Abstract
Background:
Catheter-based renal denervation (RDN) is a novel treatment for resistant hypertension (RH). A recent meta-analysis reported that RDN did not significantly reduce blood pressure (BP) based on the pooled effects with mild to severe heterogeneity. The aim of the present study was to identify and reduce clinical sources of heterogeneity and reassess the safety and efficacy of RDN within the identified homogeneous subpopulations.
Methods:
This was a meta-analysis of 9 randomized clinical trials (RCTs) among patients with RH up to June 2016. Sensitivity analyses and subgroup analyses were extensively conducted by baseline systolic blood pressure (SBP) level, antihypertensive medication change rates, and coronary heart disease (CHD).
Results:
In all patients with RH, no statistical differences were found in mortality, severe cardiovascular events rate, and changes in 24-h SBP and office SBP at 6 and 12 months. However, subgroup analyses showed significant differences between the RDN and control groups. In the subpopulations with baseline 24-h SBP ≥155 mmHg (1 mmHg = 0.133 kPa) and the infrequently changed medication, the use of RDN resulted in a significant reduction in 24-h SBP level at 6 months (P = 0.100 and P = 0.009, respectively). Subgrouping RCTs with a higher prevalent CHD in control showed that the control treatment was significantly better than RDN in office SBP reduction at 6 months (P < 0.001).
Conclusions:
In all patients with RH, the catheter-based RDN is not more effective in lowering ambulatory or office BP than an optimized antihypertensive drug treatment at 6 and 12 months. However, among RH patients with higher baseline SBP, RDN might be more effective in reducing SBP.
Keywords: Antihypertensive Treatment, Hypertension, Randomized Controlled Trials, Renal Denervation, Subgroup Meta-analysis
Introduction
Resistant hypertension (RH), defined as blood pressure (BP) that remains above goal in spite of the concurrent use of 3 antihypertensive agents, can be a leading risk factor for cardiovascular disease and chronic kidney disease.[1] Recently, catheter-based renal denervation (RDN) has ignited expectations for the treatment of RH. However, evidence-based study results for the effect of RDN in lowering BP in patients with RH have been controversial. The first randomized clinical trial (RCT), SYMPLICITY HTN-2,[2] demonstrated significant reductions in both systolic and diastolic BP in patients with RH. However, the initial excitement turned into skepticism when a large RCT, SYMPLICITY HTN-3,[3] using a sham procedure as placebo, failed to achieve its primary efficacy. More recently, RCTs on RDN in RH have also shown discrepant results.[4,5,6,7,8,9,10] A recent meta-analysis based on 7 RCTs in patients with RH reported that RDN with the SYMPLICITY systems did not significantly reduce BP compared to antihypertensive drugs at 6 months after the intervention.[11] Their conclusions, however, were drawn with the pooled effects with much heterogeneity. The aim of our study was to seek possible sources of clinical heterogeneity, identify homogeneous subpopulations, and reconduct a systematic review for the safety and efficacy assessment of RDN by those subpopulations. In addition to the seven trials reviewed by Fadl Elmula et al.,[11] the present study further includes two more recent RCTs and evaluates both 6- and 12-month efficacy endpoints.[4,5]
Methods
Data sources
Publications in English or Chinese were identified by searching PubMed, Medline, Embase, and the Cochrane Central Register of Controlled Trials available up to June 2016. Search terms included “hypertension” and “blood pressure” and “denervation” and “RDN”. A more detailed search strategy is described in Supplementary text 1 (91.1KB, pdf) , and a flow diagram for study selection is shown in Figure 1.
Figure 1.
Flow diagram for the study selection. RCTs: Randomized clinical trials.
Supplementary text 1: Search strategy
Study selection
We included all RCTs on human subjects that assessed the effect of RDN as the additional treatment to current antihypertensive drugs in use, and compared it with the continuation of antihypertensive drug use with or without a sham procedure. Use of the sham procedure in control group was not required for inclusion. We excluded single arm studies, meeting abstracts, letters, and case reports.
The inclusion criteria for eligible studies included (1) adult patients (>18 years old) with RH, defined as office BP >140/90 mmHg (1 mmHg = 0.133 kPa) or 24-h systolic BP (24-h SBP) >130 mmHg or 24-h daytime SBP >135 mmHg, in spite of the concurrent use of 3 antihypertensive agents of different classes at optimal dose amounts, including a diuretic;[1] (2) patients who underwent RDN using percutaneous catheters and radiofrequency probes; and (3) BP records measured as ambulatory BP or/and office BP at baseline and 6-month follow-up, or BP change from baseline to 6-month follow-up.
Outcomes
The primary outcomes were 24-h SBP and office SBP changes from baseline to 6-month and 12-month follow-ups, severe cardiovascular events rate, and all-cause mortality. The severe cardiovascular events included myocardial infarction, new-onset heart failure, stroke, hypertension crisis, angina needing a coronary stent, embolic event resulting in end-organ damage, and hospitalization for atrial fibrillation. The secondary outcomes were changes in 24-h diastolic BP (24-h DBP), office diastolic blood pressure (office DBP) at 6-month follow-up, and adverse events of RDN.
Data extraction, synthesis, and quality assessment
The following was extracted: the name of the first author, publication year, region, study design, total participants, number of participants receiving RDN, number of participants in the control group, trial inclusion and exclusion criteria, type of catheter used, method of BP measurement, maximal length of follow-up, office systolic and diastolic BP, 24-h SBP and DBP, daytime ambulatory SBP at baseline, 6-, 12-, 36-month follow-ups in both groups, and procedural complications. The methodological quality of RCTs was assessed independently by two reviewers using Jadad Scale.[12] The Jadad Scale is an assessment score based on the degree of participant randomization, application of the blinding method, and report of study withdrawals and dropouts. A threshold of ≥4 points is regarded as a high-quality study.[12] The risk of bias was assessed independently by two reviewers using the Cochrane Collaboration's tool. This tool evaluates each study in the following six specific domains: adequate random sequence generation, allocation sequence concealment, blinding of subjects/outcome assessors, incomplete outcome data, free of selective outcome reporting, and free of other bias. Every domain was scored to be high risk of bias, low risk of bias, or unclear. The overall assessment of each RCT was graded as “low risk” (if all the domains were assessed as low risk of bias), “unclear” (if there exists at least one domain unclear), or “high risk” otherwise.[13] With no disagreement between the reviewers in the list of studies included in the meta-analysis and their quality assessment, a third reviewer was waived.
Statistical analysis
We used a random effects model to combine the studies given significant heterogeneity in the treatment effects. The heterogeneity was statistically evaluated by the I2 statistic, where values of 0–24.9%, 25–49.9%, 50–74.9%, and 75–100% indicated no, mild, moderate, and severe heterogeneity, respectively.[14,15] Extensive subgroup analyses were carried out to minimize possible sources of clinical heterogeneity by the (1) baseline SBP level, (2) frequency of antihypertensive medication changes, (3) race, and (4) coronary heart disease (CHD) prevalence. For survival outcomes, relative risk (RR) was used to assess the effect of treatment, while mean difference (MD) was used for continuous outcomes, along with the corresponding 95% confidence interval (CI). Two-tailed P < 0.05 was considered statistically significant. All statistical analyses were carried out using Review Manager 5.3 software (Nordic Cochrane Center, Copenhagen, Denmark).
Results
Study characteristics
We identified 9 RCTs that met the inclusion criteria with a total of 1068 patients [Table 1]. All had similar inclusion criteria, except for one trial[6] [Supplementary Table 1] that enrolled mild RH patients who had 24-h SBP/DBP level just above 140/78 mmHg at baseline. In two trials, only ambulatory BP measurements[5,6] were available, while both office and ambulatory BP were collected in the seven remaining trials. The maximum length of follow-up was at least 6 months in all studies, and up to 12 months in three trials[16,17,18] and 36 months in one trial.[19] There were five other trials[2,3,5,6,9] designed not to alter antihypertensive medication during the follow-up. In contrast, the baseline antihypertensive medication was allowed to be modified in four trials.[4,7,8,10] Three trials[6,7,10] included only white population and one trial[9] included only Asian population. Regarding the patients given the antihypertensive drugs at baseline and 6-month follow-up, their characteristics are described in Supplementary Table 2. Only one trial[7] had reported that more antihypertensive drugs were used after 6 months in the control group, on average, than the RDN group (+0.3 drugs). Regarding the baseline BP severity, there were three studies[2,3,9] with baseline office SBP ≥175 mmHg, over 10–25 mmHg higher than that of other trials. Baseline level of 24-h SBP was not available in SYMPLICITY HTN-2.[2] The other two studies[3,9] with baseline 24-h SBP ≥155 mmHg were about 7–24 mmHg higher than that of other trials. As shown in Table 2, the Cochrane Collaboration's assessment suggested that most studies were at a high risk due to lack of blinding. However, the methodological quality of all included studies was rated as “high” [i.e., Jadad scale was ≥5 in Table 2].
Table 1.
Study and patient characteristics at baseline
| Study (all RCTs) | Region | BP assessment | Intervention description (catheter) | Control | Maximum length of follow-up (months) | Number of participants (R/C) | Mean age (years) (R/C) | Race | Coronary heart disease (%) (R/C) | Type 2 diabetes mellitus (%) (R/C) | eGFR (ml·min−1·1.73 m−2) (R/C) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White (%) (R/C) | Black (%) (R/C) | Asian (%) (R/C) | |||||||||||
| DENERHTN 2015[8] (NCT01570777) | French | O and A | Adjusted drugs and Simplicity RDN System (Medtronic, Mountain View, CA, USA) | A standardized stepped-care antihypertensive treatment | 6 | 101 (48/53) | 55.2/55.2 | 79/77 | -/- | -/- | 30.2/20.8 | 12/26 | 88/90 |
| DENERVHTA 2016[4] (NCT02039492) | Spain | O and A | Simplicity RDN System (Medtronic, Galway, Ireland) | Spironolactone as add-on therapy | 6 | 24 (11/13) | 61.9/64.9 | 100/85 | -/- | -/- | 18/23 | 36/62 | 74.6/85 |
| OSLO 2014[10] (NCT01673516) | Norway | O and A | Simplicity RDN System (Ardian, Mountain View, CA, USA) | Adjusted drug treatment | 6 | 19 (9/10) | 57/62.5 | 100/100 | 0/0 | 0/0 | 11/60 | 22/30 | 78/77 |
| Prague-15 2015,[7] 2016,[16] 2017[20] (NCT01560312) | Czech republic | O and A | Simplicity RDN System (Medtronic Inc., Mountain View, CA, USA) | Spironolactone as add-on therapy | 24 | 106 (52/54) | 56/59 | 100/100 | 0/0 | 0/0 | 6/7 | 22/17 | 84/80 |
| ReSET 2016[5] (NCT01762488) | Denmark | A | Simplicity RDN system (Medtronic) | An invasive sham procedure, and NDC | 6 | 69 (36/33) | 54.3/57.1 | 97/97 | -/- | -/- | 6/15 | 63/33 | -/- |
| SYMPLICITY-FLEX 2015[6] (NCT01656096) | Germany | A | Symplicity Flex RDN System (Medtronic) | An invasive sham procedure, and NDC | 6 | 67 (32/35) | 64.5/57.4 | 100/100 | 0/0 | 0/0 | 60/47 | 54/36 | 79/84 |
| SYMPLICITY HTN-Japan 2015[9] (NCT01644604) | Japan | O and A | Symplicity™ RDN system (Medtronic, Santa Rosa, CA, USA) | NDC | 6 | 41 (22/19) | 59.5/56 | 0/0 | 0/0 | 100/100 | -/- | 36.4/63.2 | 70/70 |
| SYMPLICITY HTN-2 2010,[2] 2012,[17] 2014,[18] (NCT00888433) | Europe, Australia and New Zealand | O and A | Symplicity RDN system (Ardian, Mountain View, CA, USA) | NDC | 36 | 106 (52/54) | 58/58 | 98/96 | -/- | -/- | 19/7 | 40/28 | 77/86 |
| SYMPLICITY HTN-3 2014,[3] 2015,[19] (NCT01418261) | United States | O and A | Symplicity RDN system (Medtronic) | An invasive sham procedure, and NDC | 12 | 535 (364/171) | 57.9/56.2 | 24.8/29.2 | 73/69.6 | 0.6/0 | 36.5/31.5 | 47/20.9 | 73/74 |
A: Ambulatory blood pressure measurement; C: Control group; eGFR: Estimated glomerular filtration rate; HR: Heart rate; O: Office blood pressure measurement; R: Catheter-based renal denervation group; RCTs: Randomized controlled trials; NDC: No antihypertensive drugs change; BP: Blood pressure; RDN: Renal denervation; -: Not applicable.
Supplementary Table 1.
The inclusion and exclusion criteria of included studies
| Clinical trails | Inclusion criteria | Exclusion criteria |
|---|---|---|
| DENERHTN | Men or women aged 18–75 years referred for RH, defined by supine office SBP of ≥140 mmHg or DBP of more than or equal to 90 mmHg despite a stable medication regimen of maximum tolerated doses of three or more antihypertensive drugs of different classes (including a diuretic drug), with suitable renal artery anatomy on CT angiogram, magnetic resonance angiogram, or renal angiogram done within the previous year | Secondary hypertension (ruled out by standardized screening in the past 2 years) and an eGFR of <40 ml·min−1·1.73 m−2 |
| DENERVHTA | Patients aged at least 18 years and 80 years or less with an office SBP at least 150 mmHg and a 24-h SBP at least 140 mmHg despite a prescribed therapeutic schedule with an appropriate combination of three or more full-dose antihypertensive drugs, including a diuretic, and maintained for the last 3 months, were eligible to participate in the trial. Moreover, only patients with main renal arteries with a diameter wide enough (4 mm) to enable denervation were included | Exclusion criteria included inability to perform either imaging tests; secondary hypertension, with appropriate tests being performed according to investigator criteria (with special focus on primary aldosteronism that was ruled out by both plasmatic aldosterone and renin activity determinations after stopping interfering medications as well as by CT or MRI); eGFR <45 ml·min−1·1.73 m−2; patients currently on treatment with an aldosterone receptor blocker or who had previously received one of such class of drugs and had been withdrawn because of lack of efficacy and/or adverse effects; patients unlikely compliant with treatment. Other exclusion criteria comprised prerandomization serum potassium level at least 5.5 mmol/L, pregnant women, significant valvular heart disease, or the occurrence of a major vascular event (myocardial infarction, unstable angina, or stroke) within 6 months before the study enrolment |
| OSLO | RH was defined as uncontrolled hypertension (office SBP >140 mmHg), despite regular intake of maximally tolerated doses of ≥3 antihypertensive drugs including a diuretic. In addition, patients had to qualify by having mean ambulatory daytime SBP >135 mmHg immediately patients could be 18–80 years of age with normal renal arteries at CT or MRI examination within 2 years before participation | Patients with secondary and spurious hypertension, and some patients with high serum aldosterone levels (primary hyperaldosteronisme without tumor or with high aldosterone/renin activity ratio) who responded to treatment with spironolactone, were identified and excluded. Patients with estimated glomerular filtration rate <45 ml·min−1·1.73 m−2 (MDRD formula), urine albumin/creatinine ratio >50 mg/mmol or type 1 diabetes mellitus could not be included in line with the hitherto single published randomized study of BP-lowering effects of RDN |
| Prague-15 | RH with office systolic BP of >140 mmHg; SBP of >130 mmHg during 24-h ambulatory BP monitoring; treatment with at least three antihypertensive medications, including diuretics, at optimal doses; age of >18 years; signed informed consent | Any secondary form of hypertension; noncompliance with medical treatment; presence of any chronic renal disease (serum creatinine level of >200 mmol/L); pregnancy; history of myocardial infarction or stroke in the previous 6 months; The presence of severe valvular stenotic disease; anatomical abnormality or a variant structure of either renal artery, including aneurysm, stenosis, a reference diameter of <4 mm, and a length of <20 mm; an increased bleeding risk (thrombocytopenia of <50,000 platelets/ml of blood and an INR of >1.5) |
| ReSET | Aged 30–70 years; one month of stable antihypertensive treatment with at least three antihypertensive agents including a diuretic (or in case of diuretics intolerance a minimum of three nondiuretic antihypertensive drugs); daytime ABPM SBP ≥145 mmHg (preceded by 14 days of scheduled drug intake showing at least 85% adherence) | General: Noncompliant personality (abuse and mental illness); pregnancy/inadequate contraception in fertile women; known allergy to iodine-containing radiograph contrast agent; comorbidity: Secondary hypertension; malignant disease; congestive heart failure NYHA 3–4; chronic renal failure stage 4–5 (eGFR ≤30 ml·min−1·1.73 m−2); stable angina pectoris CCS class 2–4; unstable angina pectoris; coronary artery disease with indication for coronary intervention; recent myocardial infarction or coronary intervention (<6 months); permanent atrial fibrillation; orthostatic syncope (<6 months); symptomatic peripheral artery disease; paraclinical: clinically significant abnormal electrolytes and liver function tests; hemoglobin <7.0 mmol/L; abnormal thyroidea function; macroscopic hematuria; ECG: Atrioventricular block Grades 2 and 3; Echocardiography: Left ventricular ejection fraction <50%; significant valvular disease; computed axial tomography |
| angiography and selective angiography of renal arteries; pronounced calcification in iliaco-aortic or renal arteries; multiple renal arteries: accessory renal arteries estimated to carry >10% of the kidney’s blood supply (small polar arteries accepted) and being undersized for ablation procedure; renal artery diameter <4 mm; renal artery length (from ostium to first major side branch) <20 mm; renal artery disease (stenosis, fibromuscular dysplasia, prior intervention and dissection) | ||
| SYMPLICITY HTN-Flex | RH and mildly elevated BP. Eligible patients between 18 and 75 years of age were randomized to RDN or a sham procedure. RH with mildly elevated BP was defined as (1) a stable antihypertensive drug regimen of ≥3 agents of different classes, including a diuretic (except when not tolerated/contraindicated) at optimal dosage without change in the 4 weeks preceding randomization and (2) mean day-time systolic BP on 24-h ABPM between 135 and 149 mmHg or mean daytime DBP between 90 and 94 mmHg | ABPM values below or above the predefined ranges mentioned above, unsuitable anatomy for RDN, severe renal artery stenosis, estimated glomerular filtration rate <45 ml·min−1·1.73 m−2 (modification of diet in renal disease formula), change in BP medication in the 4 weeks preceding randomization, unwillingness to adhere to unchanging BP medication during the study period of 6 months, pregnancy, and severe comorbidities with limited life expectancy |
| SYMPLICITY HTN-Japan | Eligible patients were at least 20 and ≤80 years old at the time of informed consent. Subjects were required to have uncontrolled hypertension defined as office SBP ≥160 mmHg while on a stable anti-hypertensive regimen of at least 3 anti-hypertensive drug classes at maximum tolerated dose including a diuretic for a minimum of 6 weeks before enrollment; 24-h average ambulatory SBP was required to be ≥135 mmHg. Subjects were excluded if their eGFR was <45 ml·min−1·1.73 m−2, using the modified calculation method for Japanese subjects | Anatomical exclusions included main renal arteries <4 mm in diameter or <20 mm treatable length (i.e., free of visible anatomic abnormality or atheroma), multiple renal arteries for which the main renal artery was estimated to supply <75% of the kidney, renal artery stenosis (>50%) or renal artery aneurysm in either renal artery, history of prior renal artery intervention including balloon angioplasty or stenting and unilateral (functional or morphological) kidney. Other exclusions included >1 in patient hospitalization for a hypertensive crisis not related to confirmed nonadherence to medication within the past year, type 1 diabetes mellitus and ≥1 episodes of orthostatic hypotension not related to medication changes. Secondary causes of hypertension were also excluded (primary aldosteronism, pheochromocytoma, Cushing’s disease, coarctation of the aorta, hypothyroidism, hyperthyroidism, or hyperparathyroidism) |
| SYMPLICITYHTN-2 | Patients aged 18–85 years with an SBP of 160 mmHg or more (≥150 mmHg in patients with type 2 diabetes), despite compliance with three or more antihypertensive drugs | An eGFR (based on the MDRD criteria 12) of <45 ml·min−1·1.73 m−2, type 1 diabetes, contraindications to MRI, substantial stenotic valvular heart disease, pregnancy or planned pregnancy during the study, and a history of myocardial infarction, unstable angina, or cerebrovascular accident in the previous 6 months |
| SYMPLICITYHTN-3 | Patients with severe RH were prospectively enrolled in the study. On initial screening, patients were required to have a SBP of 160 mmHg or higher (average of three measurements at an office visit [hereafter referred to as office BP] while the patient was seated) and to be taking maximally tolerated doses of three or more antihypertensive medications of complementary classes, one of which had to be a diuretic at an appropriate dose. No changes in antihypertensive medication in the previous 2 weeks were allowed. For the next 2 weeks, patients recorded their BP at home (hereafter referred to as home BP) in the morning and in the evening and kept a diary indicating their adherence to medical therapy. Then, a confirmatory screening visit occurred, during which the SBP of 160 mmHg or higher was confirmed, adherence to medications was documented, and automated 24-h ambulatory blood pressure monitoring was performed to ensure a SBP of 135 mmHg or higher | Secondary causes of hypertension and >1 hospitalization for a hypertensive emergency in the previous year. Renal-artery stenosis of >50%, renal-artery aneurysm, prior renal-artery intervention, multiple renal arteries, a renal artery of <4 mm in diameter, or a treatable segment of <20 mm in length |
1 mmHg = 0.133 kPa. BP: Blood pressure; CCS: Canadian Cardiovascular Society; CT: Computed tomography; DBP: Diastolic blood pressure; ECG: Electrocardiogram; eGFR: Estimated glomerular filtration rate; INR: International normalized ratio; MRI: Magnetic resonance imaging; NYHA: New York Heart Association; RDN: Renal denervation; RH: Resistant hypertension; SBP: Systolic blood pressure; ABPM: Ambulatory BP measurement; MDRD: Modification of Diet in Renal Disease.
Supplementary Table 2.
The characteristics of antihypertensive at baseline and 6 months
| Clinical trials | Number of drugs at baseline (R/C) | Number of drugs at 6 months (R/C) | Rate of drugs change (R/C) | Antihypertensive drug classes and their distribution at baseline | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACEI or ARB (R/C) | ACEI (R/C) | ARB (R/C) | CCB (R/C) | Diuretics (R/C) | Aldosterone antagonist (R/C) | β-blocker (R/C) | Direct renin inhibitors (R/C) | α-blocker (R/C) | Centrally acting sympatholytics (R/C) | Vasodilators (R/C) | ||||
| DENERHTN | 3/3 | 5.3/5.4 | - (-/-) | 100/100 | -/- | -/- | 100/100 | 100/100 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| DENERVHTA | 4.3/3.9 | -/- | 29 (27/30) | 100/92 | -/- | -/- | 100/100 | 91/69 | 0/0 | 55/77 | 0/0 | 55/39 | 18/8 | 0/0 |
| OSLO | 5.1/5.0 | 4.9/5.2 | 31.5 (11.1/50.0) | 100/100 | -/- | -/- | 100/100 | 89/70 | 33/60 | 56/90 | 22/0 | 56/20 | 56/40 | 0/20 |
| Prague-15 | 5.1/5.4 | 5.0/5.6 | -(-/-) | 100/100 | 89/89 | -/- | -/- | 100/100 | 27/24 | 66/69 | 0/0 | 54/46 | 54/61 | 0/0 |
| ReSET | 4.1/4.2 | 4.1/4.2 | 39 (46/33) | 53/45 | 53/45 | 0/0 | 86/85 | 53/85 | 61/61 | 81/76 | 3/6 | 11/22 | 17/6 | 0/0 |
| SYMPLICITY-FLEX | 4.4/4.3 | -/- | 21 (-/-) | -/- | 51/56 | 46/47 | 69/64 | 100/92 | 3/6 | 91/94 | 3/8 | 21/14 | 26/28 | 6/11 |
| SYMPLICITY HTN-Japan | 4.9/4.9 | 4.9/4.9 | 7.3 (9.1/5.3) | -/- | 9.1/15.8 | 100/94.7 | 95.5/94.7 | 100/100 | 45.5/36.8 | 81.8/68.4 | 0/0 | 22.7/42.1 | 0/0 | 0/0 |
| SYMPLICITY HTN-2 | 5.2/5.3 | -/- | 13 (20.4/5.9) | 96/94 | -/- | -/- | 79/83 | 89/91 | 17/17 | 83/69 | 0/0 | 33/19 | 52/52 | 15/17 |
| SYMPLICITY HTN-3 | 5.1/5.2 | 5.0/5.2 | 39 (-/-) | -/- | 49.2/41.5 | 50.0/53.2 | 69.8/73.1 | 99.7/100 | 22.5/28.7 | 85.2/86.0 | 7.1/7.0 | 11.0/13.5 | 49.2/43.9 | 36.8/45 |
ACEI: Angiotensin-converting enzyme inhibitors; ARB: Angiotensin II receptor blocker; C: Control group; CCB: Calcium channel blockers; R: Renal denervation group.
Table 2.
Assessment of the methodological quality (Jadad scale) and risk of bias (Cochrane collection) of included studies
| Study | Jadad score | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| DENERHTN | 5 | L | L | H | L | L | L | L | H |
| DENERVHTA | 5 | L | L | H | H | L | L | L | H |
| OSLO | 5 | L | L | H | U | L | L | L | H |
| Prague-15 | 5 | L | L | H | L | L | L | L | H |
| ReSET | 6 | L | U | L | L | L | L | L | U |
| SYMPLICITY-FLEX | 7 | L | L | L | L | L | L | L | L |
| SYMPLICITY HTN-2 | 5 | L | L | H | H | L | L | H | H |
| SYMPLICITY HTN-3 | 7 | L | L | L | L | L | L | L | L |
| SYMPLICITY HTN-Japan | 5 | L | L | H | H | L | L | L | H |
H: High risk; L: Low risk, U: Unclear.
Whole group analysis of 24-h systolic blood pressure and office systolic blood pressure at 6-month follow-up
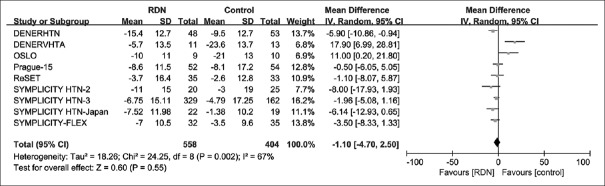
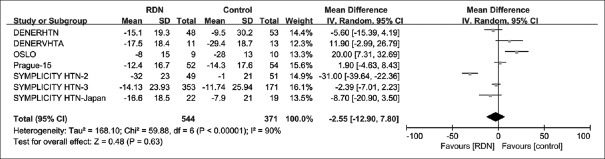
We meta-analyzed BP outcomes from a total of nine RCTs. Summary of analysis results of the nine individual studies is provided in Table 3. The pooled effect of RDN, as the difference from the baseline BP level between RDN and control groups, was MD = −1.1 mmHg [95% CI: −4.7–2.5 mmHg; P = 0.55; Figure 2] for 24-h SBP, and MD = −2.55 mmHg [95% CI: −12.90–7.80 mmHg; P = 0.63; Figure 3] for office SBP. Given much heterogeneity in 24-h SBP (I2 = 67%) and office SBP (I2 = 90%), this study further conducted subgroup analyses and sensitivity analyses.
Table 3.
Change in office and ambulatory BP (mmHg)
| Clinical trails | 0 month (R/C) | Change at 6 months (R/C) | Change at 12 months (R/C) | Change at 24 months (R/C) | Change at 36 months (R/C) |
|---|---|---|---|---|---|
| DENERHTN | |||||
| Office SBP | 159.3/155.9 | −15.1/−9.5 | -/- | -/- | -/- |
| Office DBP | 93.3/91.4 | −9.1/−6.0 | -/- | -/- | -/- |
| 24-h SBP | 151.6/146.8 | −15.4/−9.5 | -/- | -/- | -/- |
| 24-h DBP | 90.2/88.8 | –9.7/–6.6 | -/- | -/- | -/- |
| DENERVHTA | |||||
| Office SBP | 168/171.2 | −17.5/−29.4 | -/- | -/- | -/- |
| Office DBP | 89.6/90.2 | −7.5/−12.7 | -/- | -/- | -/- |
| 24-h SBP | 149.2/155.4 | −5.7/−23.6 | -/- | -/- | -/- |
| 24-h DBP | 81.3/80.9 | -3.7/-10.2 | -/- | -/- | -/- |
| OSLO | |||||
| Office SBP | 156/160 | −8/−28 | -/- | -/- | -/- |
| Office DBP | 91/89 | −2.8/–10.8 | -/- | -/- | -/- |
| 24-h SBP | 151/149 | −10/−21 | -/- | -/- | -/- |
| 24-h DBP | -/- | –6.9/−10.8 | -/- | -/- | -/- |
| Prague-15 | |||||
| Office SBP | 159/155 | −12.4/−14.3 | −13.4/−11.3 | −17.7/−14.1 | -/- |
| Office DBP | 92/89 | −7.4/−7.3 | -/- | -/- | -/- |
| 24-h SBP | 149/147 | −8.6/−8.1 | −6.4/−8.2 | −9.1/−10.9 | -/- |
| 24-h DBP | 86/84 | –5.7/−4.5 | -/- | -/- | -/- |
| ReSET | |||||
| Office SBP | 160/166 | -/- | -/- | -/- | -/- |
| Office DBP | 95/90 | -/- | -/- | -/- | -/- |
| 24-h SBP | 152/153 | −3.7/−2.6 | -/- | -/- | -/- |
| 24-h DBP | 91/89 | −1.7/−2.6 | -/- | -/- | -/- |
| SYMPLICITY-FLEX | |||||
| Office SBP | -/- | -/- | -/- | -/- | -/- |
| Office DBP | -/- | -/- | -/- | -/- | -/- |
| 24-h SBP | 140.2/140.4 | −7.0/−3.5 | -/- | -/- | -/- |
| 24-h DBP | 78.2/80.6 | −2.8/−2.1 | -/- | -/- | -/- |
| SYMPLICITY HTN-Japan | |||||
| Office SBP | 181.0/178.7 | –16.6/–7.9 | -/- | -/- | -/- |
| Office DBP | -/- | –5.9/1.0 | -/- | -/- | -/- |
| 24-h SBP | 164.7/163.3 | –7.52/–1.38 | -/- | -/- | -/- |
| 24-h DBP | -/- | −4.2/−0.4 | -/- | -/- | -/- |
| SYMPLICITY HTN-2 | |||||
| Office SBP | 178/178 | –32/–1 | −28.1/- | -/- | −32.7/- |
| Office DBP | 97/98 | −12/0 | −9.7/- | -/- | −13.6/- |
| 24-h SBP | -/- | –11/–3 | -/- | -/- | -/- |
| 24-h DBP | -/- | –7/−1 | -/- | -/- | -/- |
| SYMPLICITY HTN-3 | |||||
| Office SBP | 179.7/180.2 | –14.13/–11.74 | −18.9/−21.4 | -/- | -/- |
| Office DBP | 96.5/98.9 | −6.6/−4.6 | -/- | -/- | -/- |
| 24-h SBP | 159.1/159.5 | –6.75/–4.79 | -/- | -/- | -/- |
| 24-h DBP | 88.0/90.9 | –4.1/−3.1 | -/- | -/- | -/- |
1 mmHg = 0.133 kPa. -: Not applicable; R: Catheter-based renal denervation group; C: Control group; SBP: Systolic blood pressure; DBP: Diastolic blood pressure; BP: Blood pressure.
Figure 2.
Forest plot for mean difference in 24-h SBP at 6-month follow-up. SBP: Systolic blood pressure; CI: Confidence interval; RDN: Renal denervation.
Figure 3.
Forest plot for mean difference in office SBP at 6-month follow-up. SBP: Systolic blood pressure; CI: Confidence interval; office SBP: Office systolic blood pressure; RDN: Renal denervation.
Subgroup analysis of 24-h systolic blood pressure at 6-month follow-up
By systolic blood pressure at baseline
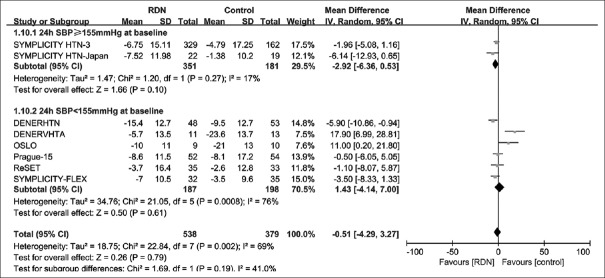
Subgroup analysis results by baseline 24-h SBP level (≥155 mmHg or <155 mmHg) are shown in Figure 4. In the subpopulation with baseline 24-h SBP ≥155 mmHg, the pooled effect of RDN was marginally significant (MD = −2.92 mmHg; 95% CI: −6.36–0.53 mmHg; P = 0.10). There was no significant difference between two groups in the subpopulation with baseline 24-h SBP <155 mmHg (P = 0.61).
Figure 4.
Forest plot for mean difference in 24-h SBP at 6-month follow-up by baseline SBP subgroup. 24-h SBP ≥155 mmHg (1 mmHg = 0.133 kPa) at baseline: an average 24 h blood pressure level at baseline >155 mmHg; 24-h SBP <155 mmHg at baseline: An average 24 h blood pressure level at baseline <155 mmHg; SBP: Systolic blood pressure; CI: Confidence interval; RDN: Renal denervation.
By frequency of medication changes
Among the nine trials, three trials[2,6,9] with the medication change rate below 25% were categorized as the “infrequent medication change” group. The remaining six trials[3,4,5,7,8,10] categorized as the “frequent medication change” group. In the infrequent medication change subgroup, the use of RDN resulted in a significant reduction in 24-h SBP level at 6 months [MD = −4.88; 95% CI: −8.54–−1.22 mmHg; P = 0.009; Figure 5]. Whereas, in the frequent medication change subgroup, the difference between RDN and control was not statistically significant [MD = 1.41; 95% CI: –3.61–6.44 mmHg; P = 0.58; Figure 5].
Figure 5.
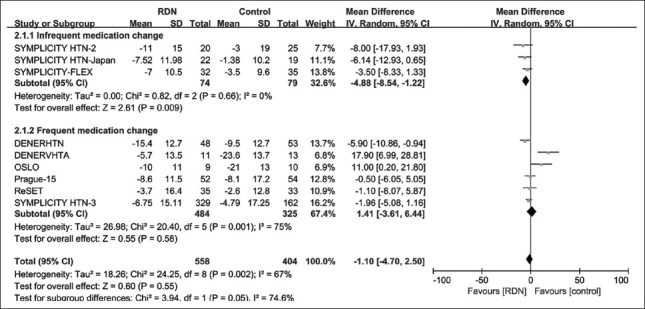
Forest plot for mean difference in 24-h SBP at 6-month follow-up by medication change rate subgroup. Frequent medication change: the medication change rate >25%; Infrequent medication change: The medication change rate <25%; SBP: Systolic blood pressure; CI: Confidence interval; RDN: Renal denervation.
By race
In both Asian[9] and white[6,7,10] subpopulations, the effect of RDN was not significantly different in lowering 24-h SBP [I2 = 66% in white subgroup, Supplementary Figure 1 (605KB, tif) ].
Forest plot for mean difference in 24-h SBP at 6-month follow-up by race subgroup. Asia: Including 100% Asian population; White: Including 100% white population. SBP: Systolic blood pressure; CI: Confidence interval; RDN: Renal denervation.
Improvement in heterogeneity
For 24-h SBP and with all 9 trials included, I2 = 67%. By restricting to trials with an average baseline SBP ≥155 mmHg,[3,9] the heterogeneity reduced to I2 = 17%. The restriction to the trials with medication change rate <25%[2,6,9] resulted in no heterogeneity (I2 = 0%). By restricting the trials with white subpopulation,[6,7,10] the heterogeneity remained similar at 66%.
Subgroup analysis of office systolic blood pressure at 6-month follow-up
By systolic blood pressure at baseline
Subgroup analysis results by baseline office SBP level (≥175 mmHg or <175 mmHg) are shown in Supplementary Figure 2 (668.9KB, tif) . There was no statistical difference in changes at 6-month follow-up in both subgroups.
Forest plot for mean difference in office SBP at 6-month follow-up by baseline SBP subgroup. Office SBP ≥175 mmHg at baseline: office blood pressure level at baseline >155 mmHg; Office SBP <175 mmHg at baseline: Office blood pressure level at baseline <175 mmHg; CI: Confidence interval; RDN: Renal denervation; SBP: Systolic blood pressure.
By frequency of medication changes
In the infrequent medication change subgroup, the use of RDN resulted in a marginally significant reduction in office SBP level at 6 months [MD = −20.28; 95% CI: −42.12–1.55 mmHg; P = 0.07; Supplementary Figure 3 (669.8KB, tif) ]. Whereas, in the frequent medication change subgroup, RDN did not significantly reduce office SBP level [MD = 3.47; 95% CI: −3.82–10.77 mmHg; P = 0.35; Supplementary Figure 3 (669.8KB, tif) ].
Forest plot for mean difference in office SBP at 6-month follow-up by medication change rate subgroup. Frequent medication change: The medication change rate >25%; Infrequent medication change: The medication change rate <25%; CI: Confidence interval; RDN: Renal denervation; SBP: Systolic blood pressure.
By prevalence of coronary heart disease
Six trials[2,3,4,7,8,10] reported the percentage of patients with CHD [Table 1]. From these reports, we have noticed that the prevalence of CHD varied considerably not only between the reviewed trials but also within the trial. As an example of the between-trial comparison, 60% of RDH patients (and 47% of control) had CHD in the SYMPLICITY-FLEX trial, whereas 6% of RDN patients (and 7% of control) had CHD in the Prague-15 trial. The OSLO trial was a good example of the within-trial comparison, where the trial was conducted under the most unbalanced study design with respect to the CHD prevalence (i.e., 11% of RDN and 60% of control patients had CHD). Therefore, we viewed the prevalent CHD as a potential source of clinical heterogeneity in trials and have identified three homogeneous subpopulation by (1) balanced CHD prevalence between RDN and control,[3,7] (2) a higher prevalent CHD in RDN,[2,8] and (3) a higher prevalent CHD in control.[4,10]
In the subgroup of the higher prevalent CHD in control, the control treatment was significantly better than RDN in office SBP reduction at 6 months [MD = 16.59; 95% CI: 6.94–26.25 mmHg; P < 0.001; Supplementary Figure 4 (805.2KB, tif) ]. In contrast, in the other subgroups by the higher CHD prevalence in RDN and the balanced CHD prevalence subgroups, there was no significant difference between the two therapies [Supplementary Figure 4 (805.2KB, tif) ].
Forest plot for mean difference in office SBP at 6-month follow-up by CHD subgroup. Balanced CHD prevalence: balanced coronary heart disease prevalence between both groups; High CHD prevalence in Control: CHD prevalence in control group was higher than that in RDN group; High CHD prevalence in RDN: CHD prevalence in RDN group was higher than that in control group; CI: Confidence interval; CHD: Coronary heart disease; RDN: Renal denervation; SBP: Systolic blood pressure.
Improvement in heterogeneity
For office SBP, when all seven trials[2,3,4,7,8,9,10] were included, there was severe heterogeneity (I2 = 90%). Even if we restrict the pooled analysis to trials with an average baseline SBP ≥175 mmHg[2,3,9] or trials with medication change rate <25%,[2,9] the heterogeneity level still remained high (I2 = 94% and I2 = 88%). However, when the prevalence of CHD was considered, the heterogeneity reduced to 0% in the subgroup defined as a higher CHD prevalence in controls, and I2 = 9% in the subgroup defined as a balanced CHD prevalence.
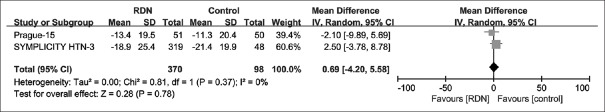
Whole group analysis of office systolic blood pressure at 12-month follow-up
The office SBP, at 12-month follow-up, was available for the pooled analysis of two trials.[16,19] There was no significant difference between the two groups [MD = 0.69 mmHg; 95% CI: −4.2–5.58; P = 0.78; Figure 6], and no heterogeneity of the pooled effect (I2 = 0%; P = 0.37).
Figure 6.
Forest plot for mean difference in office SBP at 12-month follow-up. CI: Confidence interval; SBP: Systolic blood pressure; RDN: Renal denervation.
Whole group analysis of 24-h diastolic blood pressure and office diastolic blood pressure at 6-month follow-up
Regarding DBP outcomes, this analysis showed that the BP decrease was not statistically significant [MD = 0.14; 95% CI: −1.72–2.00 mmHg; P = 0.88; Supplementary Figure 5 (485.8KB, tif) ] in 24-h DBP as well as office DBP [MD = −2.63; 95% CI: −6.70–1.44 mmHg; P = 0.21; Supplementary Figure 6 (419.7KB, tif) ].
Forest plot for mean difference in 24-h DBP at 6-month follow-up. DBP: Diastolic blood pressure; CI: Confidence interval; RDN: Renal denervation.
Forest plot for mean difference in office DBP at 6-month follow-up. CI: Confidence interval; DBP: Diastolic blood pressure; RDN: Renal denervation.
Sensitivity analysis
By excluding one trial at a time, we assessed sensitivity of meta-analysis. Although the approach reduced the level of heterogeneity to some extent in some cases, in most cases, it could not resolve the issue. Summary of sensitivity analysis is provided in Supplementary Tables 3–6.
Supplementary Table 3.
Sensitivity analysis of 24-h SBP at 6 months
| Clinical trials | Number of patients (R/C) | Pooled effect size in mmHg (95% CI) | I2 (%) | Phet | Pz |
|---|---|---|---|---|---|
| All trials pooled | 558/404 | −1.10 (−4.70, 2.50) | 67 | 0.002 | 0.55 |
| Excluded trial | |||||
| DENERHTN | 510/351 | −0.29 (−4.28, 3.71) | 68 | 0.003 | 0.89 |
| DENERVHTA | 547/391 | −2.67 (−5.25, −0.10) | 36 | 0.14 | 0.04 |
| OSLO | 549/394 | −2.10 (−5.48, 1.27) | 62 | 0.01 | 0.22 |
| Prague-15 | 506/350 | −1.06 (−5.18, 3.07) | 71 | 0.001 | 0.62 |
| ReSET | 523/371 | −1.00 (−5.02, 3.02) | 71 | 0.001 | 0.63 |
| SYMPLICITY HTN-2 | 538/379 | −0.51 (−4.29, 3.27) | 69 | 0.002 | 0.79 |
| SYMPLICITY HTN-3 | 229/242 | −0.64 (−5.22, 3.93) | 71 | 0.001 | 0.78 |
| SYMPLICITY HTN-Japan | 536/385 | −6.14 (−12.93, 0.65) | 69 | 0.002 | 0.84 |
| SYMPLICITY-FLEX | 526/369 | −0.56 (−4.77, 3.65) | 71 | 0.0001 | 0.79 |
SBP: Systolic blood pressure; CI: Confidence interval; C: Control group; Phet: Significance for the heterogeneity test; Pz: Significance for the pooled effect size; R: Renal denervation group.
Supplementary Table 6.
Sensitivity analysis of office DBP at 6 months
| Clinical trials | Number of patients (R/C) | Pooled effect size in mmHg (95% CI) | I2 (%) | Phet | Pz |
|---|---|---|---|---|---|
| All trials pooled | 544/371 | −2.63 (−6.70, 1.44) | 80 | <0.0001 | 0.21 |
| Excluded trial | |||||
| DENERHTN | 496/318 | −2.34 (−7.35, 2.66) | 83 | <0.0001 | 0.36 |
| DENERVHTA | 533/358 | −3.46 (−7.67, 0.74) | 81 | <0.0001 | 0.11 |
| OSLO | 535/361 | −3.72 (−7.75, 0.32) | 79 | 0.0002 | 0.07 |
| Prague-15 | 492/317 | −2.99 (−7.73, 1.74) | 82 | <0.0001 | 0.22 |
| SYMPLICITY HTN-2 | 495/320 | −1.37 (−4.21, 1.47) | 46 | 0.1 | 0.35 |
| SYMPLICITY HTN-3 | 191/200 | −2.45 (−7.87, 2.98) | 82 | <0.0001 | 0.38 |
| SYMPLICITY HTN-Japan | 522/352 | −1.87 (−6.44, 2.70) | 82 | <0.0001 | 0.42 |
DBP: Diastolic blood pressure; CI: Confidence interval; C: Control group; Phet: Significance for the heterogeneity test; Pz: Significance for the pooled effect size; R: Renal denervation group.
Supplementary Table 4.
Sensitivity analysis of office SBP at 6 months
| Clinical trials | Number of patients (R/C) | Pooled effect size in mmHg (95% CI) | I2 (%) | Phet | Pz |
|---|---|---|---|---|---|
| All trials pooled | 544/371 | −2.55 (−12.90, 7.80) | 90 | <0.0001 | 0.63 |
| Excluded trial | |||||
| DENERHTN | 496/318 | −1.95 (−14.03, 10.12) | 92 | <0.0001 | 0.75 |
| DENERVHTA | 533/358 | −4.59 (−15.65, 6.48) | 91 | <0.0001 | 0.42 |
| OSLO | 535/361 | −6.05 (−16.29, 4.18) | 89 | <0.0001 | 0.25 |
| Prague-15 | 492/317 | −3.21 (−16.04, 9.62) | 91 | <0.0001 | 0.62 |
| SYMPLICITY HTN-2 | 495/320 | 1.74 (−4.94, 8.42) | 70 | 0.005 | 0.61 |
| SYMPLICITY HTN-3 | 191/200 | −2.35 (−16.51, 11.81) | 92 | <0.0001 | 0.75 |
| SYMPLICITY HTN- Japan | 522/352 | −1.53 (−13.20, 10.13) | 92 | <0.0001 | 0.8 |
CI: Confidence interval; C: Control group; Phet: Significance for the heterogeneity test; Pz: Significance for the pooled effect size; R: Renal denervation group; SBP: Systolic blood pressure.
Supplementary Table 5.
Sensitivity analysis of 24-h DBP at 6 months
| Clinical trials | Number of patients (R/C) | Pooled effect size in mmHg (95% CI) | I2 (%) | Phet | Pz |
|---|---|---|---|---|---|
| All trials pooled | 558/404 | 0.14 (−1.72, 2.00) | 58 | 0.01 | 0.88 |
| Excluded trial | |||||
| DENERHTN | 510/351 | −0.32 (−2.21, 1.56) | 52 | 0.04 | 0.74 |
| DENERVHTA | 547/391 | −0.36 (−2.03, 1.32) | 47 | 0.07 | 0.68 |
| OSLO | 549/394 | −0.17 (−2.07, 1.74) | 59 | 0.02 | 0.86 |
| Prague-15 | 506/350 | 0.36 (−1.77, 2.49) | 63 | 0.009 | 0.74 |
| ReSET | 523/371 | 0.06 (−2.02, 2.15) | 63 | 0.009 | 0.95 |
| SYMPLICITY HTN-2 | 538/379 | 0.48 (−1.36, 2.31) | 57 | 0.02 | 0.61 |
| SYMPLICITY HTN-3 | 229/242 | 0.39 (−1.89, 2.67) | 61 | 0.01 | 0.74 |
| SYMPLICITY HTN-Japan | 536/385 | 0.56 (−1.35, 2.47) | 57 | 0.02 | 0.56 |
| SYMPLICITY HTN-Flex | 526/369 | 0.31 (−1.90, 2.51) | 63 | 0.008 | 0.79 |
DBP: Diastolic blood pressure; CI: Confidence interval; C: Control group; Phet: Significance for the heterogeneity test; Pz: Significance for the pooled effect size; R: Renal denervation group.
Severe cardiovascular events and mortality
In five RCTs,[2,3,5,7,10] severe cardiovascular events were reported. The conclusions from these trials were consistent with our meta-analysis in that there was no statistical difference in the risk of severe cardiovascular events rate between two groups [RR = 1.43; 95% CI: 0.84–2.45; P = 0.19; I2 = 0; [Supplementary Figure 7 (426.1KB, tif) ]. Only one trial studied mortality as outcome measure,[3] where there was no significant difference in the risk of mortality rate between two groups (RR = 0.97; 95% CI: 0.09–10.61; P = 0.98).
Forest plot for severe cardiovascular events at 6-month follow-up. Severe cardiovascular events: Myocardial infarction, new-onset heart failure, stroke, hypertension crisis, angina needing a coronary stent, embolic event resulting in end-organ damage, and hospitalization for atrial fibrillation; CI: Confidence interval; RDN: Renal denervation.
Other adverse effect
In general, adverse events rarely occurred, while a pseudoaneurysm was the most frequent adverse event among them [Supplementary Table 7].
Supplementary Table 7.
The adverse effect of RDN reported by included studies
| Clinical trails | Adverse effect of RDN |
|---|---|
| DENERHTN | Lumbar pain in two patients and mild groin hematoma in one patient |
| DENERVHTA | Mild groin hematoma (n = 3) and transient symptomatic hypotension (n = 3) |
| OSLO | One patient in the RDN group had a myocardial infarction 5 months after the procedure. Four patients had mild-to-moderate hematomas at the femoral access site for RDN. One patient had bradycardia and received atropin injection during RDN. Four patients in the drug-adjusted group and one patient in the RDN group had symptomatic hypotension. Two patients experience sexual dysfunction after increasing the dosage of spironolactone in the drug-adjusted group |
| Prague-15 | Spasms after application of radiofrequency energy, four patients Dissection of renal artery, one patient Postpunctual pseudoaneurysm, two patients Arteriovenous fistula, one patient Laryngospasm after analgosedation, one patient Asymptomatic bradycardia after procedure, two patients Phlebitis associated with peripheral line, one patient |
| ReSET | Femoral hematoma |
| SYMPLICITYHTN-2 | There were no serious complications related to the device or procedure. Minor periprocedural events included one femoral artery pseudoaneurysm, one post-procedure hypotension, one urinary tract infection and one case of back pain. Seven patients had transient intraprocedural bradycardia requiring atropine. Renal function was unchanged at 6 months. There were 5 hypertensive emergencies three patients in RDN group and 2 in control group. Other events requiring admission included one case of nausea and edema, one hypertensive crisis, one TIA, one hypotensive episode, and one coronary stent for angina |
| SYMPLICITYHTN-3 | Major adverse events: 5/361 versus 1/171 Composite safety endpoint at 6 months: 14/354 versus 10/171 Death: 2/352 versus 1/171 Myocardial infarction 6/352 versus 3/171 Increase in serum creatinine of >50% from baseline 5/352 versus 1/171 Embolic event resulting in end-organ damage: 1/352 versus 0/171 Vascular complication requiring treatment: 1/352 versus 0/171 Hypertensive crisis or emergency: 9/352 versus 9/171 Stroke: 4/352 versus 2/171 Hospitalization for new-onset heart failure: 9/352 versus 3/171 Hospitalization for atrial fibrillation: 5/352 versus 1/171 New renal-artery stenosis of >70% 1/332 versus 0/165 |
| SYMPLICITY HTN-Japan | No major adverse events were reported |
| SYMPLICITY-FLEX | There were no deaths, other serious adverse events, or vascular complications |
RDN: Renal denervation; TIA: Transient ischemic attack.
Discussion
Elevated sympathetic nervous system activity is crucial for the development and progression of systemic hypertension, by regulating renin release, tubular sodium reabsorption, and renal blood flow.[21] Afferent sympathetic nerves from the kidney contribute to regulation of whole-body sympathetic activity.[22] An early clinical evaluation has demonstrated that catheter-based RDN could lower BP in patients with RH by decreasing renal norepinephrine spillover, halving of renin activity, increasing renal plasma flow, and reducing central sympathetic drive.[23] However, RCTs designed to confirm the early clinical evaluation results have shown controversial results. Moreover, the results of Fadl Elmula et al.'s meta-analysis based on 7 trials revealed highly significant heterogeneity.[11] The present study, based on 9 RCTs, attempted to deal with the heterogeneity and find main reasons for the discrepant results among RCTs by conducting extensive subgroup and sensitivity analyses.
We considered both 24-h SBP and office SBP as the primary outcomes. Pooling all nine RCTs available, there was no difference between RDN and control in lowering SBP. However, an interesting finding is that, in the subpopulation with baseline 24-h SBP ≥155 mmHg, the effect of RDN was significantly better than control. Given that the patients with a higher BP tend to have an over-active sympathetic nerve system,[24] this finding might be explained by that the catheter-based RDN works better in decrease the sympathetic nerve activity than usual antihypertensive drugs. The role of the catheter-based RDN has been proven that it blocks the pathway of sympathetic nerve activation through a reduction whole-body norepinephrine spillover, which results in a sustained BP reduction.[23] In contrast, antihypertensive drugs that directly block the sympathetic activity directly were rarely used due to obvious adverse effects. This mechanism may also explain the nonsignificant RDN effect in mild RH (daytime SBP between 135 and 149 mmHg or daytime ambulatory DBP between 90 and 94 mmHg).[6] Another interesting finding is that, with patients changing their antihypertensive medications less frequently, this meta-analysis showed a significantly better effect on lowering 24-h SBP by the 6-month follow-up. We suspected that the reason for inconsistent conclusions by the individual nine studies (and the extremely high heterogeneity in the pooled analysis) might be because antihypertensive drugs were too frequently changes in most studies. Conducting the subgroup analysis with low medication change rates resolved this issue.
As demographic discrepancy in the study populations might contribute to inconsistent findings, we conducted subgroup analysis by race. Our results were consistent: no significant BP reduction at 6-month follow-up has been found in both Asian and white subpopulations.
There are a few limitations in this study. First, in subgroup analyses, the sample size was relatively small. However, significant differences were detected, which had not been observed in the pooled analyses with much larger sample sizes due to the severe heterogeneity. Second, due to lack of the longer follow-up details available, effects within 6 and 12 months after the intervention have been assessed. It is worth assessing a long-term effect (longer than 1-year follow-up), given that, in SYMPLICITY HTN-3, the 12-month office SBP change was greater than that observed at 6 months in RDN group. Further studies based on randomized controlled trials are needed to assess a successful ablation of RDN, while completely solving these issues and accounting for various BP levels and the sympathetic neural activity at the same time. This analysis focused on the catheter-based RDN. There are a few more RCTs still ongoing, which aim to evaluate the effect of RDN on decreasing BP [Supplementary Table 8]. The results of the clinical studies will affect the future of RDN.
Supplementary Table 8.
The official title and ClinicalTrials.gov identifier of ongoing RCTs comparing RDN with control group
| Ongoing RCTs | Official title | ClinicalTrials.gov identifier |
|---|---|---|
| DEPART | Denervation of Renal Sympathetic Activity and Hypertension Study | NCT01522430 |
| SYMPLICITY-4 | Renal Denervation in Patients with Uncontrolled Hypertension - SYMPLICITY HTN-4 | NCT01972139 |
| RDNP-2012-01 | Renal Denervation for Resistant Hypertension | NCT01865240 |
| RDNP-2012-2 | Renal Denervation for Uncontrolled Hypertension | NCT02016573 |
| PaCE | A Pragmatic Randomized Clinical Evaluation of Renal Denervation for Treatment Resistant Hypertension | NCT01895140 |
| INSPiRED | Investigator-Steered Project on Intravascular Renal Denervation for Management of Drug-Resistant Hypertension | NCT01505010 |
| EnligHTN IV | Multi-center, Randomized, Single-blind, Sham Controlled Clinical Investigation of Renal Denervation for Uncontrolled Hypertension | NCT01903187 |
| SYMPATHY | Renal Sympathetic Denervation as a New Treatment for Therapy Resistant Hypertension - A Multicenter Randomized Controlled Trial | NCT01850901 |
| Allegro-HTN | Renal Denervation by Allegro System in Patients with Resistant Hypertension | NCT01874470 |
| WAVE_IV | Wave IV Study: Phase II Randomized Sham Controlled Study of Renal Denervation for Subjects with Uncontrolled Hypertension | NCT02029885 |
RCTs: Randomized controlled trials; RDN: Renal denervation.
Supplementary information is linked to the online version of the paper on the Chinese Medical Journal website.
Financial support and sponsorship
This work was supported by a grant from the National Nature Science Foundation of China (No. 81570668).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Qiang Shi
References
- 1.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation. 2008;117:e510–26. doi: 10.1161/CIRCULATIONAHA.108.189141. doi: 10.1161/CIRCULATIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 2.Symplicity HTN- Investigators, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): A randomised controlled trial. Lancet. 2010;376:1903–9. doi: 10.1016/S0140-6736(10)62039-9. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–401. doi: 10.1056/NEJMoa1402670. doi: 10.1056/NEJMoa1402670. [DOI] [PubMed] [Google Scholar]
- 4.Oliveras A, Armario P, Clarà A, Sans-Atxer L, Vázquez S, Pascual J, et al. Spironolactone versus sympathetic renal denervation to treat true resistant hypertension: Results from the DENERVHTA study – A randomized controlled trial. J Hypertens. 2016;34:1863–71. doi: 10.1097/HJH.0000000000001025. doi: 10.1097/HJH.0000000000001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathiassen ON, Vase H, Bech JN, Christensen KL, Buus NH, Schroeder AP, et al. Renal denervation in treatment-resistant essential hypertension. A randomized, SHAM-controlled, double-blinded 24-h blood pressure-based trial. J Hypertens. 2016;34:1639–47. doi: 10.1097/HJH.0000000000000977. doi: 10.1097/HJH.0000000000000977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desch S, Okon T, Heinemann D, Kulle K, Röhnert K, Sonnabend M, et al. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;65:1202–8. doi: 10.1161/HYPERTENSIONAHA.115.05283. doi: 10.1161/HYPERTENSIONAHA.115.05283. [DOI] [PubMed] [Google Scholar]
- 7.Rosa J, Widimský P, Toušek P, Petrák O, Curila K, Waldauf P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: Six-month results from the Prague-15 study. Hypertension. 2015;65:407–13. doi: 10.1161/HYPERTENSIONAHA.114.04019. doi: 10.1161/HYPERTENSIONAHA.114.04019. [DOI] [PubMed] [Google Scholar]
- 8.Azizi M, Sapoval M, Gosse P, Monge M, Bobrie G, Delsart P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): A multicentre, open-label, randomised controlled trial. Lancet. 2015;385:1957–65. doi: 10.1016/S0140-6736(14)61942-5. doi: 10.1016/S0140-6736(14)61942-5. [DOI] [PubMed] [Google Scholar]
- 9.Kario K, Ogawa H, Okumura K, Okura T, Saito S, Ueno T, et al. SYMPLICITY HTN-Japan – First randomized controlled trial of catheter-based renal denervation in Asian patients. Circ J. 2015;79:1222–9. doi: 10.1253/circj.CJ-15-0150. doi: 10.1253/circj.CJ-15-0150. [DOI] [PubMed] [Google Scholar]
- 10.Fadl Elmula FE, Hoffmann P, Larstorp AC, Fossum E, Brekke M, Kjeldsen SE, et al. Adjusted drug treatment is superior to renal sympathetic denervation in patients with true treatment-resistant hypertension. Hypertension. 2014;63:991–9. doi: 10.1161/HYPERTENSIONAHA.114.03246. doi: 10.1161/HYPERTENSIONAHA.114.03246. [DOI] [PubMed] [Google Scholar]
- 11.Fadl Elmula FE, Jin Y, Yang WY, Thijs L, Lu YC, Larstorp AC, et al. Meta-analysis of randomized controlled trials of renal denervation in treatment-resistant hypertension. Blood Press. 2015;24:263–74. doi: 10.3109/08037051.2015.1058595. doi: 10.3109/08037051.2015.1058595. [DOI] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. doi: 0.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 16.Rosa J, Widimský P, Waldauf P, Lambert L, Zelinka T, Táborský M, et al. Role of adding spironolactone and renal denervation in true resistant hypertension: One-year outcomes of randomized PRAGUE-15 study. Hypertension. 2016;67:397–403. doi: 10.1161/HYPERTENSIONAHA.115.06526. doi: 10.1161/HYPERTENSIONAHA.115.06526. [DOI] [PubMed] [Google Scholar]
- 17.Esler MD, Krum H, Schlaich M, Schmieder RE, Böhm M, Sobotka PA Symplicity HTN-Investigators. Renal sympathetic denervation for treatment of drug-resistant hypertension: One-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976–82. doi: 10.1161/CIRCULATIONAHA.112.130880. doi: 10.1161/CIRCULATIONAHA.112.130880. [DOI] [PubMed] [Google Scholar]
- 18.Bakris GL, Townsend RR, Flack JM, Brar S, Cohen SA, D’Agostino R, et al. 12-month blood pressure results of catheter-based renal artery denervation for resistant hypertension: The SYMPLICITY HTN-3 trial. J Am Coll Cardiol. 2015;65:1314–21. doi: 10.1016/j.jacc.2015.01.037. doi: 10.1016/j.jacc.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 19.Esler MD, Böhm M, Sievert H, Rump CL, Schmieder RE, Krum H, et al. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur Heart J. 2014;35:1752–9. doi: 10.1093/eurheartj/ehu209. doi: 10.1093/eurheartj/ehu209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosa J, Widimsky P, Waldauf P, Zelinka T, Petrak O, Taborsky M, et al. Renal denervation in comparison with intensified pharmacotherapy in true resistant hypertension: 2-year outcomes of randomized PRAGUE-15 study. Journal Of Hypertension. 2017 doi: 10.1097/HJH.0000000000001257. doi: 10.1097/HJH.0000000000001257. [DOI] [PubMed] [Google Scholar]
- 21.DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev. 1997;77:75–197. doi: 10.1152/physrev.1997.77.1.75. [DOI] [PubMed] [Google Scholar]
- 22.Stella A, Zanchetti A. Functional role of renal afferents. Physiol Rev. 1991;71:659–82. doi: 10.1152/physrev.1991.71.3.659. [DOI] [PubMed] [Google Scholar]
- 23.Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med. 2009;361:932–4. doi: 10.1056/NEJMc0904179. doi: 10.1056/NEJMc0904179. [DOI] [PubMed] [Google Scholar]
- 24.Smith PA, Graham LN, Mackintosh AF, Stoker JB, Mary DA. Relationship between central sympathetic activity and stages of human hypertension. Am J Hypertens. 2004;17:217–22. doi: 10.1016/j.amjhyper.2003.10.010. doi: 10.1016/j.amjhyper.2003.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary text 1: Search strategy
Forest plot for mean difference in 24-h SBP at 6-month follow-up by race subgroup. Asia: Including 100% Asian population; White: Including 100% white population. SBP: Systolic blood pressure; CI: Confidence interval; RDN: Renal denervation.
Forest plot for mean difference in office SBP at 6-month follow-up by baseline SBP subgroup. Office SBP ≥175 mmHg at baseline: office blood pressure level at baseline >155 mmHg; Office SBP <175 mmHg at baseline: Office blood pressure level at baseline <175 mmHg; CI: Confidence interval; RDN: Renal denervation; SBP: Systolic blood pressure.
Forest plot for mean difference in office SBP at 6-month follow-up by medication change rate subgroup. Frequent medication change: The medication change rate >25%; Infrequent medication change: The medication change rate <25%; CI: Confidence interval; RDN: Renal denervation; SBP: Systolic blood pressure.
Forest plot for mean difference in office SBP at 6-month follow-up by CHD subgroup. Balanced CHD prevalence: balanced coronary heart disease prevalence between both groups; High CHD prevalence in Control: CHD prevalence in control group was higher than that in RDN group; High CHD prevalence in RDN: CHD prevalence in RDN group was higher than that in control group; CI: Confidence interval; CHD: Coronary heart disease; RDN: Renal denervation; SBP: Systolic blood pressure.
Forest plot for mean difference in 24-h DBP at 6-month follow-up. DBP: Diastolic blood pressure; CI: Confidence interval; RDN: Renal denervation.
Forest plot for mean difference in office DBP at 6-month follow-up. CI: Confidence interval; DBP: Diastolic blood pressure; RDN: Renal denervation.
Forest plot for severe cardiovascular events at 6-month follow-up. Severe cardiovascular events: Myocardial infarction, new-onset heart failure, stroke, hypertension crisis, angina needing a coronary stent, embolic event resulting in end-organ damage, and hospitalization for atrial fibrillation; CI: Confidence interval; RDN: Renal denervation.