Abstract
Systemic inflammation, as evidenced by the Glasgow prognostic score (GPS), predicts cancer-specific survival in various cancer types. The aim of this study was to evaluate the significance of the GPS in the therapeutic outcome of the patient following surgical resection for hepatocellular carcinoma. In total, 144 patients underwent surgical resection for hepatocellular carcinoma. For the assessment of systemic inflammatory response using the GPS, patients were classified into three groups: Patients with normal serum albumin (<3.5 g/dl) and normal serum C-reactive protein (CRP) (≤1.0 mg/dl) were classified as GPS 0 (n=76), those with low serum albumin (<3.5 g/dl) or elevated serum CRP (>1.0 mg/dl) were classified as GPS 1 (n=58), and those with low serum albumin (<3.5 g/dl) and elevated serum CRP (>1.0 mg/dl) were classified as GPS 2 (n=10). Retrospectively, the relationship between patient characteristics including GPS, disease-free as well as overall survival were investigated. In disease-free survival, GPS 2 (P=0.019), with a tumor number ≥3 (P=0.004), and positive portal or venous invasion (P=0.034) were independent predictors of cancer recurrence in multivariate analysis. In overall survival, GPS 1 (P=0.042), GPS 2 (P<0.001) and positive portal or venous invasion (P<0.001) were independent predictors of poor patient outcome according to multivariate analysis. To conclude, the GPS in patients with hepatocellular carcinoma is an independent prognostic predictor after hepatic resection.
Keywords: Glasgow prognostic score, prognosis, hepatocellular carcinoma, hepatic resection
Introduction
Hepatocellular carcinoma (HCC) is one of the most prevalent malignancies worldwide, the seventh most common cancer, and the third leading cause of cancer-related deaths (1). Although operative mortality and morbidity of hepatic resection in patients with HCC have been minimized by advances in surgical techniques, instruments, and perioperative management, therapeutic outcomes remain unsatisfactory due to the high incidence of recurrence (2,3). Therefore, it is important to clarify the predictor of HCC recurrence and prognosis for decision making in additional adjuvant treatment after hepatic resection, preoperatively. Several investigators have reported tumor size, vascular invasion, intrahepatic metastasis, tumor markers, excessive blood loss, and allogenic blood transfusion as factors associated with recurrence of HCC (4–7). Unlike other solid malignancies, the prognosis of HCC is not solely dependent on tumor burden but also adversely influenced by impaired liver function secondary due to the underlying pathogenic condition (8).
The presence of the systemic inflammatory response is associated with poor therapeutic outcome in patients with malignant tumors. Several recent investigators reported that the systemic inflammatory response by the combination of serum C-reactive protein (CRP) and albumin concentrations, i.e., Glasgow prognostic score (GPS), predicts cancer-specific survival (9–14). We previously reported the GPS as a predictor of long-term therapeutic outcome for hepatobiliary malignancies, including carcinoma of the ampulla of Vater (15), gallbladder cancer (16), and unresectable colorectal cancer liver metastasis (17). However, there have been only few reports on the relationship between the GPS and long-term outcome after hepatic resection for HCC (18,19). In this study, we retrospectively evaluated whether the GPS predicts disease-free or overall survival after hepatic resection for HCC.
Patients and methods
Between January 2002 and December 2011, 159 patients underwent primary hepatic resection for HCC at the Department of Surgery, The Jikei University Hospital (Tokyo, Japan). Of these, 15 patients were excluded, nine due to additional procedures for other malignancies, six due to lack of data, leaving the remaining 144 patients for the study. Generally, the extent of hepatic resection was determined based on retention rate of indocyanine green at 15 min (ICGR15) before surgery and in reference to the hepatic reserve as described by Miyagawa et al (20).
Hemogram and chemistry profile were routinely measured for each patient preoperatively. For the assessment of systemic inflammatory response using the GPS, the patients were classified into three groups: Patients with normal albumin (≥3.5 g/dl) and normal CRP (≤1.0 mg/dl) as GPS 0, those with low albumin (<3.5 g/dl) or elevated CRP (>1.0 mg/dl) as GPS 1, and both low albumin (<3.5 g/dl) and elevated CRP (>1.0 mg/dl) as GPS 2. Patient characteristics were classified into two groups for log-rank test and the Cox proportional hazard regression model. Patient age was classified as <65 or ≥65 years. Body mass index (BMI) was classified as <25 or ≥25. Preoperative ICGR15 was classified as ≤10% or >10%. Preoperative serum α-fetoprotein (AFP) was classified as ≤20 or >20 ng/ml. The type of resection was classified as anatomical resection (extended lobectomy, lobectomy, segmentectomy or subsegmentectomy) or non-anatomical limited partial resection. Duration of operation was classified as <360 or ≥360 min. Blood loss was classified as <1,000 or ≥1,000 g. Maximal tumor diameter was classified as ≤2, >2 ≤5, or >5 cm.
We investigated the relation between clinicopathologic variables and GPS by univariate analysis. The factors consisted of the following 17 factors: Age, sex, BMI, hepatitis virus status, Child classification, preoperative ICGR15, preoperative serum AFP, type of resection, duration of operation, blood loss, red cell concentrate (RC) transfusion, fresh frozen plasma (FFP) transfusion, maximal tumor diameter, tumor number, portal or venous invasion, coexistent disease, and incidence of postoperative complications.
Next, we investigated the relation between clinicopathologic variables and disease-free as well as overall survival after hepatic resection for HCC by univariate and multivariate analyses. The factors consisted of the following 16 factors: Age, sex, BMI, hepatitis virus status, Child classification, preoperative ICGR15, preoperative serum AFP, type of resection, duration of operation, blood loss, RC transfusion, FFP transfusion, maximal tumor diameter, tumor number, portal or venous invasion, and the GPS grade.
Recurrence of HCC was defined as newly detected hypervascular hepatic or extrahepatic tumors by ultrasonography, computed tomography, magnetic resonance image or angiography, with or without increase in serum a-fetoprotein, or protein induced by vitamin K absence or antagonist-II. For recurrent HCC in the liver, repeated hepatic resection, local ablation therapy or transarterial chemoembolization was given based on hepatic functional reserve judged mainly by ICGR15 and the size, number as well as location of the recurrent tumors. Extrahepatic recurrence was mainly treated conservatively.
Pulmonary complications were defined as postoperative pneumonia; postoperative respiratory failure with pyrexia, dyspenia, and a pulmonary infiltrate on chest X-ray; or pleural effusion that required thoracentesis. Bile leakage was defined as a discharge of fluid with an increased bilirubin concentration (at least 3 times the serum bilirubin concentration measured at the same time) via the intra-abdominal drains on or after postoperative day 3 or as the need for radiologic intervention (i.e., interventional drainage) and relaparotomy for biliary collections and bile peritonitis, respectively (21). Surgical site infection was defined as surgical wound infection that affect superficial tissues (skin and subcutaneous layer) and the deeper tissues (deep incisional or organ-space) of the incision according to the Centers for Disease Control and Prevention definitions (22).
This retrospective study was approved by the Ethics Committee of Jikei University School of Medicine (no. 21–121).
Statistical analysis
Patients' characteristics were analyzed using the non-paired Student's t-test and Chi-square test. Survival analyses were performed using Kaplan-Meier method, the Log-rank test, and the Cox proportional regression model with backward elimination stepwise approach. All P-values were considered statistically significant when the associated probability was less than 0.05. These analyses were conducted using IBM® SPSS statistics version 20.0 (IBM Japan, Tokyo, Japan).
Results
Patient characteristics, and univariate analysis of the association between clinicopathologic variables and Glasgow prognostic score
Patient characteristics, univariate analysis of the relationship between clinicopathologic variables and the GPS grade are outlined in Table I. Among the study population, GPS consisted of GPS 0 in 76, GPS 1 in 58 (low albumin; n=8, elevated CRP; n=50), and GPS 2 in 10 patients, respectively. In this study, the five-year disease-free and overall survival rates after hepatic resection for HCC were 32.9 and 74.5%, respectively. In univariate analysis of the relationship between clinicopathologic variables and the GPS grade, maximal tumor diameter was positively associated with the GPS grade (P=0.002). Ratio of patients with Child classification B tended to be positively associated with the GPS grade, which however did not achieved significance (P=0.053). Coexistent diabetes mellitus was positively associated with the GPS grade (P=0.008). Post-operative complications developed in 43 of 144 patients (29.9%), consisting of pulmonary complications in 11 (7.6%), bile leakage in 12, (8.3%), massive ascites in 7 (4.9%), and surgical site infection (SSI) in 11 patients (7.6%), respectively. The GPS grade was negatively associated with the incidence of SSI (P=0.021). Other complications were comparable among the three GPS grades. In-hospital mortality was 3 of 144 patients (2.1%), consisting of postoperative liver failure in two (1.4%) and sepsis in one patient (0.7%), respectively.
Table I.
Patients' characteristics, and univariate analysis of clinicopathologic variables in relation to Glasgow prognostic score after elective hepatic resection for hepatocellular carcinoma.
| Glasgow prognostic score | |||||
|---|---|---|---|---|---|
| P-value (univariate) | |||||
| Factor | All patients | 0 (n=76) | 1 (n=58) | 2 (n=10) | |
| Age (years) | 63 (57–71)a | 63 (56–68) | 66 (58–72) | 66 (62–71) | 0.246 |
| Sex (male:female) | 125:19 | 66:10 | 49:9 | 10:0 | 0.408 |
| Body mass index | 23.3 (21.3–25.4) | 22.9 (21.2–25.4) | 23.3 (21.5–25.0) | 25.3 (23.8–26.9) | 0.159 |
| Hepatitis virus (HBV:HCV:none) | 38:55:51 | 23:32:21 | 13:21:24 | 2:2:6 | 0.218 |
| Child classification (A:B) | 134:10 | 74:2 | 52:6 | 8:2 | 0.053 |
| ICGR15 (%) | 12 (8–19) | 12 (8–16) | 13 (9–23) | 13 (8–21) | 0.149 |
| Serum α-fetoprotein (ng/ml) | 13.7 (5–119.5) | 11 (4.8–26.2) | 38 (6.7–330.0) | 21.5 (10.9–36.7) | 0.183 |
| Type of resection (anatomical:partial) | 78:66 | 43:33 | 30:28 | 5:5 | 0.824 |
| Duration of operation (min) | 371 (262–480) | 377 (239–480) | 368 (285–473) | 315 (263–452) | 0.692 |
| Intraoperative blood loss (g) | 682 (308–1403) | 622 (325–1136) | 1,018 (352–1,845) | 360 (183–760) | 0.125 |
| RC transfusion (present:absent) | 33:111 | 13:63 | 18:40 | 2:8 | 0.160 |
| FFP transfusion (present:absent) | 26:118 | 12:64 | 12:46 | 2:8 | 0.755 |
| Tumor diameter (cm) | 3.2 (2.2–5.5) | 3.0 (2.2–4.2) | 4.0 (2.4–7.4) | 4.0 (2.6–8.2) | 0.002 |
| Tumor number (single:double:triple or more) | 114:13:17 | 62:9:5 | 43:4:11 | 9:0:1 | 0.161 |
| Portal or venous invasion (positive:negative) | 33:111 | 15:61 | 16:42 | 2:8 | 0.549 |
| Coexistent disease | |||||
| Hypertension (present:absent) | 64:80 | 34:42 | 27:31 | 3:7 | 0.621 |
| Diabetes mellitus (present:absent) | 40:104 | 19:57 | 14:44 | 7:3 | 0.008 |
| Hyperlipidemia (present:absent) | 18:126 | 10:66 | 6:52 | 2:8 | 0.673 |
| Postoperative complications | |||||
| Pulmonary complications (present:absent) | 11:133 | 5:71 | 4:54 | 2:8 | 0.312 |
| Massive ascites (present:absent) | 7:137 | 3:73 | 4:54 | 0:10 | 0.558 |
| Biliary leakage (present:absent) | 12:132 | 3:73 | 7:51 | 2:8 | 0.093 |
| Surgical site infection (present:absent) | 11:133 | 4:72 | 4:54 | 3:7 | 0.021 |
median (25–75 percentile). GPS, Glasgow prognostic score; HBV, hepatitis B virus; HCV, hepatitis C virus; ICGR15, retention rate of indocyanine green at 15 min; RC, red cell concentrate; FFP, fresh frozen plasma.
Univariate analysis of clinicopathologic variables in relation to disease-free as well as overall survival after hepatic resection for hepatocellular carcinoma
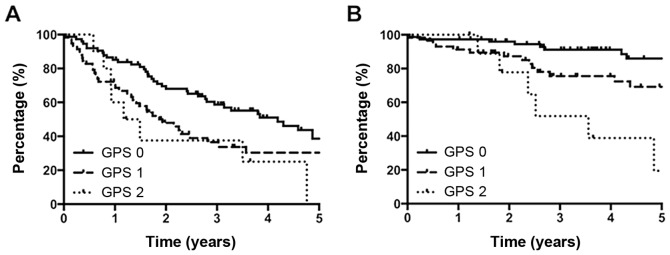
Table II lists univariate analysis of the relationship between the clinicopathologic variables and disease-free as well as overall survival after hepatic resection for HCC. In univariate analysis of disease-free survival, ≥65 years of age (P=0.015), >20 ng/ml of preoperative serum AFP (P=0.015), tumor number of triple or more (P=0.003), positive portal or venous invasion (P=0.048), and GPS 2 (Fig. 1A; P=0.036) demonstrated significant correlating with cancer recurrence. In overall survival, >5 cm of maximal tumor diameter (P=0.011), tumor number of triple or more (P=0.017), positive portal or venous invasion (P<0.001), and GPS 1 (Fig. 1B; P=0.022) and GPS 2 (P<0.001) were significantly associated with poor outcome. More than 200 ng/ml of preoperative serum AFP tended to be associated with poor overall survival, which however was not significant (P=0.070).
Table II.
Univariate analysis of clinicopathologic variables in relation to disease-free and overall survival after elective hepatic resection for hepatocellular carcinoma.
| Disease-free survival | Overall survival | ||||
|---|---|---|---|---|---|
| Factor | N | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value |
| Age (years) | |||||
| ≥65 | 66 | 1.660 (1.123–2.660) | 0.015 | 1.590 (0.7966–3.249) | 0.186 |
| <65 | 78 | Ref | Ref | ||
| Sex | |||||
| Female | 19 | 1.159 (0.5641–2.438) | 0.672 | 1.610 (0.5709–5.571) | 0.322 |
| Male | 125 | Ref | Ref | ||
| Body mass index | |||||
| ≥25 | 43 | 0.836 (0.5344–1.319) | 0.451 | 0.6755 (0.3258–1.493) | 0.355 |
| <25 | 101 | Ref | Ref | ||
| Hepatitis virus | |||||
| HBV | 38 | 0.732 (0.413–1.297) | 0.285 | 0.838 (0.318–2.203) | 0.719 |
| HCV | 55 | 1.170 (0.725–1.888) | 0.521 | 1.294 (0.579–2.893) | 0.530 |
| None | 51 | Ref | Ref | ||
| Child's classification | |||||
| B | 10 | 1.350 (0.5885–3.412) | 0.441 | 2.367 (0.8056–16.04) | 0.095 |
| A | 134 | Ref | Ref | ||
| ICGR15 | |||||
| >10 | 86 | 1.217 (0.8024–1.860) | 0.358 | 1.402 (0.6954–2.795) | 0.351 |
| ≤10 | 58 | Ref | Ref | ||
| Serum α-fetoprotein (ng/ml) | |||||
| >20 | 60 | 1.407 (0.9260–2.210) | 0.015 | 1.878 (0.9483–3.964) | 0.070 |
| ≤20 | 84 | Ref | Ref | ||
| Type of resection | |||||
| Anatomical | 78 | 0.9672 (0.6364–1.468) | 0.874 | 1.755 (0.8796–3.519) | 0.114 |
| Partial | 66 | Ref | Ref | ||
| Duration of operation (min) | |||||
| ≥360 | 77 | 1.198 (0.7917–1.826) | 0.393 | 0.8851 (0.4420–1.768) | 0.729 |
| <360 | 67 | Ref | Ref | ||
| Intraoperative blood loss (g) | |||||
| ≥1,000 | 54 | 1.400 (0.9207–2.223) | 0.114 | 1.712 (0.8590–3.612) | 0.123 |
| <1,000 | 90 | Ref | Ref | ||
| RC transfusion | |||||
| Present | 33 | 1.443 (0.8914–2.549) | 0.127 | 1.657 (0.7659–4.135) | 0.181 |
| Absent | 111 | Ref | Ref | ||
| FFP transfusion | |||||
| Present | 26 | 1.357 (0.7836–2.526) | 0.255 | 1.794 (0.8126–4.952) | 0.131 |
| Absent | 118 | Ref | Ref | ||
| Tumor diameter (cm) | |||||
| >5 | 38 | 1.156 (0.831–1.607) | 0.389 | 2.061 (1.179–3.601) | 0.011 |
| >2, ≤5 | 75 | 0.963 (0.727–1.275) | 0.791 | 1.129 (0.666–1.914) | 0.653 |
| ≤2 | 31 | Ref | Ref | ||
| Tumor number | |||||
| Triple or more | 17 | 2.472 (1.352–4.522) | 0.003 | 2.853 (1.207–6.744) | 0.017 |
| Double | 13 | 1.180 (0.563–2.473) | 0.660 | 0.791 (0.186–3.367) | 0.751 |
| Single | 114 | Ref | Ref | ||
| Portal or venous invasion | |||||
| Positive | 33 | 1.591 (1.010.2.925) | 0.048 | 4.483 (3.279.18.43) | <0.001 |
| Negative | 111 | Ref | Ref | ||
| Glasgow prognostic score | |||||
| 2 | 10 | 2.258 (1.054.4.836) | 0.036 | 7.446 (2.656.20.879) | <0.001 |
| 1 | 58 | 1.503 (0.963.2.346) | 0.073 | 2.530 (1.146.5.585) | 0.022 |
| 0 | 76 | Ref | Ref | ||
HBV, Hepatitis B virus; HCV, hepatitis C virus; ICGR15, retention rate of indocyanine green at 15 min; RC, red cell concentrate; FFP, fresh frozen plasma; GPS, Glasgow prognostic score; CI, confidence interval.
Figure 1.
In statistical analysis, GPS 1 or 2 was an independent risk factor of poor disease-free survival (A), and overall survival (B) after hepatic resection for patients with hepatocellular carcinoma.
Multivariate analysis of clinicopathologic variables in relation to disease-free as well as overall survival after hepatic resection for hepatocellular carcinoma
Table III lists multivariate analysis of the relationship between the clinicopathologic variables and disease-free as well as overall survival after hepatic resection for HCC. In multivariate analysis of disease-free survival, tumor number of triple or more (P=0.004), positive portal or venous invasion (P=0.034), and GPS 2 (P=0.019) were independent risk factors of cancer recurrence. In overall survival, positive portal or venous invasion (P=0.034), and GPS 1 (P=0.042) or GPS 2 (P<0.001) were independent risk factors of poor outcome.
Table III.
Multivariate analysis of clinicopathologic variables in relation to disease-free and overall survival after elective hepatic resection for hepatocellular carcinoma.
| Disease-free survival | Overall survival | ||||
|---|---|---|---|---|---|
| Factor | N | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value |
| Tumor number | |||||
| Triple or more | 17 | 2.468 (1.333–4.571) | 0.004 | – | – |
| Double | 13 | 1.383 (0.653–2.933) | 0.397 | – | – |
| Single | 114 | Ref | – | – | |
| Portal or venous invasion | |||||
| Positive | 33 | 1.680 (1.041–2.713) | 0.034 | 4.494 (2.226–9.073) | <0.001 |
| Negative | 111 | Ref | Ref | ||
| Glasgow prognostic score | |||||
| 2 | 10 | 2.527 (1.163–5.490) | 0.019 | 8.012 (2.818–22.784) | <0.001 |
| 1 | 58 | 1.392 (0.882–2.198) | 0.156 | 2.277 (1.029–5.039) | 0.042 |
| 0 | 76 | Ref | Ref | ||
GPS, Glasgow prognostic score; CI, confidence interval.
Discussion
Recent studies have reported the prognostic significance of the preoperative inflammation-based scores, such as the GPS, the neutrophil to lymphocyte ratio (23), the platelet to lymphocyte ratio (24), the Prognostic index (25), and the Prognostic nutritional index (26). An inflammatory response to the tumor leads to tumor proliferation and metastasis due to inhibition of apoptosis and promotion of angiogenesis (27–30). The GPS is a clinically useful inflammation-based prognostic scoring system for predicting the postoperative prognosis of the patients with colorectal cancer (9), esophageal cancer (10), bladder cancer (11), pancreatic head cancer (12), renal clear cell cancer (13), non-small cell lung cancer (14), and HCC (18,19). The GPS is evaluated using only serum CRP and serum albumin, which are common laboratory parameters for assessment of pre- and postoperative patients status. Therefore the GPS could stratify the patients with malignant tumors on prognosis after surgical resection easily, less-expensive, and less-invasive. Most HCC patients suffered from chronic hepatitis or cirrhosis, and synthesis of protein such as CRP and albumin are impaired.
Disease-free survival is probably affected by remnant liver volume and remnant liver functional reserve. We hope to show the relationship between remnant liver volume and therapeutic outcome after hepatic resection for HCC in future study.
In this study, tumor number of 3 or more, positive portal or venous invasion, and GPS 2 were independent risk factors of cancer recurrence in multivariate analysis. Positive portal or venous invasion, and GPS 1 and 2 were also independent risk factors of poor overall survival in multivariate analysis. To the best of our knowledge, this is the first study that patients were clearly stratified on risk of poor overall survival after hepatic resection for HCC with respect to grade of the GPS. Moreover, on postoperative infectious complications, the incidence of surgical site infection was greater, and that of biliary leakage tended to correlate with the GPS grade in univariate analysis. Therefore, the GPS may be able to stratify the patients with HCC on risks of postoperative complications as well as cancer recurrence and prognosis after hepatic resection.
The GPS upon diagnosis in patients with HCC was an independent predictor in disease-free and overall survival after hepatic resection. Measurement of GPS may help decision making in the post-operative management of patients with HCC.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Poon RT, Fan ST, Lo CM, Ng IO, Liu CL, Lam CM, Wong J. Improving survival results after resection of hepatocellular carcinoma: A prospective study of 377 patients over 10 years. Ann Surg. 2001;234:63–70. doi: 10.1097/00000658-200107000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poon RT, Ng IO, Fan ST, Lai EC, Lo CM, Liu CL, Wong J. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: A study of a prospective cohort. J Clin Oncol. 2001;19:3037–3044. doi: 10.1200/JCO.2001.19.12.3037. [DOI] [PubMed] [Google Scholar]
- 4.Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D'Angelica MI, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617–623. doi: 10.1097/SLA.0b013e31819ed22f. [DOI] [PubMed] [Google Scholar]
- 5.Arii S, Tanaka J, Yamazoe Y, Minematsu S, Morino T, Fujita K, Maetani S, Tobe T. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer. 1992;69:913–919. doi: 10.1002/1097-0142(19920215)69:4<913::AID-CNCR2820690413>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 6.Yanaga K. Current status of hepatic resection for hepatocellular carcinoma. J Gastroenterol. 2004;39:919–926. doi: 10.1007/s00535-004-1422-x. [DOI] [PubMed] [Google Scholar]
- 7.Shiba H, Ishida Y, Wakiyama S, Iida T, Matsumoto M, Sakamoto T, Ito R, Gocho T, Furukawa K, Fujiwara Y, Hirohara S, et al. Negative impact of blood transfusion on recurrence and prognosis of hepatocellular carcinoma after hepatic resection. J Gastrointest Surg. 2009;13:1636–1642. doi: 10.1007/s11605-009-0963-y. [DOI] [PubMed] [Google Scholar]
- 8.Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: A systematic review of 72 studies. Liver Int. 2009;29:502–510. doi: 10.1111/j.1478-3231.2008.01957.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMillian DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22:881–886. doi: 10.1007/s00384-006-0259-6. [DOI] [PubMed] [Google Scholar]
- 10.Crumley AB, McMillan DC, McKernan M, Going JJ, Shearer CJ, Stuart RC. An elevated C-reactive protein concentration, prior to surgery, predicts poor cancer-specific survival in patients undergoing resection for gastro-oesophageal cancer. Br J Cancer. 2006;94:1568–1571. doi: 10.1038/sj.bjc.6603150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hilmy M, Bartlett JM, Underwood MA, McMillan DC. The relationship between the systemic inflammatory response and survival in patients with transitional cell carcinoma of the urinary bladder. Br J Cancer. 2005;92:625–627. doi: 10.1038/sj.bjc.6602406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jamieson NB, Glen P, McMillan DC, McKay CJ, Foulis AK, Carter R, Imrie CW. Systemic inflammatory response predicts outcome in patients undergoing resection for ductal adenocarcinoma head of pancreas. Br J Cancer. 2005;92:21–23. doi: 10.1038/sj.bjc.6602305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamb GW, Aitchison M, Ramsey S, Housley SL, McMillan DC. Clinical utility of the Glasgow Prognostic Score in patients undergoing curative nephrectomy for renal clear cell cancer: Basis of new prognostic scoring systems. Br J Cancer. 2012;106:279–283. doi: 10.1038/bjc.2011.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara M, Matsuzaki Y, Shimuzu T, Tomita M, Ayabe T, Enomoto Y, Onitsuka T. Preoperative serum C-reactive protein level in non-small cell lung cancer. Anticancer Res. 2007;27:3001–3004. [PubMed] [Google Scholar]
- 15.Shiba H, Misawa T, Fujiwara Y, Futagawa Y, Furukawa K, Haruki K, Iwase R, Wakiyama S, Ishida Y, Yanaga K. Glasgow prognostic score predicts therapeutic outcome after pancreaticoduodenectomy for carcinoma of the ampulla of vater. Anticancer Res. 2013;33:2715–2721. [PubMed] [Google Scholar]
- 16.Shiba H, Iwase R, Futagawa Y, Futagawa Y, Furukawa K, Haruki K, Iwase R, Iida T, Yanaga K. Glasgow prognostic score predicts therapeutic outcome after surgical resection for gallbladder cancer. World J Surg. 2015;39:753–758. doi: 10.1007/s00268-014-2844-0. [DOI] [PubMed] [Google Scholar]
- 17.Furukawa K, Shiba H, Haruki K, Fujiwara Y, Iida T, Mitsuyama Y, Ogawa M, Ishida Y, Misawa T, Yanaga K. The Glasgow prognostic score is valuable for colorectal cancer with both synchronous and metachronous unresectable liver metastases. Oncol Lett. 2012;4:324–328. doi: 10.3892/ol.2012.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishizuka M, Kubota K, Kita J, Shimoda M, Kato M, Sawada T. Impact of an inflammation-based prognostic system on patients undergoing surgery for hepatocellular carcinoma: A retrospective study of 398 Japanese patients. Am J Surg. 2012;203:101–106. doi: 10.1016/j.amjsurg.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 19.Horino K, Beppu T, Kuroki H, Mima K, Okabe H, Nakahara O, Ikuta Y, Chikamoto A, Ishiko T, Takamori H, Baba H. Glasgow prognostic score as a useful prognostic factor after hepatectomy for hepatocellular carcinoma. Int J Clin Oncol. 2013;18:829–838. doi: 10.1007/s10147-012-0451-3. [DOI] [PubMed] [Google Scholar]
- 20.Miyagawa S, Makuuchi M, Kawasaki S, Kakazu T. Criteria for safe hepatic resection. Am J Surg. 1995;169:589–594. doi: 10.1016/S0002-9610(99)80227-X. [DOI] [PubMed] [Google Scholar]
- 21.Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, et al. Bile leakage after hepatobiliary and pancreatic surgery: A definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for disease control and prevention (CDC) hospital infection control practices advisory committee. Am J Infect Control. 1999;27:97–134; 96. doi: 10.1016/S0196-6553(99)70088-X. [DOI] [PubMed] [Google Scholar]
- 23.Ding PR, An X, Zhang RX, Fang YJ, Li LR, Chen G, Wu XJ, Lu ZH, Lin JZ, Kong LH, et al. Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis. 2010;25:1427–1433. doi: 10.1007/s00384-010-1052-0. [DOI] [PubMed] [Google Scholar]
- 24.Shirai Y, Shiba H, Sakamoto T, Horiuchi T, Haruki K, Fujiwara Y, Futagawa Y, Ohashi T, Yanaga K. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery. 2015;158:360–365. doi: 10.1016/j.surg.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 25.Kasymjanova G, MacDonald N, Agulnik JS, Cohen V, Pepe C, Kreisman H, Sharma R, Small D. The predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancer. Curr Oncol. 2010;17:52–58. doi: 10.3747/co.v17i4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanda M, Fujii T, Kodera Y, Nagai S, Takeda S, Nakao A. Nutritional predictors of postoperative outcome in pancreatic cancer. Br J Surg. 2011;98:268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 27.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 28.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 30.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]