Abstract
Objective
To determine whether thigh muscle strength predicts knee replacement (KR) risk, independent of radiographic severity and pain.
Methods
Osteoarthritis Initiative participants with KR at 12–60 month (M) follow-up (cases) were each matched with one control (no KR throughout 60M) by age, sex, height, body mass index, baseline radiographic stage, and location of joint space narrowing. Isometric knee extensor and flexor strength were recorded biennially. The strength examination prior to KR (≤2 years) was termed T0, that two years prior to T0 T−2, and that four years prior T−4. Muscle strength between cases and controls was compared using paired t-tests and conditional logistic regression adjusted for pain.
Results
136 of 4796 participants (60% women, age 65±9 years, BMI 29±4 kg/m2) received a KR during follow-up, had at least T0 strength data, and a matched control. Knee extensor strength at T0 (primary outcome) was significantly lower in female cases than controls (p<0.001; pain-adjusted odds ratio [ORp] 1.72, 95% confidence interval [CI] 1.16 to 2.56), but no difference was seen in men (p=0.451; ORp 0.80, 95%CI 0.50 to 1.27). Results were similar for knee flexor strength at T0, and for longitudinal change in extensor and flexor strength between T0 and T−2. Thigh muscle strength at T−2 or T−4, or change between T−2 and T−4, did not predict KR risk in men or women.
Conclusion
Thigh muscle strength predicted KR risk in women, but not in men. These results may identify a window for modifying risk of KR surgery in women.
Keywords: knee, osteoarthritis, joint replacement, muscle strength
Knee osteoarthritis (KOA) represents the most prevalent form of OA, with symptomatic KOA affecting an estimated 10% of men and 13% of women over the age of 60 years (1). Knee OA is associated with considerable pain, reduced physical function and impaired quality of life, and is responsible for over 600,000 knee replacements (KRs) in the United States annually (2) – a procedure driving a large portion of costs involved in managing KOA (3, 4). The health and economic burden of KOA is expected to rise significantly above the current $9 billion spent annually in the United States, largely due to expanding indications for KR, particularly among younger adults (5).
Identifying predictors of KR would be advantageous for studying targeted disease-modifying therapies as well as directing research and treatment in these patients. The few longitudinal evaluations of KR risk factors indicate that increased severity of radiographic disease, pain and disability (6, 7), as well as accelerated cartilage loss (8), bone marrow lesions (9) and willingness to consider surgery (10) are predictors of subsequent joint replacement surgery.
Thigh muscle strength may also be implicated in risk of KR given the previously reported association between knee extensor strength and KOA incidence (11) and progression (12, 13). Women with strength deficits appear to be at particular risk of KOA progression (12, 13), possibly due to the strength capacity in women being lower and thus closer to a threshold for risk (14). While appearing to play a role in incident KOA and KOA progression in women, surprisingly few studies have examined thigh muscle weakness in relation to KR risk. These previous studies have evaluated knee extensor weakness among many other variables and only included those with end-stage KOA and a small sample of KR subjects (n=40) (15), and did not adequately adjust for radiographic disease severity and pain (16), both strong predictors of KR surgery (6, 7); pain also being an important determinant of muscle strength (17). The specific impact of thigh muscle weakness on KR risk is unknown.
Using a longitudinal case/control (KR versus no KR) design, with matching for baseline radiographic severity, sex, and other demographic factors known to increase the risk of KR, the distinct influence thigh muscle weakness has on KR risk can be established, with and without adjustment of pain. Importantly, muscle weakness is amenable to non-pharmacological intervention (18). Identifying muscle weakness as a risk factor for KR, independent of radiographic severity, may thus provide avenues to modify risk of KR surgery. Therefore, the purpose of this study was to determine whether thigh muscle strength, or change in strength, predict risk of KR in men and women, independent of radiographic OA status and pain. Based on reported sex-differences in the relationship of muscle strength with OA incidence and progression (11–13), we hypothesized that thigh muscle strength would predict risk of KR in women but not in men.
METHODS
Study Design
This study was ancillary to the Osteoarthritis Initiative (OAI), an ongoing multi-center longitudinal cohort study designed to identify biomarkers and risk factors for KOA incidence and progression (http://www.oai.ucsf.edu/) (19). As part of the OAI, participants aged between 45 and 79 years with, or at risk of, symptomatic KOA in at least one knee were recruited at four centers in the United States. The study was approved by the local Institutional Review Boards at each of the sites, and all participants gave informed consent (19). OAI participants were examined annually over four years using imaging and clinical outcomes, which included specific questions in regard to receiving a KR in the preceding 12 months (M). Knee replacement was confirmed by radiography, or from hospital records when radiographs were not available.
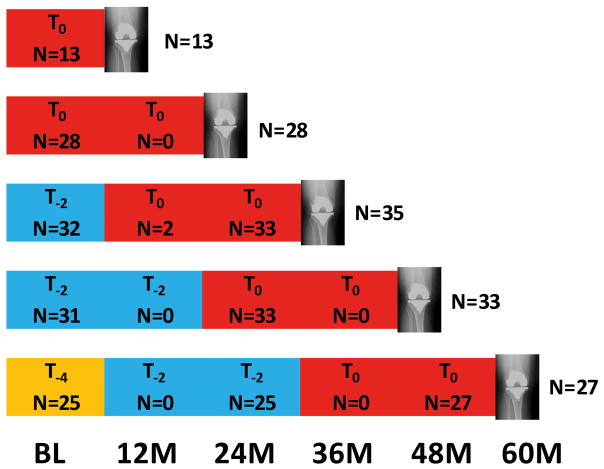
To be eligible as a case, a total KR had to be recorded at any annual follow-up, and thigh muscle strength must have been documented in either of the final two years prior to KR (OAI outcomes release 5). Thigh muscle strength was evaluated biennially (i.e. baseline, 24M, 48M); however, participants who were eligible for this measurement but for whom a valid baseline measurement was not obtained were assessed at 12M or 36M follow-up. The muscle strength examination prior to occurrence of KR (≤2 years) was termed T0, that two years prior to T0 termed T−2, and that 4 years prior T−4. KRs detected at 12M and 24M had one prior biennial visit (T0). KRs detected at 36M or 48M had up to two prior biennial measurements (T0 and T−2), and those observed at 60M had up to three previous biennial measurements (T0 through T−4) (Figure 1). If both knees of one participant were replaced, we included the knee replaced at an earlier time point. If both knees were replaced at the same time, we included the one with the lower radiographic disease stage (Kellgren and Lawrence [KL] grade) at baseline.
Figure 1.
Study design. Number of participants with knee replacement (cases) at each annual follow-up and corresponding number of cases with strength data at each follow-up. For example, at the 36 month follow-up, 35 knee replacement cases were identified. Of these, 35 had muscle strength data available at T0, while 32 had muscle strength data available at T−2.
Control knees were selected from those without self-reported KR and without evidence of KR on radiographs between baseline and 60M. Knees were excluded from being controls if the contralateral knee received a KR during the study. Controls had to have strength measurements available at time points corresponding with those of the KR cases. Cases and controls were matched 1:1 by sex, age (±5years), height (±5cm), body mass index (BMI) (±3kg/m2), KR limb (dominant [preferred leg to kick a ball] versus non-dominant), central reading baseline KL grade (strata 0, 1, 2 and 3/4), as well as presence of baseline compartmental involvement (i.e. medial/lateral radiographic joint space narrowing [JSN]). KL and JSN grades from release 0.6 from the central readings of the fixed flexion radiographs were used for this purpose (19).
Measurement of thigh muscle strength
The maximal isometric forces for knee extensor and flexor strength at each follow-up reported in the OAI database (clinical data BL/Y1/Y2/Y3/Y4: 0.2.2/1.2.1/3.2.1/5.2.1/6.2.1) were used. These measurements were performed by trained and certified research technicians using the Good Strength Chair (Metitur Oy, Jyvaskyla, Finland), which has established test-retest reliability (Pearson product-moment correlations 0.88 to 0.92) (21). Participants were positioned in sitting, with the back upright and legs hanging over the edge of the chair. Straps were placed over the waist and thigh to stabilize the pelvis and lower-limb, and participants were instructed to hold onto the arm rests. The knee of the test leg was placed in 60deg flexion (confirmed with a goniometer) for testing. A transducer/load cell was attached to the lever arm, which was in turn secured to the test leg with a strap 2cm proximal to the calcaneus. After two warm up trials at 50% maximal effort, three measurements of the maximal isometric force (N) of knee extensors (quadriceps) and knee flexors (hamstrings) were taken of each leg, by pulling and pushing against the pad, respectively. Standardised encouragement was provided, and the highest result of the three trials was used for analyses. Three female participants (one case and two controls) had flexor strength values <10N (two at T0, one at T−2), whereas extensor strength was within a normal range (77–374N), suggesting that the measurement of flexor strength was inaccurate. These three observations and their matched pair result were therefore excluded from analyses.
We used the isometric strength measurements directly (and not moments) to estimate muscle strength, because differences in anthropometrics were accounted for by matching cases and control for body height and BMI. Also, both the lever arm between the load cell and joint centre and that between the muscle tendons and joint centre depend on body size and may be assumed to be roughly proportional, as previously described (17). The length of the lever arm (knee joint centre to load cell) was recorded in a subset of OAI participants at different follow-ups. When matched cases and controls both had a lever arm length recorded (if lever arm recorded at more than one follow-up, the median length was used), we calculated the torque (force [N] x lever arm [m] = Nm) per body weight to conduct sensitivity analyses.
Statistical Analysis
After confirming normality (Kolmogorov-Smirnoff), paired t-tests between case/control pairs and case-control conditional logistic regression odds ratios (ccOR) per standard deviation were conducted independently for men and women. A ccOR >1 represents greater odds of a KR occurring in the presence of muscle weakness or in the presence of greater strength loss over time. Analysis of muscle strength was completed for each follow-up (T0, T−2 and T−4) as well as for the change in muscle strength over time from T−2 to T0, and from T−4 to T−2. Knee extensor strength in the two years prior to KR (T0) was selected as the primary outcome, as it has been previously associated with KR risk (15). The secondary outcome was knee flexor strength at T0. Knee extensor and flexor strength at T−2 and T−4, as well as change in strength from T−2 to T0 and T−4 to T−2 were considered exploratory. Robustness of the muscle strength comparisons was assessed by performing additional adjustment for the effects of pain at each observational period (ccORp). These observations were made for standard categories of pain frequency status in the past year, commonly used to classify symptomatic KOA (no pain/infrequent pain/frequent pain). Sensitivity analyses were performed by: i) repeating T0 analyses for knees with strength measures in the final year prior to KR versus those made a year earlier; and ii) repeating the original analyses using torque per body weight (Nm/kg) as the outcome measure on the subset of matched pairs with moment arm length data. Receiver operating characteristic (ROC) curves were produced for the strongest KR predictors; muscle strength values that represent a cut-off point for an increased KR risk were identified using the smallest Euclidean distance (i.e., minimizing false positive rate and maximizing true positive rate) (Supplementary File 1). Finally, because matching between cases and controls was performed at baseline, and radiographic status at T0 may differ between cases and controls, we further evaluated the associations of muscle weakness and KR risk independent of radiographic status, by performing a stratified analysis restricted to knees with baseline KL grade 3–4 (i.e., knees with limited potential for radiographic progression from baseline to T0). All analyses were performed with SPSS, version 20.0. P values <0.05 were considered significant.
RESULTS
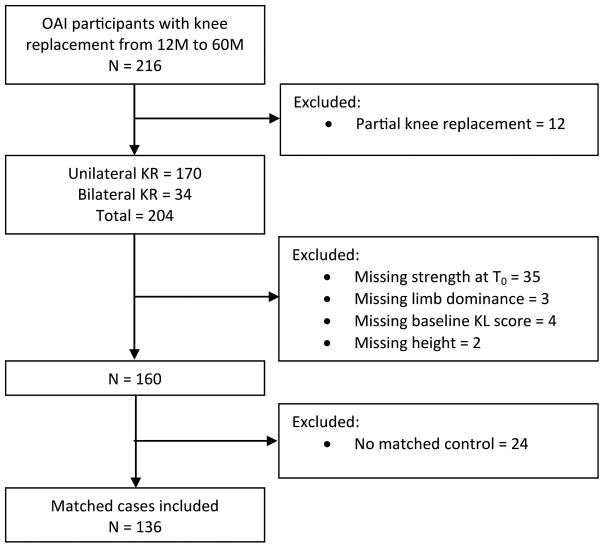
251 knees of 216 OAI participants received a KR between 12M and 60M. After excluding participants with partial KRs (n=12) (i.e., medial or lateral tibiofemoral unicompartmental KR or isolated patellofemoral KR) to maximize the homogeneity of the KR cases, and missing demographic or strength data at T0 (n=44), 160 participants with a KR remained (Figure 2). Of these, 136 participants (60% women; age 65±9 years, BMI 29±4 kg/m2) had a matched control. Of the case/control pairs, 36 (26%) were KL grade 0–2, and 100 (74%) were KL grade 3–4. Demographic and baseline radiographic outcomes are presented for women and men in Table 1. Of the case knees selected (one per participant), 13 were replaced at 12M, 28 at 24M, 35 at 36M, 33 at 48M, and 27 at 60M (Figure 1). The distribution of strength assessments over the three follow-up periods for men and women appear in Tables 2 and 3.
Figure 2.
Flowchart of extraction of matched cases with knee replacement from the OAI.
Table 1.
Baseline characteristics of KR cases and controls
| Women
|
Men
|
|||
|---|---|---|---|---|
| KR Cases (n=81) | Non-KR Controls (n=81) | KR Cases (n=55) | Non-KR Controls (n=55) | |
|
|
||||
| Age, years | 64.5±8.2 | 64.6±7.8 | 65.9±9.3 | 65.8±8.8 |
| BMI, kg/m2 | 29.06±4.64 | 28.86±4.46 | 29.6±3.8 | 29.5±3.4 |
| Height, m | 1.61±0.06 | 1.61±0.06 | 1.76±0.06 | 1.76±0.06 |
| Weight, kg | 75.5±13.1 | 75.2±12.2 | 92.4±14.5 | 92.1±13.1 |
| KR on dominant leg | 43 (53) | NA | 22 (40) | NA |
| Kellgren & Lawrence | ||||
| Grade 0 | 6 (7) | 6 (7) | 2 (4) | 2 (4) |
| Grade 1 | 1 (1) | 1 (1) | 3 (5) | 3 (5) |
| Grade 2 | 18 (22) | 18 (22) | 6 (11) | 6 (11) |
| Grade 3–4 | 56 (69) | 56 (69) | 44 (80) | 44 (80) |
| Medial JSN presence | 53 (65) | 53 (65) | 47 (85) | 47 (85) |
| Lateral JSN presence | 14 (17) | 14 (17) | 6 (11) | 6 (11) |
Values are mean ± standard deviation, or number (%).
n, number; BMI, body mass index; kg, kilograms; m, meters; KR, knee replacement; JSN, joint space narrowing; NA, not applicable.
Table 2.
Women: Thigh muscle strength (mean ± standard deviation) in KR cases vs matched non-KR controls
| Extension T0 (n=81) |
Extension T−2 (n=47) |
Extension T−4 (n=15) |
Flexion T0 (n=79) |
Flexion T−2 (n=46) |
Flexion T−4 (n=15) |
Extension T−2→T0 (n=47) |
Extension T−4→T−2 (n=14) |
Flexion T−2→T0 (n=46) |
Flexion T−4→T−2 (n=14) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| KR Cases (N) | 214 ± 68 | 247 ± 78 | 232 ± 83 | 85 ± 32 | 100 ± 31 | 107 ± 28 | −42 ± 57 | −8 ± 51 | −16 ± 30 | −12 ± 22 |
| Controls (N) | 261 ± 84 | 258 ± 94 | 267 ± 92 | 101 ± 41 | 103 ± 38 | 112 ± 54 | 12 ± 82 | 14 ± 75 | −0.8 ± 29 | −3 ± 44 |
| P (paired t) | <0.001 | 0.511 | 0.332 | 0.003 | 0.698 | 0.739 | <0.001 | 0.370 | 0.004 | 0.556 |
| ccOR (95% CI) | 1.85 (1.28, 2.63) | 1.15 (0.76, 1.79) | 1.43 (0.69, 2.94) | 1.75 (1.18, 2.63) | 1.11 (0.67, 1.85) | 1.14 (0.55, 2.33) | 2.71 (1.38, 5.23) | 1.47 (0.65, 3.34) | 1.73 (1.06, 2.82) | 1.24 (0.62, 2.48) |
| ccORp (95% CI) | 1.72 (1.16, 2.56) | 1.02 (0.46, 1.61) | 1.10 (0.46, 2.63) | 1.47 (0.96, 2.27) | 1.04 (0.59, 1.82) | 1.00 (0.46, 2.13) | 4.30 (1.34, 13.79) | 1.49 (0.63, 3.49) | 1.64 (0.86, 3.12) | 0.91 (0.40, 2.07) |
T0 = muscle strength examination prior to occurrence of knee replacement (≤ 2 years); T−2 = muscle strength examination two years prior to T0; T−4 = muscle strength examination four years prior to T0 (only available for 60M follow-up knee replacements).
A ccOR >1 represents greater odds of a KR occurring in the presence of muscle weakness or in the presence of greater strength loss over time.
n, number; N, Newtons; KR, knee replacement; ccOR, conditional logistic regression odds ratios adjusted baseline matching variables; ccORp: ccOR after adjusting out the effects of pain at the start of each interval; CI, confidence interval. ORs are based on standardized measures (per standard deviation of muscle strength at each interval). All p values <0.05 are presented in italics.
Table 3.
Men: Thigh muscle strength (mean ± standard deviation) in KR cases vs matched non-KR controls
| Extension T0 (n=55) |
Extension T−2 (n=41) |
Extension T−4 (n=10) |
Flexion T0 (n=55) |
Flexion T−2 (n=41) |
Flexion T−4 (n=10) |
Extension T−2→T0 (n=41) |
Extension T−4→T−2 (n=9) |
Flexion T−2→T0 (n=41) |
Flexion T−4→T−2 (n=9) |
|
|---|---|---|---|---|---|---|---|---|---|---|
| KR Cases (N) | 388 ± 130 | 398 ± 125 | 455 ± 159 | 164 ± 71 | 178 ± 83 | 207 ± 72 | −19 ± 68 | 5 ± 54 | −20 ± 47 | −15 ± 25 |
| Controls (N) | 370 ± 110 | 402 ± 124 | 375 ± 132 | 147 ± 66 | 168 ± 80 | 147 ± 85 | −39 ± 70 | −60 ± 49 | −19 ± 44 | −14 ± 45 |
| P (paired t) | 0.451 | 0.886 | 0.161 | 0.199 | 0.529 | 0.143 | 0.151 | 0.043 | 0.935 | 0.973 |
| ccOR (95% CI) | 0.88 (0.63,1.23) | 1.03 (0.67, 1.59) | 0.41 (0.11, 1.54) | 0.78 (0.53, 1.15) | 0.86 (0.54, 1.37) | 0.45 (0.14, 1.47) | 0.70 (0.42, 1.15) | 0.17 (0.01, 2.21) | 1.02 (0.65, 1.60) | 1.01 (0.44, 2.36) |
| ccORp (95% CI) | 0.80 (0.50, 1.27) | 0.74 (0.39, 1,37) | 0.19 (0.02, 2.33) | 0.75 (0.47, 1.20) | 0.61 (0.31, 1.19) | 0.25 (0.03, 2.33) | 0.56 (0.28, 1.11) | 0.07 (0.00, 5.25) | 0.91 (0.46, 1.77) | 0.70 (0.20, 2.39) |
T0 = muscle strength examination prior to occurrence of knee replacement (≤ 2 years); T−2 = muscle strength examination two years prior to T0; T−4 = muscle strength examination four years prior to T0 (only available for 60M follow-up knee replacements).
A ccOR >1 represents greater odds of a KR occurring in the presence of muscle weakness or in the presence of greater strength loss over time.
n, number; N, Newtons; KR, knee replacement; ccOR, conditional logistic regression odds ratios adjusted baseline matching variables; ccORp: ccOR after adjusting out the effects of pain at the start of each interval. ORs are based on standardized measures (per standard deviation of muscle strength at each interval). All p values <0.05 are presented in italics.
Extensor and flexor strength in women
In women, the primary and secondary outcomes of T0 extensor and flexor strength were significantly lower in KR cases compared to their matched non-replaced controls. For extensor strength, this was irrespective of pain (p<0.001 for paired t-test, p=0.001 for the unadjusted, and 0.007 for the pain-adjusted regression model), but for flexor strength, significance was lost when adjusting for pain (paired t-test p=0.003, and p=0.006 for the unadjusted, and 0.073 for the adjusted regression model). Adjusted and unadjusted OR and 95% CI are shown in Table 2. No differences were observed in the exploratory outcomes of T−2 and T−4 strength. However, extensor and flexor strength of female cases deteriorated more strongly between T−2 to T0, the decline being significantly greater than in non-replaced controls (p<0.001 for paired t-test, p=0.004 for the unadjusted, and 0.014 for the adjusted regression model). Longitudinal change in flexor strength was also greater in KR cases than non-replaced controls (p=0.004 for paired t-test, p=0.028 for the unadjusted regression model), but did not remain significant after adjusting for pain (p=0.130) (Table 2). Between T−4 and T−2 there was no association between quadriceps strength decline and KR risk. The optimal knee extensor strength threshold for differentiating those with an elevated KR risk was 210N or 0.90 Nm/kg; or any loss of knee extensor strength over two years (T−2 to T0) (ROC curves with associated sensitivity and specificity values for optimal cut-off points are shown in the Supplementary File 1).
Extensor and flexor strength in men
In contrast to women, male cases tended to have greater thigh strength than their matched controls at each follow-up. However, no significant differences were observed in knee extensor or flexor strength between male cases and controls at T0, T−2, T−4, or T−2 to T0 strength decline with or without adjusting for pain (Table 3). In men who underwent KR, extensor strength actually increased by a mean 5N from T−4 to T−2 compared to controls who got weaker (paired t-test p=0.043). However, there were only nine matched pairs in this analysis, limiting the generalizability of this finding, and significance was lost in the unadjusted (p=0.174) and pain-adjusted regression models (p=0.225). Additional adjustment for physical function (WOMAC) at the relevant time period prior to KR had a minimal impact on the results of all regression analyses for both men and women (data not shown).
Stratification of T0: muscle strength in the first versus second year prior to KR
For women, 44 and 37 matched case-control pairs had T0 strength measures in the year prior to KR and between 1–2 years prior to KR, respectively. The corresponding number of men was 29 and 26, respectively. Significant differences in knee extensor and flexor strength were observed between female cases and controls only in the year prior to KR (Table 4). For knee extensor strength in female cases versus controls in the year prior to KR, the ccOR and ccORp was 3.23 (95% CI: 1.67 to 6.25; p=0.001), and 3.23 (95% CI: 1.49 to 7.14; p=0.003), respectively. While for knee flexor strength the ccOR and ccORp was 2.13 (95% CI: 1.16 to 3.85; p=0.014), and 1.79 (95% CI: 0.95 to 3.33; p=0.071), respectively. There were no significant differences between male cases and controls (Table 4). In all analyses (primary and T0 stratification), all associations were similar when the sample was restricted to KL grade 3–4 knees. Only the pain adjusted association between knee extensor weakness in the year prior to KR and case-control status lost significance (ccORp 0.50, 95% CI: 0.25 to 1.02), reinforcing that, in general, the associations between muscle strength and KR were independent of radiographic disease severity.
Table 4.
Stratification of T0 thigh muscle strength according to whether T0 was recorded at ≤ 1y or at 1–2y prior to KR.
| Women
|
Men
|
|||||||
|---|---|---|---|---|---|---|---|---|
| First year prior to KR
|
Second year prior to KR
|
First year prior to KR
|
Second year prior to KR
|
|||||
| Extension (n=44) | Flexion (n=44) | Extension (n=37) | Flexion (n=35) | Extension (n=29) | Flexion (n=29) | Extension (n=26) | Flexion (n=26) | |
|
|
||||||||
| KR Cases (N) | 206 ± 66 | 83 ± 34 | 224 ±70 | 89 ± 30 | 379 ± 125 | 153 ± 67 | 399 ± 136 | 176 ± 75 |
| Controls (N) | 281 ± 78 | 101 ± 38 | 238 ± 85 | 101 ± 45 | 363 ± 114 | 143 ± 57 | 377 ± 107 | 150 ± 76 |
| P (paired t) | <0.001 | 0.007 | 0.493 | 0.156 | 0.667 | 0.597 | 0.520 | 0.205 |
| ccOR (95% CI) | 3.23 (1.67, 6.25) | 2.13 (1.16, 3.85) | 1.18 (0.75, 1.85) | 1.41 (0.58, 2.33) | 0.91 (0.58, 1.41) | 0.86 (0.51, 1.47) | 0.83 (0.49, 1.43) | 0.69 (0.38, 1.25) |
| ccORp (95% CI) | 3.23 (1.49, 7.14) | 1.79 (0.95, 3.33) | 1.08 (0.61, 1.89) | 1.23 (0.68, 2.22) | 0.96 (0.48, 1.92) | 0.75 (0.33, 1.69) | 0.67 (0.34, 1.32) | 0.70 (0.37, 1.32) |
T0 = muscle strength examination prior to occurrence of knee replacement (≤ 2 years).
A ccOR >1 represents greater odds of a KR occurring in the presence of muscle weakness or in the presence of greater strength loss over time.
n, number; N, Newtons; KR, knee replacement; ccOR, conditional logistic regression odds ratios adjusted baseline matching variables; ccORp: ccOR after adjusting out the effects of pain at the start of each interval. ORs are based on standardized measures (per standard deviation of muscle strength at each interval). All p values <0.05 are presented in italics.
Subsample with muscle torque normalized for body weight as outcome
Moment arm length was available for approximately half of all female (n=44) and one third of all male (n=21) matched pairs. The extensor and flexor strength (N) differences observed, remained largely unchanged when torque per body weight (Nm/kg) was used as the outcome measure. Knee extensor strength at T0 remained significantly different between matched pairs in women, with and without adjustment for pain, and the size of the effect was similar to analysis of strength (ccOR 2.78, 95% CI: 1.41 to 5.56, p=0.003; ccORp 2.78, 95% CI: 1.32 to 5.89, p=0.008). For flexor strength at T0, ccOR was 2.13 (95% CI 1.16 to 3.85) (p=0.015) and ccORp was 1.89 (95% CI 1.01 to 3.57) (p=0.047). The change in extensor torque from T−2 to T0 in women remained significant (ccOR 6.09, 95% CI: 1.44 to 25.77, p=0.014), irrespective of pain (ccORp 8.81, 95% CI: 1.63 to 47.48, p=0.011), while the change in flexor torque in women was not significant before or after adjusting for pain (ccOR 1.69, 95% CI: 0.92 to 3.12, p=0.093; ccORp 1.72, 95%CI: 0.92 to 3.21, p=0.088). The difference observed in the change in male extensor strength from T−4 to T−2 no longer existed when torque was the outcome (paired t-test p=0.264).
DISCUSSION
This is the first study to test the hypothesis that women who subsequently undergo KR, but not men, display lower muscle strength than non-KR controls, matched for baseline radiographic disease stage and pain. Using a longitudinal matched case-control design, we find that, indeed, knee extensor weakness in women predicts KR, independent of age, BMI, disease severity, and pain. Specifically, knee extensor weakness in the year prior to KR, and longitudinal deterioration in knee extensor strength over a 2 year observation period prior to KR predicted KR risk. Further, the association became stronger when time points within 1 year prior to KR rather than those within 1–2 years prior to KR were considered. Knee flexor weakness and deterioration in knee flexor strength at the same time points predicted KR in women, but significance was not maintained after adjusting for pain. In men, no associations were observed between thigh muscle weakness and subsequent KR.
Until now, associations observed between knee extensor strength and KR risk have been attenuated by a lack of adjustment for radiographic OA severity and pain (15, 16) – both established risk factors for KR (6, 7) that also adversely affect muscle strength (17, 22). Indeed, from longitudinal data of the OAI itself, radiographic OA status was reported to be the strongest predictor of KR (7). By performing a matched case-control evaluation, we are able to identify knee extensor and flexor weakness as risk factors for KR in women, independent of radiographic disease severity, age and BMI. Additionally, differences in pain did not explain differences in KR risk for knee extensor weakness within the year prior to KR. The current paper therefore is the first to identify extensor muscle weakness as a risk factor independent of radiographic severity and pain. It also is the first to look at the trajectory of strength loss prior to KR, finding the association with KR to be stronger the closer the measurement has been obtained to the time point of KR.
Women with strength deficits appear to be particularly vulnerable to develop incident symptomatic KOA (23) and are at increased risk of symptomatic and radiographic progression to a greater extent than men (12, 13, 24). The distinct relationship between muscle strength and risk of KR in women, but not in men, extends these findings, showing that these sex-specific relationships also apply for progression to a hard, and economically important clinical endpoint. Fewer men than women were included in our sample of matched cases and controls; however this did not appear to influence the lack of an association observed between muscle weakness and KR risk. Indeed, in most analyses, male cases tended to display greater extensor strength than their matched control. The clear discrepancy between the impact of muscle weakness on KR risk in men and women may relate to women having a lower absolute strength capacity, therefore potentially being closer to a threshold below which risk of OA progression is increased (14). Women had approximately 60% less strength than men, consistent with previous sex-specific evaluations in KOA populations (57% less in women) (12). Women are also known to be at an increased risk of prevalent KOA (25). It may be that the biochemical and biomechanical differences (i.e. hormonal and anthropometric factors), thought to play a role in increased KOA development in women (26, 27), interact with muscle and thereby increase the risk of OA progression in women with inadequate strength. Knee extensor weakness may also drive functional limitations to a greater extent in women than in men, advancing the knee to a critical clinical state necessitating KR intervention.
The robustness of our findings using muscle strength (Newtons) was verified in a subanalysis using torque per body mass (Nm/kg). Using torque per body mass, while generally not altering significance of associations, tended to increase the effect of knee extensor and flexor weakness in relation to KR risk. To be able to assess the true relationship between muscle strength and KOA it is important to account for differences in body size, as a positive relationship between strength and body size is expected. While this is not performed routinely, results did not differ markedly when adjusted for body size in the current study as we had previously matched case-control pairs for BMI (±3kg/m2) and height (±5cm).
Knee replacement is an outcome driven largely by a severely compromised clinical state (disability, pain and poor quality of life). The complexity of joint replacement as an outcome measure is highlighted by research demonstrating the influence of socioeconomic factors on KR risk and how patient perceptions of the need for surgery affect willingness to consider surgery (10). Despite this, KR represents a clinically important endpoint responsible for a large portion of costs associated with managing KOA (3, 4). While knee extensor weakness may alter local joint contact stress, lead to increased impulse loading and contribute to structural progression of KOA (12, 28), thigh muscle strength appears to be a stronger determinant of functional limitations and disability in KOA (29, 30). It is likely that the association between thigh muscle weakness and KR risk in women is driven by the negative impact of muscle weakness on function and pain. Together, evidence that thigh muscle strength is more strongly associated with disability and pain than radiographic status (17), and findings that muscle strengthening exercises result in improvements in OA pain, function and quality of life (18), support this proposition.
Muscle strengthening is advocated as a cornerstone for the management of KOA by clinical guidelines (31). The effectiveness of strengthening exercises to improve strength in KOA populations has been demonstrated by a large systematic review (32). Based on the results of the current study, knee extensor and flexor weakness are promising targets to modify risk of subsequent KR. Our findings that knee extensor strength loss from T−2 to T0, and weakness in the year prior to KR, increase the risk of KR in women, indicates the importance of maintaining a certain degree of strength. The optimal knee extensor strength threshold for differentiating those with and without KR risk appears to be approximately 200N or 0.9 Nm/kg; or prevention of any loss of knee extensor strength over two years. This threshold is one third less than the mean 300N knee extensor strength in older women without knee symptoms or OA (KL grade 0) (17). Thus, there appears to be a considerable window for women below this threshold to obtain realistic strength gains and potentially lower the risk of KR. It is important to acknowledge that improvements in clinical state with strengthening regimens are not well-maintained once strengthening exercise is ceased (33). Patients need to be counseled about the need for continued strength maintenance. Education regarding the elevated risk of KR in the presence of strength loss may provide additional motivation for adherence. It appears that the negative affect of muscle weakness on KR risk does not extend to a longer observation interval preceding KR (i.e. T−4, or change from T−4 to T−2). Similar patterns have been observed in the trajectory of cartilage loss prior to KR (8). While the potential of muscle strengthening regimens to delay KR is yet to be explored, optimizing muscle strength, even in instances of anticipated KR, is recommended as pre-operative strength contributes considerably to a favorable post-operative outcome (34).
Knee flexor weakness in the year prior to KR, and loss from T−2 to T0, was also found to elevate the risk of subsequent KR, but not when accounting pain. These findings, along with the ORs being closer to one, indicate that knee flexor weakness had less impact on KR risk than knee extensor weakness. Although evaluation of muscle in KOA typically focuses on knee extensors, adequate knee flexor function is required to produce an internal abduction moment to maintain dynamic equilibrium in the frontal plane (35). Clinically, knee flexor weakness in conjunction with knee extensor weakness predicted poor physical function in KOA (30). The relationship we observed between knee flexor weakness and elevated KR risk prior to adjustment for pain may reflect general deconditioning. Although knee flexor function is important for dynamic knee stability, the primary muscle impairment implicated in KR appears to be knee extensors.
A limitation of the current study is that different numbers of participants were available for time periods of different duration for observing muscle strength prior to KR. Secondly, strength was only assessed isometrically. Strong correlations between isometric, isotonic and isokinetic measures suggest similar results would occur irrespective of method of strength assessment (36). Thirdly, although we matched for baseline radiographic disease severity, BMI and age, and adjusted for pain, other imaging or clinical features not assessed may mediate the relationship between muscle strength and KR risk we observed. The cut-off points established potentially provide a guide for clinicians to identify patients most at risk of KR; however, such muscle strength threshold values should be verified in other cohorts. Future studies should also evaluate the efficacy of muscle strengthening interventions to arrest the progression of disease to KR.
In conclusion, we have identified isometric thigh muscle weakness as a predictor of KR risk in women, but not in men, independent of radiographic disease severity and pain. Specifically, knee extensor strength deficits in the year prior to KR, and deterioration in extensor strength over a two year interval prior to KR increased the risk of KR. Because muscle weakness is amenable to non-pharmacological intervention, these results identify a window for modifying KR risk. The potential of muscle strengthening interventions to delay KR has thus to be explored.
Supplementary Material
Acknowledgments
Adam Culvenor is supported by a European Union Initial Training Network fellowship (FP7-PEOPLE-2013-ITN; KNEEMO, grant agreement number 607510). We would like to thank the participants, the study investigators and the staff at the clinical centers for generating the image datasets used in this analysis. Data acquisition of part of this study was funded by the Osteoarthritis Initiative, a public–private partner-ship comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the Osteoarthritis Initiative study Investigators. Private funding partners of the OAI include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer, Inc. Private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health. The sponsors were not involved in the design and conduct of this particular study, in the analysis and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Funding: Data acquisition of part of this study was funded by the Osteoarthritis Initiative, a public–private partner-ship comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259;N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the Osteoarthritis Initiative study Investigators. Private funding partners of the OAI include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer, Inc. Private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health. This research has also received funding from the European Union Seventh Framework Programme (FP7-PEOPLE-2013-ITN; KNEEMO) under grant agreement number 607510. The sponsors were not involved in the design and conduct of this particular study, in the analysis and interpretation of the data, and in the preparation, review, or approval of the manuscript.
Footnotes
Potential conflict of interest:
Felix Eckstein is CEO of Chondrometrics GmbH, a company providing MR image analysis services to academic researchers and to industry. He has provided consulting services to Merck Serono, Mariel Therapeutics, Servier, and Bioclinica/Synarc, has prepared educational sessions for Medtronic, and has received research support from Pfizer, Eli Lilly, Merck Serono, GlaxoSmithKline, Centocor R&D, Wyeth, Novartis, Stryker, Abbvie, Kolon, Synarc, Ampio, BICL and Orthotrophix. Wolfgang Wirth has a part time employment with Chondrometrics GmbH and is a co-owner of Chondrometrics GmbH. No other authors declare a conflict of interest.
References
- 1.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Clin Geriatr Med. 2010;26:355–69. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the Unites States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg. 2012;94A:201–7. doi: 10.2106/JBJS.J.01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paxton EW, Namba RS, Maletis GB, Khatod M, Yue EJ, Davies M, et al. A prospective study of 80,000 total joint and 5000 anterior cruciate ligament reconstruction procedures in a community-based registry in the United States. J Bone Joint Surg. 2010;92A:117–32. doi: 10.2106/JBJS.J.00807. [DOI] [PubMed] [Google Scholar]
- 4.Katz JN, Mahomed NN, Baron JA, Barrett JA, Fossel AH, Creel AH, et al. Association of hospital and surgeon procedure volume with patient-centred outcomes of total knee replacement in a population-based cohort of patients age 65 years and older. Arthritis Rheum. 2007;56:568–74. doi: 10.1002/art.22333. [DOI] [PubMed] [Google Scholar]
- 5.Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10:437–41. doi: 10.1038/nrrheum.2014.44. [DOI] [PubMed] [Google Scholar]
- 6.Conaghan PG, D’Agostino MA, Le Bars M, Baron G, Schmidely N, Wakefield R, et al. Clinical and ultrasonographic predictors of joint replacement for knee osteoarthritis: results from a large, 3-year, prospective EULAR study. Ann Rheum Dis. 2010;69:644–7. doi: 10.1136/ard.2008.099564. [DOI] [PubMed] [Google Scholar]
- 7.Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: Preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16:494–500. doi: 10.1016/j.knee.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckstein F, Boudreau RM, Wang Z, Hannan MJ, Wirth W, Cotofana S, et al. Trajectory of cartilage loss within 4 years of knee replacement - a nested case-control study from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2014;22:1542–9. doi: 10.1016/j.joca.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raynauld JP, Martel-Pelletier J, Haraoui B, Choquette D, Dorais FM, Wildi LM, et al. Risk factors predictive of joint replacement in a 2-year multicentre clinical trial in knee osteoarthritis using MRI: results from over 6 years of observation. Ann Rheum Dis. 2011;70:1382–8. doi: 10.1136/ard.2010.146407. [DOI] [PubMed] [Google Scholar]
- 10.Hawker GA, Guan J, Croxford R, Coyte PC, Glazier RH, Harvey BJ, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54:3212–20. doi: 10.1002/art.22146. [DOI] [PubMed] [Google Scholar]
- 11.Oiestad BE, Juhl CB, Eitzen I, Thorlund JB. Knee extensor muscle weakness is a risk factor for development of knee osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:171–7. doi: 10.1016/j.joca.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Segal NA, Glass NA, Torner J, Yang M, Felson DT, Sharma L, et al. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage. 2010;18:769–75. doi: 10.1016/j.joca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckstein F, Hitzl W, Duryea J, Kwoh CK, Wirth W. Baseline and longitudinal change in isometric muscle strength prior to radiographic progression in osteoarthritic and pre-osteoarthritic knees - data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2013;21:682–90. doi: 10.1016/j.joca.2013.02.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ploutz-Snyder LL, Manini T, Ploutz-Snyder RJ, Wolf DA. Functionally relevant thresholds for quadriceps femoris strength. J Gerontol Biol Sci Med Sci. 2002;57A:B144–52. doi: 10.1093/gerona/57.4.b144. [DOI] [PubMed] [Google Scholar]
- 15.Zeni JA, Axe MJ, Snyder-Mackler L. Clinical predictors of elective joint replacement in persons with end-stage knee osteoarthritis. BMC Musculoskelet Disord. 2010;11:86. doi: 10.1186/1471-2474-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riddle DL, Kong X, Jiranek WA. Factors associated with rapid progression to knee arthroplasty: Complete analysis of three-year data from the osteoarthritis initiative. J Bone Spine. 2012;79:298–303. doi: 10.1016/j.jbspin.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Ruhdorfer A, Wirth W, Hitzl W, Nevitt M, Eckstein F. Association of thigh muscle strength with knee symptoms and radiographic disease stage of osteoarthritis: data from the osteoarthritis initiative. Arthritis Care Res. 2014;66:1344–53. doi: 10.1002/acr.22317. [DOI] [PubMed] [Google Scholar]
- 18.Pelland L, Brosseau L, Wells G, MacLeay L, Lambert J, Lamothe C, et al. Efficacy of strengthening exercises for osteoarthritis (Part I): A meta-analysis. Phys Therap Rev. 2004;9:77–108. [Google Scholar]
- 19.Eckstein F, Wirth W, Nevitt M. Recent advances in osteoarthrits imaging - the Osteoarthritis Initiative. Nat Rev Rheumatol. 2012;8:622–30. doi: 10.1038/nrrheum.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oni JK, Hochfelder J, Dayan A. Isolated patellofemoral arthroplasty. Bull Hosp Jt Dis. 2014;72:97–103. [PubMed] [Google Scholar]
- 21.Curb JD, Ceria-Ulep CD, Rodriguez BL, Grove J, Guralink J, Willcox BJ, et al. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006;54:737–42. doi: 10.1111/j.1532-5415.2006.00700.x. [DOI] [PubMed] [Google Scholar]
- 22.Sattler M, Dannhauer T, Hudelmaier M, Wirth W, Sänger AM, Kwoh CK, et al. Side differences of thigh muscle cross-sectional areas and maximal isometric muscle force in bilateral knees with the same radiographic disease stage, but unilateral frequent pain - data from the osteoarthritis initiative. Osteoarthritis Cartilage. 2012;20:532–40. doi: 10.1016/j.joca.2012.02.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal NA, Torner JC, Felson DT, Niu J, Sharma L, Lewis CE, et al. Effects of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Care Res. 2009;61:1210–7. doi: 10.1002/art.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glass NA, Torner JC, Frey Law LA, Wang K, Yang T, Nevitt MC, et al. The relationship between quadriceps muscle weakness and worsening of knee pain in the MOST cohort: a 5-year longitudinal study. Osteoarthritis Cartilage. 2013;21:1154–9. doi: 10.1016/j.joca.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan R. The prevalence of knee osteoarthritis in the elderly. Arthritis Rheum. 1987;30:914–8. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 26.Nevitt MC, Felson DT. Sex hormones and the risk of osteoarthritis in women: epidemiological evidence. Ann Rheum Dis. 1996;55:673–6. doi: 10.1136/ard.55.9.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanghi D, Srivastava RN, Singh A, Kumari R, Mishra R, Mishra A. The association of anthropometric measures and osteoarthritis knee in non-obese subjects: a cross sectional study. Clinics (Sao Paulo) 2011;66:275–9. doi: 10.1590/S1807-59322011000200016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jefferson RJ, Collins JJ, Whittle MW, Radin EL, O’Conner JJ. The role of the quadriceps in controlling impulsive forces around heel strike. J Engineer Med. 1990;204:21–8. doi: 10.1243/PIME_PROC_1990_204_224_02. [DOI] [PubMed] [Google Scholar]
- 29.McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis. 1993;52:258–62. doi: 10.1136/ard.52.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma L, Cahue S, Song J, Hayes K, Pai YC, Dunlop D. Physical functioning over three years in knee osteoarthritis: role of psycosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359–70. doi: 10.1002/art.11420. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Ardern N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–62. doi: 10.1016/j.joca.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 32.Lange AK, Vanwanseele B, Singh MA. Strength training for treatment of osteoarthritis of the knee: a systematic review. Arthritis Rheum. 2008;59:1488–94. doi: 10.1002/art.24118. [DOI] [PubMed] [Google Scholar]
- 33.van Baar M, Dekker J, Oostendorp R, Bijl D, Voorn TB, Bijlsma JW. Effectiveness of exercise in patients with osteoarthritis of hip or knee: nine months’ follow up. Ann Rheum Dis. 2001;60:1123–30. doi: 10.1136/ard.60.12.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizner RL, Petterson SC, Stevens JE, Axe MJ, Snyder-Mackler L. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheum. 2005;32:1533–9. [PubMed] [Google Scholar]
- 35.Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991;9:113–9. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 36.Jameson TD, Knight KL, Ingersoll CD, Edwards JE. Correlation of isokinetic, isometric, isotonic strength measurements with a one-leg vertical jump. Isokinetics Exerc Sci. 1997;6:203–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.