Abstract
The present study was designed to assess the protein expression of the autophagy-associated genes, Beclin-1 and microtubule-associated protein 1 light chain 3 (LC3)-II, as well as the association with clinicopathological features in papillary thyroid carcinoma (PTC). A total of 50 subjects were recruited, including 50 human PTC samples and paired adjacent noncancerous tissue samples. The protein expression of Beclin-1 and LC3-II was analyzed using immunohistochemistry and western blotting. Beclin-1 and LC3-II expression in PTC tissues significantly reduced compared with normal tissues (P<0.05). Expression of Beclin-1 and LC3-II was associated with lymph node metastasis of PTC (P<0.05), but had no association with age, gender, tumor size, tumor number and Tumor-Node-Metastasis stage (P>0.05). Expression of Beclin-1 and LC3-II were positively correlated (r=0.327;P=0.020) in PTC. In conclusion, the activity of autophagy was declined in PTC; this decrease in autophagic capacity may be associated with tumorigenesis and the development of PTC.
Keywords: beclin-1, microtubule-associated protein 1 light chain 3, papillary thyroid carcinoma, autophagy
Introduction
The prevalence of thyroid carcinoma has been increasing worldwide. Thyroid carcinoma has become the most common malignant tumor of the endocrine system; it is ranked second among all female tumors, and in particular there has been a 5.7-fold increase in papillary thyroid carcinoma (PTC) incidence (1,2). The standard therapy for PTC involves surgical resection, chemotherapeutics and radiotherapy (3,4). However, there are few effective treatment measures for patients with metastatic thyroid carcinoma. Autophagy is involved in numerous physiopathological processes and in cancer autophagy has become a novel target for the investigation of tumorigenesis (5–7).
Beclin-1 and microtubule-associated protein light chain 3 (LC3) genes serve an important role in mammalian autophagy. Beclin-1 interacts with several cofactors to regulate the lipid kinase Vps-34 protein and promote formation of Beclin-1-Vps34-Vps15 core complexes, thereby inducing autophagy (8). Beclin-1 also acts as a tumor suppressor. In mice, when heterozygous disruption occurs, autophagy is reduced, cellular proliferation increased and spontaneous tumor development occurs (9,10).
LC3 is comprised of LC3-I and LC3-II. LC3-II correlates with autophagy, being recruited into autophagosomes. Autophagy is used by organisms as a defense strategy to face environmental stress. LC3-I is formed by removal of the C-terminal 22 amino acids from newly synthesized LC3, followed by conversion of a fraction of LC3-I into LC3-II. LC3-II is the first mammalian protein identified that specifically associates with autophagosome membranes (11,12).
In the present study, Beclin-1 and LC3-II protein levels were detected in human PTC and adjacent normal tissues, and the changes and clinical significance of autophagy were investigated in PTC.
Materials and methods
Patients and samples
A total of 50 human PTC samples and their paired adjacent noncancerous tissue samples were obtained with informed consent from patients of Tangshan Worker Hospital (Tangshan, China) from April to November 2014. The patients in the present study comprised 36 women and 14 men, with an age range of 17–67 years (median, 47.12±15.37 years). The diagnosis of PTC was confirmed by histopathology. The most representative PTC and their adjacent normal tissues were selected for study. The tissue specimens were snap-frozen immediately in liquid nitrogen following resection from the patients, and the samples were frozen until the time of protein extraction. None of the patients had received preoperative chemotherapy, radiation therapy or other biological therapy prior to the operation. All samples were deposited in the Central Laboratory of the Tangshan Worker Hospital. Diagnosis and histological typing of thyroid cancer was performed according to the World Health Organization 2004 thyroid histology classification standards (13). All cases of PTC were staged according to the 2009 Tumor-Node-Metastasis (TNM) classification (14). The histopathological features included a papillary structure, ground glass-like nuclei, nuclear overlap, folding of the nuclear membrane and nuclear inclusion bodies. The present study was approved by the Institutional Ethics Review Board of Tangshan Worker Hospital.
Immunohistochemical analysis
Rabbit anti-human Beclin-1 polyclonal antibody (sc-11427), rabbit anti-human LC3-II polyclonal antibody (sc-28266) and rabbit anti-human β-actin polyclonal antibody (sc-130656) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Immunohistochemical staining was performed on 4-µm thick sections of the most representative tumor paraffin block. Sections were dewaxed in 3% H2O2 for 10 min. Antigen retrieval was performed with citrate buffer in a microwave for 3 min. Following blocking with 5% Normal Goat Serum For Blocking (PH0424; Phygene Life Sciences, Fuzhou, China) at room temperature for 15 min, the primary antibodies (1:100) were subsequently applied overnight at 4°C. Following washing in TBS three times, the sections were incubated with a biotin-conjugated secondary goat anti-rabbit antibody (1:1,000; ab6720; Abcam, Cambridge, UK) at 37°C for 1 h. Following treatment with 3,3′-diaminobenzidine, sections were counterstained with hematoxylin, dehydrated through graded alcohols, cleared with dimethyl benzene and mounted with resin.
Immunostaining was semiquantitatively evaluated by 2 independent observers. Beclin-1 and LC3-II were considered positive by cytoplasmic and/or cytomembrane staining. A total of 10 high power fields were randomly observed, and 100 cells in each view were counted using a BX-60 microscope (Olympus Corporation, Tokyo, Japan). Comprehensive evaluation was according to the intensity and percentage of the stained tumor cells. The staining intensity was classified into 4 grades: 0 (no staining), 1 (yellow), 2 (deep yellow) and 3 (brown). The percentage of positive cells was scored in 4 grades: 0 (0–10%), 1 (11–25%), 2 (26–50%) and 3 (51–100%). The immunohistochemical expression level was based on the total points. Total points = staining intensity total + total percentage of positive cells. The specimens were classified into two groups: Negative expression, 0–1 points; positive expression, 2–6 points.
Western blotting
Protein was extracted from nitrogen frozen tissue fragments of samples. The tissues were homogenized in 1 ml radioimmunoprecipitation assay buffer, treated with protease inhibitor cocktail, incubated for 20 min on ice and subsequently centrifuged at 12,000 × g for 15 min at 4°C. The supernatant was collected, boiled for 15 min and preserved at −80°C. Proteins were separated by 10% SDS-PAGE (Beyotime Institute of Biotechnology, Haimen, China), followed by electroblotting to a nitrocellulose membrane (EMD Millipore, Billerica, MA, USA), After 1 h incubation in blocking solution (TBS containing 0.1% Tween-20 and 5% nonfat milk), membranes were incubated with primary antibodies, anti-beclin-1 (1:1,000) or anti-LC3-II (1:1,000) overnight at 4°C. Anti-β-actin antibody (1:1,000) was used as a loading control. Subsequently, membranes were incubated with a secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit antibody; 1:1,000; ab6721; Abcam) 37°C for 2 h. Following washing three times with TBS for 15 min at room temperature, the membranes were treated with a chemiluminescence detection kit (Fast Western Blot kit, ECL Substrate; #35055; Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Protein bands were quantified using densitometry.
Statistical analysis
Statistical evaluation was performed using the SPSS version 13.0 software package (SPSS, Inc., Chicago, IL, USA). The association between Beclin-1 and LC3-II protein expression and clinicopathological features was analyzed by χ2 test. Spearman's test was used to evaluate the correlation between Beclin-1 and LC3-II protein expressions. P<0.05 was considered to indicate a statistically significant difference.
Results
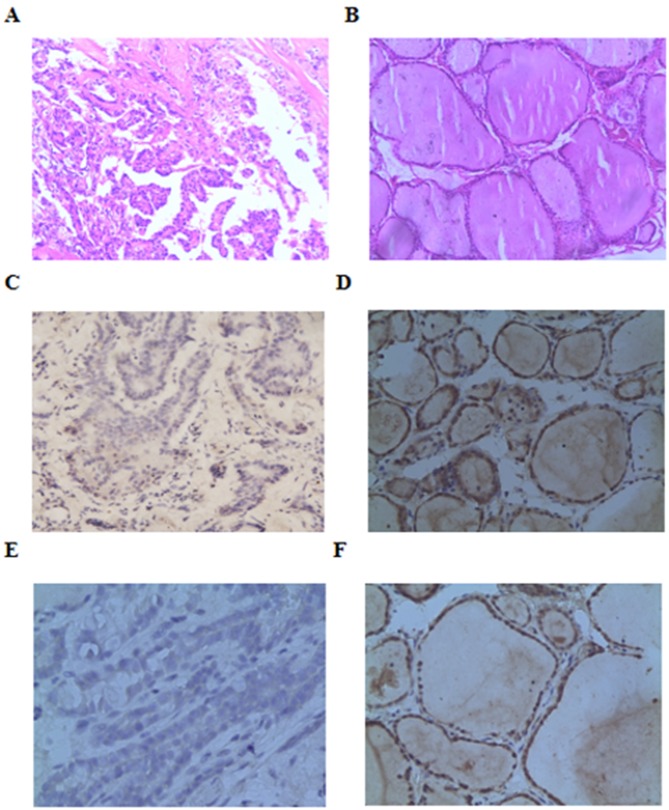
Beclin-1 and LC3-II immunohistochemistry demonstrated 18 patients with positive expression of Beclin-1 protein and 15 patients with positive expression of LC3-II protein in PTC (Fig. 1). Western blot analysis was performed to confirm the specificity of Beclin-1 and LC3-II antibody, As presented in Fig. 2, lanes 1 and 2 exhibited faint bands for Beclin-1 and LC3-II in PTC samples, and normal thyroid tissue samples yielded stronger bands for Beclin-1 and LC3-II. Beclin-1 and LC3-II expression in PTC tissues was significantly reduced compared with in normal tissue (Table I). Expression of Beclin-1 and LC3-II was associated with lymph node metastasis in PTC, but had no association with age, sex, tumor size, number of tumors and TNM stage (Table II).
Figure 1.
Representative cell images. Hematoxylin and eosin staining for samples of (A) PTC and (B) normal thyroid tissues. Immunohistochemical photomicrographs of Beclin-1 and LC3-II in tissue samples from PTC. Negative (C) Beclin-1 and (E) LC3-II immunostaining in PTC tissues. Positive (D) Beclin-1 and (F) LC3-II immunoreactivity in normal cells. All images show magnification, ×200. PTC, papillary thyroid carcinoma; LC3-11, microtubule-associated protein 1 light chain 3.
Figure 2.
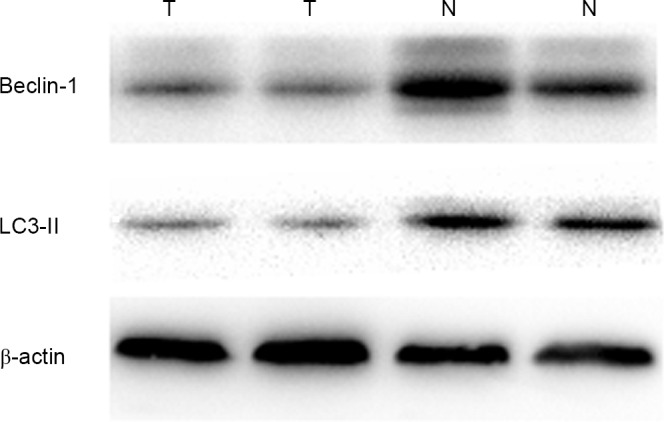
Expression of Beclin-1 and LC3-II in papillary thyroid carcinoma and matched normal tissues. Relatively low Beclin-1 and LC3-II protein expression was observed in PTC. LC3-11, microtubule-associated protein 1 light chain 3.
Table I.
Expression of Beclin-1 and LC3-II in PTC and normal tissue (n=50).
| Beclin-1 | LC3-II | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Cases | Negative | Positive | χ2 | P-value | Negative | Positive | χ2 | P-value |
| PTC | 50 | 32 | 18 | 19.869 | <0.001 | 35 | 15 | 29.743 | <0.001 |
| Normal | 50 | 10 | 40 | 8 | 42 | ||||
LC3-II, microtubule-associated protein 1 light chain 3; PTC, papillary thyroid carcinoma.
Table II.
Association between Beclin-1 and LC3-II protein expression levels and clinicopathological characteristics of papillary thyroid carcinoma.
| Beclin-1 | LC3-II | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Cases | Negative | Positive | χ2 | P-value | Negative | Positive | χ2 | P-value |
| Age, years | 3.766 | 0.067 | 0.004 | 0.948 | |||||
| <45 | 17 | 14 | 3 | 12 | 5 | ||||
| ≥45 | 33 | 18 | 15 | 23 | 10 | ||||
| Gender | 0.001 | 0.979 | 0.019 | 1.000 | |||||
| Male | 14 | 9 | 5 | 10 | 4 | ||||
| Female | 36 | 23 | 13 | 25 | 11 | ||||
| Tumor size, cm | 2.335 | 0.126 | 2.068 | 0.215 | |||||
| <1 | 29 | 16 | 13 | 18 | 11 | ||||
| ≥1 | 21 | 16 | 5 | 17 | 4 | ||||
| Tumor number | 3.979 | 0.056 | 0.680 | 0.507 | |||||
| Single | 36 | 20 | 16 | 24 | 12 | ||||
| Multiple | 14 | 12 | 2 | 11 | 3 | ||||
| TNM stage | 1.666 | 0.398 | 3.488 | 0.087 | |||||
| I and II | 43 | 26 | 17 | 28 | 15 | ||||
| III and IV | 7 | 6 | 1 | 7 | 0 | ||||
| Lymph node involvement | 5.640 | 0.026 | 6.320 | 0.019 | |||||
| Present | 16 | 14 | 2 | 15 | 1 | ||||
| Absent | 34 | 18 | 16 | 20 | 14 | ||||
LC3-II, microtubule-associated protein 1 light chain 3; PTC, papillary thyroid carcinoma; TNM, tumor-node-metastasis.
Spearman's test was used to evaluate the correlation between Beclin-1 and LC3-II protein expression in PTC. The results revealed Beclin-1 and LC3-II expression was positively correlated in PTC (r=0.327; P=0.020).
Discussion
Autophagy is an evolutionarily conserved processes that regulates cell fate. Under certain circumstances, autophagy constitutes a stress adaptation that avoids cell death. Portions of the cytoplasm are sequestered within double-membrane cytosolic vesicles termed autophagosomes, the autophagosomes are delivered to a lysosome, degradation into amino acids occurs and nucleotides are released into the cytoplasm. Under various nutrient limitations, autophagy may become essential for viability (15–17). Emerging evidence has correlated impaired autophagy with tumor progression (18). Autophagy is considered to play a dual role in the process of tumor formation. A lack of autophagy genes would destroy the environmental balance and may lead to the occurrence of tumors; furthermore, autophagy can promote tumor cells resistance to stress, meaning improved survival of cancer cells (19). It is thought that autophagy is a type of anti-tumor mechanism (20).
Beclin-1 is an autophagy-specific protein that regulates autophagosome formation. The human Beclin-1 gene is located on chromosome 17q21. In human pancreatic cancer cells, wogonin is able to activate Beclin-1 and the phosphoinositide 3-kinase (PI3K) signaling pathway, inducing reactive oxygen species-mediated autophagy (21). Beclin-1 also serves a role in the occurrence and development of tumors by regulating autophagy. Research has revealed that Beclin-1 protein is almost undetectable in human breast cancer cell lines (22). In human thyroid papillary carcinoma cell lines TPC-1 and 8505-C low levels of autophagosomes were observed (23). However, certain experiments have demonstrated the opposite result. In the present study, it was observed that the expression level of Beclin-1 was significantly downregulated in human PTC tissues compared with the normal thyroid tissue; inducing autophagy decline serves a role in the incidence and development of PTC (24,25). Analysis of the association between Beclin-1 protein and general clinical and pathological factors demonstrated that Beclin-1 protein expression decreased more significantly in patients with lymph node metastases, but had no association with age, sex, tumor size, the number of tumors and TNM stage. The decline of autophagy activity may be involved in tumor metastasis. In pcDNA3.1-Bec transfected CaSki cells, the expression of Beclin-1 protein was upregulated, and led to arrest in the G0/G1 phase of the cell cycle (26). In CaSki cells, the apoptosis signaling induced by anti-cancer drugs may be enhanced by overexpression of Beclin-1. The results suggest that Beclin-1 serves a significant role in the regulation of potent anti-tumor activity (26). In a study of tongue squamous cell carcinoma cell lines SCC9 and SCC15, it was demonstrated that knockdown of Beclin-1 promoted proliferation, migration and invasion, while overexpression of Beclin-1 inhibited proliferation and migration in the two lines cell (27). The data also confirmed that Beclin-1 inhibited TSCC xenograft growth in vivo (27). These results indicate that autophagy-regulating gene Beclin-1 may be a potential target for cancer gene therapy.
In the present study, reduced expression of LC3-II protein was observed in human PTC tissues compared with normal thyroid tissue. The analysis of the association between LC3-II protein and general clinical and pathological factors revealed that LC3-II protein expression was inversely correlated with lymph node metastases, but had no association with age, sex, tumor size, number of tumors and TNM stage. Beclin-1 and LC3-II were positively correlated in PTC. This finding may present novel strategies for the development of therapies for PTC. LC3 is a yeast autophagy gene autophagy-related protein 8 homolog in mammalian cells, and is a specific autophagic marker in mammalian cells (28). LC3 is processed from LC3-I to the membrane-bound form LC3-II. LC3-II is located on the membrane of autophagosomes (29). The results of a previous study of triple-negative breast cancer suggested that expression of LC3 in triple-negative breast cancer was associated with increased distant metastases, and LC3 negativity was a significant independent prognostic factor of disease-free survival (30). Beclin-1 and LC3 autophagic genes are altered in several human types of cancer (31). The lowest expression of LC3-II protein is observed in melanoma metastases; LC3 messenger RNA significantly decreased with tumor progression, and the expression of LC3-II protein was inversely correlated to thickness, ulceration and mitotic rate (32).
In conclusion, compared with normal thyroid tissue, Beclin-1 and LC3-II expression in PTC was reduced, particularly in patients with lymph node metastasis. Furthermore, it was observed that clinical characteristics, including patient age, sex, tumor size and the number of tumors did not affect Beclin-1 and LC3-II expression. The results of the present study indicate that a decline autophagy activity may be associated with PTC metastasis. However, the regulation of autophagy is a two-way process, and its regulatory mechanism in tumors was not completely clear. As a form of programmed cell death, autophagy is regulated by common signaling pathways with apoptosis, including PI3K/Akt and B-cell lymphoma 2 family members (33). The results of the present study may provide initial evidence for the further investigation of Beclin-2 and LC3-II in PTC. Autophagy may be a novel target for the prevention or treatment of PTC.
References
- 1.Qian BY, He M, Gao M, Chen KX. Thyroid cancer incidence in the city of Tianjin during 2002–2006 and its secular trend in recent 26 year. Chin J Gen Surg. 2011;4:275–278. [Google Scholar]
- 2.La Vecchia C, Malvezzi M, Bosetti C, Garavello W, Bertuccio P, Levi F, Negri E. Thyroid cancer mortality and incidence: A global overview. Int J Cancer. 2015;136:2187–2195. doi: 10.1002/ijc.29251. [DOI] [PubMed] [Google Scholar]
- 3.Grant CS. Recurrence of papillary thyroid cancer after optimized surgery. Gland Surg. 2015;4:52–62. doi: 10.3978/j.issn.2227-684X.2014.12.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong GC, Song M, Park HJ, Min JJ, Bom HS, Cho SG, Park KS, Kang SR, Kim J, Song HC, Kwon SY. Iodine uptake patterns on post-ablation whole body scans are related to elevated serum thyroglobulin levels after radioactive iodine therapy in patients with papillary thyroid carcinoma. Nucl Med Mol Imaging. 2016;50:329–336. doi: 10.1007/s13139-016-0421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perlmutter DH. The role of autophagy in alpha-1-antitrypsin deficiency: A specific cellular response in genetic diseases associated with aggregation-prone proteins. Autophagy. 2006;2:258–263. doi: 10.4161/auto.2882. [DOI] [PubMed] [Google Scholar]
- 7.Terman A, Brunk UT. Autophagy in cardiac myocyte homeostasis, aging, and pathology. Cardiovasc Res. 2005;68:355–365. doi: 10.1016/j.cardiores.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Fogel AI, Dlouhy BJ, Wang C, Ryu SW, Neutzner A, Hasson SA, Sideris DP, Abeliovich H, Youle RJ. Role of membrane association and Atg14-dependent phosphorylation in beclin-1-mediated autophagy. Mol Cell Biol. 2013;33:3675–3688. doi: 10.1128/MCB.00079-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pirtoli L, Cevenini G, Tini P, Vannini M, Oliveri G, Marsili S, Mourmouras V, Rubino G, Miracco C. The prognostic role of Beclin 1 protein expression in high-grade gliomas. Autophagy. 2009;5:930–936. doi: 10.4161/auto.5.7.9227. [DOI] [PubMed] [Google Scholar]
- 11.Klionsky DJ, Abdalla FC, Abeliovich H, Abraham RT, Acevedo-Arozena A, Adeli K, Agholme L, Agnello M, Agostinis P, Aguirre-Ghiso JA, et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLellis RA, Lloyd R, Heitz P, Eng C, editors. Pathology and Genetics of Tumours of Endocrine Organs (IARC WHO Classification of Tumours) 1st. IARC Press; Lyon: 2004. [Google Scholar]
- 14.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. 7th. Wiley-Blackwell; Oxford: 2009. [Google Scholar]
- 15.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 16.Thorburn A. Apoptosis and autophagy: Regulatory connections between two supposedly different processes. Apoptosis. 2008;13:1–9. doi: 10.1007/s10495-007-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizushima N, Klionsky DJ. Protein turnover via autophagy: Implications for metabolism. Annu Rev Nutr. 2007;27:19–40. doi: 10.1146/annurev.nutr.27.061406.093749. [DOI] [PubMed] [Google Scholar]
- 18.Helgason GV, Holyoake TL, Ryan KM. Role of autophagy in cancer prevention, development and therapy. Essays Biochem. 2013;55:133–151. doi: 10.1042/bse0550133. [DOI] [PubMed] [Google Scholar]
- 19.Katheder NS, Khezri R, O'Farrell F, Schultz SW, Jain A, Rahman MM, Schink KO, Theodossiou TA, Johansen T, Juhász G, et al. Microenvironmental autophagy promotes tumour growth. Nature. 2017;541:417–420. doi: 10.1038/nature20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morselli E, Galluzzi L, Kepp O, Vicencio JM, Criollo A, Maiuri MC, Kroemer G. Anti- and pro-tumor functions of autophagy. Biochim Biophys Acta. 2009;1793:1524–1532. doi: 10.1016/j.bbamcr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Li SJ, Sun SJ, Gao J, Sun FB. Wogonin induces Beclin-1/PI3K and reactive oxygen species-mediated autophagy in human pancreatic cancer cells. Oncol Lett. 2016;12:5059–5067. doi: 10.3892/ol.2016.5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Riwei, Wu Aiguo. Autophagy gene Beclinl and breast cancer. Chin J Gen Surg. 2012;21:591–596. [Google Scholar]
- 23.Lin CI, Whang EE, Abramson MA, Jiang X, Price BD, Donner DB, Moore FD, Jr, Ruan DT. Autophagy: A new target for advanced papillary thyroid cancer therapy. Surgery. 2009;146:1208–1214. doi: 10.1016/j.surg.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Xu H, Ma H. Beclin 1 is highly expressed in papillary thyroid carcinoma and correlates with lymph node metastasis. Acta Chir Belg. 2013;113:175–181. doi: 10.1080/00015458.2013.11680907. [DOI] [PubMed] [Google Scholar]
- 25.Yeşil C, Kandemir O, Haksever H, Dabakoğlu T. Is BECLIN-1 Immunoreactivity more effective than HBME-1 in diagnosis of papillary thyroid cancer? Acta Chir Belg. 2015;115:299–305. doi: 10.1080/00015458.2015.11681116. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Liu JH, Jin L, Lin SM, Yang Y, Sui YX, Shi H. Over-expression of the Beclin1 gene upregulates chemosensitivity to anti-cancer drugs by enhancing therapy-induced apoptosis in cervix squamous carcinoma CaSki cells. Cancer Lett. 2010;294:204–210. doi: 10.1016/j.canlet.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Weng J, Wang C, Wang Y, Tang H, Liang J, Liu X, Huang H, Hou J. Beclin1 inhibits proliferation, migration and invasion in tongue squamous cell carcinoma cell lines. Oral Oncol. 2014;50:983–990. doi: 10.1016/j.oraloncology.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Kraft C, Kijanska M, Kalie E, Siergiejuk E, Lee SS, Semplicio G, Stoffel I, Brezovich A, Verma M, Hansmann I, et al. Binding of the Atg1/ULK1 kinase to the ubiquitin-like protein Atg8 regulates autophagy. EMBO J. 2012;31:3691–3703. doi: 10.1038/emboj.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang H, Cheng D, Liu W, Peng J, Feng J. Protein kinase C inhibits autophagy and phosphorylates LC3. Biochem Biophys Res Commun. 2010;395:471–476. doi: 10.1016/j.bbrc.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He JH, Luo RZ, Cai MY, Li M, Lu JB, Yuan ZY. Decreased expression of light chain 3 (LC3) increased the risk of distant metastasis in triple-negative breast cancer. Med Oncol. 2013;30:468. doi: 10.1007/s12032-013-0468-0. [DOI] [PubMed] [Google Scholar]
- 31.Miracco C, Cevenini G, Franchi A, Luzi P, Cosci E, Mourmouras V, Monciatti I, Mannucci S, Biagioli M, Toscano M, et al. Beclin 1 and LC3 autophagic gene expression in cutaneous melanocytic lesions. Hum Pathol. 2010;41:503–512. doi: 10.1016/j.humpath.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Marino ML, Pellegrini P, Di Lernia G, Djavaheri-Mergny M, Brnjic S, Zhang X, Hägg M, Linder S, Fais S, Codogno P, De Milito A. Autophagy is a protective mechanism for human melanoma cells under acidic stress. J Biol Chem. 2012;287:30664–30676. doi: 10.1074/jbc.M112.339127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boya P, González-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N, Métivier D, Meley D, Souquere S, Yoshimori T, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]