Abstract
The expression of O6-methylguanine DNA methyltransferase (MGMT) in different grade gliomas were analyzed in relation to its therapeutic effect and impact on disease prognosis. In total, 62 patients with glioma, who were admitted by neurosurgery and received surgical treatment and postoperative conventional chemoradiation, were selected for this study. Expression of MGMT was greater with an increase in brain glioma grade. Gender, age, tumor size and Karnofsky performance status (KPS) score did not differ with MGMT expression (P>0.05). Expression of MGMT in normal brain tissue was slightly significantly different than expression of MGMT in glioma tissue (P<0.05). The short-term efficacy and survival time of the MGMT-negative expression group were better than those of MGMT-positive expression. MGMT was only treated as an index to monitor tumor recurrence or metastasis and a reference to judge the prognosis of patients. The expression level of MGMT in glioma had no relation with age, gender, tumor size, surgical approach and KPS score. For glioma patients with positive expression of MGMT, antineoplastic drugs of alkylating agent class should be avoided.
Keywords: glioma, MGMT, resistance gene
Introduction
Glioma is not only the most common primary intracranial tumor (1), but also the most intractable in the treatment of neurosurgery tumors. Incidence rates of about 5 in 100,000 have been reported with a rising trend (2). The treatment of glioma has shown great improvement for patients who are diagnosed early with excision often used as treatment approach (3). However, surgical treatment has a poor effect for the malignant growth of tumor cells, leading to high recurrence and mortality rates (4). In order to decrease the recurrence and mortality rate, patients receive antineoplastic drugs post-operatively (5). However, data show that patients diagnosed with glioma, have a survival time of only 12–15 months, and the 5-year survival rate <5% (6).
O6-methylguanine DNA methyltransferase (MGMT) is a DNA repair protein, found in humans and many prokaryotic organisms. Research has shown that for the patients with glioma, antineoplastic drug treatments by alkylating agents may result in drug resistance, and MGMT is the main reason and therefore, not an ideal treatment for patients (7). Some reports indicate that the effect of positive MGMT expression is inferior to that of negative expression during chemotherapy for the patient with glioma (8). Thus, MGMT activity is higher, and the drug resistance to antineoplastic drugs of alkylating agent class is greater. If MGMT expression is lower, the alkylating class of antineoplastic drugs is more sensitive, with a better efficacy. We analyzed the expression of MGMT in newly diagnosed glioma patients to increase the sensitivity of patients with glioma to antineoplastic drugs of alkylating agent class so as to significantly improve the prognosis and reduce the reoccurrence.
Materials and methods
In the study, 62 patients with glioma, who were admitted by neurosurgery from January 2011 to January 2013, were selected. Post-surgical treatment was required and the pathology results were diagnosed as glioma. A total of 33 males (aged 25–72 years; average 42.7 years) and 29 females (aged 23–71 years; average 40.1 years) were included. There were 15 cases of grade I, 16 cases of grade II, 14 cases of grade III and 17 cases of grade IV glioma. As a control group we used brain tissue from 12 patients with cerebral hemorrhage caused by high blood pressure (aged 40–55 years; average 48.1 years). It was required that the time between incidence and operative treatment was not more than 6 h.
This study was approved by the Ethics Committee of Tongde Hospital of Zhejiang Province. Signed written informed consents were obtained from all participants before the study.
Inclusion criteria
In the study were: ⅰ) Age ≥18 years old and Karnofsky performance status (KPS) >60 scores; ⅱ) women of non-gestation or non-lactation; ⅲ) normal heart, brain, liver, lung and kidney; ⅳ) all patients received surgical treatment of tumor resection, and the postoperative pathological diagnosis results could be confirmed; ⅴ) complications such as intracranial infection and intracranial hematoma did not occur after operation; ⅵ) favorable paraffin-embedded tissue; and ⅶ) patients with good compliance, complete data of cases and coordination of follow-up.
Therapeutic regimen
All patients had surgical treatment, and the tumor was resected as much as possible on the precondition of retaining neurological function. In the study, 62 patients were given chemotherapy combining with radiotherapy after operation. After 2–3 weeks, the patients were given conformal radiotherapy of photon knife (Elekta, Stockholm, Sweden), with at least one course of treatment. On the third day of radiotherapy, 42-day chemotherapy by the dose of 75 mg/m2 temozolomide (TMZ) (Biosharp, Hefei, China) was started. After 4 weeks of combination of chemotherapy and radiotherapy, for 2-periods a single-drug chemotherapy of trimethylamine (TMA) was carried out. During the process of chemoradiotherapy, if the patients had encephaledema, intracranial pressure was decreased by dehydration and diuresis treatments. Blood routine examination was reviewed once a week, and hepatorenal function was tested at fixed period. Symptomatic treatment such as liver protection also was conducted according to the test situation and clinical symptoms.
Immunohistochemical methods
SP immunohistochemical method was applied and the steps proceeded according to instructions. In cell nucleus and/or intracytoplasm, the yellow or claybank indicated positive MGMT. The specific judgement methods of positive MGMT were as follows: under the scope of high power lens (400 times), 10 representative areas that gathered glioma cells were selected, the rate of positive cells was observed, which was divided into four grades: i) Negative (−), without positive cells or <10%; ii) weakly positive (+), positive cells among 10 scopes of 10–24%; iii) intermediately positive (++), positive cells of 25–50%; and iv) strongly positive (+++), positive cells >50%.
Observation methods
The time from initial chemotherapy to the last return visit, or death was tracked, namely, the overall survival (OS). Related data were recorded and Kaplan-Meier survival curve was drawn. The level of myelosuppression was determined by WHO grade. According to the subjective feelings and clinical manifestation of patients, gastrointestinal symptoms were judged.
Statistical analysis
SPSS 16.0 (SPSS, Inc., Chicago, IL, USA) was used to analyze the data. The χ2 test was applied to detect the difference of positive rate for different grades of glioma. P<0.05 meant that the difference had statistical significance. Fishers exact probability test was used to judge the relation between MGMT expression and gender and age for the patients with glioma. Spearmans correlation was taken to analyze the expression of MGMT in different grades of glioma; Kaplan-Meier survival curve presented the relation between the survival time and of the patient tissues, and log-rank detection was also conducted at the same time.
Results
MGMT expression in different grades of glioma
As shown in Table I and Fig. 1, the negative expression of MGMT reached 66.7% in grade I, 50% in grade II, 42.9% in grade III and 29.4% in grade IV. MGMT expression increased in high-grade glioma, and the expression gradually decreased in low-grade glioma. By statistical analysis, it was confirmed that the expression of MGMT had no relation with WHO grade (P>0.05).
Table I.
MGMT distribution in different grades of glioma.
| MGMT expression | I | II | III | IV | Total |
|---|---|---|---|---|---|
| − | 10 | 8 | 6 | 5 | 29 |
| + | 5 | 5 | 2 | 5 | 17 |
| ++ | 0 | 3 | 4 | 4 | 11 |
| +++ | 0 | 0 | 2 | 3 | 5 |
| Total | 15 | 16 | 14 | 17 | 62 |
MGMT, O6-methylguanine DNA methyltransferase.
Figure 1.
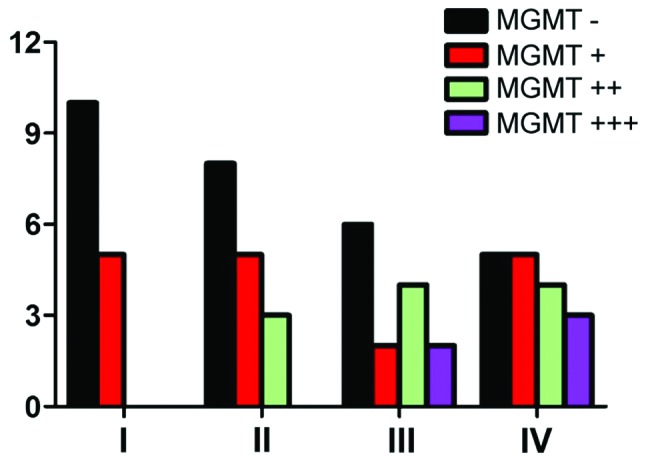
MGMT distribution in different grades of glioma. MGMT, O6-methylguanine DNA methyltransferase.
MGMT expression and biological characteristics
Fig. 2 shows the expression of MGMT among males was 52.9%, while the positive expression of MGMT among females was 53.6% (P>0.05) and no correlation with age (Fig. 3). There were nine cases with negative expression of MGMT in patients whose KPS score was <80 and 20 cases in patients whose score was >80 (P>0.05) (Fig. 4). The expression of MGMT was not related to the method of excision (P>0.05) (Fig. 5). In patients whose tumor volume was <50 cm3, 44.8% had negative and 55.2% had positive expression rate and the difference had no statistical significance (P>0.05) (Fig. 6). The negative expression in low-grade glioma (grade I and II) was 58.1% and the positive rate was 41.9%, while the negative expression in high-grade (grade III and IV) was 35.5% and the positive expression was 54.5% (Fig. 7). The positive expression of MGMT in low-grade glioma was significantly lower than that of high-grade (P<0.05). However, gender, age, tumor size, surgical method and KPS score had no statistical difference with MGMT among the 62 patients (P>0.05).
Figure 2.
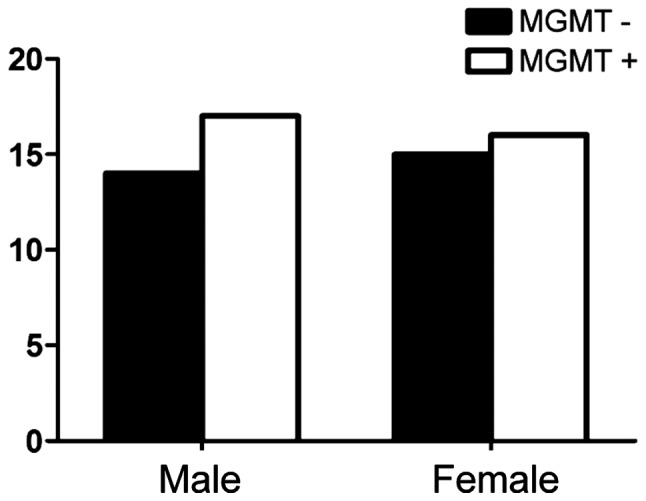
Relationship between MGMT expression and gender. MGMT, O6-methylguanine DNA methyltransferase.
Figure 3.
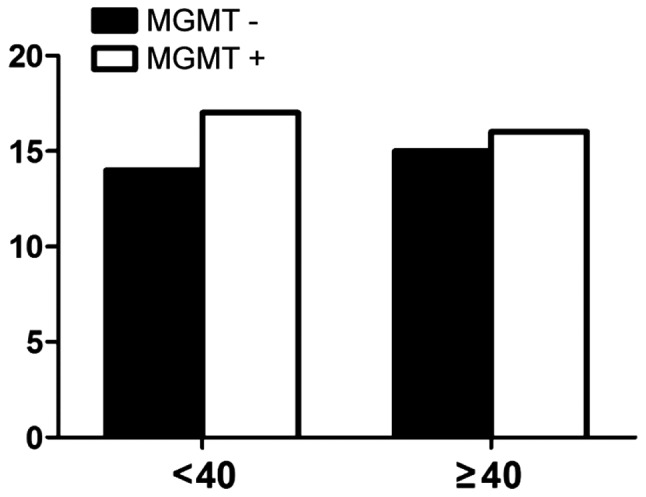
Relationship between MGMT expression and age. MGMT, O6-methylguanine DNA methyltransferase.
Figure 4.
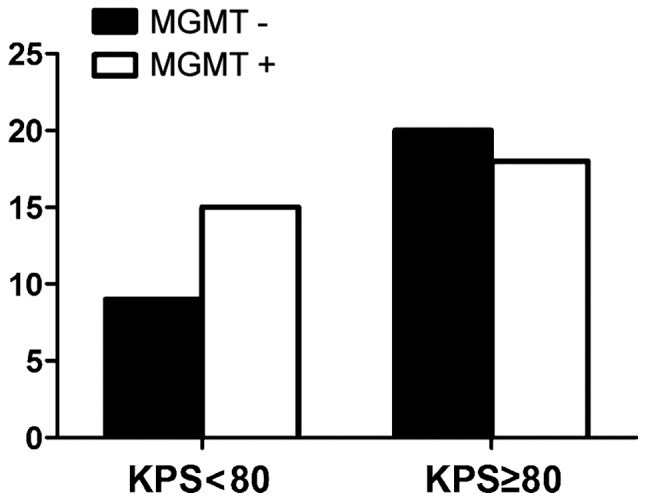
Relationship between MGMT expression and pre-operative KPS score. MGMT, O6-methylguanine DNA methyltransferase; KPS, Karnofsky performance status.
Figure 5.
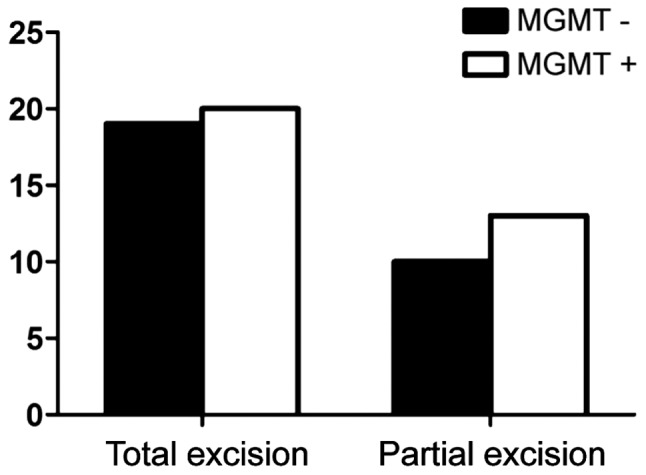
Relationship between MGMT expression and excision method. MGMT, O6-methylguanine DNA methyltransferase.
Figure 6.
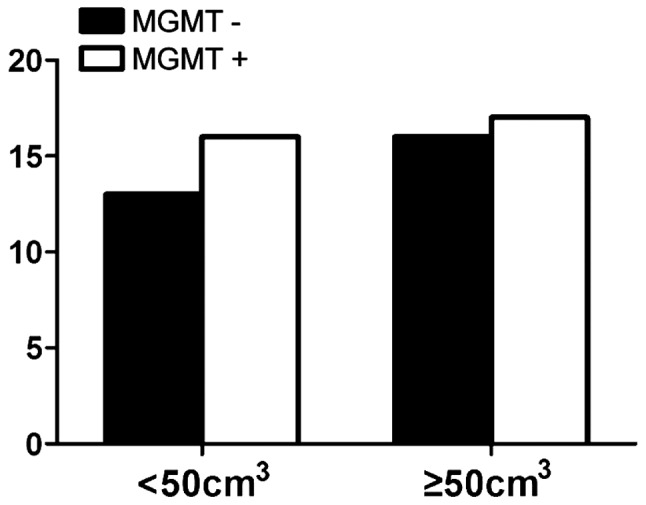
Relationship between MGMT expression and tumor volume. MGMT, O6-methylguanine DNA methyltransferase.
Figure 7.
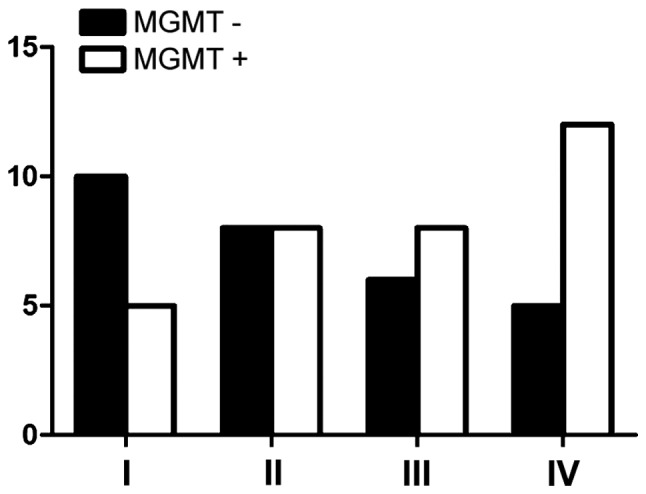
Positive expression of MGMT in different grades. MGMT, O6-methylguanine DNA methyltransferase.
Western blotting
Protein expression of MGMT in normal controls was the lowest, while protein expression increased with increasing glioma grade especially for grade IV (Fig. 8). The expression of MGMT was 3.2-fold that of the control group, and the difference had statistical significance (P<0.01), but the expression of each tumor grade showed no relation with WHO tumor grade (P>0.05).
Figure 8.
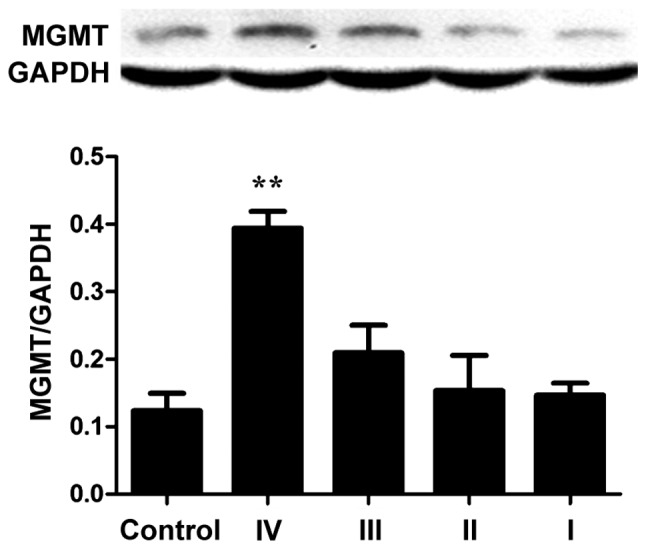
Protein expression of MGMT in different grades. MGMT, O6-methylguanine DNA methyltransferase. **P<0.05 compared to control.
Evaluation of short-term remission
After treatment, the patients were divided into complete remission and partial remission as according to the short-term evaluation standard of WHO. In this experiment, the objective efficacy for patients with negative MGMT protein expression was better than that of MGMT-positive expression group. In MGMT negative expression group, the effective rate reached 72.4% (21/29), while the effective rate of MGMT-positive expression group was only 18.2% (5/33), with statistical significance (P<0.05).
Survival time
The 3-year Kaplan-Meier survival of patients with MGMT-negative expression was 65.5% (19 cases), while the survival rate of patients with MGMT-positive expression was 45.5% (15 cases), but the difference had no statistical significance by log-rank detection (P>0.05) (Fig. 9).
Figure 9.
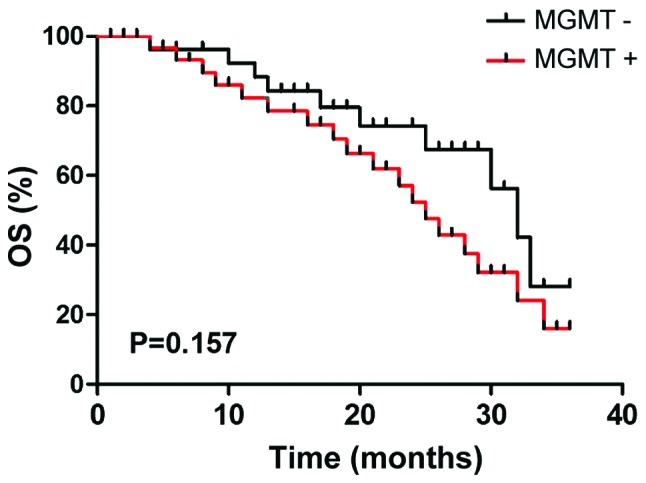
Comparison of the patient survival curve between MGMT negative expression group and MGMT positive expression group. MGMT, O6-methylguanine DNA methyltransferase.
Discussion
Glioma often occurs in the neurogliocyte of neuroderm, which is a most common malignant tumor with high recurrence, fatality and low recovery rates. The tumor cell has obvious effect, high invasiveness and infiltrative growth. Tumor cell proliferation is fast and expands to other brain tissue. Currently, the main therapy for glioma is a comprehensive treatment including neurosurgical operation, chemotherapy and radiotherapy. However, the prognosis is not ideal (9–11).
Radiotherapy is a vital step in the process of glioma treat-ment, and the main chemotherapeutic agent is from the alkylating class of drugs. These drugs can alkylate DNA and form crosslinks, leading to high cytotoxicity, effect on DNA replication leading to tumor cell death. MGMT is distributed in different tissues, with brain tissue among the lowest. The expression of MGMT in normal tissue is lower than that of tumor tissue, whose activity is often taken as an important index for observing the prognosis of patients. MGMT can act on DNA crosslinks and restore DNA alkylation, reducing the toxic effect of alkane chemotherapy drugs and causing MGMT inactivation, so that patients develop drug resistance. As a result, the expression level of MGMT can influence the drug resistance of an alkylating agent in tumor cells (12). However, besides individual variation, the main reason that influences the chemotherapeutic effect for tumor also depends on drug resistance (13). The survival time of the patients with glioma is usually only 12–15 months, up to 90% of patients die due to drug tolerance of the tumor cells (14). Therefore, before the treatment on glioma, it is important to identify high expression of MGMT for the chemotherapy prognosis of patients. For the patients with glioma, the current first line of chemotherapeutics is alkylating agent TMZ and new nitrosourea drugs. Some clinical research has confirmed that the treatment effect of radiotherapy combined with TMZ was better than that of single radiotherapy for patients with glioma who received tumor excision, which can increase the survival rate of patients from 10 to 26% and improve disease prognosis (15).
In this study, gender, age, tumor size, surgical method and KPS score had no statistical difference in MGMT expression. This suggests that MGMT could not be used as an independent reference for the individual chemotherapy regimen in clinic. At the same time, it was confirmed that the difference between positive expression of MGMT and WHO grade of glioma had statistical significance. The expression of MGMT in glioma tissue was higher than that of normal tissue and with an increase in WHO grade, the positive expression of MGMT was higher. Research by Yuan et al showed that expression of MGMT in the patients with lower level malignancy increased, while the expression was decreased in the patients with high-grade malignancy (16–18). Other research has also confirmed that there was no obvious correlation between the positive expression of MGMT and WHO grade (18). However, the conclusions were based on limited cases.
Our results show that the survival time of the MGMT-negative expression group was longer than that of MGMT-positive expression group, but the difference showed no statistical significance by log-rank detection. It suggests that for glioma patients with positive expression of MGMT, antineoplastic drugs of alkylating agent class should be avoided, so as to provide patients with a better personalized treatment (19–21).
References
- 1.Blumenthal DT, Dvir A, Lossos A, Tzuk-Shina T, Lior T, Limon D, Yust-Katz S, Lokiec A, Ram Z, Ross JS, et al. Clinical utility and treatment outcome of comprehensive genomic profiling in high grade glioma patients. J Neurooncol. 2016;130:211–219. doi: 10.1007/s11060-016-2237-3. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, van den Bent MJ, Mason WP, Weller M, Mirimanoff RO, Cairncross JG. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group: Changing paradigms - an update on the multidisciplinary management of malignant glioma. Oncologist. 2006;11:165–180. doi: 10.1634/theoncologist.11-2-165. [DOI] [PubMed] [Google Scholar]
- 3.Kaina B, Christmann M, Naumann S, Roos WP. MGMT: Key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents. DNA Repair (Amst) 2007;6:1079–1099. doi: 10.1016/j.dnarep.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Hsu CY, Lin SC, Ho HL, Chang-Chien YC, Hsu SP, Yen YS, Chen MH, Guo WY, Ho DM. Exclusion of histiocytes/endothelial cells and using endothelial cells as internal reference are crucial for interpretation of MGMT immunohistochemistry in glioblastoma. Am J Surg Pathol. 2013;37:264–271. doi: 10.1097/PAS.0b013e318267b061. [DOI] [PubMed] [Google Scholar]
- 5.Jacinto FV, Esteller M. MGMT hypermethylation: A prognostic foe, a predictive friend. DNA Repair (Amst) 2007;6:1155–1160. doi: 10.1016/j.dnarep.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Bocangel D, Sengupta S, Mitra S, Bhakat KK. p53-mediated down-regulation of the human DNA repair gene O6-methylguanine-DNA methyltransferase (MGMT) via interaction with Sp1 transcription factor. Anticancer Res. 2009;29:3741–3750. [PMC free article] [PubMed] [Google Scholar]
- 7.Molnár J, Engi H, Hohmann J, Molnár P, Deli J, Wesolowska O, Michalak K, Wang Q. Reversal of multidrug resitance by natural substances from plants. Curr Top Med Chem. 2010;10:1757–1768. doi: 10.2174/156802610792928103. [DOI] [PubMed] [Google Scholar]
- 8.Tugcu B, Postalci LS, Gunaldi O, Tanriverdi O, Akdemir H. Efficacy of clinical prognostic factors on survival in patients with glioblastoma. Turk Neurosurg. 2010;20:117–125. doi: 10.5137/1019-5149.JTN.2461-09.4. [DOI] [PubMed] [Google Scholar]
- 9.Yoshino A, Ogino A, Yachi K, Ohta T, Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N, Sano E, et al. Gene expression profiling predicts response to temozolomide in malignant gliomas. Int J Oncol. 2010;36:1367–1377. doi: 10.3892/ijo_00000621. [DOI] [PubMed] [Google Scholar]
- 10.Lombardi G, Pace A, Pasqualetti F, Rizzato S, Faedi M, Anghileri E, Nicolotto E, Bazzoli E, Bellu L, Villani V, et al. Predictors of survival and effect of short (40 Gy) or standard-course (60 Gy) irradiation plus concomitant temozolomide in elderly patients with glioblastoma: A multicenter retrospective study of AINO (Italian Association of Neuro-Oncology) J Neurooncol. 2015;125:359–367. doi: 10.1007/s11060-015-1923-x. [DOI] [PubMed] [Google Scholar]
- 11.Suryadevara CM, Verla T, Sanchez-Perez L, Reap EA, Choi BD, Fecci PE, Sampson JH. Immunotherapy for malignant glioma. Surg Neurol Int. 2015;6(Suppl 1):S68–S77. doi: 10.4103/2152-7806.151341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen ZP, Malapetsa A, McQuillan A, Marcantonio D, Bello V, Mohr G, Remack J, Brent TP, Panasci LC. Evidence for nucleotide excision repair as a modifying factor of O6-methylguanine-DNA methyltransferase-mediated innate chloroethylnitrosourea resistance in human tumor cell lines. Mol Pharmacol. 1997;52:815–820. doi: 10.1124/mol.52.5.815. [DOI] [PubMed] [Google Scholar]
- 13.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: Standard of care and future directions. J Clin Oncol. 2007;25:4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 14.Bush ZM, Longtine JA, Cunningham T, Schiff D, Jane JA, Jr, Vance ML, Thorner MO, Laws ER, Jr, Lopes MB. Temozolomide treatment for aggressive pituitary tumors: Correlation of clinical outcome with O6-methylguanine methyltransferase (MGMT) promoter methylation and expression. J Clin Endocrinol Metab. 2010;95:E280–E290. doi: 10.1210/jc.2010-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nitta M, Muragaki Y, Maruyama T, Ikuta S, Komori T, Maebayashi K, Iseki H, Tamura M, Saito T, Okamoto S, et al. Proposed therapeutic strategy for adult low-grade glioma based on aggressive tumor resection. Neurosurg Focus. 2015;38:E7. doi: 10.3171/2014.10.FOCUS14651. [DOI] [PubMed] [Google Scholar]
- 16.Yuan Q, Matsumoto K, Nakabeppu Y, Iwaki T. A comparative immunohistochemistry of O6-methylguanine-DNA methyltransferase and p53 in diffusely infiltrating astrocytomas. Neuropathology. 2003;23:203–209. doi: 10.1046/j.1440-1789.2003.00504.x. [DOI] [PubMed] [Google Scholar]
- 17.Esteller M, Garcia-Foncillas J, Andion E, Goodman SN, Hidalgo OF, Vanaclocha V, Baylin SB, Herman JG. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354. doi: 10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 18.Rimel BJ, Huettner P, Powell MA, Mutch DG, Goodfellow PJ. Absence of MGMT promoter methylation in endometrial cancer. Gynecol Oncol. 2009;112:224–228. doi: 10.1016/j.ygyno.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavon I, Zrihan D, Zelikovitch B, Fellig Y, Fuchs D, Soffer D, Siegal T. Longitudinal assessment of genetic and epigenetic markers in oligodendrogliomas. Clin Cancer Res. 2007;13:1429–1437. doi: 10.1158/1078-0432.CCR-06-2050. [DOI] [PubMed] [Google Scholar]
- 20.Bobola MS, Blank A, Berger MS, Silber JR. O6-methylguanine-DNA methyltransferase deficiency in developing brain: Implications for brain tumorigenesis. DNA Repair (Amst) 2007;6:1127–1133. doi: 10.1016/j.dnarep.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rapkins RW, Wang F, Nguyen HN, Cloughesy TF, Lai A, Ha W, Nowak AK, Hitchins MP, McDonald KL. The MGMT promoter SNP rs16906252 is a risk factor for MGMT methylation in glioblastoma and is predictive of response to temozolomide. Neuro Oncol. 2015;17:1589–1598. doi: 10.1093/neuonc/nov064. [DOI] [PMC free article] [PubMed] [Google Scholar]


