Abstract
Epicutaneous immunization of T cell receptor (TCR) transgenic (Tg) mice whose CD4+ T cells are specific for the Ac1-11 fragment of myelin basic protein (MBP) with Ac1-11 elicits T cells with dominant suppressor/regulatory activity that confers protection against Ac1-11-induced experimental autoimmune encephalomyelitis. We now report that such disease-resistant MBP TCR Tg mice also harbor a sizeable fraction of peripheral CD4+ T cells lacking surface expression of the Tg TCR β chain and expressing diverse, endogenously rearranged TCR β chains. Ex vivo incubation at physiological temperature caused the loss of neo-β-chain expression and reversion to the MBP αβ TCR+ phenotype. The presence of recombination activating gene 1 and 2 proteins in CD4+ T cells with revised TCRs was consistent with effective V(D)J recombination activity. The emergence of these cells did not depend on the thymic compartment. We conclude that in mice epicutaneously immunized with an autoantigen, peripheral specific T cells are susceptible to multiple mechanisms of tolerance.
Keywords: CD4 T cells, tolerance, skin, recombination
The direct application of purified protein antigen onto the epidermis of mice, or epicutaneous immunization (ECi), is capable of inducing a potent adaptive immune response biased toward the so-called type 2 of CD4+ T helper (Th) cell response, that is, helper T cells with prominent secretion of antiinflammatory cytokines (1–3). Hypothesizing that ECi with purified forms of autoantigens might then interfere with the development of Th1 CD4+ T cell-driven proinflammatory autoimmune disorders, we previously applied the Ac1-11 fragment of myelin basic protein (MBP) to the skin of mice transgenic (Tg) for the rearranged α and β chain of the Ac1-11:I-Au complex-specific MBP T cell receptor (TCR) (MBP αβ TCR Tg mice) (4, 5). Because of the principle of allelic exclusion (6–10) that operates at the TCR chain loci, such engineered mice harbor a T cell repertoire dominated by the MBP αβ TCR, yet not strictly monoclonal, because allelic exclusion is not absolute, particularly at the TCR α-chain locus. TCR chains encoded by endogenously rearranged TCR genes can thus be found on T cells from TCR Tg mice. MBP TCR Tg mice, as well as other TCR Tgs (11), are commonly used to study experimental autoimmune encephalomyelitis (EAE), a chronic proinflammatory autoimmune pathology that targets the white matter of the CNS and serves as an animal model for the human disease multiple sclerosis (12). We have observed that MBP TCR Tg mice epicutaneously immunized with pure Ac1-11 in the absence of any adjuvant are resistant to EAE that can be normally induced upon standard immunization with Ac1-11 plus adjuvant (13). Interestingly, rather than involving immune deviation from the classical Th1 profile that prevails in mice with clinical signs of EAE, disease protection was mediated by CD4+ T cells that acquired regulatory/suppressor capacity. Such suppressor T cells did not produce major antiinflammatory cytokines and could confer protection to unmanipulated hosts upon adoptive transfer, indicating that suppression was dominant. Importantly, ECi with other CNS antigens could protect non-TCR Tg mice from relapsing-remitting forms of EAE, as well (13).
We report here that, in addition to induction of CD4+ T cells with suppressive activity, ECi of MBP TCR Tg mice with only purified Ac1-11 endowed peripheral, specific CD4+ T cells with the capacity to replace the MBP TCR β chain by a diverse set of endogenously produced β chains, leading to revision of the surface-expressed TCR. The data point to a model where the epicutaneous delivery of autoantigens makes peripheral T cells susceptible to multiple mechanisms of tolerance to self-determinants.
Materials and Methods
Mice. MBP TCR Tg mice (MBP TCR Tg+/–) express the rearranged genes encoding the α (Vα4+) and β (Vβ8.2+) chains of an autoreactive, I-Au-restricted TCR specific for the acetylated N-terminal peptide (Ac1-11) of MBP. MBP TCR Tg (Thy1.1 or Thy1.2) mice were mated to TCR Cα–/– or to rag2–/– mice to generate MBP TCR Cα–/– and MBP TCR rag2–/– mice, respectively. Four-week-old to 1-month-old adult thymectomized B10.PL(H2u) mice were purchased from The Jackson Laboratory. All mice were bred and housed in the pathogen-free mouse facility at the Section of Immunobiology at Yale University.
Epicutaneous (Skin) Patch Immunization, EAE Induction, and Monitoring of Clinical Signs of Disease. The procedures are fully described in ref. 13. Very briefly, the backs of mice were shaved, and, one day later, Ac1-11, myelin oligodendroglial glycoprotein (MOG) 31–55, or ovalbumin (Grade V, Sigma) at 10 μg/ml in 100 μl of PBS or PBS alone was loaded onto an occlusive patch (Duo-DERM Extra Thin, Convatec, Princeton) and applied to the shaved area. The patch was left in place for 1 week, and the procedure was repeated for a second week. For EAE induction, Ac1-11 (3 μg/ml in PBS) was mixed with an equal volume of complete Freund's adjuvant, and 50 μl was injected s.c. in each flank of MBP TCR Tg mice that were previously skin-patched. Pertussis toxin (0.2 μg) (Biological Laboratories, Campbell, CA) was given i.v. at the time of immunization and again 2 days later. Mice were scored daily for clinical signs of disease, and a numerical score was assigned to mice based on the severity of the disease symptoms: 0, no disease; 1, weak tail; 2, weak tail and partial hind limb paralysis; 3, total hind limb paralysis; 4, both hind limb and forelimb paralysis; 5, death. Mice with a score of 4 were euthanized. All procedures involving mice were approved by Yale University's Institutional and Animal Care and Use Committee.
Antibodies and FACS Sorting. Cells were stained with anti-CD4-FITC conjugate or anti-CD4-phycoerythrin (PE) (Caltag, South San Francisco, CA); anti-CD4-CyChrome; anti-Vβ8.1/2-FITC/PE; anti-TCRCβ-PE; anti-CD69-PE; anti-CD44-PE; anti-Vβ2, 3, 4, 6, 7, 10, 13, 14-FITC; or anti-CD62L-FITC/PE (BD Biosciences). Clonotypic antibody 3H12 was a kind gift from Juan Lafaille (Skirball Institute of Biomolecular Medicine, New York). The clonotypic mAb 19G was produced in C.A.J.'s laboratory. Cells were incubated with antibodies at 4°C for 30 min, washed, and sorted on a FACStarplus or FACSVantage instrument (Becton Dickinson). Sorted cells were examined on a FACSScan analyzer (Becton Dickinson). For occasional donor/recipient T cell discrimination, anti-Thy 1.1 (CD90)-biotin, streptavidin-FITC, and anti-Thy1.2 (CD90.2)-PE antibodies were used (BD Biosciences).
Cell Culture and Proliferation Assay. For phenotypical analysis ex vivo, sorted CD4+Vβ8.2– T cells were incubated at 37°C at a density of 5 × 106 cells per well in a 12-well tissue culture plate in Click's Eagle–Hanks' amino acid (EHAA) medium (Life Technologies, Grand Island, NY) supplemented with 5% FCS without antigen or exogenous cytokines. Cells were removed at various time points (4–72 h) for analysis by flow cytometry-coupled immunofluorescence, in particular for detection of components of the transgene-encoded MBP TCR. For T cell proliferation assay, sorted CD4+Vβ8.2– or CD4+Vβ8.2+ cells from spleen and lymph nodes of Ac1-11 epicutaneously immunized mice or CD4+Vβ8.2+ cells from naïve MBP TCR Tg mice were plated at 5 × 104 cells per well in the presence of 2 × 105 irradiated syngeneic (B10.PL) spleen cells per well as antigen-presenting cells in Click's EHAA medium supplemented with 5% FCS. The Ac1-11 peptide was added at concentrations ranging from 0 to 100 μg/ml. After 48 h of incubation at 37°C, cells were pulsed with 1 μCi (1 Ci = 37 GBq) of [3H]thymidine and harvested 18–24 h later. Counts were determined in a β-plate scintillation counter.
Adoptive Transfer of Sorted T Cells. CD4+Vβ8.2– T cells from pooled spleen and lymph nodes from naïve (as controls) or Ac1-11 epicutaneously immunized mice were washed in sterile PBS two to three times. Cells (1 × 107) were resuspended in a final volume of 200 μl and administered i.v. to MBP TCR Tg Cα–/– recipients. Five days later, EAE was induced by standard procedure. In some experiments, naïve CD4+Vβ8.2+ T cells were sorted from Thy1.2+ MBP TCR Tg mice and transferred into B10.PL (Thy1.1+) hosts.
Western Blotting. Cell lysates were obtained from sorted CD4+Vβ8.2+ or CD4+Vβ8.2– cells from Ac1-11 epicutaneously immunized mice or controls. Aliquots (10 μg) of each cell lysate were run on SDS/PAGE under reducing conditions and transferred to a transblot transfer medium nitrocellulose membrane (Bio-Rad). Blocking was with 5% milk for 2 h at room temperature. Membranes were incubated for 3 h with polyclonal antibodies against recombination-activating gene (RAG) 1 and RAG2 proteins by using specific polyclonal antibodies (a gift from D. Schatz, Yale University). The blots were washed three times with PBS plus 0.1% Tween 20. Protein bands were visualized by using secondary horseradish peroxidase-conjugated antibodies and the ECL detection system (Amersham Biosciences) according to the manufacturer's instructions.
Results
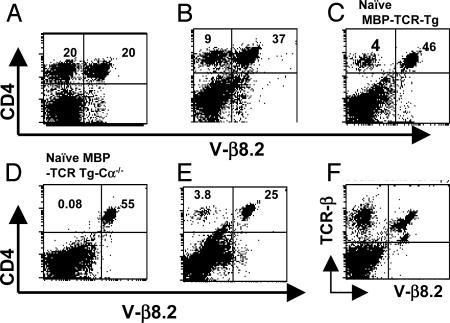
Endogenous TCR β-Chain Expression in Peripheral CD4+ T Cells from MBP TCR Tg Mice. MBP TCR Tg mice that are epicutaneously immunized with the cognate (Ac1-11) peptide are resistant to EAE induction. MBP TCR Tg mice that lack surface expression of the endogenously rearranged TCR α chains (MBP TCR Tg Cα–/– mice) are well known for their spontaneous and rapid development of EAE, and these mice could also be protected from disease upon ECi. Such resistance relied on the induction of CD4+ T cells with dominant suppressive/regulatory activity (13). Disease-resistant MBP TCR Tg or MBP TCR Tg Cα–/– mice will be referred to hereafter as Ac1-11 ECi mice. The phenotypic analysis of peripheral blood, spleen, or lymph node CD4+ T cells from Ac1-11 ECi mice revealed that a sizeable fraction of them stained negative for Vβ8.2, that is, for the V domain used by the transgene-encoded TCR β chain (Fig. 1 A and B). This fraction varied from mouse to mouse and could comprise 50% of CD4+ T cells, or more, over time. This percentage was in sharp contrast with the low frequency (2–4%) of CD4+Vβ8.2– T cells detected in unmanipulated MBP TCR Tg mice and with the virtual absence of these cells in MBP TCR Tg Cα–/– mice (Fig. 1 C and D). The accumulation of CD4+Vβ8.2– T cells was detected exclusively in mice that were epicutaneously immunized with Ac1-11 and immunized with Ac1-11 plus adjuvant for disease induction. In addition, the increase in frequency of CD4+Vβ8.2– T cells was never observed in MBP TCR Tg mice that were epicutaneously immunized with vehicle (PBS), an irrelevant antigen (ovalbumin), or a CNS antigenic peptide other than Ac1-11 (such as MOG 35-55) before Ac1-11 challenge (Fig. 1E), indicating that the detection of a substantial fraction of CD4+Vβ8.2– T cells in EAE-resistant MBP TCR Tg mice required both ECi and challenge with the cognate antigenic peptide. The CD4+Vβ8.2– T cell fraction remained readily detectable for at least 3 months after the attempt to induce EAE. The simultaneous use of the anti-Cβ-specific H57 and anti-Vβ8.2 F23.2 mAbs showed that spleen and lymph node cells isolated from Ac1-11 ECi mice contained both Cβ+Vβ8.2– and Cβ+Vβ8.2+ cells (Fig. 1F). Again, Cβ+Vβ8.2– cells were scarce and absent in unmanipulated MBP TCR Tg mice and MBP TCR Tg Cα–/– mice, respectively (data not shown). Collectively, the results document the presence of a substantial population of peripheral T cells with surface expression of endogenous (H57+Vβ8.2–), rather than Tg (Vβ8.2+), TCR β chain(s) in EAE-resistant, Ac1-11 ECi MBP TCR Tg mice.
Fig. 1.
Surface expression of endogenously rearranged TCR β chains in CD4+ T cells from MBP TCR Tg mice epicutaneously immunized with Ac1-11 and treated for EAE induction. (A and B) FACS analysis of peripheral blood, spleen, or lymph node cells of disease-resistant Ac1-11 ECi mice after being stained with mAbs to CD4 and to the Tg β chain (anti-Vβ8.2). (C) Unmanipulated MBP TCR Tg mice produce 2–5% of CD4 T cells expressing the endogenous β chain (CD4+Vβ8.2–). (D) Unmanipulated MBP TCR Tg Cα–/– mice produce virtually no CD4+Vβ8.2– T cells. (E) MBP TCR Tg mice that were epicutaneously immunized with a control CNS peptide or treated with PBS before disease induction and survived did not display any increase in CD4+Vβ8.2– T cells. (F) Spleen and lymph node CD4 T cells from Ac1-11 ECi mice were stained with mAbs specific for the constant region of the TCR β chain (H57) or for the Tg β chain (F23.2).
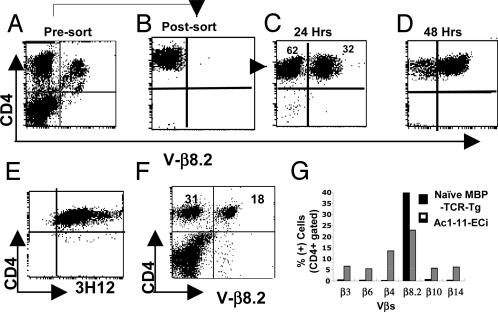
Cultured CD4+Vβ8.2– Peripheral T Cells Gain Surface Expression of the MBP TCR. The detection of a large fraction of CD4+ T cells with surface expression of endogenously rearranged TCR β chains could reflect at least two phenomena. One possibility is that the rare CD4+Vβ8.2– T cells present in untreated MBP TCR Tg mice are induced to proliferate extensively, and their detection follows the expansion of a minor, preexisting population. On the other hand, MBP TCR β-chain expression may be altered in CD4+ T cells from mice that were epicutaneously immunized and challenged with Ac1-11. The first possibility was rather improbable for two reasons. First, CD4+Vβ8.2– T cells from Ac1-11 ECi mice were not blasting, as assessed by examination of their side scatter/forward scatter flow cytometry profile (data not shown). Second, the frequency of Ac1-11:I-Au complex reactive T cells would have to be high among the few T cells lacking expression of the Tg MBP TCR for such a massive expansion to occur upon challenge. Most importantly, we observed that sorted CD4+Vβ8.2– T cells from spleen/lymph nodes of Ac1-11 ECi mice acquired a Vβ8.2+ surface phenotype after overnight incubation at 37°C in complete media in the absence of antigen or of any exogenous cytokines (Fig. 2 A–C). By 48 h of culture, most cells were Vβ8.2+ and, remarkably, were positive when stained with the MBP TCR-specific 3H12 clonotypic mAb (Fig. 2 D and E), indicating that ex vivo incubation at physiological temperature induced them to express the complete MBP αβ TCR on their surface. We also observed that, over time, the CD4+Vβ8.2– population increased with concomitant decrease in the CD4+Vβ8.2+ population (Fig. 2F). The data are most compatible with the idea that MBP TCR β-chain expression is repressed in peripheral CD4+H57+Vβ8.2– T cells from Ac1-11 ECi mice and, therefore, that these cells have undergone TCR revision in vivo.
Fig. 2.
CD4+Vβ8.2– T cells acquire surface expression of the MBP αβ TCR ex vivo.(A and B) Cells from Ac1-11 ECi mice with 50% or more CD4 T cells lacking surface expression of the Tg β chain were sorted into CD4+Vβ8.2+ (A) or CD4+Vβ8.2– (B) populations. (C) Sorted CD4+Vβ8.2– T cells reexpressed the Tg (Vβ8.2+) TCR β chain after 24 h of ex vivo incubation at physiological temperature. (D and E) Sorted CD4+Vβ8.2– T cells gained a Vβ8.2+ phenotype after 48 h at 37°C in complete media (D) stained positive for the 3H12 clonotypic mAb (E), demonstrating that they express the MBP αβ TCR. (F) In Ac1-11 Eci mice, the increase in Vβ8.2– cell frequency among CD4+ cells was associated with a concomitant decrease in the Vβ8.2+ frequency. (G) CD4+Vβ8.2– cells used a variety of endogenously rearranged TCR β chains.
To determine whether the repertoire of endogenously rearranged TCR β chains expressed by CD4+Vβ8.2– T cells was diverse or rather restricted, we first performed flow cytometry-coupled immunostaining using a panel of anti-Vβ-specific mAbs. The results showed that multiple variable domains were used by the β chain of endogenous origin in CD4+ T cells with revised TCR from Ac1-11 ECi mice. Fig. 2G shows, for instance, that the Vβ3, Vβ4, Vβ6, Vβ10, and Vβ14 domains were all used by substantial fractions of peripheral CD4+ T cells from Ac1-11 ECi mice. Thus, in CD4+Vβ8.2– T cells, distinct TCR β chains could be endogenously synthesized and used to form TCRs able to traffic intracellularly and get expressed on the cell surface. Expression of endogenous Vβ chains was further confirmed by detection of transcripts (data not shown).
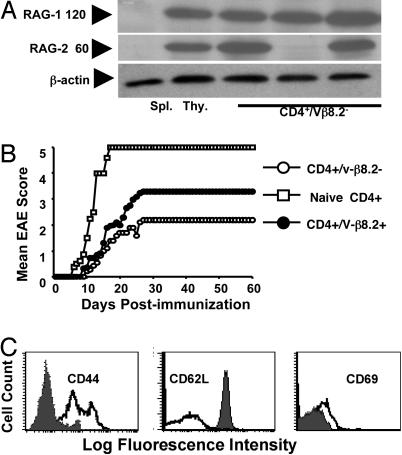
CD4+Vβ8.2– T Cells Express the RAG1 and RAG2 Proteins. Because the TCR gene rearrangement process requires both rag1 and rag2 gene products for sequence-specific DNA recognition and DNA cleavage and postcleavage functions (14, 15), we examined whether we could detect RAG expression in CD4+ T cells from Ac1-11 ECi mice that had acquired expression of endogenously rearranged TCR β chains. We carried out RAG1- and RAG2-specific Western blot analysis of lysates from sorted CD4+ T cells isolated from Ac1-11 ECi mice and of thymic and splenic cells from unmanipulated animals as controls. Although WT spleen cells and thymocytes were respectively negative and positive for RAG protein expression, peripheral CD4+Vβ8.2– T cells from the same mice were found to express substantial amounts of RAG1 and RAG2 proteins (Fig. 3A). CD4+Vβ8.2+ T cells from Ac1-11 ECi mice, however, showed either no expression or, occasionally, trace amounts of RAG proteins (data not shown). There were rare CD4+Vβ8.2– T cell samples with detectable expression of RAG1 but not RAG2. RAG protein expression was typically observed for CD4+Vβ8.2– T cell samples from one-third to half of disease-resistant Ac1-11 ECi mice analyzed at a given time point. In contrast, spleen/lymph node cells from MOG-ECi mice or unmanipulated MBP TCR Tg mice remained negative for RAG1/2 protein expression (Fig. 3A). These observations demonstrate that expression of the RAG proteins, which is turned off upon intrathymic positive selection and is normally absent in mature peripheral T lymphocytes (16, 17), was induced in peripheral CD4+ T cells that surface-express endogenously rearranged TCR β chains in Ac1-11 ECi mice.
Fig. 3.
CD4+Vβ8.2– cells from Ac1-11 ECi mice express RAG1/2 proteins, display a suppressive potential in vivo, and harbor an “effector/memory” mouse T cell phenotype. (A) RAG1 (120 kDa) and RAG2 (60 kDa) proteins are expressed in CD4+Vβ8.2– cells as shown by Western blot analysis of sorted cells from Ac1-11 ECi mice. (Seven of 16 mice expressed RAG1, 5 of 16 expressed RAG2, and 5 of 16 expressed both RAG1 and RAG2.) Sorted CD4+Vβ8.2– cells from spleen and lymph nodes of control MOG ECi mice (three mice per group) or control naïve MBP TCR Tg mice (three mice per group) expressed neither RAG1 nor RAG2. An anti-β-actin mAb was used for control. Spleen and thymic cells from naïve mice were included as negative and positive controls, respectively. (B) Both CD4+Vβ8.2– and CD4+Vβ8.2+ cells display suppressive activity in vivo. 1 × 106 CD4+Vβ8.2–, CD4+Vβ8.2+, or naïve CD4+ T cells from MBP TCR Tg Cα–/– mice were adoptively transferred into naïve MBP TCR Tg Cα–/– recipients, and disease was induced 3 days later. (C) Sorted CD4+Vβ8.2– cells from spleen and lymph nodes of Ac1-11 ECi (open histograms) or unmanipulated MBP TCR Tg (filled histograms) mice were stained with mAbs to CD44, CD62L, and CD69 molecules.
Both CD4+Vβ8.2– and CD4+Vβ8.2+ T Cells Confer Protection Against EAE. We then asked whether receptor revision was necessary for disease resistance to occur in Ac1-11 ECi mice. Sorted CD4+Vβ8.2– and CD4+Vβ8.2+ T cells from Ac1-11 ECi mice were adoptively transferred into young MBP TCR Tg Cα–/– hosts. Upon disease induction, MBP TCR Tg Cα–/– mice that received naïve MBP TCR Tg CD4+ T cells developed rapid and severe disease. In contrast, recipients of either CD4+Vβ8.2– or CD4+Vβ8.2+ epicutaneously immunized T cells showed signs of protection, with a greater resistance in the case of the CD4+Vβ8.2– T cell transfer (Fig. 3B). Thus, although CD4+Vβ8.2– T cells seemed to have a slightly enhanced suppression potential under the conditions used, CD4+Vβ8.2+ T cells were protective as well. That is, there was no strict dependence on receptor revision for purified CD4 T cells from Ac1-11 mice to confer disease resistance upon adoptive transfer. To further examine whether receptor revision is required for protective activity, we turned to a more stringent assay. We used MBP TCR Tg rag2–/– mice that exhibit spontaneous EAE and a fulminate disease course; all such mice develop severe clinical signs by 5 weeks of age and succumb very rapidly (ref. 5; unpublished data). We reasoned that if receptor revision was a requirement for ECi treatment to confer protection, we would repeatedly fail to observe any protection against EAE in epicutaneously immunized MBP TCR Tg rag2–/– mice whose T cells are devoid of any V(D)J rearrangement potential. Conversely, the detection of disease resistance in such mice would strongly argue against a true requirement for receptor revision in order for ECi-induced protection to occur. We observed that two of eight Ac1-11 ECi MBP TCR Tg rag2–/– mice remained free of clinical signs of disease over the time of observation (up to 3 months). In contrast, all mice died rapidly from EAE when epicutaneously immunized with MOG 35-55 (Table 1). The data support the notion that the ECi-induced protection against disease does not depend on TCR revision.
Table 1. Sphingolipid content of WT and Galgt1 Siat9 double-null brains.
| Sphingolipid | WT 1 | WT 2 | Galgt1 Siat9 DN 1 | Galgt1 Siat9 DN 2 |
|---|---|---|---|---|
| Cer | 1,200 | 610 | 730 | 300 |
| HexosylCer | 4,400 | 4,700 | 4,700 | 7,600 |
| LactosylCer | <200 | <200 | 3,700 | 2,700 |
| SM4s | 2,200 | 2,000 | 2,800 | 2,700 |
| SM3 (LacCer II3-sulfate) | <40 | <40 | 450 | 480 |
Quantitation of ceramide (Cer), hexosylceramide (HexosylCer), lactosylceramide (LactosylCer), SM4s, and SM3 in lipid extracts from two brains from WT mice (WT 1 and 2) and two brains from Galgt1 Siat9 double-null mice (Galgt1Siat9DN 1 and 2). Values are in picomoles of lipid per milligram of wet weight.
CD4+Vβ8.2– Peripheral T Cells Harbor an Antigen-Experienced Mouse T Cell Phenotype. To gain insight into the nature of CD4+Vβ8.2– T cells from Ac1-11 ECi mice, we analyzed the expression of a few cell surface markers. Unlike sorted CD4+Vβ8.2+ T cells from unmanipulated MBP TCR Tg mice, spleen and lymph node CD4+Vβ8.2– T cells from Ac1-11 ECi mice displayed augmented expression of CD44 and reduced expression levels of L-selectin (CD62L) (Fig. 3C). CD4+Vβ8.2+ T cells from Ac1-11 ECi mice also displayed modified CD44 and CD62L expression (data not shown). This phenotype is reminiscent of that of antigen-experienced mouse T lymphocytes (18) and is consistent with the assumption that T cells expressing the CD4+Vβ8.2– phenotype have been involved in antigen recognition in vivo. Occasionally, increased CD69 surface expression could also be observed among CD4+Vβ8.2– T cells (Fig. 3C).
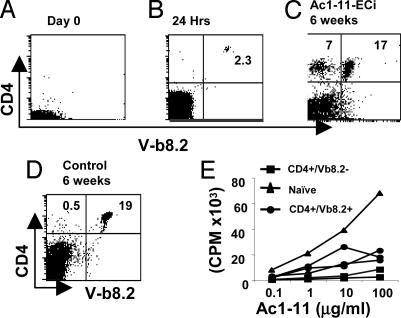
Peripheral T Cells with Revised TCR Do Not Require the Thymic Microenvironment. Because developing thymocytes appear susceptible of modifying their receptor by de novo V(D)J rearrangements in response to self-determinants (19–21), a process often referred to as editing by reference to antigen-receptor modifications during B-cell maturation in bone marrow (22–26), we specifically asked whether the emergence of cells with revised TCR depended on the thymic compartment. To examine this issue, we sorted naïve CD4+Vβ8.2+ T cells from normal MBP TCR Tg mice and transferred them into regular B10.PL hosts that were thymectomized and sublethally irradiated (Fig. 4A). FACS analysis showed that donor cells were detectable in the peripheral blood at 24 h posttransfer (Fig. 4B). Six to ten days later, the recipient mice were epicutaneously immunized with Ac1-11 or a control peptide for 2 weeks and then immunized with Ac1-11 plus adjuvant. Five of 10 mice died (data not shown). Two of the five disease-resistant mice clearly had a substantial fraction of T cells that were CD4+Vβ8.2– at 6 weeks postchallenge (Fig. 4C). Upon isolation by sorting and incubation at 37°C, these cells switched again to a Vβ8.2+3H12+ phenotype (data not shown). In contrast, we repeatedly failed to detect CD4+Vβ8.2– T cells in the few mice that could survive disease induction in the MOG 35-55 ECi control group (Fig. 4D). Thus, receptor revision can occur among peripheral MBP TCR Tg T cells transferred into thymectomized, irradiated hosts. This result indicates that the thymic microenvironment itself is not required for the emergence of CD4+ T cells with revised TCR in Ac1-11 ECi mice.
Fig. 4.
CD4+Vβ8.2– cells from Ac1-11 ECi mice do not depend on the thymic compartment for their emergence in vivo and do not react to Ac1-11 stimulation in vitro.(A and B) Peripheral blood of naïve thymectomized B10.PL mice that were sublethally irradiated before transfer (day 0) (A) or 24 h after they were adoptively transferred with 1 × 107 sorted CD4+Vβ8.2+ cells from unmanipulated MBP TCR Tg mice (B). Six to 10 days later, recipient mice were epicutaneously immunized with Ac1-11 or a control peptide for 2 weeks, and disease was induced. (C and D) Peripheral blood cells obtained from Ac1-11 ECi (C) or control (D) mice were stained with mAbs to CD4 and Vβ8.2. (E) Sorted populations of CD4+Vβ8.2– or CD4+Vβ8.2+ cells from Ac1-11 ECi mice or CD4+Vβ8.2+ cells (5 × 104 cells per well) from naïve MBP TCR Cα–/– mice were stimulated in vitro with varying concentrations of Ac1-11 and irradiated antigen-presenting cells (2 × 105 cells per well).
CD4+Vβ8.2– T Cells from Ac1-11 ECi Mice Are Not Reactive to Ac1-11:I-Au Stimulation. To determine whether CD4+Vβ8.2– T cells are responsive to Ac1-11, we isolated CD4+Vβ8.2– and CD4+Vβ8.2+ T cell subsets from Ac1-11 ECi mice along with naïve CD4+ T cells from unmanipulated MBP TCR Tg mice and challenged them in vitro with serial doses of Ac1-11 in the presence of syngeneic (B10.PL) antigen-presenting cells. Although CD4+Vβ8.2+ T cells gave a detectable proliferative response, they were clearly hyporesponsive when compared with the response of naïve MBP TCR Tg T cells (Fig. 4E). This hyporesponsiveness was consistent with our previous observation that purified CD4+ T cells from Ac1-11 ECi mice, which are able to transfer protection to naïve hosts, were only marginally responsive to specific stimulation in culture (13). CD4+Vβ8.2– T cells, however, did not divide in response to Ac1-11 stimulation under the conditions used, although they could respond to anti-CD3 stimulation (data not shown). The data indicate that freshly isolated CD4+Vβ8.2– T cells from Ac1-11 ECi mice do not proliferate in response to the Ac1-11:I-Au complex in vitro.
Discussion
MBP TCR Tg mice epicutaneously immunized with only the cognate (Ac1-11) peptide before challenge for disease induction (Ac1-11 plus adjuvant) are protected against full EAE. Such resistance relies on the induction of CD4+ suppressor T cells capable of conferring protection to naïve hosts upon adoptive transfer (13). We document here the presence of a substantial fraction of peripheral blood, spleen, or lymph node CD4+ T cells that lack expression of the Tg TCR β chain (CD4+Vβ8.2– T cells) in ≈30–50% of Ac1-11 ECi MBP TCR Tg mice that are resistant to EAE. The emergence of CD4+Vβ8.2– cells required a double in vivo encounter with Ac1-11 because these cells were not detected in MBP TCR Tg mice that were epicutaneously immunized with Ac1-11 and not challenged for EAE induction. In addition, MBP TCR Tg mice epicutaneously immunized with MOG 35-55 (or ovalbumin) developed full EAE after Ac1-11-mediated disease induction and were devoid of peripheral CD4+Vβ8.2– T cells. The H57+ phenotype of CD4+Vβ8.2– T cells demonstrated that these cells surface-express non-Tg, that is, endogenously rearranged, TCR β chain(s).
The presence of a sizeable fraction of peripheral CD4+Vβ8.2– T cells expressing a diverse set of non-Tg β chains in disease-resistant MBP TCR Tg mice could be explained by at least two mechanisms. One can imagine that CD4+Vβ8.2– T cells that are detected at low frequency in unmanipulated MBP TCR Tg mice are induced to proliferate extensively in vivo. Alternatively, it is possible that under the conditions used, Tg CD4+ T cells revise their surface-expressed αβ TCR. Evidence supporting the second possibility is twofold. First, Western blot data demonstrated that the lymphoid lineage specific components of the V(D)J recombination machinery (RAG1/2), whose expression is normally turned off in mature peripheral T lymphocytes, were induced in CD4+Vβ8.2– T cells from Ac1-11 ECi mice. Second, freshly isolated CD4+Vβ8.2– T cells acquired a MBP αβ TCR+ phenotype (3H12+) ex vivo after few hours in culture without further manipulation. Indeed, the possibility that CD4+Vβ8.2– T cells could have originated from the sustained expansion of few such cells in response to ECi/challenge of MBP TCR Tg mice with Ac1-11 would imply that CD4+Vβ8.2– T cells react to Ac1-11. One may then expect them to respond to Ac1-11 stimulation in vitro because they are not modifying their TCR composition. Clearly, this was not the case, even in the presence of exogenous IL-2. Hence, the corpus of data supports the occurrence of RAG-mediated receptor revision in CD4+Vβ8.2– T cells from disease-resistant mice. Such a TCR revision phenomenon (27) among peripheral T cells was perhaps best characterized by studying the fate of Tg CD4+Vβ5+ T cells exposed in vivo to a mouse mammary tumor virus 8-encoded peripheral superantigen (20, 28–31). In our system, maintenance of TCR revision appeared to require factor(s) present in vivo, because acquisition of the 3H12+ phenotype occurred relatively rapidly ex vivo. We speculate that the presence of Ac1-11 could be a contributing factor, as sorted CD4+Vβ8.2– T cells incubated with syngeneic antigen-presenting cells plus Ac1-11 in vitro did not gain the 3H12+ phenotype (data not shown). Although RAG1 and RAG2 protein expression was often codetected in sorted CD4+Vβ8.2– T cells, it happened that only RAG1 was readily detectable. The reason for this difference is unclear, but, interestingly, a similar phenomenon has been reported for human mature CD4+CD3-low T cells that display RAG reexpression and initiate secondary V(D)J rearrangements (32).
Sorted CD4+Vβ8.2– T cells from Ac1-11 ECi mice, that is, CD4+ T cells bearing endogenously rearranged TCR β chains, effectively mediate suppression of T cells that cause EAE in vivo. It is reasonable to assume that among CD4+Vβ8.2– T cells with revised TCR, there were many T cells with TCR synonymous to those expressed by the non-TCR Tg CD4+ T cells that protect unmanipulated MBP TCR Tg mice from the spontaneous occurrence of disease (33) and that also display a diverse TCR repertoire (34). Thus, the protective potential of CD4+Vβ8.2– T cells with revised TCR observed in vivo in transfer experiments is not unexpected. Indeed, the high incidence (≈66%) of spontaneous EAE among MBP TCR Tg mice lacking only endogenous TCR β chains (MBP TCR Tg Cβ–/–) proved that cells with high suppressive potential in MBP TCR Tg mice are not subject to tight β-chain allelic exclusion (33).
When we first observed TCR chain replacement in Ac1-11 ECi mice that were resistant to EAE induction, we wondered whether TCR revision was a requirement for protection. This scenario appears unlikely, because in a given group of simultaneously manipulated mice, at most one-third to one-half of EAE-resistant mice displayed a substantial fraction of CD4+Vβ8.2– T cells at the time of analysis. The reason for this frequency is unclear, but it was not related to the sex or age of the mice (data not shown). It is possible that TCR revision occurs in most mice, but with a differential efficiency because of subtle variability in efficiency of Ac1-11 diffusion from the skin patch and/or during s.c. immunization. More importantly, MBP TCR Tg rag2–/– mice that normally display a fulminate disease course with 100% mortality by 5 weeks of age (5) could show long-term protection from EAE when epicutaneously immunized with Ac1-11.
In summary, the ECi of MBP TCR Tg mice with the CNS autoantigen Ac1-11 before immunization not only elicits T cells with dominant suppressor/regulatory activity that confer protection against Ac1-11-induced EAE, but also causes CD4+ T cells to repress surface expression of the autoreactive TCR β chain and to express a broad repertoire of endogenously rearranged TCR β chains because of RAG reexpression. The emergence of T cells with such revised TCRs was not dependent on the thymic compartment. Thus, the epicutaneous administration of pure autoantigens renders peripheral specific T cells susceptible to multiple mechanisms of tolerance in a nonmutually exclusive fashion.
Acknowledgments
This work is dedicated to the memory of Dr. Charles A. Janeway, Jr. We thank E. Robinson for assistance with sequencing, L. Corbett (Yale University) and Dr. D. Schatz for the RAG antibodies, Dr. J. Lafaille (SIBM, New York) for the 3H12 antibody, Dr. A. Etgen and Wook-Jin Chae for reading the manuscript, and F. Manzo for assistance with manuscript preparation. We also thank L. Gorelik, E. Tran, and A. Bothwell for discussion; Dr. O. Henegariu, P. Preston-Hurlburt, and J. Shanabrough for technical assistance; and C. Annicelli and J. Apicelli for help with mice. This work was supported by the Howard Hughes Medical Institute, National Institutes of Health (NIH) Minority Supplement, and NIH Grants Al14579 and Po1A136529 to (C.A.J.). R.A.F. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations: TCR, T cell receptor; ECi, epicutaneous immunization; MBP, myelin basic protein; Tg, transgenic; EAE, experimental autoimmune encephalomyelitis; RAG, recombination-activating gene; PE, phycoerythrin; MOG, myelin oligodendroglial glycoprotein.
References
- 1.Wang, L. F., Lin, J. Y., Hsieh, K. H. & Lin, R. H. (1996) J. Immunol. 156, 4077–4082. [PubMed] [Google Scholar]
- 2.Wang, L. F., Sun, C. C., Wu, J. T. & Lin, R. H. (1999) Clin. Exp. Allergy 29, 271–279. [DOI] [PubMed] [Google Scholar]
- 3.Herrick, C. A., MacLeod, H., Glusac, E., Tigelaar, R. E. & Bottomly, K. (2000) J. Clin. Invest. 105, 765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardardottir, F., Baron, J. L. & Janeway, C. A., Jr. (1995) Proc. Natl. Acad. Sci. USA 92, 354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafaille, J. J., Nagashima, K., Katsuki, M. & Tonegawa, S. (1994) Cell 78, 399–408. [DOI] [PubMed] [Google Scholar]
- 6.Uematsu, Y., Ryser, S., Dembic, Z., Borgulya, P., Krimpenfort, P., Berns, A., von Boehmer, H. & Steinmetz, M. (1988) Cell 52, 831–841. [DOI] [PubMed] [Google Scholar]
- 7.von Boehmer, H. (1988) Annu. Rev. Immunol. 6, 309–326. [DOI] [PubMed] [Google Scholar]
- 8.von Boehmer, H. (1990) Annu. Rev. Immunol. 8, 531–556. [DOI] [PubMed] [Google Scholar]
- 9.Malissen, M., Trucy, J., Jouvin-Marche, E., Cazenave, P. A., Scollay, R. & Malissen, B. (1992) Immunol. Today 13, 315–322. [DOI] [PubMed] [Google Scholar]
- 10.Khor, B. & Sleckman, B. P. (2002) Curr. Opin. Immunol. 14, 230–234. [DOI] [PubMed] [Google Scholar]
- 11.Lafaille, J. J. (2004) J. Autoimmun. 22, 95–106. [DOI] [PubMed] [Google Scholar]
- 12.Steinman, L. (1999) Neuron 24, 511–514. [DOI] [PubMed] [Google Scholar]
- 13.Bynoe, M. S., Evans, J. T., Viret, C. & Janeway, C. A., Jr. (2003) Immunity 19, 317–328. [DOI] [PubMed] [Google Scholar]
- 14.Fugmann, S. D., Lee, A. I., Shockett, P. E., Villey, I. J. & Schatz, D. G. (2000) Annu. Rev. Immunol. 18, 495–527. [DOI] [PubMed] [Google Scholar]
- 15.Grawunder, U., Schatz, D. G., Leu, T. M., Rolink, A. & Melchers, F. (1996) J. Exp. Med. 183, 1731–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turka, L. A., Schatz, D. G., Oettinger, M. A., Chun, J. J., Gorka, C., Lee, K., McCormack, W. T. & Thompson, C. B. (1991) Science 253, 778–781. [DOI] [PubMed] [Google Scholar]
- 17.Borgulya, P., Kishi, H., Uematsu, Y. & von Boehmer, H. (1992) Cell 69, 529–537. [DOI] [PubMed] [Google Scholar]
- 18.Dutton, R. W., Bradley, L. M. & Swain, S. L. (1998) Annu. Rev. Immunol. 16, 201–223. [DOI] [PubMed] [Google Scholar]
- 19.McGargill, M. A., Derbinski, J. M. & Hogquist, K. A. (2000) Nat. Immunol. 1, 336–341. [DOI] [PubMed] [Google Scholar]
- 20.Fink, P. J. & McMahan, C. J. (2000) Immunol. Today 21, 561–566. [DOI] [PubMed] [Google Scholar]
- 21.Mayerova, D. & Hogquist, K. A. (2004) J. Immunol. 172, 851–856. [DOI] [PubMed] [Google Scholar]
- 22.McGargill, M. A. & Hogquist, K. A. (2000) Immunol. Lett. 75, 27–31. [DOI] [PubMed] [Google Scholar]
- 23.Nemazee, D. (2000) Annu. Rev. Immunol. 18, 19–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nemazee, D. (2000) Adv. Immunol. 74, 89–126. [DOI] [PubMed] [Google Scholar]
- 25.Kouskoff, V. & Nemazee, D. (2001) Life Sci. 69, 1105–1113. [DOI] [PubMed] [Google Scholar]
- 26.Nemazee, D. & Hogquist, K. A. (2003) Curr. Opin. Immunol. 15, 182–189. [DOI] [PubMed] [Google Scholar]
- 27.Mostoslavsky, R. & Alt, F. W. (2004) Trends Immunol. 25, 276–279. [DOI] [PubMed] [Google Scholar]
- 28.McMahan, C. J. & Fink, P. J. (1998) Immunity 9, 637–647. [DOI] [PubMed] [Google Scholar]
- 29.McMahan, C. J. & Fink, P. J. (2000) J. Immunol. 165, 6902–6907. [DOI] [PubMed] [Google Scholar]
- 30.Cooper, C. J., Orr, M. T., McMahan, C. J. & Fink, P. J. (2003) J. Immunol. 171, 226–233. [DOI] [PubMed] [Google Scholar]
- 31.Ali, M., Weinreich, M., Balcaitis, S., Cooper, C. J. & Fink, P. J. (2003) J. Immunol. 171, 6290–6296. [DOI] [PubMed] [Google Scholar]
- 32.Lantelme, E., Palermo, B., Granziero, L., Mantovani, S., Campanelli, R., Monafo, V., Lanzavecchia, A. & Giachino, C. (2000) J. Immunol. 164, 3455–3459. [DOI] [PubMed] [Google Scholar]
- 33.Olivares-Villagomez, D., Wang, Y. & Lafaille, J. J. (1998) J. Exp. Med. 188, 1883–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivares-Villagomez, D., Wensky, A. K., Wang, Y. & Lafaille, J. J. (2000) J. Immunol. 164, 5499–5507. [DOI] [PubMed] [Google Scholar]