Abstract
Patent ductus arteriosus (PDA) is a common congenital heart disease that results when the ductus arteriosus, a muscular artery, fails to remodel and close after birth. A syndromic form of this disorder, Char syndrome, is caused by mutation in TFAP2B, the gene encoding a neural crest-derived transcription factor. Established features of the syndrome are PDA, facial dysmorphology, and fifth-finger clinodactyly. Disease-causing mutations are missense and are proposed to be dominant negative. Because only a small number of families have been reported, there is limited information on the spectrum of mutations and resulting phenotypes. We report the characterization of two kindreds (K144 and K145) with Char syndrome containing 22 and 5 affected members, respectively. Genotyping revealed linkage to TFAP2B in both families. Sequencing of TFAP2B demonstrated mutations in both kindreds that were not found among control chromosomes. Both mutations altered highly conserved bases in introns required for normal splicing as demonstrated by biochemical studies in mammalian cells. The abnormal splicing results in mRNAs containing frameshift mutations that are expected to be degraded by nonsense-mediated mRNA decay, resulting in haploinsufficiency; even if produced, the protein in K144 would lack DNA binding and dimerization motifs and would likely result in haploinsufficiency. Examination of these two kindreds for phenotypes that segregate with TFAP2B mutations identified several phenotypes not previously linked to Char syndrome. These include parasomnia and dental and occipital-bone abnormalities. The striking sleep disorder in these kindreds implicates TFAP2B-dependent functions in the normal regulation of sleep.
Keywords: human genetics, parasomnia, congenital heart defects, Char
The ductus arteriosus is a muscular artery connecting the pulmonary artery and the aorta during fetal life, shunting blood away from the lungs. It normally occludes shortly after birth. Failure of ductal closure results in patent ductus arteriosus (PDA), one of the most common congenital heart defects, affecting 1 in 2,000 to 1 in 5,000 full-term infants and constituting ≈5–7% of all congenital heart defects (1–3). The process of closure of the ductus arteriosus starts with intimal thickening in the fetus in the second trimester of gestation, with subendothelial accumulation of matrix proteins accompanied by detachment of endothelial cells from the internal elastic lamina, followed by migration of smooth muscle cells into the subendothelial region (4, 5). The final steps include contraction of smooth muscle regulated by oxygen tension and by alteration in the levels of vasoactive hormones (6–8). The molecular mechanisms underlying this complex process are largely unknown. Premature delivery commonly results in PDA, which can be corrected by cyclooxygenase inhibitors. Similarly, targeted disruption of the prostaglandin E2 receptor gene, EP4, results in PDA (9). In humans, loci for dominant and recessive forms of PDA have been mapped (10, 11, **).
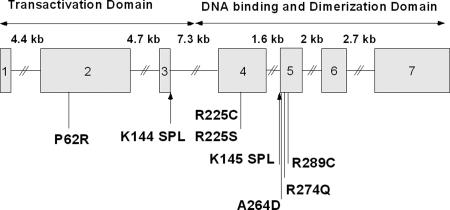
One such disorder is Char syndrome, an autosomal dominant trait characterized by PDA, facial dysmorphism, and skeletal abnormalities of the hand (clinodactyly). Char is caused by mutations in TFAP2B, the gene encoding a neural crest-derived transcription factor (12). To date, five kindreds, each with four to seven mutation carriers, have been reported, along with a mutation in an isolated case (Fig. 1) (11, 12). The TFAP2B mutations in Char syndrome to date are all missense. Biochemical studies have supported a dominant-negative mechanism of TFAP2B mutants (11, 12).
Fig. 1.
Structure of TFAP2B. The genomic structure of TFAP2B is depicted, with exons as boxes; the size of introns is indicated above the diagram, and the location and consequence of mutations found in Char kindreds are indicated. “SPL” denotes splice site mutations found in K144 and K145.
TFAP2B is expressed in the neural crest (13) and is a member of the AP2 transcription factor family, a family of retinoic acid-responsive genes that play an important role in development, apoptosis, cell-cycle control, and complex morphogenic processes (14, 15).
The protein consists of an amino-terminal P/Q-rich transcriptional activation domain and a helix–span–helix domain that mediates dimerization and DNA binding (Fig. 1).
TFAP2B binds to the consensus sequence GCCN3/4GGC, stimulates transcription of a number of downstream target genes, and interacts with a number of other transcription factors.
Here we report the characterization of two Char kindreds. We show that in both, disease is caused by splice site mutations that are inferred to cause haploinsufficiency, in contrast to the previously shown dominant-negative mechanism. In addition, we demonstrate a number of previously unreported phenotypes that segregate with these mutations, including a striking sleep disorder.
Methods
Family Studies. Investigation of kindred 144 (K144) and kindred 145 (K145) was performed under a protocol approved by the Human Investigation Committee of the Yale University School of Medicine. All study participants provided informed consent. Genomic DNA was prepared from venous blood of 40 participating members of K144 and 5 members of K145 by standard procedures. Individuals were classified as having Char syndrome if they had surgical or echocardiographic diagnosis of PDA after term birth or had both typical facial dysmorphism and clinodactyly of the fifth finger without PDA (16–19). The echocardiographic diagnosis of PDA was made by pulsed Doppler echocardiographic evidence of continuous turbulent flow in the right pulmonary artery from the precordial and suprasternal notch approaches. One subject who was born at 34 weeks of gestation had PDA and was prospectively classified as phenotype unknown. This individual had no syndromic features and was the offspring of phenotypically normal parents.
Genotyping and Analysis of Linkage. We tested linkage to the Char critical region (20). Eleven polymorphic tetra- and dinucleotide repeat markers spanning 48 cM across the Char critical region were genotyped in study participants by PCR using specific fluorescence-labeled oligonucleotide primers and genomic DNA as templates. The amplified products were fractionated by electrophoresis on an Applied Biosystems 377 instrument equipped with gene scanner 2.1 software.
Analysis of linkage was performed on a Sun Sparcstation 20 (Sun Microsystems, Mountain View, CA). Pairwise and multipoint analysis was performed by using linkage 5.1 (21). The model of the trait locus specified a mutant gene frequency of 0.0001, a penetrance of 90%, and a phenocopy prevalence of 0.0001.
Mutation Screening. Mutations were sought in TFAP2B by single-strand conformational polymorphism, denaturing HPLC, and direct sequencing. The coding exons and their flanking exon–intron boundaries were amplified by PCR using genomic DNA from affected and unaffected members of the kindred, as well as 100 normal unrelated subjects, as templates. Identified variants were confirmed by sequencing of both DNA strands in independent amplifications of multiple affected members of each kindred.
Splicing Assay. A 211-bp segment containing wild-type exon 3 and the flanking intron sequences of TFAP2B was amplified by using specific primers; a second segment was prepared that was identical except for the mutation identified in K144. These segments were subcloned into the splicing vector pSPL3 (Life Technologies, Grand Island, NY), which places this segment between exons and splice sites for β-globin. Constructs were sequenced to ensure fidelity. For exon trapping, COS7 cells were transiently transfected with 3 μg of plasmid DNA by using lipofectamin (Life Technologies). Cells were harvested 36 h later and RNA was extracted by using TRIzol reagent (Life Technologies). First-strand cDNA synthesis was performed by using Omniscript RT (Qiagen, Valencia, CA). Forward and reverse primers from the globin exons were used to amplify the spliced mRNA. The amplified PCR products were fractionated on agarose gel, purified, and subjected to DNA sequencing.
Results
Characterization of PDA Kindreds. K144 was ascertained by means of an asymptomatic 22-year-old female who was referred for evaluation of a heart murmur before a planned pregnancy. A large PDA was diagnosed, and surgical ligation was recommended. Her family history revealed two living sisters with term PDA, both treated with surgical ligation, and a brother who died in the neonatal period from heart failure with coarctation of aorta, bicuspid aortic valve, and a large PDA. Examination of these three living siblings with PDA revealed facial dysmorphic features in one and finger clinodactyly in all, providing evidence of Char syndrome. Based on these findings, the extended family was examined for PDA and Char phenotypes. Echocardiography and physical examination was performed in 37 additional family members (Fig. 2a), which identified five additional subjects with PDA. All of these five had facial dysmorphic features and finger clinodactyly (Table 1, which is published as supporting information on the PNAS web site). In sum, nine kindred members with PDA were diagnosed between the neonatal period and age 30, and all were born at term as the product of normal gestation. In addition, there were 13 subjects who had dysmorphology and clinodactyly without PDA. Given the known incomplete penetrance of PDA in Char syndrome (11), these subjects were also classified as affected. The facial dymorphism varied and included hypertelorism, broad forehead, nasal abnormality (flat nasal bridge with upturned nares or curved nose), short philtrum, thick or patulous lips, mild ptosis, and strabismus. All affected members had at least two of the three features of Char syndrome: PDA, dysmorphic facial appearance, and clinodactyly. One obligate carrier (subject II-5) was nonpenetrant for all features. There were no kindred members with isolated dysmorphology or clinodactyly, indicating a clear separation of subjects with and without Char in this kindred.
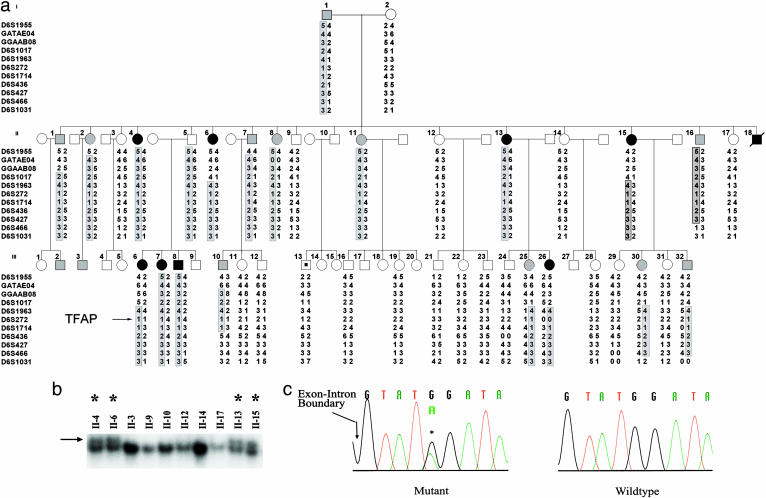
Fig. 2.
Splice site mutation in TFAP2B in K144. (a) Linkage of Char syndrome to TFAP2B. The structure of K144 is shown. Solid symbols indicate members of the kindred with PDA, shaded symbols denote members of the kindred without PDA but with typical facial and hand features of Char syndrome, and open symbols denote unaffected members. (Clinical details are provided in Table 1.) The symbol with a dot in the center indicates a subject with PDA at premature birth who was considered phenotype unknown for linkage analysis. Genotypes for 11 microsatellite markers spanning the TFAP2B locus are shown. Chromosome segments that cosegregate with Char syndrome are enclosed by boxes. (b) Single-strand conformational polymorphism analysis. The results of single-strand conformational polymorphism analysis of exon 3 and its flanking exon–intron boundaries are shown in affected (denoted by asterisk) and unaffected family members. A previously uncharacterized variant found in affected members is indicated by the arrow. (c) DNA sequence of splice junction of exon 3 and intron 3. DNA sequences of an affected member of K144 (Left) and a wild-type subject (Right) are shown. A heterozygous substitution (G to A) at position +5 of the splice donor site of intron 3 is indicated by an asterisk.
Finally, one additional family member (subject III-13; Fig. 2a) had surgery for PDA shortly after premature birth. This patient had no dysmorphology or clinodactyly and was the offspring of unaffected parents and had no affected siblings. He was classified as phenotype unknown for genetic analysis.
The segregation of Char syndrome in the kindred was compatible with autosomal dominant transmission; PDA showed incomplete penetrance, whereas the dysmorphic faces and clinodactyly showed evidence of high penetrance (Table 1 and Fig. 2a).
K145 was ascertained by means of a 6-month-old infant, the product of an uncomplicated term delivery, who presented with congestive heart failure. She was found to have a large PDA. Evaluation of the kindred revealed that the father, an additional sibling, and the father's sister had term PDA, dysmorphic faces, and clinodactyly typical of Char syndrome. All had undergone corrective procedures to close the ductus. The pattern of transmission was consistent with autosomal dominant transmission with high penetrance (Fig. 3a).
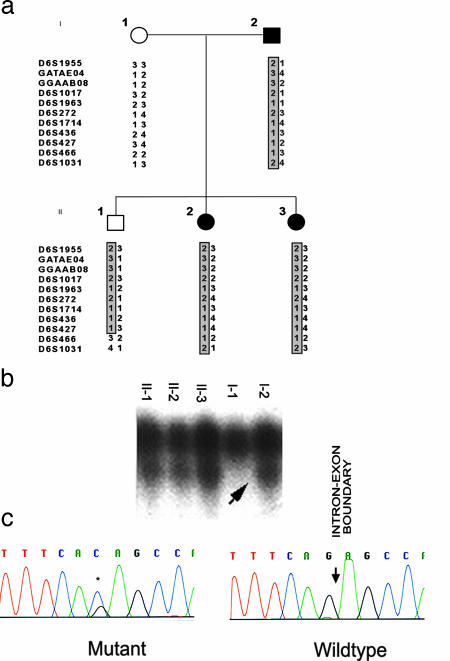
Fig. 3.
Splice site mutation in K145. (a) The structure of K145 and genotypes across the TFAP2B locus are shown as in Fig. 2. Affected members all share the same haplotype, as does one unaffected member. (b) Single-strand conformational polymorphism analysis of the segment containing the intron 4–exon 5 splice junction identifies a variant in affected kindred members. (c) DNA sequence analysis of amplified segment in b identifies a heterozygous (G-to-C) splice acceptor mutation at the last base of intron 4.
Linkage to TFAP2B. Analysis of linkage was performed in these kindreds, genotyping 11 microsatellite markers on chromosome 6p that span 48 cM across the interval containing the TFAP2B locus (Figs. 2a and 3a). Strong evidence of linkage of this chromosome segment to Char was detected in K144 (multipoint lod score 7.48). The meiotic recombination events in affected subjects localized the disease gene to a 9.2 million-bp interval flanked by loci D6S1017 and D6S436; this interval contains TFAP2B. In K145, all of the affected individuals shared the same haplotype spanning the Char critical region, consistent with linkage (Fig. 3a).
Splice Site Mutation in TFAP2B. We next screened TFAP2B for mutation in both kindreds, as described in Methods. We identified one previously unreported DNA-sequence variant in the affected subjects of each kindred (Figs. 2b and 3b). These variants cosegregated with the affected phenotype and were absent among 200 unrelated control chromosomes. The mutation in K144 introduced a single base substitution (G to A) at position +5 of the splice donor site of intron 3 (Fig. 2c). G is present at this position in 84% of all eukaryotic splice donors, whereas A is present in only 5%; moreover, G at this position in TFAP2B is conserved among species from sea squirt (Ciona intestinalis) to human (www.ncbi.nlm.nih.gov/BLAST/Blast.cgi), a span of ≈550 million years (22). No other variant was observed in TFAP2B. Finally, splice site prediction programs (e.g., www.fruitfly.org/seq_tools/splice.html) predict that this mutation will result in aberrant splicing. These features all suggest that this mutation will disrupt normal splicing of exons 3 and/or 4 (Fig. 1; see below).
Direct sequencing of the TFAP2B gene in K145 revealed a single previously unreported variant, a G-to-C transition of the last base of intron 4 (Fig. 3 b and c); G at the last base of an intron is virtually completely conserved among eukaryotic splice junctions and is essential for normal splicing (23). The mutation in K145 was shared among all affected individuals in the kindred (Fig. 3b). This variant was not found in 200 control chromosomes. There is no doubt that this mutation will disrupt the normal splicing of TFAP2B, disrupting splicing of exon 5.
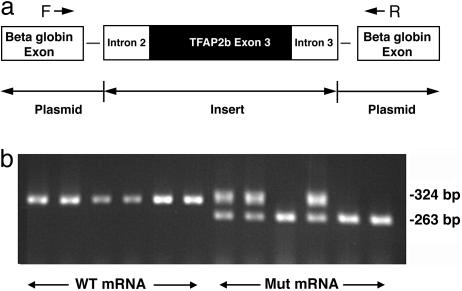
Disrupted Splicing Due to Mutation in K144. It appears highly likely that the mutation in K144 disrupts splicing, and we tested this hypothesis directly by assay in mammalian cells. We cloned the wild type and the mutant segment encoding exon 3 and flanking intronic sequence into the vector pSPL3, which places exon 3 between two β-globin exons along with their respective splice donor and acceptor sites (Fig. 4a). After transfection into COS7 cells, this segment is transcribed, and splicing occurs by means of the normal cellular machinery. After extraction of RNA and generation of cDNA by reverse transcription, primers inside the two β-globin exons are used to amplify the products; amplification of a properly spliced transcript will result in a 324-bp product (263 bp from the β-globin exons plus 61 bp from exon 3 of TFAP2B). Six independent transfections with the wild-type segment from TFAP2B all yielded a single product that, when sequenced, showed proper splicing of exon 3 between the two β-globin exons. In contrast, six independent transfections with an insert that differs only by the presence of the mutation found in K144 all revealed abnormal splicing: either complete exon skipping fusing the two β-globin exons or a mixture of properly spliced products with skipped exon products (Fig. 4b). This finding was reproduced with three independent clones of the mutant segment of TFAP2B (data not shown). This finding confirms the functional significance of the mutation in K144.
Fig. 4.
Aberrant splicing of exon 3 due to TFAP2B mutation. (a) Inserts containing wild-type or mutant exon 3 and 150 bp of the flanking introns were inserted in the cloning site of the vector SPL3. The insert in each case is flanked by β-globin exons and their splice junctions. (b) Total cellular RNA was extracted from COS7 cells and reverse-transcribed, and the product was used to direct PCR using primers from the two β-globin exons as described in Methods. The products were fractionated on 1.2% agarose gel, and the results are shown for wild-type and mutant TFAP2B constructs. The wild-type construct consistently produces a 384-bp product that contains all three exons properly spliced. The mutant construct produces, either instead of or in addition to this fragment, a product of 263 bp that contains only the two β-globin exons.
Altered Sleep and Bone and Dental Abnormalities Segregate with TFAP2B Mutations. K144 has a very large number of mutation carriers (n = 23). With the identification of the functional mutation in this kindred, we are able to determine whether there are any additional phenotypes that significantly segregate with the disease-causing mutation.
The most striking of these phenotypes was parasomnia, presenting primarily as sleepwalking. This disorder came to our attention when the index case described the habit of sleepwalking, associated with food-seeking behavior. This pattern dated to age 8. Direct inquiry of all family members identified 12 additional family members with similar features. The characteristics were distinctive. Although the age of onset varied from 8 to 30 years, the frequency did not diminish after adolescence, with episodes typically recurring three to four times per week. All 13 members with this trait carried the TFAP2B mutation, and this trait was absent among family members without the mutation [χ2 (1df) = 19, P = 10-5]. The severity of this problem had led to polysomnography studies in several affected members; in each, the study indicated sleep disruption but excluded sleep apnea.
In K145, the affected father and his three children reportedly sleep only 2–3 h each night, without signs of daytime fatigue. Although polysomnography was not performed, the father and one of his daughters underwent medical therapy and tonsillectomy for a presumptive diagnosis of sleep apnea but have not responded to these therapies.
Ten of 13 family members with the TFAP2B mutation in K144 had a protuberant occipital bone with growth of overlaying coarse hair, whereas none of the six members directly studied who are wild type had this trait (P = 0.003, Fisher's exact test). In each case, the border of the occiput was well defined by a sharp elevated ridge, suggestive of craniosynostosis.
Hypodontia, the absence of secondary teeth with the abnormal retention of primary teeth, was identified in 14 adult genotype-positive members of the family and none of the genotype-negative family members [Fig. 5, which is published as supporting information on the PNAS web site; χ 2 (1 df) = 20, P < 10-5]. The affected individuals retain their primary teeth and either partially or completely lack secondary teeth.
Finally, in addition to finger clinodactyly, affected individuals of both kindreds had varying degrees of clinodactyly of the fourth and fifth toes, and syndactyly of these digits was seen in four family members.
Discussion
We have demonstrated splice site mutations in TFAP2B in two kindreds with Char syndrome. These mutations segregate with disease in the kindreds and are absent in control subjects, and their functional significance is indicated by both the high conservation of the mutated bases and the demonstrated requirement of the wild-type sequence for normal splicing.
In contrast to the prior reports of dominant-negative TFAP2B mutations in Char syndrome, the mechanism of disease in these two kindreds is likely to be haploinsufficiency. For example, we showed that the K144 mutation results in skipping of the 61 bp of exon 3. The spliced product would fuse exons 1 and 2 to exons 4–7, resulting in a frameshift mutation after the 169 aa encoded in exons 1 and 2; beyond this point would be seven mutant amino acids followed by a premature termination codon. These transcripts will be susceptible to nonsense-mediated mRNA decay (NMD) and, therefore, should be null alleles (24). Similar frameshifts and premature termination result if the mutation leads to skipping of exon 4 or both exon 3 and exon 4. Even if this transcript were to escape degradation, because the encoded protein would lack the dimerization and DNA-binding domains, it is unlikely to have dominant-negative effects. Similarly, the mutation in K145 is predicted to result in transcripts that would be degraded by NMD. These mutations are consequently most consistent with haploinsufficiency as the genetic mechanism.
Although counterintuitive, there is precedent for both dominant-negative and haploinsufficient mutations causing the same phenotype. For example, this phenomenon is believed to occur in Holt–Oram syndrome, in which both dominant-negative and loss-of-function mutations in TBX5 are found (25). In these cases, it remains an open question whether both mechanisms operate in vivo or whether, for example, the dominant-negative effect seen in vitro is less relevant in vivo.
We have reported previously unreported phenotypes that segregate with TFAP2B mutations and that should be considered a part of Char syndrome. Although some of these traits, such as hypodontia, have previously been reported in a few cases (11), the finding of significant cosegregation in K144 provides convincing evidence that the trait is attributable to the mutation and not a chance finding. Several of these traits are similar to phenotypes that have been previously linked to mutations in other transcription factors expressed in the neural crest. For example, mutation in MSX2 leads to craniosynostosis type 2 (26) [MIM:604757], and mutation in MSX1 results in hypodontia (27) like that seen in both of our Char kindreds. The finding that TFAP2B mutation results in these phenotypes further implicates the neural crest in these developmental processes in ectoderm.
The sleep abnormality observed in both kindreds is particularly interesting. Sleepwalking, the predominant feature of the sleep disorder in K144, represents a subgroup of parasomnias and consists of a series of complex behaviors that are initiated during slow-wave sleep (28). Factors disrupting slow-wave sleep, such as sleep apnea, are known to predispose people to this trait; however, formal sleep studies in several severely affected subjects in K144 eliminated this diagnosis. The incidence of sleepwalking in adults is estimated to have a prevalence of 0.1–0.6% (29), so the high frequency of parasomnia in K144, as well as its cosegregation with the TFAP2B mutation, is highly significant. Although genetic factors have been implicated in sleepwalking, particularly when the trait persists in adulthood, no genes have been previously identified that contribute to this trait (30, 31).
Although there is no direct evidence regarding the mechanism of the sleep disorder in these kindreds, one might speculate that abnormalities in adenosine metabolism could contribute to both PDA and sleep disorder. Increased adenosine levels contribute to wakefulness and are known to modulate sleep (32, 33); adenosine is also a potent vasodilator of the ductus arteriosus during oxygen-induced vasoconstriction (34). It would consequently be of interest to examine adenosine levels and metabolism in these patients as well as to examine the TFAP2B+/- mouse (14) and members of other Char kindreds for altered sleep; further clinical investigation of the sleep abnormality in these patients will be of interest.
Finally, with the exception of a few isolated cases (17), the previously reported kindreds or individuals with Char have apparently had isolated hand anomalies. Accordingly, Char has often been referred to as a hand–heart syndrome (11). The toe clinodactyly and syndactyly in many affected members of K144 demonstrates a role of TFAPB2 in foot development, as well. Similar results have been seen in animal models (35). This finding is in accord with observations in other limb–heart syndromes, such as Rubinstein–Taybi (mutation in CREBBP) (36, 37), ulnar–mammary (mutation in TBX3) (38, 39), and Simpson–Golabi–Behmel (mutation in glypican-3) (40, 41), which all feature concordant hand and foot anomalies.
Supplementary Material
Acknowledgments
We are deeply grateful to the patients and families for their participation in the study and to Stanley Diede and Karin Hagel for their generous help in examination of family members. We thank David Geller and Maria Lalioti for advice and helpful dicussions.
Abbreviations: PDA, patent ductus arteriosus; Kn, kindred n.
Footnotes
Mani, A., Lynch, H. T., Begleiter, M. L., Porter, T. R., Rezaie, T. & Lifton, R. P. (1998) Circulation 98, I-596 (abstr.).
References
- 1.Mitchell, S. C., Korones, S. B. & Berendes, H. W. (1971) Circulation 43, 323-332. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman, J. I. & Christianson, R. (1978) Am. J. Cardiol. 42, 641-647. [DOI] [PubMed] [Google Scholar]
- 3.Ferencz, C., Rubin, J. D., McCarter, R. J., Brenner, J. I., Neill, C. A., Perry, L. W., Hepner, S. I. & Downing, J. W. (1985) Am. J. Epidemiol. 121, 31-36. [DOI] [PubMed] [Google Scholar]
- 4.Gittenberger-de Groot, A. C. & Strengers, J. L. (1988) Int. J. Cardiol. 19, 153-166. [DOI] [PubMed] [Google Scholar]
- 5.Gittenberger-de Groot, A. C., Strengers, J. L., Mentink, M., Poelmann, R. E. & Patterson, D. F. (1985) J. Am. Coll. Cardiol. 6, 394-404. [DOI] [PubMed] [Google Scholar]
- 6.Heymann, M. A., Rudolph, A. M. & Silverman, N. H. (1976) N. Engl. J. Med. 295, 530-533. [DOI] [PubMed] [Google Scholar]
- 7.Clyman, R. I., Mauray, F., Roman, C., Rudolph, A. M. & Heymann, M. A. (1980) J. Pediatr. 97, 455-461. [DOI] [PubMed] [Google Scholar]
- 8.Clyman, R. I., Wong, L., Heymann, M. A. & Rudolph, A. M. (1978) Prostaglandins 15, 325-331. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen, M., Camenisch, T., Snouwaert, J. N., Hicks, E., Coffman, T. M., Anderson, P. A., Malouf, N. N. & Koller, B. H. (1997) Nature 390, 78-81. [DOI] [PubMed] [Google Scholar]
- 10.Mani, A., Meraji, S. M., Houshyar, R., Radhakrishnan, J., Ahangar, M., Rezaie, T. M., Taghavinejad, M. A., Broumand, B., Zhao, H., Nelson-Williams, C. & Lifton, R. P. (2002) Proc. Natl. Acad. Sci. USA 99, 15054-15059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satoda, M., Zhao, F., Diaz, G. A., Burn, J., Goodship, J., Davidson, H. R., Pierpont, M. E. & Gelb, B. D. (2000) Nat. Genet. 25, 42-46. [DOI] [PubMed] [Google Scholar]
- 12.Zhao, F., Weismann, C. G., Satoda, M., Pierpont, M. E., Sweeney, E., Thompson, E. M. & Gelb, B. D. (2001) Am. J. Hum. Genet. 69, 695-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilger-Eversheim, K., Moser, M., Schorle, H. & Buettner, R. (2000) Gene 260, 1-12. [DOI] [PubMed] [Google Scholar]
- 14.Moser, M., Pscherer, A., Roth, C., Becker, J., Mucher, G., Zerres, K., Dixkens, C., Weis, J., Guay-Woodford, L., Buettner, R. & Fassler, R. (1997) Genes Dev. 11, 1938-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moser, M., Ruschoff, J. & Buettner, R. (1997) Dev. Dyn. 208, 115-124. [DOI] [PubMed] [Google Scholar]
- 16.Slavotinek, A., Clayton-Smith, J. & Super, M. (1997) Am. J. Med. Genet. 71, 229-232. [PubMed] [Google Scholar]
- 17.Zannolli, R., Mostardini, R., Matera, M., Pucci, L., Gelb, B. D. & Morgese, G. (2000) Am. J. Med. Genet. 95, 201-203. [DOI] [PubMed] [Google Scholar]
- 18.Temple, I. K. (1992) Clin. Dysmorphol. 1, 17-21. [PubMed] [Google Scholar]
- 19.Sletten, L. J. & Pierpont, M. E. (1995) Am. J. Med. Genet. 57, 27-30. [DOI] [PubMed] [Google Scholar]
- 20.Satoda, M., Pierpont, M. E., Diaz, G. A., Bornemeier, R. A. & Gelb, B. D. (1999) Circulation 99, 3036-3042. [DOI] [PubMed] [Google Scholar]
- 21.Shimkets, R. A., Warnock, D. G., Bositis, C. M., Nelson-Williams, C., Hansson, J. H., Schambelan, M., Gill, J. R., Jr., Ulick, S., Milora, R. V., Findling, J. W., et al. (1994) Cell 79, 407-414. [DOI] [PubMed] [Google Scholar]
- 22.Bejerano, G., Pheasant, M., Makunin, I., Stephen, S., Kent, W. J., Mattick, J. S. & Haussler, D. (2004) Science 304, 1321-1325. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro, M. B. & Senapathy, P. (1987) Nucleic Acids Res. 15, 7155-7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker, K. E. & Parker, R. (2004) Curr. Opin. Cell Biol. 16, 293-299. [DOI] [PubMed] [Google Scholar]
- 25.Basson, C. T., Huang, T., Lin, R. C., Bachinsky, D. R., Weremowicz, S., Vaglio, A., Bruzzone, R., Quadrelli, R., Lerone, M., Romeo, G., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 2919-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jabs, E. W., Muller, U., Li, X., Ma, L., Luo, W., Haworth, I. S., Klisak, I., Sparkes, R., Warman, M. L., Mulliken, J. B., et al. (1993) Cell 75, 443-450. [DOI] [PubMed] [Google Scholar]
- 27.Vastardis, H., Karimbux, N., Guthua, S. W., Seidman, J. G. & Seidman, C. E. (1996) Nat. Genet. 13, 417-421. [DOI] [PubMed] [Google Scholar]
- 28.Diagnostic Classification Steering Committee (1990) International Classification of Sleep Disorders: Diagnostic and Coding Manual (American Sleep Disorders Association, Rochester, MN), Vol. 1.
- 29.Partinen, M. & Hubline, C. (2000) in Principles and Practice in Sleep Medicine, eds. Kryger, M. H., Roth, T. & Dement W. C. (Saunders, Philadelphia), pp. 558-579.
- 30.Abe, K., Amatomi, M. & Oda, N. (1984) Am. J. Psychiatry 141, 800-801. [DOI] [PubMed] [Google Scholar]
- 31.Bakwin, H. (1970) Lancet 2, 446-447. [DOI] [PubMed] [Google Scholar]
- 32.Porkka-Heiskanen, T. (1999) Ann. Med. 31, 125-129. [DOI] [PubMed] [Google Scholar]
- 33.Porkka-Heiskanen, T., Strecker, R. E., Thakkar, M., Bjorkum, A. A., Greene, R. W. & McCarley, R. W. (1997) Science 276, 1265-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mentzer, R. M., Jr., Ely, S. W., Lasley, R. D., Mainwaring, R. D., Wright, E. M., Jr., & Berne, R. M. (1985) Ann. Surg. 202, 223-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nottoli, T., Hagopian-Donaldson, S., Zhang, J., Perkins, A. & Williams, T. (1998) Proc. Natl. Acad. Sci. USA 95, 13714-13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubinstein, J. H. & Taybi, H. (1963) Am. J. Dis. Child 105, 588-608. [DOI] [PubMed] [Google Scholar]
- 37.Petrij, F., Giles, R. H., Dauwerse, H. G., Saris, J. J., Hennekam, R. C., Masuno, M., Tommerup, N., van Ommen, G. J., Goodman, R. H., Peters, D. J., et al. (1995) Nature 376, 348-351. [DOI] [PubMed] [Google Scholar]
- 38.Schinzel, A., Illig, R. & Prader, A. (1987) Clin. Genet. 32, 160-168. [DOI] [PubMed] [Google Scholar]
- 39.Bamshad, M., Lin, R. C., Law, D. J., Watkins, W. C., Krakowiak, P. A., Moore, M. E., Franceschini, P., Lala, R., Holmes, L. B., Gebuhr, T. C., et al. (1997) Nat. Genet. 16, 311-315. [DOI] [PubMed] [Google Scholar]
- 40.Simpson, J. L., Landey, S., New, M. & German, J. (1975) Birth Defects Orig. Artic. Ser. 11, 18-24. [PubMed] [Google Scholar]
- 41.Pilia, G., Hughes-Benzie, R. M., MacKenzie, A., Baybayan, P., Chen, E. Y., Huber, R., Neri, G., Cao, A., Forabosco, A. & Schlessinger, D. (1996) Nat. Genet. 12, 241-247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.