Abstract
This panel was developed to measure the functional capability of natural killer (NK) cell subsets in rhesus macaques (Macaca mulatta). It includes markers to determine the frequency of cytokine secreting and cytotoxic NK cell subpopulations in peripheral blood mononuclear cell (PBMC) samples stimulated in vitro with human 721.221 cells. NK cell subsets were defined by the expression of killer cell immunoglobulin-like receptors (KIRs) Mamu-KIR3DL01 and Mamu-KIR3DL05, and differentiation antigens CD16 and CD56. The panel can be used to assess the functional capability of NK cells in a range of normal and pathologic conditions of captive bred rhesus macaques of Indian origin. V
Key terms: NK cell functions, KIR3DL05, KIR3DL01, rhesus macaque
NEW data provided evidence that SIV-derived peptides could influence NK cell responses by modulating MHC Class I interactions with inhibitory killer cell immunoglobulin-like receptors (KIRs) of rhesus macaques. For example, Mamu-KIR3DL05 can be engaged by Mamu-A1*002 MHC Class I allele-bound SIVmac239 peptides, resulting in the inhibition of the cytolytic activity of Mamu-KIR3DL05 positive NK cells (1). Disruption of the differentiation of NK cells in rhesus macaques were reported after infection with SIVmac239 (2). However, it is not known whether all subsets of the NK cell population are equally affected. Here we developed a panel to support studies that address this issue. Our goal was to monitor the functional capability of NK cell subpopulations defined by the expression of Mamu-KIR3DL01 and Mamu-KIR3DL05 inhibitory receptors. Approximately 90% of the captive bred rhesus macaques of Indian origin express KIR3DL01 and >40% of the animals are positive for KIR3DL05 (3–5).
In rhesus macaques, NK cells are primarily defined as CD8αα+CD3-lymphocytes (6,7). The overwhelming majority (>90%) of these cells express NKG2A/C receptor. The CD16 (FcRγ III) and CD56 (N-CAM) differentiation markers define four NK cell subpopulations. In healthy animals the CD16+ NK cells are primarily cytotoxic, the CD56+ are predominantly cytokine-secreting, while CD16-CD56− NK cells can perform both functions (2). In flow cytometric methods, the presence of intracellular granzyme B and up-regulation of the degranulation marker CD107a are used as indicators of cytotoxic capability. The cytokine-secreting ability of NK cells is determined by detection of IFN-γ and TNF-α.
To assess the functional capability of the NK ell subsets we performed an 18-hour long in vitro stimulation assay using human 721.221 cells as stimulatory agents. To exclude the human cells in the gating strategy we used a nonhuman primate-specific antibody recognizing the hematopoietic marker CD45 antigen (clone D058-1283).
As we previously reported in OMIP-028 (8), the availability of antibodies recognizing certain rhesus-antigens is rather limited. For example, there is only one antibody (clone Z199) specific to NKG2A (CD159a) in humans that cross-reacts with rhesus samples. It is noteworthy that this antibody binds both the activating NKG2C and the inhibitory NKG2A receptor in rhesus macaques (9). Mamu-KIR3DL01 is recognized only by the monoclonal antibody NKVFS1. Mamu-KIR3DL05 is bound by several Mamu-A1*00201-restricted SIV-mac239 epitope tetramers, among them the GY9 epitope loaded provides the best option as far as the staining is regarded (3).
Since a subset of CD20 positive cells in the blood of rhesus macaques express CD56 (Supporting Information Fig. S1), and cytotoxic T cells express CD8α we included the CD20 and CD3 markers in the dump channel. Of the four anti-CD3 specific antibody clones (SP34-2, FN18, 10D12, and SK7) that are available commercially, we have had experience with clones SP34-2 and FN18. In our animal colony a small percentage of rhesus macaques express a CD3 isoform that is not recognized by the FN18 clone. Therefore, we tested several fluorochrome-conjugated forms of clone SP34-2 as detailed in the supplementary information (Supporting Information Fig. S2).
There are numerous CD8α chain-specific clones in a wide variety of conjugates to choose from. We selected the BV711-conjugated RPA-T8 clone, because it provided good separation and had minimal spectral overlap with the other fluorochromes in the panel. Five CD16 cross-reacting clones are available commercially in multiple fluorochrome-conjugates. We have had excellent results with clone 3G8 in the past. For this panel we tested the FITC, Pacific Blue, BV711, and PerCP-Cy5.5 forms.
We tested two clones of CD56-specific antibodies labeled with either FITC (clone B159), PE-Cy7, or PerCPCy5.5 (clone NCAM16.2). Since we reserved the PE-Cy7 conjugate for the NKG2A/C and the FITC conjugate for the IFN-γ-specific antibody we selected the PerCPCy5.5-labeled clone NCAM16.2.
The single known cross-reacting CD107a-specific antibody is available in multiple fluorochrome-labeled forms. We usually use the PE-conjugated form, however this channel was already reserved for Mamu-KIR3DL01. For this panel we evaluated the BV786 and the BV605 forms. While the BV786 conjugate did not yield the desired separation, the BV605 did. To quantify the level of intracellular granzyme B zymogen, we selected the monoclonal antibody GB11. As this protein is stored in copious amounts in cytoplasmic granules, we were able to choose a detector where cellular autofluorescence would be otherwise prohibitive for markers expressed at considerably lower quantities. We tested the BV510-labeled antibody and obtained excellent results. In rhesus macaques all circulating NK cells contain granzyme B. However, NK cells expressing the CD107a degranulation marker could be subdivided into granzyme Blow and granzyme Bhigh subpopulations (Fig. 1C and 1D: Q2 and Q3). We postulated that the cells with lower granzyme B content are the most active cytotoxic cells; therefore, the separate monitoring of the two populations is a worthwhile exercise (Fig. 1C–1E). Finally, we selected the reagents to define the frequency of cytokine producing NK cell subsets. For IFN-γ we tested the BV650 and FITC-conjugated variants of clone 4S.B3. The violet laser excitable conjugate delivered inferior staining compared to the FITC-labeled antibody, so we selected the latter one. For monitoring TNF-α secretion, we examined the PE-CF594 and the Alexa700-conjugated forms of clone MAb11.
Figure 1.
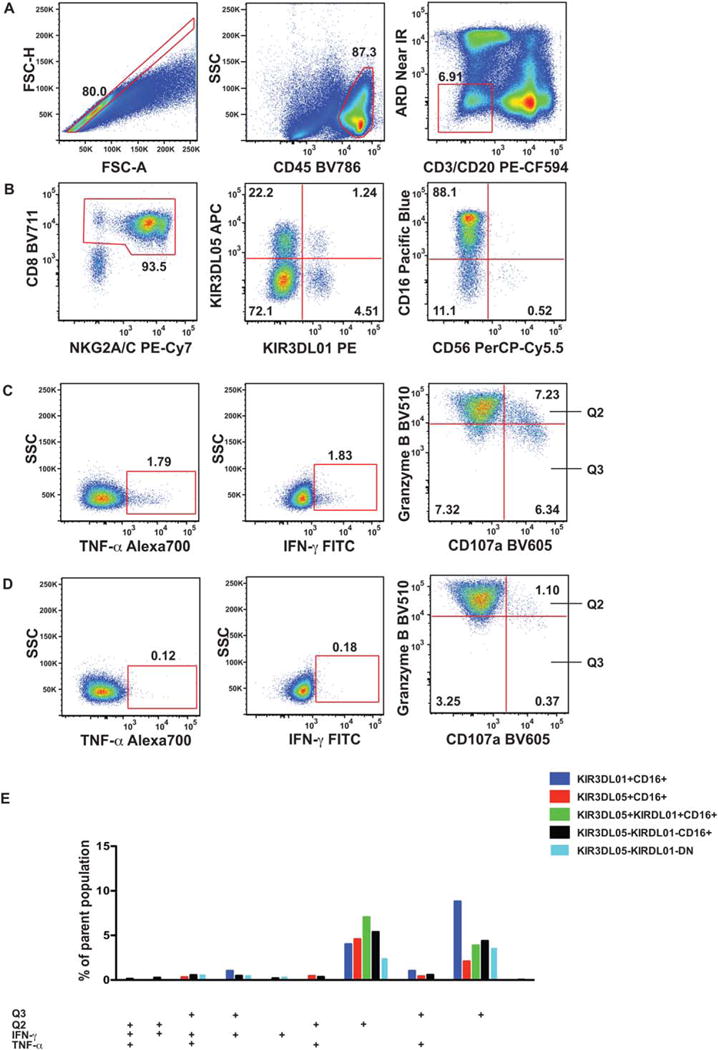
Gating strategy for OMIP-035. Ficoll-purified PBMC of an SIVmac239-infected rhesus macaque were stimulated with 721.221 cells for 18 h, then stained with the NK cell function panel as detailed in the Supporting Information. (A) With the initial sequential gating we excluded cell aggregates (single cell gate defined by forward scatter area and height), dead cells (Amine reactive dye−), T cells (CD3−), B cells (CD20−), and human 721.221 cells (CD45+) gate. CD8+ and NKG2A/C+ antigens were used to define the NK cell population. (B) Within the NK cell population, four subsets were identified using the KIR3DL01 and KIR3DL05 receptor expression pattern. All four subsets were further divided into CD16+, CD56+, and CD16-CD56− (DN) populations. (The third panel shows the KIR3D double negative population.) (C) Next, we defined the IFN-γ+, TNF-α+, CD107a+ Granzyme Bhigh (Q2), and CD107a+ Granzyme Blow (Q3) subsets using (D) the matching non-stimulated sample within the CD16+, and CD16-CD56− NK cell populations. (Panels in the C and D series shows the CD16+ subset within the KIR3D double negative population.) (E) Functional subpopulations, as defined by the combined expression pattern of IFN-γ+, TNF-α+, CD107a + Granzyme Bhigh (Q2), and CD107a+ Granzyme Blow (Q3) subsets were quantified by Boolean gating and displayed as a bar graph. Only subsets above 0.6% of the parent population, containing >10 events are displayed. None of the CD56+ populations reached this cutoff level.
As the considerably different size of CD107a + Granzymelow (Q3) subset within the KIR3DL01 + CD16+ and KIR3DL05 + CD16+ populations (2.19% vs. 9.22%) (Fig. 1E) demonstrates our analytical approach can reveal subtle functional divergence between NK cell groups.
Similarity to Published OMIPs
This rhesus macaque-specific panel shares a lot of similarities with OMIP-007 (10) and OMIP-027 (11) (Table 1). OMIP-007 was designed to analyze the maturation, homing, and activation phenotype of human KIR-defined NK cell subsets. OMIP-027 was developed to measure the functional capability of the total NK cell population in human samples. Our panel in essence is a combination and species-specific modification of the two panels. It enables the investigator to follow the function of up to five KIR3D and CD16 defined NK cell populations in individual rhesus macaques of Indian origin (Table 2).
Table 1.
Summary table for OMIP-035
Table 2.
Reagents used in OMIP-035
| SPECIFICITY | CLONE | FLUOROCHROME | PURPOSE |
|---|---|---|---|
| Live/dead | N/A | Near infrared | Viability |
| CD45 | D058-1283 | BV786a | Nonhuman primate specific hematopoietic cell lineage |
| CD3 | SP34-2 | PE-CF594 | Exclusion |
| CD20 | 2H7 | PE-CF594 | |
| CD8 | RPA-T8 | BV711 | NK subsets |
| CD16 | 3G8 | Pacific Blue | |
| CD56 | B159 | PerCP-Cy5.5 | |
| NKG2A/C | Z199 | PE-Cy7 | |
| KIR3DL01 | NKVFS1 | PE | |
| KIR3DL05 | GY9 tetramer | APC | |
| IFN-γ | 4S.B3 | FITC | NK cell function |
| TNF-α | MAb11 | Alexa700 | |
| CD107a | H4A3 | BV605 | |
| Granzyme B | GB11 | BV510 |
BV is brilliant violet.
Supplementary Material
Acknowledgments
All rhesus macaques were handled in accordance to the standards of the American Association for the Accreditation of Laboratory Animal Care (AAALAC).
Grant sponsor: NIH; Grant numbers: 5P51OD011106-54, R01 AI095098.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Literature Cited
- 1.Schafer JL, Ries M, Guha N, Connole M, Colantonio AD, Wiertz EJ, Wilson NA, Kaur A, Evans DT. Suppression of a natural killer cell response by simian immunodeficiency virus peptides. PLoS Pathog. 2015;11:e1005145. doi: 10.1371/journal.ppat.1005145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeves RK, Gillis J, Wong FE, Yu Y, Connole M, Johnson RP. CD16- natural killer cells: Enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood. 2010;115:4439–4446. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colantonio AD, Bimber BN, Neidermyer WJ, Jr, Reeves RK, Alter G, Altfeld M, Johnson RP, Carrington M, O’Connor DH, Evans DT. KIR polymorphisms modulate peptide-dependent binding to an MHC class I ligand with a Bw6 motif. PLoS Pathog. 2011;7:e1001316. doi: 10.1371/journal.ppat.1001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schafer JL, Colantonio AD, Neidermyer WJ, Dudley DM, Connole M, O’Connor DH, Evans DT. KIR3DL01 recognition of Bw4 ligands in the rhesus macaque: maintenance of Bw4 specificity since the divergence of apes and Old World monkeys. J Immunol. 2014;192:1907–1917. doi: 10.4049/jimmunol.1302883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreland AJ, Guethlein LA, Reeves RK, Broman KW, Johnson RP, Parham P, O’Connor DH, Bimber BN. Characterization of killer immunoglobulin-like receptor genetics and comprehensive genotyping by pyrosequencing in rhesus macaques. BMC Genomics. 2011;12:295–308. doi: 10.1186/1471-2164-12-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeves RK, Evans TI, Gillis J, Johnson RP. Simian immunodeficiency virus infection induces expansion of a4b7 and cytotoxic CD56 NK cells. J Virol. 2010;84:8959–8963. doi: 10.1128/JVI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster RL, Johnson RP. Delineation of multiple subpopulations of natural killer cells in rhesus macaques. Immunology. 2005;115:206–214. doi: 10.1111/j.1365-2567.2005.02147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pomplun N, Weisgrau K, Evans DT, Rakasz EG. OMIP-028: Activation panel for rhesus macaque NK cell subsets. Cytometry Part A. 2015;87A:890–893. doi: 10.1002/cyto.a.22727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaBonte ML, Choi EI, Letvin NL. Molecular determinants regulating the pairing of NKG2 molecules with CD94 for cell surface heterodimer expression. J Immunol. 2004;172:6902–6912. doi: 10.4049/jimmunol.172.11.6902. [DOI] [PubMed] [Google Scholar]
- 10.Currier JR, Eller MA. OMIP-007: Phenotypic analysis of human natural killer cells. Cytometry Part A. 2012;81A:447–449. doi: 10.1002/cyto.a.22033. [DOI] [PubMed] [Google Scholar]
- 11.Constanzo MC, Creegan M, Lal KG, Eller MA. OMIP-027: Functional analysis of human natural killer cells. Cytometry Part A. 2015;87A:803–805. doi: 10.1002/cyto.a.22719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


