Abstract
It has been previously been shown by our lab and others that persistent organic pollutants, such as polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs), are contaminants in milk produced for human consumption. To further this research we determined the concentration of 21 PCB and 14 PBDE congeners in livestock serum, mainly bovine, across California. Congeners were extracted from serum using solid phase extraction (SPE), cleaned up by silica cartridge and quantified using gas chromatography-triple quadruple mass spectrometry. We detected significant differences among species and the production class of cattle (beef or dairy). The sum of all 21 PCB congeners (ΣPCBs) in caprine and ovine sera had a mean value of 9.26 and 9.13 ng/mL, respectively, compared to 3.98 ng/mL in bovine sera. The mean value for the sum of all 14 PBDE congeners (ΣPBDEs) in caprine and ovine sera was 2.82 and 2.39 ng/mL, respectively, compared to 0.91 ng/mL in bovine sera. Mean ΣPCBs in dairy cattle was 5.92 ng/mL compared to 2.70 ng/mL in beef cattle. Mean ΣPBDEs in dairy cattle was 1.33 ng/mL compared to 0.70 ng/mL in beef cattle. There were no regional differences in the ΣPCBs or ΣPBDEs in cattle distributed across California. These results highlight the fact that livestock are still being exposed to these pollutants yet little is known about where this exposure may be coming from.
Keywords: Bovine, Caprine, Ovine, Serum, PCB, PBDE
Graphical Abstract
1. Introduction
Polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) are synthetic industrial chemicals that have become widespread persistent organic pollutants (POPs) and are resistant to biodegradation leading to their persistence in our environment. Both of these classes of POPs contain 209 different congeners with varying degree of halogenation and position of halogen atoms on their aromatic rings. One of the key characteristics shared amongst POPs are their high lipophilicity leading to their ability to accumulate in fatty animal tissue, thus leading to diet being an important source of exposure to these chemicals for humans (Schecter et al., 2010; Chan-Hon-Tong et al., 2013; Cimenci et al., 2013; Ampleman et al., 2015). Human exposure is a point of concern because epidemiologic studies have implicated PCBs and PBDEs in a variety of adverse health effects including immune system dysfunction (Jusko et al., 2012; Kramer et al., 2012), endocrine disruption (Abdelouahab et al., 2011; Silverstone et al., 2012; Valvi et al., 2012), and deficits in neurodevelopment (Schantz et al., 2003; Korrick and Sagiv, 2008; Herbstman et al., 2010; Winneke, 2011; Gascon et al., 2012; Eskenazi et al., 2013). One form of dietary exposure that has a relatively high fat content is milk. Since these toxic compounds are highly lipophilic, milk has been thought to be a prominent source of exposure for humans (Kim et al., 2008; O’Donovan et al., 2011). Many countries have analyzed bovine milk made within its borders for PCB and PBDE content and have found a multitude of congeners at quantifiable amounts (Focant et al., 2003; Durand et al., 2008; Kim et al., 2013; Lake et al., 2013). To date, there has only been one study within the United States (U.S.) looking at a small subset of PCBs in milk samples collected across the country (Schaum et al., 2003), not allowing for any region specific conclusions on PCB content or detection of PBDEs. To expand on this previous study, and since California produces approximately 20% of the total milk supply in the U.S., we analyzed milk samples made in California and discovered PCBs and PBDEs at quantifiable levels (Chen et al., 2017). As a follow up we conducted this current study to pursue these same pollutants in bovine serum, a matrix not yet investigated in the U.S in terms of PCB and PBDE contamination. A previous study from Italy has shown a difference in PCB content between ovine and bovine samples (Benedetto et al., 2016), which led us to expand the scope of this study to include ovine and caprine serum samples for assessing possible species differences.
PCBs are categorized into dioxin-like (DL) and non-dioxin-like (NDL) compounds. DL PCBs are potent activators of the aryl hydrocarbon receptor (Vondracek et al., 2005). Previous assessment of POP contamination of dairy products have primarily evaluated DL-compounds (Focant et al., 2003; Durand et al., 2008; Pizarro-Aranguiz et al., 2015) because, in the past, these compounds were considered to be the most toxic. However, NDL-PCBs dominate over DL-PCBs in biological and environmental samples (DeCaprio et al., 2005), and specifically multiple NDL-PCBs have been shown to be potent neurodevelopmental toxicants (Schantz et al., 1997; Howard et al., 2003; Yang et al., 2009; Yang and Lein, 2010; Wayman et al., 2012; Lesiak et al., 2014; Yang et al., 2014). Many of the previous studies with focus on DL-compounds also do not include assessment of PBDEs leaving out an entire class of compounds implicated as neurodevelopmental toxicants (Chen et al., 2012; Bradner et al., 2013; Behl et al., 2015; Jarema et al., 2015). PBDEs have been used extensively in products in California until their proposed ban in 2003. Following the ban in 2004, two commercial formulations, penta-BDE and octa-BDE, were phased out of production in some U.S. states after a voluntary agreement between the U.S. EPA and the sole manufacturer of these products (Dodson et al., 2012a). Despite the phase out of many PBDEs used in industry, these compounds persist in our environment due to their resistance to biodegradation (Dodson et al., 2012b; Bradman et al., 2014; Whitehead et al., 2015). Thus, we focused our study on mainly NDL-PCBs and PBDEs with potential neurodevelopmental toxicity as this is a highly sensitive endpoint of concern for these POPs. In addition, previous work assessing PBDEs and PCBs in bovine milk for human consumption detected a non-legacy PCB, or a PCB that was never intentionally synthesized for industrial purposes, PCB 11, at quantifiable levels (Chen et al., 2017). PCB 11 has recently emerged as a global pollutant and is currently produced as an unintentional byproduct of paint pigment synthesis (Choi et al., 2008; Du et al., 2008; Hu et al., 2008; Basu et al., 2009; Du et al., 2009; Hu and Hornbuckle, 2010; Heo et al., 2014); thus, we included PCB 11 in the analysis of serum samples collected in this study.
This study was performed to 1) evaluate the presence of PCBs and PBDEs in bovine, caprine and ovine sera and to 2) assess differences in pollutant profiles based on species or cattle production class (beef or dairy).
2. Materials and methods
2.1. Materials
All organic solvents used were of HPLC grade and obtained from Fisher Scientific (Fair Lawn, NJ, USA). The PCB standards (PCB-11, 28, 52, 77, 84, 91, 95, 101, 118, 131, 132, 135, 136, 138, 149, 153, 174, 175, 176, 180, 196) and PBDE standards (BDE-17, 28, 47, 49, 52, 66, 85, 95, 99, 100, 136, 153, 154, 183) were purchased from AccuStandard Inc. (New Haven, CT, USA). The 13C12 labeled 2,2′,3′,4,5-pentachlorobiphenyl (13C12-PCB-97) and 13C12 labeled 2,3′,4,4′,5-pentabromodiphenyl ether (13C12-BDE-118) were purchased from Cambridge Isotope Laboratories (Tewksbury, MA, USA). Control human serum was purchased from Golden West Biologicals (Temecula, CA, USA). Mirex was purchased from Sigma Aldrich (St. Louis, MO, USA). Solutions were diluted with isooctane to appropriate concentrations.
2.2. Sample collection
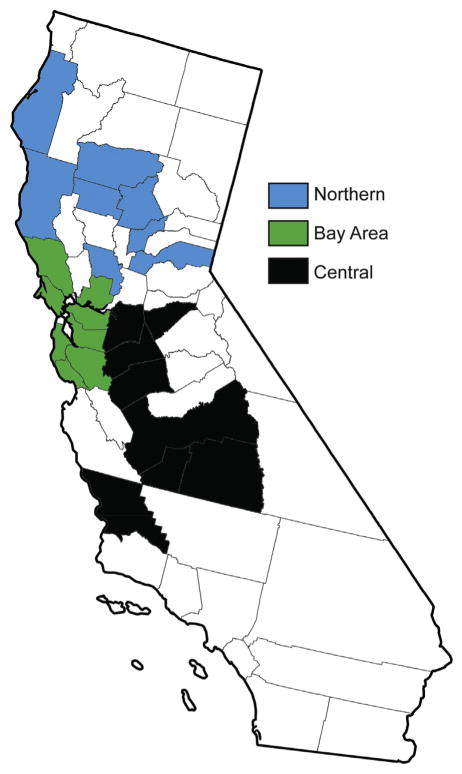
Samples were collected from August 2012 to September 2015 from locations depicted in Fig. 1. Counties were grouped into three different areas to identify any regional differences: Northern, Bay Area and Central California. All ovine and caprine serum samples were collected during appointments at the Veterinary Medical Teaching Hospital (VMTH), School of Veterinary Medicine, University of California Davis. Bovine samples were from cattle presented to the VMTH for appointments, and to the California Animal Health and Food Safety Laboratory, University of California Davis for diagnostic work-up. Additional bovine serum samples were collected in the Bay Area region under the supervision of a licensed large animal veterinarian. All samples were stored at −80 °C prior to analysis. In total, 172 serum samples were included in this study (Bovine n = 145, Caprine n = 17, Ovine n = 10). Information on location and production class was also noted when available. Location was designated by county and the various counties sampled were grouped into three regions of California based on geography and anthropogenic activities within the region. Only bovine serum samples were used in regional comparisons (Northern n = 51, Bay Area n = 46, or Central n = 53). Caprine and ovine samples were collected in the northern and bay area regions and were thus excluded from regional analyses since there was uneven regional distribution. The Bay Area was defined by any county touching the San Francisco bay and counties north of the bay were considered to be in the Northern region. All other counties were grouped into the Central region (See Table 1).
Fig. 1.
Map of California depicting the counties where serum samples were collected and how those counties were grouped into Northern (blue), Bay Area (green), and Central (black) regions. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Description of serum samples analyzed in this study.
| Production Class
|
|||
|---|---|---|---|
| Beef | Dairy | Information Unavailable | |
| Bovine | 44 (30%) | 61 (42%) | 40 (28%) |
| Caprine | N/A | N/A | 17 (100%) |
| Ovine | N/A | N/A | 10 (100%) |
2.3. Sample processing and analysis
The serum sample extraction protocol was adapted from our previously published method (Lin et al., 2013). Samples were thawed and briefly vortexed. Aliquots of 0.5 mL of serum were transferred into a disposable glass tube. Serum samples were spiked with 10 μL of an isooctane solution containing 100 ng/mL of 13C12-PCB-97 and 13C12-BDE-118. 0.5 mL of formic acid was added and then samples were vortexed for 30 s. Water Oasis HLB SPE cartridges (Milford, MA, USA) were gravimetrically conditioned with 3 mL of methanol and then 3 mL of ultrapure water containing 1% formic acid. Samples were added to the SPE cartridges and the disposable glass tubes were rinsed with 1 mL of ultrapure water with 1% formic acid. This wash was also added to the SPE cartridge and samples filtered gravimetrically. The cartridges were then dried under vacuum (~5 mm Hg) for 300 s. Waters disposable Sep-Pak® Light Silica cartridges (Milford, MA, USA) were placed underneath the SPE cartridges. 3 separate aliquots of 3 mL dichloromethane were added to the SPE cartridges while placed under vacuum (~10 mm Hg) to extract the pollutants of interest into disposable glass tubes containing 100 μL of an isooctane solution containing 100 ng/mL Mirex. The dichloromethane was then evaporated to 1.5 mL under N2 in a warm water bath (~45 °C). Samples were then vortexed for 30 s and evaporated to dryness under N2 in a warm water bath (~45 °C). The residue was reconstituted with 100 μL isooctane and vortexed for 30 s. Samples were then placed into an auto-sampler vial for GC/EI-MS/MS analysis.
Seven-point calibration curves at PBDE and PCB concentrations of 0.04, 0.1, 0.2, 0.8, 2, 4, and 10, ng/mL were prepared by adding PBDE and PCB analytical standards to 0.5 mL of control human serum. Calibration samples were processed following the same extraction method as samples.
All samples were analyzed following a previously published method (Lin et al., 2013) using a Bruker Scion TQ triple quadruple mass spectrometer (Bruker, Fremont, CA, USA) equipped with a Bruker 451 GC and CP 8400 auto-sampler and series split/splitless injector set at 280 °C. GC separation was performed by a 15 m BR-5MS, 0.25 mm i.d. column with 0.25 μm film thickness (Bruker, Fremont, CA, USA). The GC oven temperature was as follows: 1) started at 90 °C and held for 1 min; 2) increased to 220 °C at a rate of 50 °C/min and held for 1 min; 3) increased to 260 °C at a rate of 5°C/min and held for 1 min; 4) increased to 300 °C at a rate of 50 °C/min and held for 2 min. The flow rate of the carrier gas, helium, was set at 1.8 mL/min. Source temperature was set at 280 °C and transfer line temperature at 300 °C. PBDE/PCB concentrations were determined in multiple reactions monitoring (MRM) mode. MS/MS was operated in EI positive mode at 70 eV. An aliquot of 2 μL sample was injected by pulsed splitless method (split ratio of 50:1, 50 psi, 0.2 min).
2.4. Quality control
A procedural blank was run in parallel with every batch of samples using isooctane. No PCB or PBDE congeners were detected in the blanks. The accuracy of the method was assessed using three quality control (QC) samples of human control serum fortified with all PBDEs and PCBs at concentrations of 0.1, 0.8 and 4 ng/mL. QC samples were prepared following the same extraction method as described for samples and analyzed in parallel with each group of samples. For each batch of samples processed and analyzed, the determined concentration of each PBDE and PCB congener in the QC samples, as quantified by the standard curves, was required to fall within ±30% of the known concentration of the individual congener for the data to be included in the final analysis.
2.5. Data and statistical analysis
The GC-MS/MS data was processed by Bruker Mass Spectrometry Working Station version 8.2 (Bruker, Fremont, CA, USA). All analytes were quantified using the 7-point calibration curve. The peak areas were used for quantification following an internal algorithm. The limit of detection (LOD) and limit of quantification (LOQ) were defined based on signal-to-noise (S/N) ratio exceeding three and ten, respectively. Values found below the LOD were reported as “non-detected” (ND) and, for statistical purposes, were assigned a value of half the LOD. The LODs for PBDEs ranged from <0.00001 ng/mL for BDE 154 to <0.094 ng/mL for BDE 66. For PCBs, the LODs ranged from <0.001 ng/mL for PCB 176 to <0.094 ng/mL for PCB 136.
Mann-Whitney Rank Sum Test was conducted using GraphPad Prism v6.07 (San Diego, CA) to compare differences between beef and dairy bovine samples with values of p < 0.05 considered significant. Comparisons between three or more groups used a nonparametric Kruskal Wallis test using an alpha set using the Holm-Bonferroni method using Stata IC/13 (StataCorp LP, College Station, TX).
3. Results and discussion
3.1. Caprine and ovine sera contain higher concentrations of PCBs and PBDEs than bovine sera
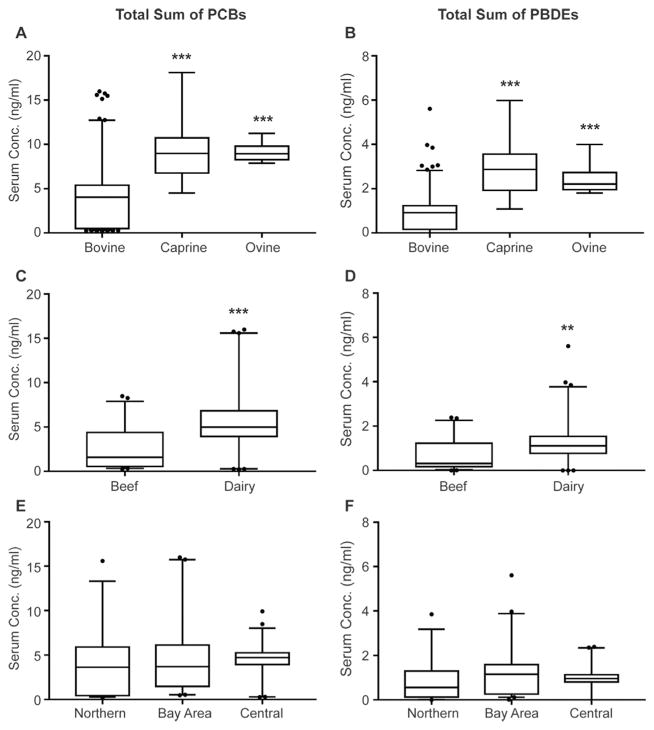
The sum of the concentrations of PCBs and PBDEs in bovine, ovine, and caprine are depicted in Fig. 2A and B and summarized in the Supplementary Material. Location and production class was not available for every bovine sample, precluding us from using these parameters when evaluating concentrations between species. Total PCBs are significantly greater in caprine and ovine sera than bovine sera with a mean of 9.26 and 9.13 ng/mL compared to 3.98 ng/mL, respectively (Fig. 2A). Total PBDEs are also significantly greater in caprine and ovine sera than bovine sera with a mean of 2.82 and 2.39 ng/mL compared to 0.91 ng/mL, respectively (Fig. 2B).
Fig. 2.
Species, production, and regional differences in the sum of total PCBs and total PBDEs in serum samples collected across California. Caprine and ovine serum have a greater amount of PCBs (A) and PBDEs (B) compared to bovine serum (n = 145 bovine, n = 17 caprine, n = 10 ovine). Dairy producing cattle have greater amounts of PCBs (C) and PBDEs (D) compared to beef cattle (n = 44 beef, n = 61 dairy). There are no regional differences in the total PCBs (E) and total PBDEs (F) (n = 36 northern, n = 41 bay area, n = 46 central). Plots are showing the 5th–95th percentile. *Significant differences with p values set at p < 0.05, **p < 0.01, and ***p < 0.001.
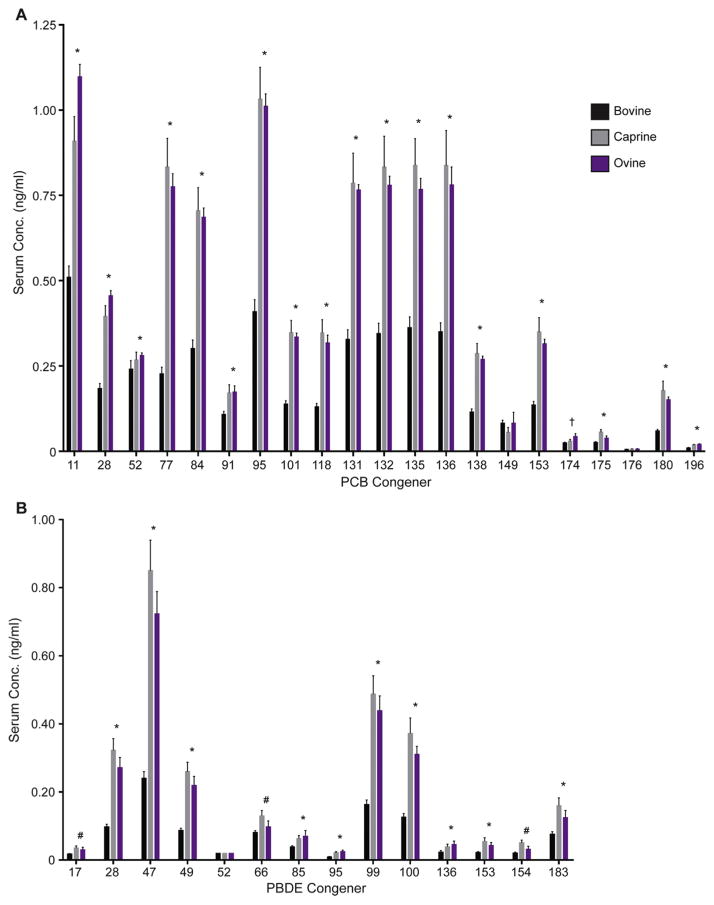
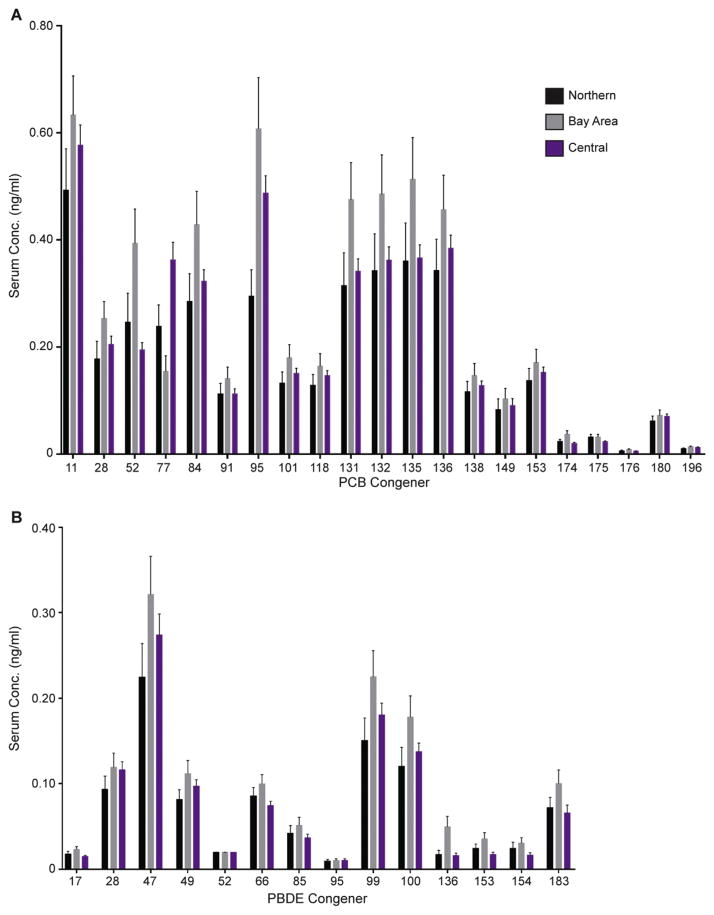
Individual congener concentrations for each species can be found in Fig. 3 and Supplementary Material. The finding of differing concentrations between species is not surprising because of known differences in biotransformation enzymes and activities between goats, sheep, and cattle (Poppi et al., 1981; Huston et al., 1986; Chen et al.,1990; Szotakova et al., 2004; Girolami et al., 2016). While PCBs and PBDEs are not highly susceptible to metabolic processes, they are subject to cytochrome P450-mediated hydroxylation, primarily performed by the CYP1A and CYP2B family in mammals (Grimm et al., 2015). Cows have higher amounts of CYP1A protein and CYP1A activity compared to sheep and goats, and also have detectable CYP1B mRNA expression and CYP2B activity while goat and sheep lack any CYP2B activity (Szotakova et al., 2004; Girolami et al., 2016). Another species difference in biotransformation mediators is the increased expression of the aryl hydrocarbon receptor (AhR) and a key protein involved in mediating AhR effects, the AhR nuclear translocator (ANTR), in cows compared to sheep (Girolami et al., 2016). The AhR can be activated by coplanar pollutants, such as DL-PCBs, and this activation increases the expression of metabolic enzymes, such as P450s (Denison et al., 2011). Therefore, it is likely that cattle are able to metabolize these pollutants at a higher rate than goats or sheep. Once PCBs are hydroxylated they undergo phase 2 metabolism, attaching even more polar groups leading to their excretion (Grimm et al., 2015). Different digestive processes, such as rumen retention time or microbial communities in the rumen amongst these species can also influence bioavailability of xenobiotics, thereby altering uptake (Agarwal et al., 2015; Ferreira et al., 2016). Other factors, such as body composition, breeding and grazing habits may also contribute to the observed differences. Cattle have a higher mature body fat fraction compared to sheep or goats (Johnson et al., 2012; Maeno et al., 2013). This increase in adipose tissue can serve as a larger reservoir for highly lipophilic POPs allowing them to sequester into fat and removing them from circulation. An additional important physiological consideration to consider in the comparison between bovine, ovine and caprine PCB and PBDE levels is the lactation status of the animals. While about 42% of the analyzed serum samples from cattle came from dairy, none of the ovine and caprine samples were collected from lactating animals. It is well documented that PCBs and PBDEs partition into bovine milk (Focant et al., 2003; O’Donovan et al., 2011; Lake et al., 2013) presenting a path of elimination of these pollutants.
Fig. 3.
Species differences in individual PCB (A) and PBDE (B) congeners. Data are presented as mean ± standard error of the mean. * Caprine and Ovine > Bovine, † Ovine > Bovine, # Caprine > Bovine. Significance was set at p < 0.05.
All livestock, bovine, ovine and caprine, can be exposed to contaminants from air-soil deposition from industrial emissions, and run-off from wastewater treatment plants. In addition, husbandry practices can expose animals to painted fences, barns and hutches, and corral pipes. Husbandry techniques also vary between ruminant species with cattle generally being most closely monitored while small ruminants are allowed more free exploration. Livestock markers are commonly used to temporarily mark livestock after certain procedures including husbandry practices; pigments in these markers including yellow and green may present a potential source for exposure to non-legacy PCBs, such as PCB 11 (Hu and Hornbuckle, 2010). Yellow and green dyes contain the greatest amount of inadvertent PCB contamination providing a potential exposure source for animals through licking and ingesting paint pigments used in markers or paint.
3.2. Dairy cows contain higher serum concentrations of PCBs and PBDEs than beef cattle
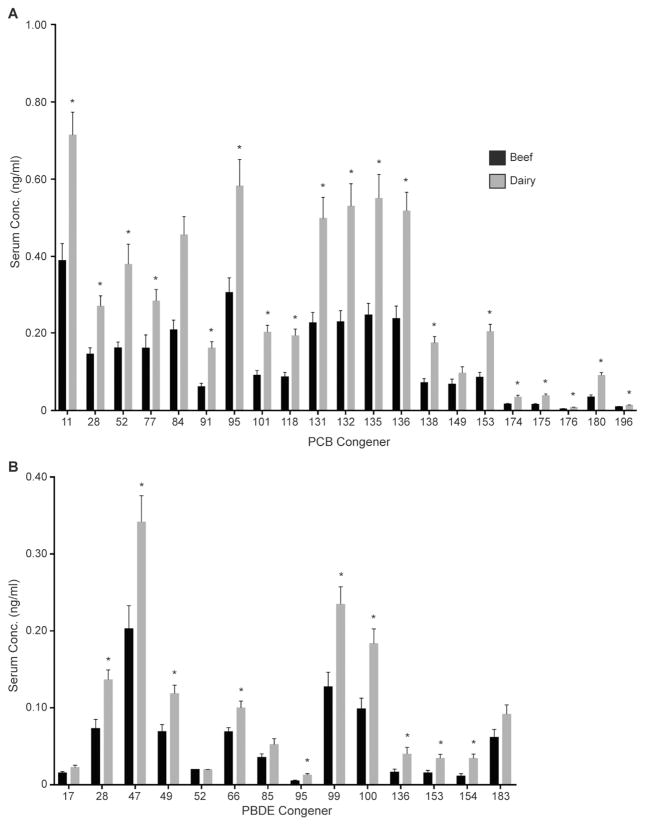
The sum of the concentrations of PCBs and PBDEs in beef and dairy bovine samples are depicted in Fig. 2C and D and summarized in the Supplementary Material. Total PCBs are significantly greater in sera from dairy cows compared to beef with a mean of 5.92 ng/mL versus 2.70 ng/mL (Fig. 2C). Total PBDEs are also significantly greater in sera from dairy cows versus beef with a mean of 1.33 ng/mL versus 0.70 ng/mL (Fig. 2D). Individual congener concentrations for each production class can be found in Fig. 4 and in Supplementary Material. The significant difference in body burden of these pollutants and the directionality of the observed difference are surprising. We expected the difference to be in the opposite direction with sera of dairy cows containing lower concentrations of PCBs and PBDEs due to the fact that lactation allows for enhanced elimination in milk.
Fig. 4.
Production class differences in individual PCB (A) and PBDE (B) congeners. Data are presented as mean ± standard error of the mean. *Significant differences with p values set at p < 0.05.
However, diet is likely a major contributor to the observed differences in PCB and PBDE serum concentrations. One study conducted in Germany strongly supports this hypothesis by finding that 99% of a cow’s PCB burden came from its diet with less than 1% coming from the air and water (McLachlan, 1993). U.S. studies confirmed contamination of animal feed with PCBs and other POPs (Sapkota et al., 2007). Dairy cows are usually kept in controlled environments and fed total mixed rations to achieve optimum performance; those rations contain known commodity ingredients which are regularly assessed for quality and nutritional content. The total mixed ration typically contains corn and or corn silage, a feed component documented to contain PCBs and PBDEs (Wang et al., 2011; Brambilla et al., 2015; Hoogenboom et al., 2015; Sun et al., 2016). In contrast, in California, beef cattle tend to be kept on pastures and generally allowed free range, but may be fed hay or other roughage when needed. There are studies reporting PCB levels in fresh grass from the United Kingdom and corn from Italy (Lake et al., 2005; Wang et al., 2011). While the locations for these feed ingredients differed, corn samples have 10–1000 times the PCB content measured in grass. This may be due, in part, to the fact that corn contains a large amount of fatty acids (Gronewald et al., 1982) which would facilitate partitioning of lipophilic compounds into seeds, and/or roots and leaves that are used in grain production (Sidhu et al., 2000). Various forms of corn that are used in animal feed have lipid percentages ranging from 3.8 to 10% while those among different grasses range from 2.0 to 4.1% (NRC, 2000). Based on these data, rations including corn, such as typical dairy cow rations, may present a greater risk for exposure to PCBs and PBDEs than pastures. However, a recent study in Italy has shown PBDE contamination in pastures and detected its carryover into cow milk (Parolini et al., 2012) showing that pastures are not free of contamination either. While PCB and PBDE concentrations in sera of beef cattle were lower than in dairy cattle, the reported concentrations are still able to make into dietary samples prepared for human consumption. This is illustrated by a study surveying meat samples at a U.S. market detecting PCBs and PBDEs in meat products derived from beef cattle (Huwe and Larsen, 2005) and multiple studies in Europe detecting PCBs and PBDEs in the animal tissues of cattle and goats (Kierkegaard et al., 2007; Ounnas et al., 2010). Unfortunately these past studies mainly looked at dioxin-like POPs, so it is difficult to make comparisons to this study, which focused mainly on the NDL congeners. It would be of great interest to assess how the levels of POPs in the sera of the animal relate to the presence of these pollutants in their meat prepared for human consumption.
With this study we can compare the levels of PCBs and PBDEs present in the sera of the dairy cattle to the results found in a previous study looking at these same congeners in supermarket milk produced in California (Chen et al., 2017) although a limitation is that these dairy cattle were not the producers of the milk analyzed. In the milk study, the most prominent congeners are PCB-138, −118 and −101. These three congeners are present at lower concentrations in bovine sera compared to the other congeners detected in this study, which makes sense and would suggest that there is deposition of specific PCBs from bovine sera to their milk. To further support this hypothesis some of the more prominent congeners in bovine sera PCB-95, −131, −132, −135 and −136 are present at lower concentrations in supermarket milk suggesting an inverse relationship between serum concentration and milk concentration.
3.3. There are no regional differences in the sum of PBDEs and PCB congeners in California
The sum of the concentrations of PCBs and PBDEs are illustrated in Fig. 2E and F and summarized in Supplementary Material. There were no statistically significant differences in the ΣPCBs or ΣPBDEs in bovine sera among the three regions of California. There was not a large enough sample size of each type of production cattle in all three regions, and since dairy cattle already have a larger burden of PCBs and PBDEs compared to beef cattle and there were already species differences this made it difficult to draw statistically valid conclusions about this region specific data. Thus we only separated the bovine sera data by samples where location was available (Fig. 5), and although no statistics were run on the individual congeners there appear to be possible regional differences which is not entirely surprising due to the varied anthropogenic activities that occur in each region.
Fig. 5.
Regional representation of individual PCB (A) and PBDE (B) congeners in bovine samples including both beef and dairy. Data are presented as mean ± standard error of the mean.
Differences in PCBs may be due to different construction materials used in each region. It is known that the caulking in buildings of many cities (Herrick et al., 2004; Robson et al., 2010; Klosterhaus et al., 2014) contain appreciable amounts of PCBs that can move into the surrounding environment by either leeching into soil, or volatilizing into the air (Herrick et al., 2007). Studies of buildings from the Bay Area that contain PCBs confirmed a PCB profile similar to Aroclor 1254 (Klosterhaus et al., 2014), a commercial mixture used prior to the ban of PCBs. Unfortunately, there are no data on PCB contamination of older or agricultural buildings in the Northern or Central regions of California. Other regional differences in pollutant profiles may also be due to the proximity of livestock to man-made waste processing facilities (Greichus and Dohman, 1980; Fernandez-Gonzalez et al., 2011).
Due to the high volume of PBDEs used in consumer products and the Bay Area being the most densely populated region of California examined in this study, it is not surprising that the individual PBDE congener concentrations tend to be highest in the Bay Area. Prior to the ban, California mainly used Penta-BDE mixtures, comprised mainly of BDE-47 and −99 (>70%), with smaller contributions from BDE-100, BDE-153, and BDE-154 (La et al., 2006; Stapleton et al., 2012). One epidemiological study assessed sera PBDEs in mothers and their children from the Bay Area of California and found that BDE-47, −99, −100, −153 made up ~90% of serum PBDE content (Eskenazi et al., 2013) consistent with the composition of flame retardants. Our data agrees with these trends with the Bay Area having the highest concentrations of BDE-47, −99, −100 and −153 compared to the Northern and Central regions (Fig. 5).
Another possibility for the regional differences observed in both PCBs and PBDEs levels could be the microbial communities and flora present in the environment. Microbes have been shown to metabolize and dehalogenate POPs, and different microbial communities have higher activities, or preference to degrade specific congeners (Furukawa and Fujihara, 2008; Zanaroli et al., 2015). There are different strains of dehalogenating microbes and each strain prospers under its own specific conditions (Hiraishi, 2008), and it is known that microbial communities vary greatly based on geographic location (Arp et al., 2014), thus these communities may be changing the POP profile in their region. Alfalfa is able to take up PCBs from soil, with enhanced uptake in the presence of nitrogen fixing bacteria (Xu et al., 2010). Maize, or corn has also been shown to uptake both PCBs and PBDEs, with PBDEs primarily staying in the roots, and being more susceptible to metabolic transformations compared to PCBs (Wang et al., 2011). Plant genera and species have different abilities for uptake and metabolism of PCBs that can vary with congeners, as shown in maize, wheat and rice (Sun et al., 2016). Important is also the relationship between plants and the microbial community in the environment as illustrated by the influence of microbial activity and corn on PCB degradation rates (Federici et al., 2012). Collectively, these studies illustrate the effect that varying microbial environments in the environment can have on PCB disposition. In addition, dietary rations change with region based on available commodities.
3.4. PCB 11, a non-legacy PCB, is present in every serum sample collected in California
PCB 11 has recently emerged as a ubiquitous pollutant in multiple regions of the world (Choi et al., 2008; Hu et al., 2008; Du et al., 2009; Heo et al., 2014), yet no study to date has reported serum PCB 11 levels in livestock. PCB 11 was present at quantifiable levels in each serum sample collected for this study. This is surprising because PCB 11 was never a part of any industrial PCB mixtures, and was not present in the caulking of buildings in the Bay Area (Klosterhaus et al., 2014). It is known that PCB 11 is a byproduct of modern pigment manufacturing processes and detected in consumer goods such as newspapers, plastic bags, magazines and napkins. It is hypothesized that PCB 11 enters the environment by waste release from paint production facilities, or by leaching into the water and soil from consumer products (Hu and Hornbuckle, 2010; Guo et al., 2014). PCB 11 is present at highest concentrations in bright yellow and green dyes, which are both colors used to mark livestock after various procedures. Once marked these dyes can be readily licked or ingested by livestock. PCB 11 can also volatilize and is recognized as a ubiquitous airborne pollutant in multiple regions around the world (Choi et al., 2008; Hu et al., 2008; Heo et al., 2014). Therefore, California livestock are likely being exposed to PCB 11 through inhalation and diet, but it is unknown which route of exposure represents the major contributor to the burdens of PCB 11 we have detected. The fact that our study did not detect geographic differences with this congener suggests that PCB 11 is a ubiquitous pollutant across California without a specific point source. This is of great concern because although PCB 11 is clearly a ubiquitous pollutant present in all forms of environmental media there is currently very little data regarding the potential toxicity of this congener.
While this study provided novel data on the content of PCBs and PBDEs in livestock sera from California there are some limitations. When observing species differences one factor we were unable to control for is obtaining caprine and ovine samples from the same locations as the bovine samples. Assessment of contamination of various feed ingredients was not part of this study; thus we were unable to control for dietary differences. Our analytical method did not include analysis for PCB 209 or BDE-209. In future studies, these congeners should be included because PCB 209 is a prominent paint pigment contaminant (Hu and Hornbuckle, 2010) and BDE-209 occurs at high levels due to firefighting activities (Shaw et al., 2013; Shen et al., 2015).
4. Conclusions
This study is the first to detect PCBs and PBDEs in serum from livestock in the U.S. and also the first study to separate out bovine samples by production class, either beef or dairy. This study is also the first to report the presence of PCB 11 in sera of all livestock evaluated. Sera of goats and sheep have higher concentration of these pollutants than cattle. Dairy cattle have higher serum PCB and PBDE concentrations than beef cattle. This detection of PCBs and PBDEs in sera of livestock in California, along with their detection in commercial milk from California is a point of concern since PCBs and PBDEs are both well-recognized as developmental neurotoxicants. The results of this study bring attention to the lack of knowledge regarding the exposure to these pollutants in livestock and the unknown associated risk factor to humans through the consumption of animal-derived product.
Supplementary Material
HIGHLIGHTS.
Caprine and ovine sera contain higher levels of PCBs and PBDEs than sera of bovine.
Dairy cows have higher levels of PCBs and PBDEs than cows used in beef production.
A non-legacy PCB, PCB 11, was detected at quantifiable levels in all livestock sera.
There are no significant regional differences in the sum concentration of PCBs and PBDEs in bovine sera in California.
Acknowledgments
This study is supported by 1R01ES020392, 2R01 ES014901, P42ES04699, P01 ES011269, and T32 ES007059 [predoctoral fellowship to Sunjay Sethi], and the U.S. Environmental Protection Agency Grant 8354320.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.chemosphere.2017.04.059.
References
- Abdelouahab N, Ainmelk Y, Takser L. Polybrominated diphenyl ethers and sperm quality. Reprod Toxicol. 2011;31:546–550. doi: 10.1016/j.reprotox.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Agarwal N, Kamra DN, Chaudhary LC. Rumen microbial ecosystem of domesticated ruminants. In: Puniya AK, Singh R, Kamra DN, editors. Rumen Microbiology: from Evolution to Revolution. Springer; India, New Delhi: 2015. pp. 17–30. [Google Scholar]
- Ampleman MD, Martinez A, DeWall J, Rawn DF, Hornbuckle KC, Thorne PS. Inhalation and dietary exposure to PCBs in urban and rural cohorts via congener-specific measurements. Environ Sci Technol. 2015;49:1156–1164. doi: 10.1021/es5048039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp A, Munyaneza JE, Crosslin JM, Trumble J, Bextine B. A global comparison of Bactericera cockerelli (Hemiptera: triozidae) microbial communities. Environ Entomol. 2014;43:344–352. doi: 10.1603/EN13256. [DOI] [PubMed] [Google Scholar]
- Basu I, Arnold KA, Venier M, Hites RA. Partial pressures of PCB-11 in air from several Great Lakes sites. Environ Sci Technol. 2009;43:6488–6492. doi: 10.1021/es900919d. [DOI] [PubMed] [Google Scholar]
- Behl M, Hsieh JH, Shafer TJ, Mundy WR, Rice JR, Boyd WA, Freedman JH, Hunter ES, 3rd, Jarema KA, Padilla S, Tice RR. Use of alternative assays to identify and prioritize organophosphorus flame retardants for potential developmental and neurotoxicity. Neurotoxicol Teratol. 2015;52:181–193. doi: 10.1016/j.ntt.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Benedetto A, Brizio P, Guaraldo P, Stella C, Cappa C, Baioni E, Spalenza V, Nebbia C, Abete MC. Dioxins, DL-PCB and NDL-PCB accumulation profiles in livers from sheep and cattle reared in North-western Italy. Chemosphere. 2016;152:92–98. doi: 10.1016/j.chemosphere.2016.02.101. [DOI] [PubMed] [Google Scholar]
- Bradman A, Castorina R, Gaspar F, Nishioka M, Colon M, Weathers W, Egeghy PP, Maddalena R, Williams J, Jenkins PL, McKone TE. Flame retardant exposures in California early childhood education environments. Chemosphere. 2014;116:61–66. doi: 10.1016/j.chemosphere.2014.02.072. [DOI] [PubMed] [Google Scholar]
- Bradner JM, Suragh TA, Caudle WM. Alterations to the circuitry of the frontal cortex following exposure to the polybrominated diphenyl ether mixture, DE-71. Toxicology. 2013;312:48–55. doi: 10.1016/j.tox.2013.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla G, Abate V, di Domenico A, Esposito M, Fulgenzi AR, Iacovella N, Serpe FP, Tassinari M. Non-dioxin-like PCB and PBDE deposition on Zea mays L. leaves: modelled contamination in milk from dairy animals fed on silage. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2015;32:864–873. doi: 10.1080/19440049.2015.1029993. [DOI] [PubMed] [Google Scholar]
- Chan-Hon-Tong A, Charles MA, Forhan A, Heude B, Sirot V. Exposure to food contaminants during pregnancy. Sci Total Environ. 2013;458–460:27–35. doi: 10.1016/j.scitotenv.2013.03.100. [DOI] [PubMed] [Google Scholar]
- Chen X, Huang C, Wang X, Chen J, Bai C, Chen Y, Chen X, Dong Q, Yang D. BDE-47 disrupts axonal growth and motor behavior in developing zebrafish. Aquat Toxicol. 2012;120–121:35–44. doi: 10.1016/j.aquatox.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Chen X, Lin Y, Dang K, Puschner B. Quantification of polychlorinated biphenyls and polybrominated diphenyl ethers in commercial cows’ milk from California by gas chromatography-triple quadruple mass spectrometry. PLoS One. 2017;12:e0170129. doi: 10.1371/journal.pone.0170129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XB, Orskov ER, Hovell FD. Excretion of purine derivatives by ruminants: endogenous excretion, differences between cattle and sheep. Br J Nutr. 1990;63:121–129. doi: 10.1079/bjn19900097. [DOI] [PubMed] [Google Scholar]
- Choi SD, Baek SY, Chang YS, Wania F, Ikonomou MG, Yoon YJ, Park BK, Hong S. Passive air sampling of polychlorinated biphenyls and organochlorine pesticides at the Korean Arctic and Antarctic research stations: implications for long-range transport and local pollution. Environ Sci Technol. 2008;42:7125–7131. doi: 10.1021/es801004p. [DOI] [PubMed] [Google Scholar]
- Cimenci O, Vandevijvere S, Goscinny S, Van Den Bergh MA, Hanot V, Vinkx C, Bolle F, Van Loco J. Dietary exposure of the Belgian adult population to non-dioxin-like PCBs. Food Chem Toxicol. 2013;59:670–679. doi: 10.1016/j.fct.2013.06.020. [DOI] [PubMed] [Google Scholar]
- DeCaprio AP, Johnson GW, Tarbell AM, Carpenter DO, Chiarenzelli JR, Morse GS, Santiago-Rivera AL, Schymura MJ Akwesasne Task Force on the E. Polychlorinated biphenyl (PCB) exposure assessment by multivariate statistical analysis of serum congener profiles in an adult Native American population. Environ Res. 2005;98:284–302. doi: 10.1016/j.envres.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012a;46:13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012b;46:13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Belton TJ, Rodenburg LA. Source apportionment of polychlorinated biphenyls in the tidal Delaware River. Environ Sci Technol. 2008;42:4044–4051. doi: 10.1021/es703047a. [DOI] [PubMed] [Google Scholar]
- Du S, Wall SI, Cacia D, Rodenburg LA. Passive air sampling for polychlorinated biphenyls in the Philadelphia metropolitan area. Environ Sci Technol. 2009;43:1287–1292. doi: 10.1021/es802957y. [DOI] [PubMed] [Google Scholar]
- Durand B, Dufour B, Fraisse D, Defour S, Duhem K, Le-Barillec K. Levels of PCDDs, PCDFs and dioxin-like PCBs in raw cow’s milk collected in France in 2006. Chemosphere. 2008;70:689–693. doi: 10.1016/j.chemosphere.2007.06.057. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjodin A, Bradman A. In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study. Environ Health Perspect. 2013;121:257–262. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici E, Giubilei MA, Covino S, Zanaroli G, Fava F, D’Annibale A, Petruccioli M. Addition of maize stalks and soybean oil to a historically PCB-contaminated soil: effect on degradation performance and indigenous microbiota. N Biotechnol. 2012;30:69–79. doi: 10.1016/j.nbt.2012.07.007. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Martinez-Carballo E, Gonzalez-Barreiro C, Rial-Otero R, Simal-Gandara J. Distribution of polychlorinated biphenyls in both products and by-products of a mussel shell incinerator facility. Environ Sci Pollut Res Int. 2011;18:1139–1146. doi: 10.1007/s11356-011-0467-7. [DOI] [PubMed] [Google Scholar]
- Ferreira L, Hervás G, Belenguer A, Celaya R, Rodrigues M, García U, Frutos P, Osoro K. Comparison of feed intake, digestion and rumen function among domestic ruminant species grazing in upland vegetation communities. J Anim Physiol Anim Nutr. 2016 doi: 10.1111/jpn.12474. http://dx.doi.org/10.1111/jpn.12474. [DOI] [PubMed]
- Focant JF, Pirard C, Massart AC, De Pauw E. Survey of commercial pasteurised cows’ milk in Wallonia (Belgium) for the occurrence of polychlorinated dibenzo-p-dioxins, dibenzofurans and coplanar polychlorinated biphenyls. Chemosphere. 2003;52:725–733. doi: 10.1016/S0045-6535(03)00127-9. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Fujihara H. Microbial degradation of polychlorinated biphenyls: biochemical and molecular features. J Biosci Bioeng. 2008;105:433–449. doi: 10.1263/jbb.105.433. [DOI] [PubMed] [Google Scholar]
- Gascon M, Fort M, Martinez D, Carsin AE, Forns J, Grimalt JO, Santa Marina L, Lertxundi N, Sunyer J, Vrijheid M. Polybrominated diphenyl ethers (PBDEs) in breast milk and neuropsychological development in infants. Environ Health Perspect. 2012;120:1760–1765. doi: 10.1289/ehp.1205266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girolami F, Spalenza V, Benedetto A, Manzini L, Badino P, Abete MC, Nebbia C. Comparative liver accumulation of dioxin-like compounds in sheep and cattle: possible role of AhR-mediated xenobiotic metabolizing enzymes. Sci Total Environ. 2016;571:1222–1229. doi: 10.1016/j.scitotenv.2016.07.150. [DOI] [PubMed] [Google Scholar]
- Greichus YA, Dohman BA. Polychlorinated biphenyl contamination of areas surrounding two transformer salvage companies, Colman, South Dakota–September 1977. Pestic Monit J. 1980;14:26–30. [PubMed] [Google Scholar]
- Grimm FA, Hu D, Kania-Korwel I, Lehmler HJ, Ludewig G, Hornbuckle KC, Duffel MW, Bergman A, Robertson LW. Metabolism and metabolites of polychlorinated biphenyls. Crit Rev Toxicol. 2015;45:245–272. doi: 10.3109/10408444.2014.999365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronewald JW, Aboukhalil W, Weber EJ, Hanson JB. Lipid-composition of a plasma-membrane enriched fraction of maize roots. Phytochemistry. 1982;21:859–862. [Google Scholar]
- Guo J, Capozzi SL, Kraeutler TM, Rodenburg LA. Global distribution and local impacts of inadvertently generated polychlorinated biphenyls in pigments. Environ Sci Technol. 2014;48:8573–8580. doi: 10.1021/es502291b. [DOI] [PubMed] [Google Scholar]
- Heo J, Kim D, Lee G. Congener profiles and source-wise phase partitioning analysis of PCDDs/Fs and PCBs in Gyeonggi-do ambient air, South Korea. Int J Environ Res Public Health. 2014;11:11065–11080. doi: 10.3390/ijerph111111065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F. Prenatal exposure to PBDEs and neurodevelopment. Environ Health Perspect. 2010;118:712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick RF, Lefkowitz DJ, Weymouth GA. Soil contamination from PCB-containing buildings. Environ Health Perspect. 2007;115:173–175. doi: 10.1289/ehp.9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick RF, McClean MD, Meeker JD, Baxter LK, Weymouth GA. An unrecognized source of PCB contamination in schools and other buildings. Environ Health Perspect. 2004;112:1051–1053. doi: 10.1289/ehp.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraishi A. Biodiversity of dehalorespiring bacteria with special emphasis on polychlorinated biphenyl/dioxin dechlorinators. Microbes Environ. 2008;23:1–12. doi: 10.1264/jsme2.23.1. [DOI] [PubMed] [Google Scholar]
- Hoogenboom RL, Klop A, Herbes R, van Eijkeren JC, Zeilmaker MJ, van Vuuren AM, Traag WA. Carry-over of polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) and polychlorinated biphenyls (PCBs) in dairy cows fed smoke contaminated maize silage or sugar beet pulp. Chemosphere. 2015;137:214–220. doi: 10.1016/j.chemosphere.2015.07.040. [DOI] [PubMed] [Google Scholar]
- Howard AS, Fitzpatrick R, Pessah I, Kostyniak P, Lein PJ. Polychlorinated biphenyls induce caspase-dependent cell death in cultured embryonic rat hippocampal but not cortical neurons via activation of the ryanodine receptor. Toxicol Appl Pharmacol. 2003;190:72–86. doi: 10.1016/s0041-008x(03)00156-x. [DOI] [PubMed] [Google Scholar]
- Hu D, Hornbuckle KC. Inadvertent polychlorinated biphenyls in commercial paint pigments. Environ Sci Technol. 2010;44:2822–2827. doi: 10.1021/es902413k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Martinez A, Hornbuckle KC. Discovery of non-aroclor PCB (3,3′-dichlorobiphenyl) in Chicago air. Environ Sci Technol. 2008;42:7873–7877. doi: 10.1021/es801823r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huston JE, Rector BS, Ellis WC, Allen ML. Dynamics of digestion in cattle, sheep, goats and deer. J Anim Sci. 1986;62:208–215. doi: 10.2527/jas1986.621208x. [DOI] [PubMed] [Google Scholar]
- Huwe JK, Larsen GL. Polychlorinated dioxins, furans, and biphenyls, and polybrominated diphenyl ethers in a U.S. meat market basket and estimates of dietary intake. Environ Sci Technol. 2005;39:5606–5611. doi: 10.1021/es050638g. [DOI] [PubMed] [Google Scholar]
- Jarema KA, Hunter DL, Shaffer RM, Behl M, Padilla S. Acute and developmental behavioral effects of flame retardants and related chemicals in zebrafish. Neurotoxicol Teratol. 2015;52:194–209. doi: 10.1016/j.ntt.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson IR, France J, Thornley JHM, Bell MJ, Eckard RJ. A generic model of growth, energy metabolism, and body composition for cattle and sheep1. J Anim Sci. 2012;90:4741–4751. doi: 10.2527/jas.2011-5053. [DOI] [PubMed] [Google Scholar]
- Jusko TA, Sonneborn D, Palkovicova L, Kocan A, Drobna B, Trnovec T, Hertz-Picciotto I. Pre- and postnatal polychlorinated biphenyl concentrations and longitudinal measures of thymus volume in infants. Environ Health Perspect. 2012;120:595–600. doi: 10.1289/ehp.1104229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierkegaard A, Asplund L, de Wit CA, McLachlan MS, Thomas GO, Sweetman AJ, Jones KC. Fate of higher brominated PBDEs in lactating cows. Environ Sci Technol. 2007;41:417–423. doi: 10.1021/es0619197. [DOI] [PubMed] [Google Scholar]
- Kim DG, Kim M, Jang JH, Bong YH, Kim JH. Monitoring of environmental contaminants in raw bovine milk and estimates of dietary intakes of children in South Korea. Chemosphere. 2013;93:561–566. doi: 10.1016/j.chemosphere.2013.06.055. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim DG, Yun SJ, Son SW. Relationship of PCDD/Fs congener profiles between beef and raw milk in South Korea. Chemosphere. 2008;70:1563–1567. doi: 10.1016/j.chemosphere.2007.08.060. [DOI] [PubMed] [Google Scholar]
- Klosterhaus S, McKee LJ, Yee D, Kass JM, Wong A. Polychlorinated biphenyls in the exterior caulk of san Francisco bay area buildings, California, USA. Environ Int. 2014;66:38–43. doi: 10.1016/j.envint.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Korrick SA, Sagiv SK. Polychlorinated biphenyls, organochlorine pesticides and neurodevelopment. Curr Opin Pediatr. 2008;20:198–204. doi: 10.1097/MOP.0b013e3282f6a4e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S, Hikel SM, Adams K, Hinds D, Moon K. Current status of the epidemiologic evidence linking polychlorinated biphenyls and non-hodgkin lymphoma, and the role of immune dysregulation. Environ Health Perspect. 2012;120:1067–1075. doi: 10.1289/ehp.1104652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La AGMJ, Hale RC, Harvey E. Detailed polybrominated diphenyl ether (PBDE) congener composition of the widely used penta-, octa-, and deca-PBDE technical flame-retardant mixtures. Environ Sci Technol. 2006;40:6247–6254. doi: 10.1021/es060630m. [DOI] [PubMed] [Google Scholar]
- Lake IR, Foxall CD, Fernandes A, Lewis M, Rose M, White O, Dowding A. Seasonal variations in the levels of PCDD/Fs, PCBs and PBDEs in cows’ milk. Chemosphere. 2013;90:72–79. doi: 10.1016/j.chemosphere.2012.07.038. [DOI] [PubMed] [Google Scholar]
- Lake IR, Foxall CD, Lovett AA, Fernandes A, Dowding A, White S, Rose M. Effects of river flooding on PCDD/F and PCB levels in cows’ milk, soil, and grass. Environ Sci Technol. 2005;39:9033–9038. doi: 10.1021/es051433a. [DOI] [PubMed] [Google Scholar]
- Lesiak A, Zhu M, Chen H, Appleyard SM, Impey S, Lein PJ, Wayman GA. The environmental neurotoxicant PCB 95 promotes synaptogenesis via ryanodine receptor-dependent miR132 upregulation. J Neurosci. 2014;34:717–725. doi: 10.1523/JNEUROSCI.2884-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Pessah IN, Puschner B. Simultaneous determination of polybrominated diphenyl ethers and polychlorinated biphenyls by gas chromatography-tandem mass spectrometry in human serum and plasma. Talanta. 2013;113:41–48. doi: 10.1016/j.talanta.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno H, Oishi K, Hirooka H. Interspecies differences in the empty body chemical composition of domestic animals. animal. 2013;7:1148–1157. doi: 10.1017/S1751731113000220. [DOI] [PubMed] [Google Scholar]
- McLachlan MS. Mass balance of polychlorinated biphenyls and other organochlorine compounds in a lactating cow. J Agric Food Chem. 1993;41:474–480. [Google Scholar]
- NRC. Nutrient Requirements of Beef Cattle. The National Academies Press; Washington, DC: 2000. 7th revised. [Google Scholar]
- O’Donovan JV, O’Farrell KJ, O’Mahony P, Buckley JF. Temporal trends in dioxin, furan and polychlorinated biphenyl concentrations in bovine milk from farms adjacent to industrial and chemical installations over a 15 year period. Vet J. 2011;190:e117–121. doi: 10.1016/j.tvjl.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Ounnas F, Feidt C, Toussaint H, Marchand P, Bizec BL, Rychen G, Jurjanz S. Polychlorinated biphenyl and low polybrominated diphenyl ether transfer to milk in lactating goats chronically exposed to contaminated soil. Environ Sci Technol. 2010;44:2682–2688. doi: 10.1021/es9036786. [DOI] [PubMed] [Google Scholar]
- Parolini M, Guazzoni N, Binelli A, Tremolada P. Polybrominated diphenyl ether contamination in soil, vegetation, and cow milk from a high-mountain pasture in the Italian Alps. Arch Environ Contam Toxicol. 2012;63:29–44. doi: 10.1007/s00244-012-9753-8. [DOI] [PubMed] [Google Scholar]
- Pizarro-Aranguiz N, Galban-Malagon CJ, Ruiz-Rudolph P, Araya-Jordan C, Maddaleno A, San Martin B. Occurrence, variability and human exposure to polychlorinated dibenzo-p-dioxins (PCDDs), polychlorinated dibenzofurans (PCDFs) and dioxin-like polychlorinated biphenyls (DL-PCBs) in dairy products from Chile during the 2011–2013 survey. Chemosphere. 2015;126:78–87. doi: 10.1016/j.chemosphere.2014.10.087. [DOI] [PubMed] [Google Scholar]
- Poppi DP, Minson DJ, Ternouth JH. Studies of cattle and sheep eating leaf and stem fractions of grasses. 3 The retention time in the rumen of large feed particles. Aust J Agric Res. 1981;1:123–137. [Google Scholar]
- Robson M, Melymuk L, Csiszar SA, Giang A, Diamond ML, Helm PA. Continuing sources of PCBs: the significance of building sealants. Environ Int. 2010;36:506–513. doi: 10.1016/j.envint.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Sapkota AR, Lefferts LY, McKenzie S, Walker P. What do we feed to food-production animals? A review of animal feed ingredients and their potential impacts on human health. Environ Health Perspect. 2007;115:663–670. doi: 10.1289/ehp.9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schantz SL, Seo BW, Wong PW, Pessah IN. Long-term effects of developmental exposure to 2,2′,3,5′,6-pentachlorobiphenyl (PCB 95) on locomotor activity, spatial learning and memory and brain ryanodine binding. Neurotoxicology. 1997;18:457–467. [PubMed] [Google Scholar]
- Schantz SL, Widholm JJ, Rice DC. Effects of PCB exposure on neuropsychological function in children. Environ Health Perspect. 2003;111:357–576. doi: 10.1289/ehp.5461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaum J, Schuda L, Wu C, Sears R, Ferrario J, Andrews K. A national survey of persistent, bioaccumulative, and toxic (PBT) pollutants in the United States milk supply. J Expo Anal Environ Epidemiol. 2003;13:177–186. doi: 10.1038/sj.jea.7500269. [DOI] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Haffner D, Patel K, Opel M, Papke O, Birnbaum L. Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas. USA. Environ Health Perspect. 2010;118:796–802. doi: 10.1289/ehp.0901347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SD, Berger ML, Harris JH, Yun SH, Wu Q, Liao C, Blum A, Stefani A, Kannan K. Persistent organic pollutants including polychlorinated and polybrominated dibenzo-p-dioxins and dibenzofurans in firefighters from Northern California. Chemosphere. 2013;91:1386–1394. doi: 10.1016/j.chemosphere.2012.12.070. [DOI] [PubMed] [Google Scholar]
- Shen B, Whitehead TP, McNeel S, Brown FR, Dhaliwal J, Das R, Israel L, Park JS, Petreas M. High levels of polybrominated diphenyl ethers in vacuum cleaner dust from California fire stations. Environ Sci Technol. 2015;49:4988–4994. doi: 10.1021/es505463g. [DOI] [PubMed] [Google Scholar]
- Sidhu RS, Hammond BG, Fuchs RL, Mutz JN, Holden LR, George B, Olson T. Glyphosate-tolerant corn: the composition and feeding value of grain from glyphosate-tolerant corn is equivalent to that of conventional corn (Zea mays L.) J Agric Food Chem. 2000;48:2305–2312. doi: 10.1021/jf000172f. [DOI] [PubMed] [Google Scholar]
- Silverstone AE, Rosenbaum PF, Weinstock RS, Bartell SM, Foushee HR, Shelton C, Pavuk M. Polychlorinated biphenyl (PCB) exposure and diabetes: results from the anniston community health survey. Environ Health Perspect. 2012;120:727–732. doi: 10.1289/ehp.1104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 2012;46:13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Pan L, Chen J, et al. Uptake, translocation, and metabolism of hydroxylated and methoxylated polychlorinated biphenyls in maize, wheat, and rice. Environ Sci Pollut Res. 2016 Oct 03; doi: 10.1007/s11356-016-7724-8. http://dx.doi.org/10.1007/s11356-016-7724-8. [DOI] [PubMed]
- Szotakova B, Baliharova V, Lamka J, Nozinova E, Wsol V, Velik J, Machala M, Neca J, Soucek P, Susova S, Skalova L. Comparison of in vitro activities of biotransformation enzymes in pig, cattle, goat and sheep. Res Vet Sci. 2004;76:43–51. doi: 10.1016/s0034-5288(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Valvi D, Mendez MA, Martinez D, Grimalt JO, Torrent M, Sunyer J, Vrijheid M. Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study. Environ Health Perspect. 2012;120:451–457. doi: 10.1289/ehp.1103862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vondracek J, Machala M, Bryja V, Chramostova K, Krcmar P, Dietrich C, Hampl A, Kozubik A. Aryl hydrocarbon receptor-activating polychlorinated biphenyls and their hydroxylated metabolites induce cell proliferation in contact-inhibited rat liver epithelial cells. Toxicol Sci. 2005;83:53–63. doi: 10.1093/toxsci/kfi009. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang S, Huang H, Zhao M, Lv J. Uptake, translocation and metabolism of polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in maize (Zea mays L.) Chemosphere. 2011;85:379–385. doi: 10.1016/j.chemosphere.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Bose DD, Yang D, Lesiak A, Bruun D, Impey S, Ledoux V, Pessah IN, Lein PJ. PCB-95 modulates the calcium-dependent signaling pathway responsible for activity-dependent dendritic growth. Environ Health Perspect. 2012;120:1003–1009. doi: 10.1289/ehp.1104833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead TP, Crispo Smith S, Park JS, Petreas MX, Rappaport SM, Metayer C. Concentrations of persistent organic pollutants in California women’s serum and residential dust. Environ Res. 2015;136:57–66. doi: 10.1016/j.envres.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winneke G. Developmental aspects of environmental neurotoxicology: lessons from lead and polychlorinated biphenyls. J Neurol Sci. 2011;308:9–15. doi: 10.1016/j.jns.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Xu L, Teng Y, Li ZG, Norton JM, Luo YM. Enhanced removal of polychlorinated biphenyls from alfalfa rhizosphere soil in a field study: the impact of a rhizobial inoculum. Sci Total Environ. 2010;408:1007–1013. doi: 10.1016/j.scitotenv.2009.11.031. [DOI] [PubMed] [Google Scholar]
- Yang D, Kania-Korwel I, Ghogha A, Chen H, Stamou M, Bose DD, Pessah IN, Lehmler HJ, Lein PJ. PCB 136 atropselectively alters morphometric and functional parameters of neuronal connectivity in cultured rat hippocampal neurons via ryanodine receptor-dependent mechanisms. Toxicol Sci. 2014;138:379–392. doi: 10.1093/toxsci/kft334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Kim KH, Phimister A, Bachstetter AD, Ward TR, Stackman RW, Mervis RF, Wisniewski AB, Klein SL, Kodavanti PR, Anderson KA, Wayman G, Pessah IN, Lein PJ. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ Health Perspect. 2009;117:426–435. doi: 10.1289/ehp.11771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Lein PJ. Polychlorinated biphenyls increase apoptosis in the developing rat brain. Curr Neurobiol. 2010;1:70–76. [PMC free article] [PubMed] [Google Scholar]
- Zanaroli G, Negroni A, Haggblom MM, Fava F. Microbial dehalogenation of organohalides in marine and estuarine environments. Curr Opin Biotechnol. 2015;33:287–295. doi: 10.1016/j.copbio.2015.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.