & Harding, R. K. (1989) Dig. Dis. Sci. 34, 390–399. [DOI] [PubMed] [Google Scholar]
- 3.El Asmar, R., Panigrahi, P., Bamford, P., Berti, I., Not, I., Coppa, G. V., Catassi, C. & Fasano, A. (2002) Gastroenterology 123, 1607–1615. [DOI] [PubMed] [Google Scholar]
- 4.Shulman, R. J., Schanler, R. J., Lau, C., Heitkemper, M., Ou, C. N. & Smith, E. O. (1998) Pediatr. Res. 44, 519–523. [DOI] [PubMed] [Google Scholar]
- 5.Stagg, A. J., Hart, A. L., Knight, S. C. & Kamm, M. A. (2004) Best Pract. Res. Clin. Gastroenterol. 18, 255–270. [DOI] [PubMed] [Google Scholar]
- 6.DeMeo, M. T., Mutlu, E. A., Keshavarzian, A. & Tobin, M. C. (2002) J. Clin. Gastroenterol. 34, 385–396. [DOI] [PubMed] [Google Scholar]
- 7.Todd, D. J., Forsberg, E. M., Greiner, D. L., Mordes, J. P., Rossini, A. A. & Bortell, R. (2004) J. Immunol. 172, 5356–5362. [DOI] [PubMed] [Google Scholar]
- 8.Camarero, C., Eiras, P., Asenio, A., Leon, F., Olivares, F., Escobar, H. & Roy, G. (2000) Acta Paediatr. 89, 285–290. [PubMed] [Google Scholar]
- 9.Caserta, L., De Magistris, L., Secondulfo, M., Caravelli, G., Riegler, G., Cuomo, G., D'Angelo, S., Naclerio, C., Valentini, G. & Carratu, R. (2003) Rheumatol. Int. 23, 226–230. [DOI] [PubMed] [Google Scholar]
- 10.Di Leo, V., Venturi, C., Baragiotta, A., Martines, D. & Floreani, A. (2003) Eur. J. Gastroenterol. Hepatol. 15, 967–973. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton, I., Cobden, I., Rothwell, J. & Axon, A. T. (1982) Gut. 23, 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovacs, T., Kun, L., Schmelczer, M., Wagner, L., Davin, J. C. & Nagy, J. (1996) Am. J. Nephrol. 16, 500–505. [DOI] [PubMed] [Google Scholar]
- 13.Meddings, J. B., Jarand, J., Urbanski, S. J., Hardin, J., Gall, D. G. (1999) Am. J. Physiol. 276, G951–G957. [DOI] [PubMed] [Google Scholar]
- 14.Feldman, M. & Schiller, L. R. (1983) Ann. Intern. Med. 98, 378–384. [DOI] [PubMed] [Google Scholar]
- 15.Ellenberg, M. (1980) Mt. Sinai J. Med. 47, 561–567. [PubMed] [Google Scholar]
- 16.Damci, T., Nuhoglu, I., Devranoglu, G., Osar, Z., Demir, M. & Ilkova, H. (2003) Eur. J. Clin. Invest. 33, 397–401. [DOI] [PubMed] [Google Scholar]
- 17.Secondulfo, M., Iafusco, D., Carratu, R., deMagistris, L., Sapone, A., Generoso, M., Mezzogiomo, A., Sasso, F. C., Carteni, M., De Rosa, R., et al. (2004) Dig. Liver Dis. 36, 35–45. [DOI] [PubMed] [Google Scholar]
- 18.Fasano, A., Baudry, B., Pumplin, D. W., Wasserman, S. S., Tall, B. D., Ketley, J. M. & Kaper, J. B. (1991) Proc. Natl. Acad. Sci. USA 88, 5242–5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uzzau, S., Lu, R., Wang, W., Fiore, C. & Fasano, A. (2001) FEMS Microbiol. Lett. 194, 1–5. [DOI] [PubMed] [Google Scholar]
- 20.Fasano, A., Fiorentini, C., Donelli, G., Uzzau, S., Kaper, J. B., Margaretten, K., Ding, X., Guandalini, S., Comstock, L. & Goldblum, S. E. (1995) J. Clin. Invest. 86, 710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang, W., Uzzau, S., Goldblum, S. E. & Fasano, A. (2001) J. Cell Sci. 113, 4425–4440. [DOI] [PubMed] [Google Scholar]
- 22.Fasano, A., Not, T., Wang, W., Uzzau, S., Berti, I., Tommasini, A. & Goldblum, S. E. (2000) Lancet 355, 1518–1519. [DOI] [PubMed] [Google Scholar]
- 23.Di Pierro, M., Lu, R., Uzzau, S., Wang, W., Margaretten, K., Pazzani, C., Maimone, F. & Fasano, A. (2001) J. Biol. Chem. 276, 19160–19165. [DOI] [PubMed] [Google Scholar]
- 24.Fasano, A., Uzzau, S., Fiore, C. & Margaretten, K. (1997) Gastroenterology 112, 839–846. [DOI] [PubMed] [Google Scholar]
- 25.Fasano, A. (2001) in Tight Junctions, eds. Cereijido, M. & Anderson, J. M. (CRC Press, Boca Raton, FL), pp. 697–722.
- 26.Kuitunen, M., Saukkonen, T., Ilonen, J., Akerblom, H. K. & Savilahti, E. (2002) Autoimmunity 35, 365–368. [DOI] [PubMed] [Google Scholar]
- 27.Clemente, M. G., De Virgiliis, S., Macatagney, R. & Fasano, A. (2003) Gut 52, 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funda, D. P., Kaas, A., Bocl, T., Tlaskalova-Hogenova, H. & Buschard, K. (1999) Diabetes Metab. Res. Rev. 15, 323–327. [DOI] [PubMed] [Google Scholar]
- 29.Collin, P., Salmi, J., Hallstrom, O., Oksa, H., Oksala, H., Maki, M. & Reunala, T. (1989) Scand. J. Gastroenterol. 24, 81–84. [DOI] [PubMed] [Google Scholar]
- 30.Kumar, V., Rajadhyaksha, M. & Wortsman, J. (2001) Clin. Diagn. Lab. Immunol. 8, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norris, J. M., Barriga, K., Klingensmith, G., Hoffman, M., Eisenbarth, G. S., Erlich, H. A. & Rewers, M. (2003) J. Am. Med. Assoc. 290, 1713–1720. [DOI] [PubMed] [Google Scholar]
- 32.Ziegler, A. G., Schmid, S., Huber, D., Hummel, M. & Bonifacio, E. (2003) J. Am. Med. Assoc. 290, 1721–1728. [DOI] [PubMed] [Google Scholar]
- 33.Fasano, A. (2003) N. Engl. J. Med. 348, 2568–2570. [DOI] [PubMed] [Google Scholar]
- 34.Paronen, J., Klemetti, P., Kantele, J. M., Savilahti, E., Perheentupa, J., Akerblom, H. K. & Vaarala, O. (1997) Diabetes 46, 583–588. [DOI] [PubMed] [Google Scholar]
- 35.Hanninen, A., Jaakkola, I. & Jalkanen, S. (1998) J. Immunol. 160, 6018–6025. [PubMed] [Google Scholar]
- 36.Yang, X. D., Michie, S. A., Roland, T., Karin, N., Steinman, L. & McDevitt, H. O. (1994) Proc. Natl. Acad. Sci. USA 91, 12604–12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fasano, A., Sztein, M., Lu, R. & Tanner, M. (2004) U.S. Patent no. 6,733,562 B1.
- 38.Auricchio, R., Paparo, F., Maglio, M., Franzese, A., Lombardi, F., Valerio, G., Nardone, G., Percopo, S., Greco, L. & Troncone, R. (2004) Diabetes 53, 1680–1683. [DOI] [PubMed] [Google Scholar]
- 39.Steele, C., Hagopian, W. A., Gitelman, S., Masharani, U., Cavaghan, M. Rother, K. I., Donaldson, D., Harlan, D. M., Bluestone, J. & Herold, K. C. (2004) Diabetes 53, 426–433. [DOI] [PubMed] [Google Scholar]