Abstract
Pancreatic ductal adenocarcinoma (PDA) is an aggressive malignant disease that is resistant to various chemotherapeutic agents and commonly relapses. Efficient elimination of metastasized PDA is critical for a positive post-surgical treatment outcome. The present study analyzed the effect of the B-cell lymphoma-2 (Bcl-2)/B-cell lymphoma extra-large (Bcl-xL) inhibitor, ABT-737, on paclitaxel-induced PDA cell death. A total of 8 PDA cell lines were subjected to immunoblotting to compare the expression of Bcl-2/Bcl-xL and other factors associated with taxane resistance, including myeloid cell leukemia 1 and βIII-tubulin (TUBB3). The viability of PDA cells was analyzed following treatment with paclitaxel alone or a combination treatment with ABT-737 and paclitaxel. Treatment with the ABT-737/paclitaxel combination induced PDA cell death at a lower concentration of paclitaxel compared with paclitaxel alone. In addition, the viable cell population at the saturation point of paclitaxel was also decreased by co-treatment with ABT-737. ABT-737 lowered the half maximal inhibitory concentration (IC50) by >2-fold in PDA cells with high Bcl-2/Bcl-xL expression, but not in PDA cells with low Bcl-2/Bcl-xL expression and high TUBB3 expression. Knockdown of Bcl-xL lowered the IC50 of paclitaxel, but knockdown of TUBB3 did not. ABT-737 sensitized PDA to paclitaxel-induced cell death, and Bcl-xL expression was a key determinant of its sensitivity. ABT-737 is potential candidate for combination chemotherapy of PDA with high Bcl-xL expression levels.
Keywords: ABT-737, B-cell lymphoma-2, B-cell extra-large, paclitaxel, pancreatic ductal adenocarcinoma
Introduction
Pancreatic ductal adenocarcinoma (PDA) is one of the most aggressive types of malignant cancer, with a 5 year survival rate of 5% (1). The majority of PDA cases are identified at a progressed stage that is too late for surgical intervention, due to a lack of symptoms and diagnostic markers at earlier stages (1). Even following surgical intervention, the 5-year survival rate is <15% without adjuvant therapy or <25% with adjuvant chemotherapy (1). Gemcitabine (GEM) -based regimens or a combined regimen of 5-fluorouracil, leucovorin, irinotecan and oxaliplatin (FOLFIRINOX) are standard, widely used first-line treatments for patients with advanced PDA (2). Second-line chemotherapy may be beneficial for patients with good performance status and tolerability to additional chemotherapy. Although a number of phase II and III second-line chemotherapy trials have been completed, sufficient evidence for efficacy has not been obtained (3). Paclitaxel is effective for advanced pancreatic cancer refractory to GEM, GEM-based regimens or FOLFIRINOX as second-line chemotherapy (4,5). In addition, nanoparticle albumin-bound paclitaxel (nab-paclitaxel; Abraxane) was previously used for first-line chemotherapy in the combination with GEM in phase I and II chemotherapy trials (6,7).
Paclitaxel binds to β-tubulin and stabilizes microtubules that induce metaphase arrest, inducing apoptotic cell death via the spindle assembly checkpoint pathway (8,9). Genetic mutations in the paclitaxel-binding region of β-tubulin may modify the sensitivity of cells to paclitaxel (10,11). Overexpression of a neuron-specific subtype βIII-tubulin (TUBB3) is involved in taxane resistance in malignant tumors (11–13), including PDA (14).
Overexpression of anti-apoptotic proteins is also associated with taxane-based therapeutics. B-cell lymphoma-2 (Bcl-2) family proteins that consist of both pro- and anti-apoptotic members regulate mitochondrial apoptotic signals and thus cell fate. Apoptotic signals are mediated by Bcl-2 homology-3-only proteins that induce oligomerization of Bcl-2-associated X protein and Bcl-2 antagonist/killer, cytochrome c release and caspase activation. The anti-apoptotic members, including Bcl-2, B-cell lymphoma extra-large (Bcl-xL), Bcl-2-like protein 2 (Bcl-w) and myeloid cell leukemia 1 (Mcl-1) antagonize this activation (15). Mcl-1 suppresses apoptosis triggered by paclitaxel or vincristine in ovarian cancer and non-small-cell lung cancer (NSCLC) cells (16). Loss-of-function mutations in the F-box and WD-40 domain protein 7 gene, which encodes an E3 ubiquitin ligase for Mcl-1 degradation, stabilizes Mcl-1 protein and contributes to taxane resistance in these tumors (17). The association between Bcl-2 family protein expression and chemosensitivity for taxane derivatives has not been fully elucidated in PDA. Furthermore, ABT-737 is a small-molecule inhibitor of Bcl-2, Bcl-xL and Bcl-w (18). Navitoclax (ABT-263) is an orally bioavailable inhibitor with a similar binding profile that is under evaluation in clinical trials (19–21). In combination with taxane-based therapy, these small molecules improved responses in a number of human malignancies (22–24).
The present study investigated the expression of Bcl-2-associated anti-apoptotic proteins, and evaluated the efficacy of combining ABT-737 with paclitaxel in PDA cell lines.
Materials and methods
Cell culture
A total of 6 PDA cell lines (PK-9, PK-8, KLM-1, PK-59, PK-45-P and PK-45-H) were obtained from the RIKEN BioResource Centre (Tsukuba, Japan). A total of two PDA cell lines (MIA-PaCa-2 and Panc-1) were purchased from the American Type Culture Collection (Manassas, VA, USA). MIA-PaCa-2 cells were maintained at 37°C under 5% CO2 in Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.); other cell lines were maintained in RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS.
Reagents and antibodies
Paclitaxel (Calbiochem; Merck KGaA, Darmstadt, Germany) and ABT-737 (Selleck Chemicals, Houston, TX, USA) were prepared at a stock concentration of 10 mM in dimethyl sulfoxide. The following antibodies were used for western blotting: Monoclonal anti-Bcl-2 (dilution, 1:250; cat. no., 05-729; EMD Millipore, Billerica, MA, USA), monoclonal anti-Mcl-1 (dilution, 1:1,000; cat. no., #5453), monoclonal anti-Bcl-xL (dilution, 1:1,000; cat. no., #2764) monoclonal anti-Bcl-w (dilution, 1:1,000; cat. no., #2764), anti-poly-(ADP-ribose) polymerase (PARP; dilution, 1:1,000; cat. no., #9542; all from Cell Signaling Technology, Inc., Danvers, MA, USA), anti-TUBB3 (dilution, 1:1,000; cat. no., MMS-435P; Covance, Inc., Princeton, NJ, USA) and mouse monoclonal anti-α-tubulin (dilution, 1:1,000; cat. no., T5168; Sigma-Aldrich; Merck KGaA). Horseradish peroxidase (HRP)-linked anti-mouse immunoglobulin (Ig) G or anti-rabbit IgG (1:3,000; NA931 or NA934, respectively; GE Healthcare Life Sciences, Chalfont, UK) were used as a secondary antibodies for western blotting.
Cell viability assay
Cells were seeded into 96-well cell culture plates at a density of 104 cells/well for 24 h. To examine the dose-response association, cells were incubated with paclitaxel (2-fold serial dilution from 1 pM to 2.5 µM) and/or ABT-737 (500 nM), or 0.1% dimethyl sulfoxide (DMSO) as a control, for 72 h at 37°C. Cell viability assays were performed using Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan), according to the manufacturer's protocol. Absorbance at 450 nm was evaluated using a Multiskan spectrum (Thermo Fisher Scientific, Inc.). IC50 was determined by fitting to a normal distribution.
Western blotting
Cells at 80–90% confluence were washed twice with cold PBS and harvested by scraping. To analyze apoptotic marker proteins, cells were treated with 100 nM paclitaxel and/or 500 nM ABT-737, or 0.1% DMSO as a control, for 24–72 h at 37°C. Adherent and floating cells were harvested followed by washing with cold PBS. Cells were collected by centrifugation (200 × g for 3 min at 4°C) and lysed with sonication (5 sec, output 4; Branson Sonifier S-150D; Branson Ultrasonics, Danbury, CT, USA) in ice-cold RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 10 mM NaF, 2 mM Na3VO4, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with Complete Protease Inhibitor cocktail (EDTA-free; Roche Diagnostics GmbH, Mannheim, Germany) and 0.5 mM PMSF. The insoluble fraction was removed by centrifugation (20,000 × g for 10 min at 4°C). Protein concentration was evaluated using a BCA Protein Assay kit (Novagen, Inc., Madison, WI, USA). Protein samples (20 µg/lane) were separated on 10% SDS-PAGE gel and then transferred onto polyvinylidene difluoride transfer membranes (Pall Corporation, Port Washington, NY, USA). The membranes were blocked with 5% non-fat dried milk (cat. no. 9999; Cell Signaling Technology, Inc.) in 0.1% Tween-20/PBS for 1 h at room temperature, and then incubated with primary antibodies overnight at 4°C and with HRP-conjugated secondary antibodies (GE Healthcare Life Sciences) for 1 h at room temperature. Signals were detected with enhanced chemiluminescence prime detection reagents (GE Healthcare Life Sciences) and processed with ChemiDoc XRS with Quantity One software (version 4.5.2; Bio-Rad Laboratories, Inc., Hercules, CA, USA), with α-tubulin as the loading control.
Knockdown of Bcl-2, Bcl-xL and TUBB3
Silencer Select Validated short interfering (si)RNA against Bcl-2 (cat. no. s224526), Bcl-xL (cat. no. s1920), TUBB3 (cat. no. s20297) and Negative Control siRNA No. 1 (cat. no., 4390843) were purchased from Ambion (Thermo Fisher Scientific, Inc.). Cells were washed once with Opti-Minimum Essential medium (Gibco; Thermo Fisher Scientific, Inc.) and then transfected with 20 nM siRNA mixed with Lipofectamine RNAiMAX transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Culture media were refreshed 6 h after transfection and cells were incubated for a further 48 h at 37°C. Cells were then replated for the cell viability assay and western blotting as previously described.
Results
Bcl-2 family and TUBB3 expression and sensitivity to ABT-737
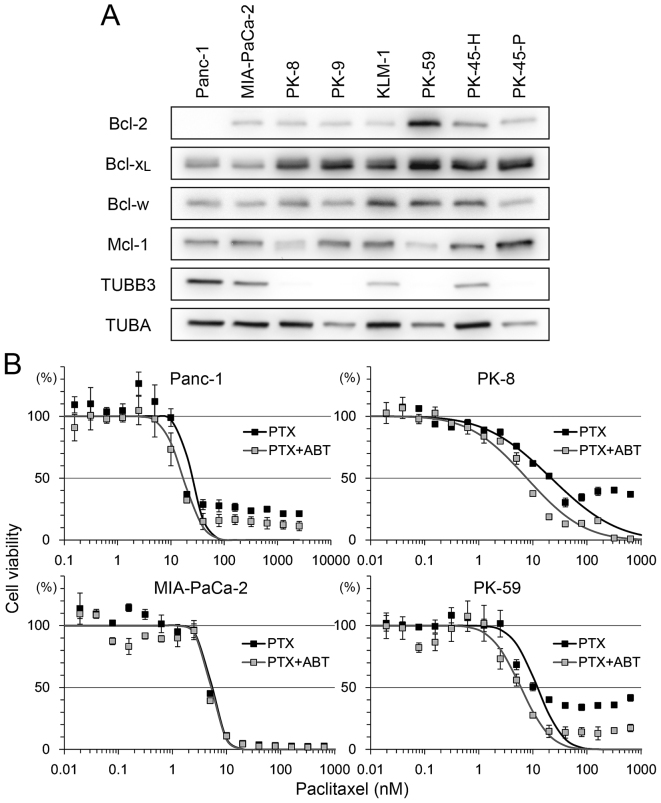
The expression level of the Bcl-2 family and TUBB3 was analyzed in 8 PDA cell lines (Fig. 1A). There was strong expression of Bcl-2 in PK-59 and moderate expression in other cell lines, but no expression in Panc-1. Bcl-xL was detectable in all cell lines, with relatively low expression in Panc-1 and MIA-PaCa-2. Bcl-w and Mcl-1 expression was detectable in all cell lines with certain variations in expression. Strong expression of TUBB3 was detected in Panc-1 and MIA-PaCa-2 but not in PK-8, PK-9, PK-59 and PK-45-P (Fig. 1A).
Figure 1.
Bcl-2 family protein expression levels in PDA and sensitivity to PTX/ABT. (A) A total of eight PDA cells lines were subjected to western blotting to detect Bcl-2, Bcl-xL, Bcl-w, Mcl-1, TUBB3 and TUBA. (B) A total of four PDA cell lines were seeded into 96-well plates and treated with 2-fold serial dilution of PTX with or without 500 nM ABT for 72 h. Cell viability was determined using Cell Counting Kit-8. Viability was normalized to control samples (treated with vehicle or ABT alone) and expressed as the mean ± standard deviation (n=5). Mortality of shifting points were fitted to a normal distribution and used to calculate the half maximal inhibitory concentration. PDA, pancreatic ductal adenocarcinoma; PTX, paclitaxel; ABT, ABT-737; Bcl-2, B-cell lymphoma-2; Bcl-xL, B-cell lymphoma extra-large; Bcl-w, Bcl-2-like protein 2; Mcl-1, myeloid cell leukemia 1; TUBB3, βIII-tubulin; TUBA, α-tubulin.
To determine the association between these factors and sensitivity to paclitaxel and ABT-737, four cell lines, Panc-1 (Bcl-2-negative, Bcl-xL-low, TUBB3-high), MIA-PaCa-2 (Bcl-2/Bcl-xL-low, TUBB3-high), PK-8 (Bcl-2-low, Bcl-xL-high, TUBB3-negative) and PK-59 (Bcl-2/Bcl-xL -high, TUBB3-negative), were selected for use in viability assays following treatment with paclitaxel and/or ABT-737. Treatment with paclitaxel decreased the viability of all four cell lines in a dose-dependent manner (Fig. 1B). Compared with paclitaxel alone, combination treatment with ABT-737 shifted the survival curve to a lower paclitaxel concentration in Panc-1, PK-8 and PK-59 cells. ABT-737 alone did not decrease the viability of PDA cell lines (data not shown), a result similar to that obtained by a previous study in melanoma cell lines (25). The IC50 of paclitaxel vs. paclitaxel/ABT-737 combination in each cell line was as follows: Panc-1 (25.05 vs. 17.14 nM), MIA-PaCa-2 (5.36 vs. 5.17 nM), PK-8 (21.87 vs. 7.75 nM) and PK-59 (12.16 vs. 6.02 nM). In addition, small populations of Panc-1, PK-8 and PK-59 cells survived following the treatment with paclitaxel at saturating concentrations (>100 nM). However, the survival population was decreased by combined treatment with paclitaxel and ABT-737 (Fig. 1B).
ABT-737 abrogates the Bcl-xL dependent anti-apoptotic effect
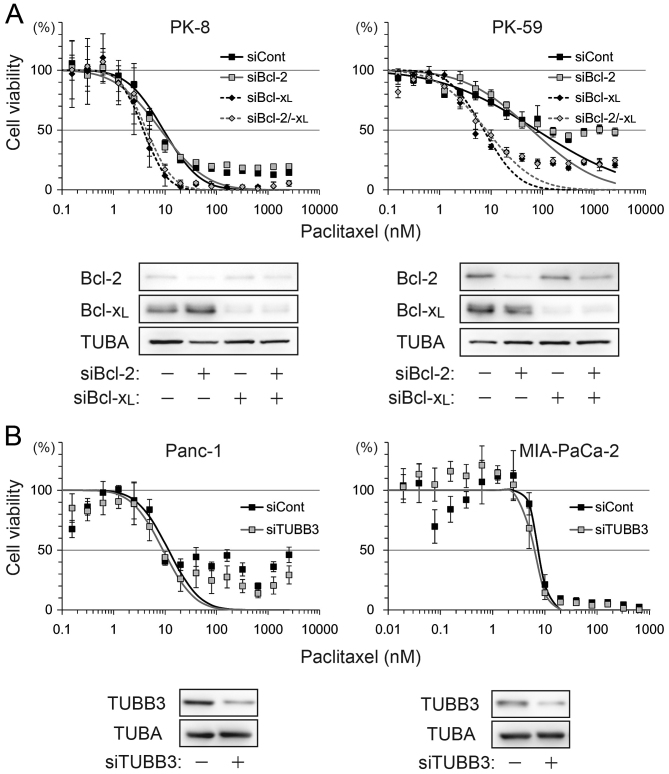
ABT-737 lowered paclitaxel-induced IC50 by >2-fold in PK-8 and PK-59 cells, but <2-fold in Panc-1 and MIA-PaCa-2 cells. To determine which factor contributed to ABT-737 sensitivity, Bcl-2, Bcl-xL and TUBB3 were depleted by siRNA transfection and subjected to a viability assay following treatment with paclitaxel alone. Compared with control siRNA, the IC50 was shifted by Bcl-xL knockdown or Bcl-2/Bcl-xL double knockdown, but not by Bcl-2 knockdown in PK-8 and PK-59 cell lines (Fig. 2A). TUBB3 knockdown in Panc-1 and MIA-PaCa-2 slightly shifted IC50 by <2-fold (Fig. 2B).
Figure 2.
Bcl-xL-dependent anti-apoptotic effect of pancreatic ductal adenocarcinoma. (A) PK-8 and PK-59 cells were transfected with control siRNA, Bcl-2 siRNA, Bcl-xL siRNA or Bcl-2 and Bcl-xL siRNA and incubated for 48 h. Cells were re-plated in 96-well plates for the Cell Counting Kit-8 viability assays and in culture dishes for western blotting to check Bcl-2 and Bcl-xL knockdown. (B) Panc-1 and MIA-PaCa-2 cells were transfected with control siRNA or TUBB3 siRNA and subjected to viability assays and western blotting. siRNA/si, short interfering RNA; Bcl-2, B-cell lymphoma-2; Bcl-xL, B-cell lymphoma extra-large; TUBB3, βIII-tubulin; TUBA, α-tubulin.
ABT-737 accelerates paclitaxel-induced cell death
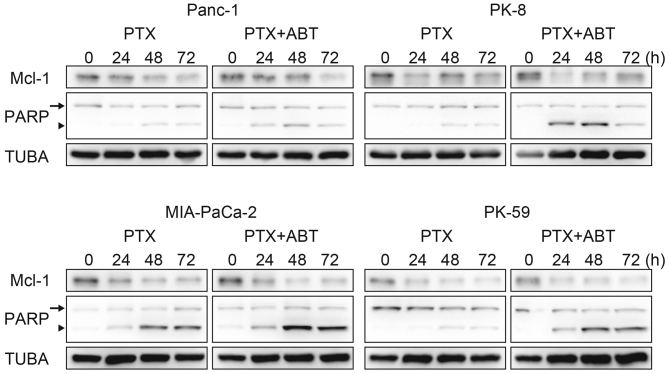
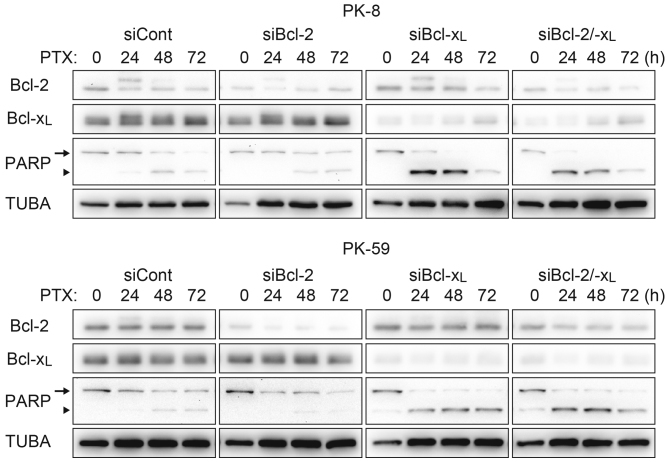
The survival population at the saturating concentration of paclitaxel was decreased by combination treatment with ABT-737 in Panc-1, PK-8 and PK-59 cells (Fig. 1A), and by Bcl-xL knockdown in PK-8 and PK-59 cell lines (Fig. 2A). Although Mcl-1 degradation during mitotic arrest was observed in all four cell lines, ABT-737 did not affect the Mcl-1 degradation kinetics (Fig. 3). Subsequently, cleavage of PARP by caspase-3 was detected as an apoptotic marker. In Panc-1, PK-8 and PK-59, cleaved PARP was detected from 48 to 72 h following treatment with paclitaxel alone. In contrast, cleaved PARP was detected from 24 h following combined treatment with paclitaxel and ABT-737. In addition, ABT-737 combination treatment increased the amount of cleaved PARP compared with treatment with paclitaxel alone (Fig. 3). Acceleration of apoptosis onset by paclitaxel/ABT-737 combination treatment was confirmed by knockdown of Bcl-2 and/or Bcl-xL. In the PK-8 and PK-59 cells, knockdown of Bcl-xL was sufficient to increase the amount of cleaved PARP 24 h following treatment with paclitaxel alone, whereas knockdown of Bcl-2 did not affect PARP cleavage compared with the control siRNA (Fig. 4).
Figure 3.
Acceleration of the apoptotic signaling pathway by ABT. Panc-1, MIA-PaCa-2, PK-8 and PK-59 cells were treated with 100 nM PTX with or without 500 nM ABT for 24, 48 or 72 h. Following treatment, adherent and floating cells were subjected to western blotting to detect Mcl-1, full length PARP (arrow) and its cleaved form (arrowhead). ABT, ABT-737; PTX, paclitaxel; Mcl-1, myeloid cell leukemia 1; PARP, Poly-(ADP-ribose) polymerase.
Figure 4.
Acceleration of the apoptotic pathway by Bcl-xL knockdown. PK-8 and PK-59 cells were transfected with siRNA against Bcl-2 and/or Bcl-xL and incubated for 48 h. Cells were re-plated in a culture dish and incubated for 24 h, and then treated with 100 nM PTX for 24, 48 or 72 h. Adherent and floating cells were subjected to western blotting to detect Bcl-2, Bcl-xL, full length PARP (arrow) and its cleaved form (arrowhead). Bcl-xL, B-cell lymphoma extra-large; siRNA/si, short interfering RNA; Bcl-2, B-cell lymphoma-2; PTX, paclitaxel; PARP, Poly-(ADP-ribose) polymerase; TUBA, α-tubulin; cont, control.
Discussion
To the best of our knowledge, the present study is the first to evaluate the efficacy of combining ABT-737 with paclitaxel in PDA. Although ABT-737 targets Bcl-2, Bcl-xL and Bcl-w, the sensitization by ABT-737 was dependent on Bcl-xL alone in the PDA cell lines investigated in the present study. In spite of other factors associated with taxane resistance, including Bcl-2 (15) and TUBB3 (11–13) expression levels, the abundance of Bcl-xL protein was the key determinant of paclitaxel sensitivity in PDA based on the results of the present study. In addition, paclitaxel-induced Mcl-1 degradation was comparable in PDA cell lines evaluated, and was independent of ABT-737 sensitivity. Abundant Bcl-xL expression contributed to an anti-apoptotic effect that raised the IC50 of paclitaxel and delayed the onset of apoptotic events. In the case of chemotherapy treatment for human malignancies, physiological concentrations of paclitaxel (26) and ABT-737 (27) were decreased by metabolic kinetics. Since ABT-737 accelerates the apoptotic signaling pathway and induces a decrease in paclitaxel dosage as observed in the present study, combination treatment with paclitaxel or other chemotherapeutics may be beneficial for the treatment of PDA. The abundance of Bcl-xL in biopsies or resected tumors may be a potential biomarker to predict the effect of administration of ABT-737.
Previously, Tan et al (28) revealed that combination treatment of NSCLC and PDA with ABT-263 and the mitogen-activated protein kinase (MEK) inhibitor G-963. ABT-263/G-963 combination treatment was revealed to be efficient in NSCLC cell lines with Kirsten rat sarcoma viral oncogene homolog mutations that constitutively activated the MEK signaling pathway. Wong et al (22) also investigated navitoclax therapy combined with paclitaxel or GEM in ovarian cancer cell lines. These combinations resulted in synergistic growth inhibition in the majority of the cell lines analyzed (22). A GEM-based regimen is considered to be the gold standard for first-line treatment of patients with advanced PDA, thus navitoclax may be a promising agent for the management of these patients.
A few unique trials targeting secreted protein acidic and rich in cysteine (SPARC) have been performed in PDA (29–31). SPARC is an extracellular matrix protein that functions as a stromal chaperone and is a target of nab-paclitaxel (29,30). Abundant SPARC expression in pancreatic cancer is associated with poor prognosis, invasion and metastasis (29,31). In addition to the cell-directed effect of nab-paclitaxel on SPARC-rich PDA, nab-paclitaxel facilitates the delivery of GEM (29). A combination of nab-paclitaxel and GEM treatment improved the median survival rate of patients with advanced disease in phase I and II studies (31,32). The results of the present study support the use of navitoclax in combination with nab-paclitaxel/GEM, as the combination may improve disease outcome in advanced PDA.
In conclusion, the present study analyzed the abundance of Bcl-2, Bcl-xL, Mcl-1 and TUBB3 in PDA cell lines. Combination treatment with ABT-737 and paclitaxel induced apoptotic cell death more efficiently compared with paclitaxel treatment alone in PDA cells with high Bcl-xL expression level. Knockdown experiments indicated that Bcl-xL, but not Bcl-2 or TUBB3, counteracted paclitaxel-induced cell death. ABT-737 is potential candidate for combination chemotherapy to treat PDA with high Bcl-xL expression levels.
Glossary
Abbreviations
- Bcl-2
B-cell lymphoma-2
- Bcl-xL
B-cell lymphoma extra-large
- GEM
gemcitabine
- IC50
half maximal inhibitory concentration
- Mcl-1
myeloid cell leukemia 1
- nab-paclitaxel
nanoparticle albumin-bound paclitaxel
- NSCLC
non-small cell lung carcinoma
- PARP
Poly-(ADP-ribose) polymerase
- PDA
pancreatic ductal adenocarcinoma
- SPARC
secreted protein acidic and rich in cysteine
- TUBB3
βIII-tubulin
References
- 1.Warshaw AL, Fernández-del Castillo C. Pancreatic Carcinoma. N Engl J Med. 1992;326:455–465. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 2.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Boige V, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23–29. doi: 10.1200/JCO.2012.44.4869. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, III, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Shukuya T, Yasui H, Boku N, Onozawa Y, Fukutomi A, Yamazaki K, Taku K, Kojima T, Machida N. Weekly Paclitaxel after failure of gemcitabine in pancreatic cancer patients with malignant ascites: A retrospective study. Jpn J Clin Oncol. 2010;40:1135–1138. doi: 10.1093/jjco/hyq117. [DOI] [PubMed] [Google Scholar]
- 5.Maeda S, Motoi F, Onogawa T, Morikawa T, Shigeru O, Sakata N, Takadate T, Naitoh T, Rikiyama T, Katayose Y, et al. Paclitaxel as second-line chemotherapy in patients with gemcitabine-refractory pancreatic cancer: A retrospective study. Int J Clin Oncol. 2011;16:539–545. doi: 10.1007/s10147-011-0220-8. [DOI] [PubMed] [Google Scholar]
- 6.Saif MW. Advancements in the management of pancreatic cancer: 2013. JOP. 2013;14:112–118. doi: 10.6092/1590-8577/1481. [DOI] [PubMed] [Google Scholar]
- 7.Zhang DS, Wang DS, Wang ZQ, Wang FH, Luo HY, Qiu MZ, Wang F, Li YH, Xu RH. Phase I/II study of albumin-bound nab-paclitaxel plus gemcitabine administered to Chinese patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2013;71:1065–1072. doi: 10.1007/s00280-013-2102-4. [DOI] [PubMed] [Google Scholar]
- 8.Blagosklonny MV. Mitotic arrest and cell fate: Why and how mitotic inhibition of transcription drives mutually exclusive events. Cell Cycle. 2007;6:70–74. doi: 10.4161/cc.6.1.3682. [DOI] [PubMed] [Google Scholar]
- 9.Rowinsky EK. The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annu Rev Med. 1997;48:353–374. doi: 10.1146/annurev.med.48.1.353. [DOI] [PubMed] [Google Scholar]
- 10.Hari M, Loganzo F, Annable T, Tan X, Musto S, Morilla DB, Nettles JH, Snyder JP, Greenberger LM. Paclitaxel-resistant cells have a mutation in the paclitaxel-binding region of beta-tubulin (Asp26Glu) and less stable microtubules. Mol Cancer Ther. 2006;5:270–278. doi: 10.1158/1535-7163.MCT-05-0190. [DOI] [PubMed] [Google Scholar]
- 11.Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E, Ranelletti FO, Ferrandina G, Scambia G. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res. 2005;11:298–305. [PubMed] [Google Scholar]
- 12.Derry WB, Wilson L, Khan IA, Luduena RF, Jordan MA. Taxol differentially modulates the dynamics of microtubules assembled from unfractionated and purified beta-tubulin isotypes. Biochemistry. 1997;36:3554–3562. doi: 10.1021/bi962724m. [DOI] [PubMed] [Google Scholar]
- 13.Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, Horwitz SB. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100:1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KM, Cao D, Itami A, Pour PM, Hruban RH, Maitra A, Ouellette MM. Class III beta-tubulin, a marker of resistance to paclitaxel, is overexpressed in pancreatic ductal adenocarcinoma and intraepithelial neoplasia. Histopathology. 2007;51:539–546. doi: 10.1111/j.1365-2559.2007.02792.x. [DOI] [PubMed] [Google Scholar]
- 15.Youle RJ, Strasser A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 16.Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 17.Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, et al. SCF (FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–109. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 19.Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, Tulpule A, Dunleavy K, Xiong H, Chiu YL, et al. Safety, pharmacokinetics, pharmacodynamics, and activity of navitoclax, a targeted high affinity inhibitor of BCL-2, in lymphoid malignancies. Lancet Oncol. 2010;11:1149–1159. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 21.Gandhi L, Camidge DR, de Oliveira M Ribeiro, Bonomi P, Gandara D, Khaira D, Hann CL, McKeegan EM, Litvinovich E, Hemken PM, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29:909–916. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong M, Tan N, Zha J, Peale FV, Yue P, Fairbrother WJ, Belmont LD. Navitoclax (ABT-263) reduces Bcl-x(L)-mediated chemoresistance in ovarian cancer models. Mol Cancer Ther. 2012;11:1026–35. doi: 10.1158/1535-7163.MCT-11-0693. [DOI] [PubMed] [Google Scholar]
- 23.Tan N, Malek M, Zha J, Yue P, Kassees R, Berry L, Fairbrother WJ, Sampath D, Belmont LD. Navitoclax enhances the efficacy of taxanes in non-small cell lung cancer models. Clin Cancer Res. 2011;17:1394–1404. doi: 10.1158/1078-0432.CCR-10-2353. [DOI] [PubMed] [Google Scholar]
- 24.Stamelos V, Robinson E, Redman CW, Richardson A. Navitoclax augments the activity of carboplatin and paclitaxel combinations in ovarian cancer cells. Gynecol Oncol. 2013;128:377–382. doi: 10.1016/j.ygyno.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe A, Yasuhira S, Inoue T, Kasai S, Shibazaki M, Takahashi K, Akasaka T, Masuda T, Maesawa C. BCL2 and BCLxL are key determinants of resistance to antitubulin chemotherapeutics in melanoma cells. Exp Dermatol. 2013;22:518–523. doi: 10.1111/exd.12185. [DOI] [PubMed] [Google Scholar]
- 26.Sonnichsen DS, Relling MV. Clinical pharmacokinetics of paclitaxel. Clin Pharmacokinet. 1994;27:256–269. doi: 10.2165/00003088-199427040-00002. [DOI] [PubMed] [Google Scholar]
- 27.Jain HV, Richardson A, Meyer-Hermann M, Byrne HM. Exploiting the synergy between carboplatin and ABT-737 in the treatment of ovarian carcinomas. PLoS One. 2014;9:e81582. doi: 10.1371/journal.pone.0081582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan N, Wong M, Nannini MA, Hong R, Lee LB, Price S, Williams K, Savy PP, Yue P, Sampath D, et al. Bcl-2/Bcl-xL inhibition increases the efficacy of MEK inhibition alone and in combination with PI3 kinase inhibition in lung and pancreatic tumor models. Mol Cancer Ther. 2013;12:853–864. doi: 10.1158/1535-7163.MCT-12-0949. [DOI] [PubMed] [Google Scholar]
- 29.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, Yeo C, Iacobuzio-Donahue C, Goggins M. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 30.Hidalgo M, Von Hoff DD. Translational therapeutic opportunities in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2012;18:4249–4256. doi: 10.1158/1078-0432.CCR-12-1327. [DOI] [PubMed] [Google Scholar]
- 31.Oikonomopoulos GM, Syrigos KN, Saif MW. Prognostic factors in pancreatic cancer. JOP. 2013;14:322–324. doi: 10.6092/1590-8577/1644. [DOI] [PubMed] [Google Scholar]
- 32.von Hoff DD, Ramanathan RK, Borad MJ, Laheru DA, Smith LS, Wood TE, Korn RL, Desai N, Trieu V, Iglesias JL, et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: A phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]