Abstract
Sodium nitrite alone is shown to ameliorate sub-lethal cyanide toxicity in mice when given from ~1 hour before until 20 minutes after the toxic dose as demonstrated by the recovery of righting ability. An optimum dose (12 mg/kg) was determined to significantly relieve cyanide toxicity (5.0 mg/kg) when administered to mice intraperitoneally. Nitrite so administered was shown to rapidly produce NO in the bloodsteam as judged by the dose dependent appearance of EPR signals attributable to nitrosylhemoglobin and methemoglobin. It is argued that antagonism of cyanide inhibition of cytochrome c oxidase by NO is the crucial antidotal activity rather than the methemoglobin-forming action of nitrite. Concomitant addition of sodium thiosulfate to nitrite-treated blood resulted in the detection of sulfidomethemoblobin by EPR spectroscopy. Sulfide is a product of thiosulfate hydrolysis and, like cyanide, is known to be a potent inhibitor of cytochrome c oxidase; the effects of the two inhibitors being essentially additive under standard assay conditions, rather than dominated by either one. The findings afford a plausible explanation for an observed detrimental effect in mice associated with the use of the standard nitrite-thiosulfate combination therapy at sub-lethal levels of cyanide intoxication.
Keywords: Complex IV, hemoglobin, methemoglobinemia, respiratory poisons, synergistic toxicity
Introduction
The combined intravenous administration of sodium nitrite and sodium thiosulfate solutions has been available as a treatment for cyanide poisoning for more than half a century. The protocol seems to have been developed to address certain occupational accidents and the suicide attempts periodically presenting in emergency rooms, or veterinary cases where cyanogenic plants have been grazed by livestock. In these instances, several times the LD50 for cyanide may have been ingested, but absorption of a lethal dose from the gastrointestinal tract can take hours. In relation to such cases it is significant that, compared to either of their sodium salts alone, the combined use of nitrite plus thiosulfate can be shown to yield increased protection provided the cyanide intoxication is more than 4 × LD50 (1). However, given the abundance of materials now used in construction that typically release HCN upon combustion, there is a growing concern with sub-lethal intoxications. For example, it has been suggested that cyanide-induced narcosis may be indirectly responsible for deaths in modern fires due to the incapacitated individuals making poor escape decisions (2). In addition, the potential use of cyanide in a wanton terroristic act may also produce a broad spectrum of exposures, including sub-lethal ones, to a significant population. The recent finding by Crankshaw et al. (3) that recovery times of sub-lethally cyanide-intoxicated mice can actually be made worse (i.e. lengthened) by delayed treatment with the standard nitrite-thiosulfate combination is troublesome as the kits are still manufactured and commercially available.
We have previously suggested that nitric oxide can efficiently displace bound cyanide from the active site of complex IV (cytochrome c oxidase) and this is probably an important component of the mechanism by which NO donors, including the nitrite anion, are antidotal to cyanide poisoning (4–5). In this paper, we have set out to demonstrate in mice that sodium nitrite alone is sufficient to offer full protection against sub-lethal cyanide intoxications and also, investigate by electron paramagnetic resonance (EPR) some of the reactions of thiosulfate with hemoglobin in whole blood that could be responsible for the reported (3) adverse outcome with the nitrite-thiosulfate combination.
Experimental Procedures
Chemicals
All reagents were ACS grade, or better, used without further purification and unless stated to the contrary, were purchased from Fisher or Sigma-Aldrich. Carbon dioxide and argon gases were obtained from Matheson Incorporated.
Animals, Exposure and Blood Collection
All animal procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (Protocol Numbers 0808101 & 1008725). Veterinary care was provided by the Division of Laboratory Animal Research of the University of Pittsburgh. Male Swiss Webster (CFW) mice weighing 35–45 g were purchased from Charles River Laboratories, Wilmington, MA. All animals were 16–20 weeks old and were housed four per cage. The mice were allowed access to food and water ad lib. Experiments commenced after the animals were allowed to adapt to their new environment for one week. A series of experiments testing the efficacy of sodium nitrite as a cyanide antidote were performed. All solutions were prepared by dilutions into sterilized saline and administered through ~0.1 mL intraperitoneal (ip) injections. In general, a group of at least 3 mice were tested for each experimental point. Efficacy was tested through recovery of righting ability and pulse oximetric measurements (see below). At the end of exposures and tests, mice were euthanized with 150 mg/kg sodium pentobarbital by ip injection.
For collection of blood samples, mice were first euthanized in an atmosphere of carbon dioxide, the thoracic cavities opened and blood drawn by cardiac puncture. The blood was expelled into the bottom of a quartz EPR tube containing 10% (w/v) EDTA through a Teflon “needle” and then frozen by immersion in liquid nitrogen. This entire process could comfortably be completed in 2 minutes. The cryogenically preserved sample was stored and subsequently transferred to the EPR spectrometer without ever having been thawed.
Righting Recovery Procedure
Lengths of time required for recovery of righting ability were determined based on some of the recommendations of Crankshaw et al. (3) regarding their measurement of the righting reflex, but adopting a simpler procedure. Following ip administration of NaCN (5.0 mg/kg) or NaCN (5.0 mg/kg) + NaNO2 (1 – 24 mg/kg) mice were placed in a transparent but dark colored plastic tube in a supine position. The time duration from the cyanide injection until the mouse flipped from the supine to a prone position in the plastic tube was taken as the endpoint.
Measurement of Oxygen Saturation, Heart Rate and Respiratory Rate
A MouseOx® Pulse Oximeter (manufactured by STARR Life Sciences Corp.) was employed with a subset of mice to record physiologic data in response to NaCN and/or NaNO2. The data were recorded and processed using the software supplied by the manufacturer. The procedure was non-invasive, requiring only the placement of a wrap-around collar clip-sensor (designed to fit) around the neck of the mouse (non-anesthetized and unshaven). The mouse was then free to roam in his cage while the sensor on the collar constantly monitored oxygen saturation, heart rate and breathing rate. The collar was placed on the mouse to record baseline data, removed during ip injections and then replaced. The collar was removed (experiment terminated) approximately 45 min to 1 hr after the initial injection. Multiple trials were conducted with four different sets of experiment condition: (i) saline, (ii) 5 mg/kg NaCN, (iii) 12 mg/kg NaNO2 and (iv) 5 mg/kg NaCN (0.05 mL) + 12 mg/kg NaNO2 (0.05 mL).
Protein Isolations and Enzyme Assay
Human hemoglobin A0 (Hb) was isolated from fresh blood obtained from a local blood bank (Central Blood Bank Manufacturing Operations, Pittsburgh) employing the ammonium sulfate crystallization procedure originally described by Drabkin (6–9). Cytochrome c oxidase was prepared as previously described (4) from intact bovine heart mitochondria using a modified Harzell-Beinert procedure (without the preparation of Keilin-Hartree particles). The enzyme was determined to be spectroscopically pure if the 444 nm to 424 nm ratio for the reduced enzyme was 2.2 or higher (10). Derivatives were prepared in 50 mM potassium phosphate, 1 mM sodium EDTA and 0.1% (w/v) lauryl maltoside, pH 7.4–7.8, to concentrations of 5–80 μM (in enzyme). Enzyme concentrations were determined as total heme a using the differential (absorption) extinction coefficient of Δε604 = 12 mM−1cm−1 for the reduced minus oxidized spectra of the mammalian and bacterial enzymes, respectively (11). Concentrations throughout are given on a per enzyme (molar) concentration basis (not per [heme a]).
Ferrocytochrome c:O2 oxidoreductase activity was determined spectrophotometrically employing the high ionic strength method of Sinjorgo et al. (12). Using this assay, we routinely obtain a turnover number with respect to cytochrome c of 340 (± 30) s−1 (260 μM O2, 0.1 M sodium phosphate, 0.1% (w/v) lauryl maltoside, pH 7.4, 22 °C) similar to that of the bovine enzyme isolated from a variety of tissues by others (12). Oxygen consumption kinetics were measured polarographically using a catalytic amount of cytochrome c (6 μM) and 5 mM sodium ascorbate as the reductant. Reactions were carried out at room temperature in 0.1 M potassium phosphate buffer, 0.1% (w/v) lauryl maltoside, pH 7.4, 22 °C, at an initial oxygen concentration of ~230 μM. All kinetic time courses for oxygen consumption (and ferrocytochrome c oxidation) were essentially linear in the range 10 – 60 s. Where required, rates were estimated from the linear-region slopes of the oxygen (or ferrocytochrome c) concentration versus time plots without applying corrections.
Electron Paramagnetic Resonance (EPR)
X-band (9 GHz) EPR spectra were recorded on a Bruker ESP 300 spectrometer equipped with an Oxford ESR 910 cryostat for ultra-low-temperature measurements. The microwave frequency was calibrated by a frequency counter and the magnetic field was calibrated with a gaussmeter. This instrument and the software (SpinCount) used to analyze the EPR spectra were graciously provided by Professor Mike Hendrich, Carnegie Mellon University. Quantification of EPR signals was performed by simulating the spectra using known (or determined) parameters for each sample in question. Simulations employed a least-squares fitting method to match the lineshape and signal intensity of a selected spectrum. Simulated spectra were expressed in terms of an absolute intensity scale, which could then be related to sample concentration through comparison with a CuII(EDTA) spin standard of known concentration.
Data Analysis
Statistical data was analyzed using KaleidaGraph™ software by ANOVA with a Tukey post-hoc test. A p-value of < 0.05 was considered statistically significant and results are reported as values ± S.E.
Results
Sodium nitrite (alone) as a cyanide antidote
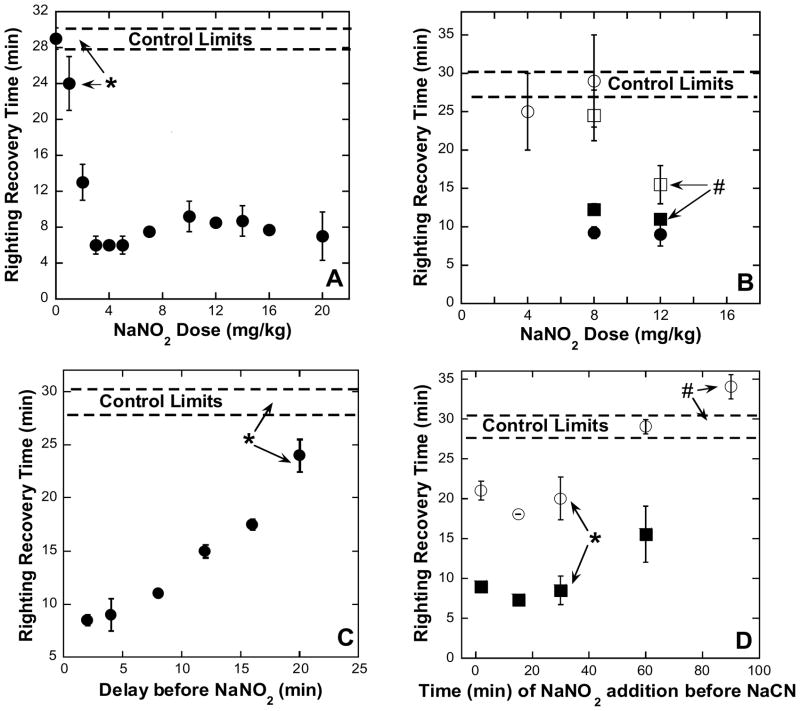
In order to determine the efficacy of sodium nitrite as an antidote to sub-lethal cyanide intoxication, without the need for any additional sulfur-donor or other cyanide-scavenging compounds, conscious mice were first administered 5.0 mg/kg NaCN (~0.1 mL saline solution, ip). Within approximately two minutes, this dose (88% of the LD50 dose, 5.7 mg/kg ip, determined by Baskin et al. (13)) induced a knockdown state in surviving animals (66%) of reproducible duration (see the control limits indicated with broken lines on the 4 panels of Figure 1). Mice used to generate a dose-response curve received NaNO2 (0 – 20 mg/kg given as ~0.1 mL saline solution, ip) 2 minutes following the cyanide injection (Figure 1A). At about 5 minutes after the initial cyanide injection, the mice were placed in a supine position and the times at which they were subsequently able to right themselves noted (see Experimental Procedures). All righting recovery times are quoted with respect to the initial cyanide injection (i.e. given at t = 0). There were some notable subsidiary observations made. In control experiments, where mice received the cyanide, but no antidote, 34% of the animals died, the vast majority (13 of 38) within 3 minutes. In experiments where mice received both cyanide and the antidote (at t = 2 min) survival was improved and only 18% of the animals (19 of 105) died irrespective of the level of antidote given (3–16 mg/kg). Note that the speed with which cyanide acts is a critical determinant here, because in another small set of experiments where 4 – 12 mg/kg NaNO2 was given 2 minutes before (i.e. ip at t = −2 min) 5.0 mg/kg NaCN, 15% of the animals (2 of 13) died; whereas if the nitrite were given 15–30 minutes before the cyanide, all (n = 13) survived. In addition, we observed that a subset of the surviving mice exhibited obvious signs of “dyskinesia” while in the knockdown state – we use this term to describe a variety of odd limb movements (trembling, repetitive motion, seizure, etc.) presumably indicating increased neurological impairment in these animals compared to those not showing the symptom. The mice exhibiting dyskinesia (open symbols in 1B and 1D) did not recover as quickly as the other non-dyskinetic animals (solid symbols); on average a 5–10 minutes longer recovery time was observed. When the antidote can be administered quickly (i.e. within 2 min) it is clear that, for 5.0 mg/kg NaCN, the minimum effective antidotal dose of NaNO2 has been found to be 3 – 4 mg/kg (ip).
Figure 1. The effect of NaNO2 on cyanide intoxication as monitored by righting recovery.
Swiss-Webster mice (males, 16–20 weeks of age) were given NaCN (5 mg/kg, 88% LD50) and, except for controls, NaNO2 (either pre- or post- cyanide), all doses being administered intraperitoneally (i.p). Open symbols indicate mice that exhibited dyskinesia (see text for details). Control limits shown represent the response of surviving animals without antidote (n = 17). Values represent means and standard errors. In general, at least 4 animals per point were used. All points were statistically significant from the controls (P < 0.05, see Methods for details) unless otherwise indicated. A: Dose-repose determination. NaNO2, (2–20 mg/kg) was administered 2 minutes post NaCN injection. Righting was timed from the moment of NaCN injection. *P = 0.1. B: Time and dose dependence of post-cyanide nitrite administration. A series of experiments varying the dose and delay time of NaNO2 administration were completed to determine the minimum dose needed for efficacy at various delayed antidote administration times. NaNO2 doses (4, 8, and 12 mg/kg) were tested at 2 different times after the administration of NaCN. Circles represent mice administered NaNO2 4 min post NaCN and squares represent mice administered NaNO2 8 min post NaCN. #P = 0.2. C: Effect of delayed nitrite administration. NaNO2 (12 mg/kg) given 2 to 20 min after NaCN. The righting recovery test began after the NaNO2 injection. *P = 0.1. D: Time dependence of nitrite administration pre-cyanide. NaNO2 (12 mg/kg) administered 2 to 90 min before 5 mg/kg NaCN. Righting recovery test began after the NaCN injection. *P = 0.07 and #P = 0.3.
Since antidotal doses that must be administered within 2 minutes are likely to be of limited usefulness, a series of experiments were carried out in order to determine minimum effective NaNO2 doses to be given at longer delay times following the initial cyanide injection. At 4 minutes after giving the NaCN (5.0 mg/kg, ip) a 4 mg/kg NaNO2 dose was clearly ineffective, but by increasing the NaNO2 dose in 4 mg/kg increments, we determined that 12 mg/kg NaNO2 was an effective minimum antidotal dose to be used at longer delay times (Figure 1B). Moreover, 12 mg/kg NaNO2 was effective in improving (i.e. decreasing) the righting recovery time significantly for both groups (+/− dyskinesia) of mice when administered up to 20 minutes following the cyanide injection (Figure 1C) although, of course, the sooner the antidote was given, the better was the outcome. We also investigated the prophylactic use of NaNO2, giving a 12 mg/kg dose at various times prior to administration of 5.0 mg/kg NaCN. It was found that this protective treatment was beneficial if the antidote were given up to ~1 hour before the cyanide injection, as judged by the improvement in righting recovery times compared with controls (Figure 1D).
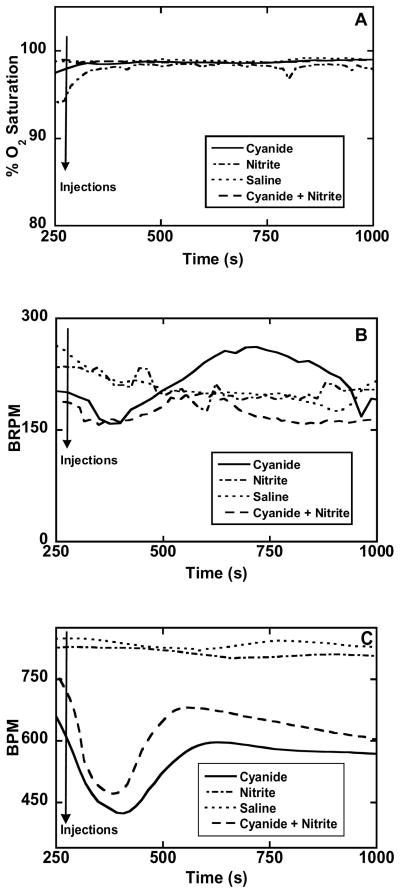
The physiological condition of a group of mice was monitored to a limited extent (blood oxygen saturation, breathing rate and heart rate) using a MouseOx® Pulse Oximeter (see Methods for details). The blood oxygen saturation is a measure of the percentage of oxygenated hemoglobin (HbO2) in arterial blood; since a collar clip-type sensor was used this essentially means HbO2 levels in the carotid arteries. Following the administration of either saline, 5.0 mg/kg NaCN, 5.0 mg/kg NaCN + 12 mg/kg NaNO2, or 12 mg/kg NaNO2 alone, no significant changes/differences were observed in percentage HbO2 levels (Figure 2A). In general, these measurements were continued for over 20 minutes following the injections, but only the data collected during the first ~12 minutes is shown. The absence of any measurable effect on arterial blood is not unexpected as cyanide intoxication prevents oxygen usage by the tissues and so, changes in venous blood levels are more likely. The principal importance of this result is that it shows the lack of any undesirable deleterious effect due to the nitrite antidote.
Figure 2. Physiological assessment of cyanide, nitrite and nitrite plus cyanide administration.
Non-invasive data using a pulse oximeter with Swiss-Webster mice (males, 16–20 weeks of age) was obtained after administration of one of the following solutions by i.p. injection: saline, 5 mg/kg NaCN, 12 mg/kg NaNO2, or 5 mg/kg NaCN + 12 mg/kg NaNO2. Data was collected for 5 minutes prior to injections (to establish baseline response) and 45–60 minutes post injections with the animals allowed to roam freely in individual cages (approximately 20 × 10 cm). Injection times are indicated by arrows: A: Percent O2 saturation of hemoglobin in the carotid arterial blood reported after every heartbeat. B: Breath Rate (breaths per minute BRPM) reported after every heartbeat. C: Cardiac pulse rate (beats per minute, BPM) reported every 1.7 seconds.
The breathing rate of cyanide-injected mice (5.0 mg/kg NaCN) initially showed a small decrease in rate, this was followed by hyperventilation (Figure 2B, solid trace) consistent with the previously reported and, therefore, expected physiological response (14). In animals also given nitrite (12 mg/kg NaNO2, ip, 1 min before cyanide) the hyperventilation was eliminated (Figure 2B, broken trace). The pulse rate of cyanide-injected mice (5.0 mg/kg NaCN) initially decreased by ~200 beats/min, returning to stable, near-control rates after about 5 minutes (Figure 2C, solid trace). Qualitatively, animals also given nitrite (12 mg/kg NaNO2, ip, 1 min before cyanide) did not exhibit much difference in cardiac response (Figure 2C, dashed line) but the overall pulse rate was not depressed as much as in the mice not given the antidote. Interestingly, the administration of nitrite alone did increase the measured pulse rate (Figure 2C, dot-dash trace) compared to saline-injected animals (Figure 2C, dotted trace) which seemed to be reflected in the noticeably increased activity levels of the mice on sodium nitrite alone compared to all the others. It should be noted that the changes observed in the responses of both breathing rate and pulse rate to the antidote and/or the toxin lie within the range of values reported to be normal for the species (respectively, 110–330 breaths/min (15–16) and 400–800 beats/min (17) depending on strain).
Interactions of sodium nitrite with hemoglobin in blood
The pulse oximetric results suggest that if changes in hemoglobin biochemistry are to be mechanistically informative regarding cyanide intoxication and the effects of antidotes, it will be minority hemoglobin species that are important. Electron paramagnetic resonance (EPR) spectroscopy is particularly useful in this regard as the two major heme components in blood, HbO2 and deoxyhemoglobin (Hb) are both EPR silent, whereas the minority components likely to be of most interest, methemoglobin (metHb) and nitrosylhemoglobin (HbNO), yield readily detectable EPR signals – we are thus in the advantageous position of seeking emergent signals against a zero background. Mice were given NaNO2 (4 – 16 mg/kg in saline, ip) then later euthanized in an atmosphere of CO2 at experimentally required time points. Within 1 minute of CO2 euthanasia, blood had been withdrawn by cardiac puncture and 250 μL dispensed into an EPR tube. The sample was then cryogenically preserved by immersion of the EPR tube in liquid nitrogen at 2 minutes following initial exposure to CO2. When required, NaCN (5.0 mg/kg in saline, ip) was administered immediately following the nitrite dose and before CO2 euthanasia.
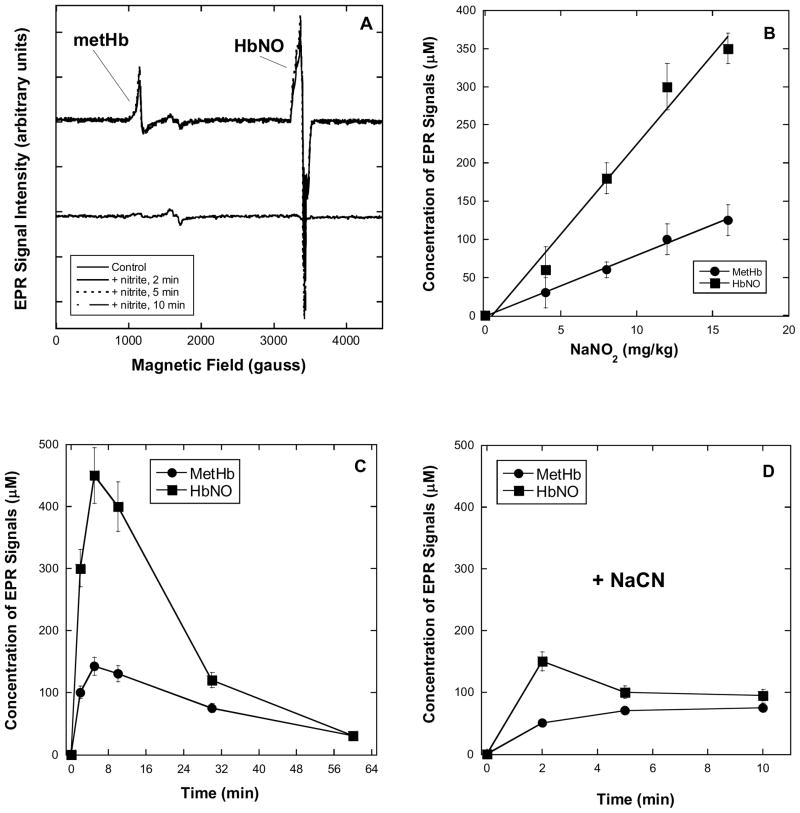
The EPR spectra of control blood samples, from animals given neither cyanide nor nitrite, exhibit only weak signals arising from transferrin at g ~1600 gauss (Figure 3A, lower trace). In the case of samples prepared with 12 mg/kg NaNO2, both metHb (g ~1100 gauss) and HbNO (g ~3400 gauss) EPR signals can be observed (Figure 3A, upper trace) at the earliest time point (2 min). The signals are dose dependent (0–16 mg/kg, Figure 3B) and, in general, the amount of HbNO in the blood (a maximum of 0.5 mM, or ~ 6% of the total hemoglobin) is roughly 2–3 times the amount of metHb (0.15 mM, or ~2% of the total hemoglobin). Followed over time, the signal intensities peaked at 10–15 minutes and measurably persisted up to 1 hour after administration of the nitrite dose (Figure 3C). The presence of both signals is actually evidence for the presence of NO in the blood rather than nitrite (HbO2 + NO → metHb + nitrate, or Hb + NO → HbNO). However, as the signals were not present in the spectra of blood samples taken from control animals, they clearly arose in a nitrite-dependent manner. The co-administration of 5.0 mg/kg NaCN and nitrite to the mice resulted in EPR spectra showing decreased signal due to metHb (Figure 3D) consistent with some binding of cyanide anion to metHb. While the product cyanomethemoglobin (metHbCN) itself yields an EPR signal, this is known to be very broad (4, 18), difficult to detect at low concentration and, consequently, it is unsurprising that it is not readily apparent in the current spectra. More interestingly, we cannot observe any binding of nitrite to metHb (EPR signals due to nitrimethemoglobin, HbNO2, are fairly sharp and would be observed at ~3000 gauss (18)) suggesting that nitrite was excluded either from the blood, or specifically from erythrocytes. In another set of experiments, where sodium nitrite was added to blood only after it had already been withdrawn by cardiac puncture, qualitatively similar EPR results were obtained (not shown). This finding indicates that the blood alone contained all the factors necessary for conversion of nitrite to NO and that nitrite itself was probably not rapidly taken up by erythrocytes.
Figure 3. EPR spectra (x-band, 20 K) of whole mouse blood obtained by cardiac puncture following CO2 euthanasia.
EPR conditions: 9.8 G modulation amplitude, 63.2 μW microwave power. Doses of nitrite were administered intraperitoneally (i.p.) 2–60 minutes prior to sacrifice. See Methods details. A: Lower trace: spectrum obtained from blood of control animals, showing no evidence for the presence of NO-derived species. Upper trace: Overlaid spectra of blood following dose of 12 mg/kg NaNO2 (i.p.) 2, 5 & 10 min prior to sacrifice showing clear evidence for the generation of NO. B: EPR signal intensity of metHb and HbNO correlate with nitrite dose. Doses of 4, 8, 12 & 16 mg/kg NaNO2 were given 10 min prior to sacrifice. C: EPR signal intensities of metHb and HbNO over time (0–60 minutes) following nitrite administration. A dose of 12 mg/kg NaNO2 was administered and the animals sacrificed at the times indicated by the x-axis. D: EPR signal intensities of metHb and HbNO over time (0–10 minutes) following administration of cyanide and nitrite. A dose of 12 mg/kg NaNO2 was administered with a 5 mg/kg dose of NaCN and the animals sacrificed at the times indicated by the x-axis. Both metHb and HbNO signal intensities were attenuated when compared with the signals obtained in the absence of NaCN (panel C). Values represent means ± standard deviation.
Sodium thiosulfate-dependent reactions with hemoglobin in blood and cytochrome c oxidase
Crankshaw et al. have reported (3) that mice sub-lethally intoxicated with cyanide and then, subsequently given the supposed antidotal combination of nitrite-thiosulfate more than 10 minutes later after the cyanide dose, were slower to recover their righting reflex than control animals which received only cyanide. The counterproductive effect seems to have been most pronounced some 20–30 minutes after the cyanide dose. The righting-reflex assessment of these authors and our righting-recovery procedure (see Methods) are testing the same kind of neuromuscular coordination capabilities. Consequently, as we do not observe any similar poor outcome in any of the experiments with sodium nitrite alone, these findings, taken together, suggest there could be an additional complication with the sodium thiosulfate.
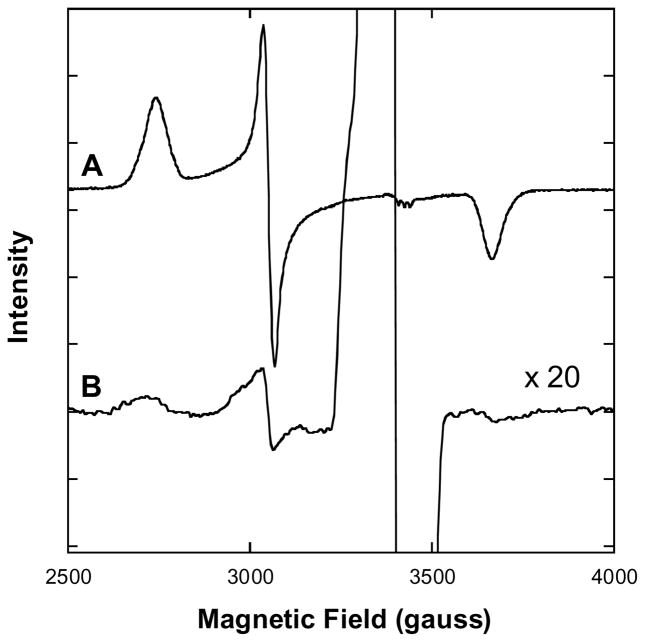
In our hands, thiosulfate essentially does not react with any form of isolated hemoglobin in aqueous buffer (data not shown) but it has been previously established that the addition of thiosulfate to biological fluids (e.g. cell media) results in its hydrolysis to sulfate and sulfide (19). After mixing sodium thiosulfate (Na2S2O3 in saline) and sodium nitrite (NaNO2 in saline) with freshly withdrawn blood (to 0.2 M and 2.0 mM, respectively) EPR signals were detectable that could be identified as arising from sulfidomethemoglobin (written metHbS, but probably containing HS− rather than S2−) (Figure 4). By comparing the EPR spectrum of isolated metHb after reaction with excess Na2S (fully converting the sample to metHbS) it is possible to quantitate the amount of metHbS found in the mouse-blood samples (see Methods). We found that the majority of the metHb produced by nitrite in the blood had become converted to metHbS (up to ~0.1 mM) over a period of 15 minutes, upon reaction with the sulfide produced by hydrolysis of thiosulfate.
Figure 4. EPR spectra (x-band, 20 K) of isolated metHb and whole mouse blood in the presence of Na2S.
EPR conditions: 9.8 G modulation amplitude, 63.2 μW microwave power. A: Purified human A0 metHb (1.3 mM in 50 mM HEPES buffer, pH 7.4) with Na2S added to 10 mM. No aquometHb or hydroxometHb was observed after the addition of Na2S (data not shown). B: NaNO2 (to 2 mM) and Na2S2O3 (to 200 mM) were added to mouse blood obtained by cardiac puncture. Samples were frozen 20 minutes after the nitrite-thiosulfate additions and cryogenically preserved for the recording of spectra at a later time.
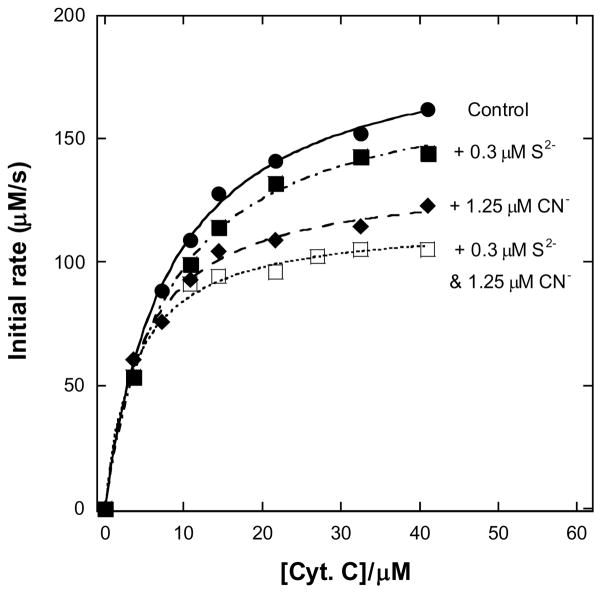
The concentration of hemoglobin tetramer in blood is ~2 mM (i.e. 8 mM in total heme) (20) so the conversion of hemoglobin to metHbS we estimate here represents a loss of < 2% of the oxygen transport capacity of the animals. This not enough to be much of a problem, as humans tolerate methemoglobinemias well up to ~20% metHb (21–23). While we cannot categorically exclude a slow accumulation of metHbS in vivo, leading to metHbS levels higher than 0.1 mM, there is another more plausible mechanism for the toxic effect of thiosulfate. Sulfide inhibits cytochrome c oxidase, displaying an affinity for the enzyme similar to that of cyanide (24–25). The addition of cyanide and sulfide to the isolated enzyme, during turnover under routine assay conditions, results in an essentially additive inhibition (Figure 5). It follows that production of sulfide in the bloodstream of an animal already poisoned with cyanide is highly undesirable.
Figure 5. Dual inhibition of cytochrome c oxidase (complex IV) turnover (spectrophotometric measurements at 550 nm) by cyanide and sulfide.
Michaelis-Menten plots showing inhibition of ferrocytochrome c oxidation. Reaction conditions were 1.2 nM enzyme in 0.1 M aqueous potassium phosphate buffer, pH 7.4, 1.0 mM in EDTA, 0.05% (w/v) in lauryl maltoside, 22 °C. About one third of the data points have been omitted for clarity, but the fits shown are to all the data in each case.
Discussion
Many groups have confirmed that cyanide exerts its toxicity primarily on the central nervous system (26–27) through inhibition of cytochrome c oxidase (28–31). We have used righting recovery, a determinant of neuromuscular coordination, to investigate the efficacy of sodium nitrite on acute, but sub-lethal, doses of sodium cyanide (5.0 mg/kg, 88% LD50 ip). At these doses, sodium nitrite (12 mg/kg ip) has been shown to be antidotal when given up to 20 minutes following (Figure 1C) or 1 hour before (Figure 1D) the cyanide. In fact, while sodium nitrite has typically been used in conjunction with sodium thiosulfate as part of what was the standard therapy, the present results are in keeping with those originally reported by researchers developing what became the Eli Lilly Cyanide Antidote Kit (1). That is, at cyanide doses up to 4 × LD50 in dogs (slow absorption after sub-dermal injections) nitrite alone was protective and only at doses > 4 × LD50 did there appear to be any demonstrated benefit of using thiosulfate in combination with nitrite (1). The combination therapy is clearly most beneficial in those acute-chronic cases in which huge quantities of cyanogenic material has been ingested, but smoke-inhalation injury, where doses of ~2 × LD50 and upwards are irrelevant, is probably of greater concern nowadays (32).
It has been widely accepted that the mechanism by which nitrites are antidotal to cyanide intoxication is due to the metHb-inducing activity of nitrite, leading to cyanide scavenging by subsequent metHbCN formation (33). However, it has also been pointed out that the generation of significant metHb levels lags behind the antidotal action of amyl nitrite (34) and that inorganic nitrite is still effective even if the MetHb formation is suppressed (30, 35). Undoubtedly, cyanide binds to metHb and, if there is an increase in the metHb level in the presence of cyanide, there will unavoidably be a concomitant increase in the amount of metHbCN present in the blood. Indeed, we see evidence of this (cf. Figures 3C and 3D) but it need not necessarily represent the main, or even a significant, ameliorative process (36). Specifically, at the cyanide dose used in this study (5.0 mg/kg, or 4.1 mmol NaCN per 40 g mouse) the circulating concentration of cyanide should be maximally ~1.3 mM (assuming up to 3 mL total blood per mouse). However, following the administration of nitrite (12 mg/kg NaNO2, without cyanide) we observe a maximum of 0.15 mM metHb (Figure 3C). Therefore, nitrite-induced levels of metHb we have detected are an order of magnitude below the projected cyanide concentration; supporting the position that metHb generation is not the primary antidotal action of nitrite.
We have previously shown that NO can overcome the inhibition of the crucial target, cytochrome c oxidase, by cyanide (4–5). Importantly, although they did not attribute the beneficial action to reversal of cytochrome c oxidase inhibition, Baskin et al. (13) showed that the NO-donor molecule diethylamine NONOate is an effective cyanide antidote. Other groups have also shown an antagonistic effect of NO and/or nitrite toward cyanide intoxication not linked to metHb formation (37–40). It follows that the mechanism by which sodium nitrite probably ameliorates cyanide intoxication involves release of NO – certainly there are many systems in vivo able to effect the conversion of nitrite to NO in the bloodstream and vasculature (41–44). The present EPR spectra in which we have observed nitrite-dependent formation of metHb (HbO2 + NO → metHb + nitrate) and HbNO (Hb + NO → HbNO) in mouse blood strongly support this hypothesis.
There are some additional observations to be made with respect to the EPR data. First, while we reproducibly observed 2–3 times more HbNO formation than metHb, we cannot assert that this represents the in vivo ratio. Tissue hypoxia follows death quickly and there was a 2 minute delay between sacrificing the animals and preserving the samples, during which time much redistribution of electrons and NO between hemoglobin species could take place. That there was NO production is, however, unambiguous. Second, comparing the upper data sets in Figures 3C and 3D, it appears that in the presence of cyanide, less HbNO was generated. Since cyanide is a poor ligand for ferrous heme, it should not interfere with formation of HbNO from Hb. This suggests that cyanide actually partially inhibits the conversion of nitrite to NO in the blood/vasculature – a clue to the identity of at least one site involved in the NO production. In addition to ferric heme-containing systems, we note that xanthine oxidoreductase has been shown to produce nitric oxide from nitrite (45–47). This enzyme is found in human plasma with an average activity of 2.1±0.8 ×10−3 U/mL (48) and can be inactivated by cyanide (49).
The intriguing observation by Crankshaw et al. (3) that the combined intravenous use of nitrite-thiosulfate (at the doses recommended as part of the standard therapy) actually made matters worse when administered at 20 minutes or later in sub-lethally cyanide intoxicated mice requires some plausible explanation. Hashwa & Pfenning (19) demonstrated and we can confirm (not shown) the hydrolysis of thiosulfate to sulfate and sulfide catalyzed by bovine serum albumin. These earlier authors showed that 10% of the thiosulfate was hydrolyzed to sulfide in 30 minutes at pH 7.0 and 20°C. In our thiosulfate-treated blood experiment (Figure 4) within 20 minutes, we find 0.1 mM sulfide in a form (metHbS) suggesting it could be transported systemically in the animal. At the level of cyanide intoxication employed (5.0 mg/kg NaCN) both here and elsewhere (3) the initial concentration of cyanide in the animals is ~1 mM. Following administration of the nitrite-thiosulfate combination in the standard therapy, this cyanide concentration will decrease while the sulfide concentration increases – seemingly resulting in the sulfide concentration exceeding the cyanide concentration within a matter of ~15 minutes. Sulfide is a potent inhibitor of cytochrome c oxidase (Figure 5) with a Ki that we have determined to be 85 nM with respect to the present preparation, in the range of values summarized by Cooper and Brown (50) (40–200 nM); that is, comparably inhibitory to cyanide (51) (50 –100 nM). Consequently, it is not surprising that there are conditions under which thiosulfate may exacerbate rather than ameliorate cyanide intoxication. That it can be antidotal at all is, perhaps, more remarkable, but suggests that its ability to donate sulfur to rhodanese during the catalytic conversion of cyanide to the much less toxic thiocyanate must in some circumstances outweigh the detrimental generation of sulfide. Note that the reported efficacy of the nitrite-thiosulfate combination at very high intoxication levels with slow absorption of the cyanide (1) can now be seen to be another argument against the potency of nitrite as a cyanide antidote being due to any metHb-inducing effect. The metHb formed would bind sulfide as well as cyanide, so giving nitrite and thiosulfate together does not make any sense if nitrite-dependent metHb formation is considered the antidotal effect of the nitrite.
Acknowledgments
The authors wish to thank Prof. Mike Hendrich and his students for all their help with the EPR spectroscopy.
Funding Support: Supported by the National Institutes of Health CounterACT Program (U01 NS063732 to JP, LLP & BRP).
Abbreviations
- EDTA
ethylenediaminetetraacetate
- EPR
electron paramagnetic resonance
- Hb
hemoglobin
References
- 1.Chen KK, Rose CL, Clowes GHA. Comparative values of several antidotes in cyanid poisoning. Am J Med Sci. 1934;188:767–781. [Google Scholar]
- 2.Chaturvedi AK, Smith DR, Canfield DV. Blood carbon monoxide and hydrogen cyanide concentrations in the fatalities of fire and non-fire associated civil aviation accidents, 1991–1998. Forensic Sci Int. 2001;121:183–188. doi: 10.1016/s0379-0738(01)00400-5. [DOI] [PubMed] [Google Scholar]
- 3.Crankshaw DL, Goon DJ, Briggs JE, Delong D, Kuskowski M, Patterson SE, Nagasawa HT. A novel paradigm for assessing efficacies of potential antidotes against neurotoxins in mice. Toxicol Lett. 2007;175:111–117. doi: 10.1016/j.toxlet.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pearce LL, Bominaar EL, Hill BC, Peterson J. Reversal of cyanide inhibition of cytochrome c oxidase by the auxiliary substrate nitric oxide: an endogenous antidote to cyanide poisoning? J Biol Chem. 2003;278:52139–52145. doi: 10.1074/jbc.M310359200. [DOI] [PubMed] [Google Scholar]
- 5.Pearce LL, Lopez Manzano E, Martinez-Bosch S, Peterson J. Antagonism of nitric oxide toward the inhibition of cytochrome c oxidase by carbon monoxide and cyanide. Chem Res Toxicol. 2008;21:2073–2081. doi: 10.1021/tx800140y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonini E, Brunori M. Hemoglobin and myoglobin in their reactions with ligands. North-Holland; Amsterdam: 1971. Chapter 3: the derivatives of ferric hemoglobin and myoglobin; pp. 40–54. [Google Scholar]
- 7.Bonaventura C, Godette G, Tesh S, Holm DE, Bonaventura J, Crumbliss AL, Pearce LL, Peterson J. Internal electron transfer between hemes and Cu(II) bound at cysteine beta93 promotes methemoglobin reduction by carbon monoxide. J Biol Chem. 1999;274:5499–5507. doi: 10.1074/jbc.274.9.5499. [DOI] [PubMed] [Google Scholar]
- 8.Drabkin DL. Spectrophotometric studies. XIV. The Crystallographic and optical properties of the hemoglobin of man in comparison with those of other species. J Biol Chem. 1946;164:703–723. [PubMed] [Google Scholar]
- 9.Fago A, Crumbliss AL, Peterson J, Pearce LL, Bonaventura C. The case of the missing NO-hemoglobin: spectral changes suggestive of heme redox reactions reflect changes in NO-heme geometry. Proc Natl Acad Sci U S A. 2003;100:12087–12092. doi: 10.1073/pnas.2032603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson Q, Palmer G, Wharton D. J Biol Chem. 1965;240:915–920. [PubMed] [Google Scholar]
- 11.van Gelder BF. On cytochrome c oxidase: I. The extinction coefficients of cytochrome a and cytochrome a3. Biochim Biophy Acta. 1966;118:36–46. doi: 10.1016/s0926-6593(66)80142-x. [DOI] [PubMed] [Google Scholar]
- 12.Sinjorgo KM, Durak I, Dekker HL, Edel CM, Hakvoort TB, van Gelder BF, Muijsers AO. Bovine cytochrome c oxidases, purified from heart, skeletal muscle, liver and kidney, differ in the small subunits but show the same reaction kinetics with cytochrome c. Biochim Biophy Acta. 1987;893:251–258. doi: 10.1016/0005-2728(87)90046-6. [DOI] [PubMed] [Google Scholar]
- 13.Baskin SI, Nealley EW, Lempka JC. Cyanide toxicity in mice pretreated with diethylamine nitric oxide complex. Human & Experimental Toxicology. 1996;15:13–18. doi: 10.1177/096032719601500103. [DOI] [PubMed] [Google Scholar]
- 14.ATSDR. Toxicological Profile for Cyanide. Agency for Toxic Substances and Disease Registry, Diviosion of Toxicology; Atlanta, GA: 2006. [PubMed] [Google Scholar]
- 15.Travis EL, Down JD, Hall L, Vojnovic B, Holmes SJ. Factors affecting the breathing rate of mice as used for studies of radiation damage to lungs. Br J Radiol. 1981;54:50–53. doi: 10.1259/0007-1285-54-637-50. [DOI] [PubMed] [Google Scholar]
- 16.Mattson DL. Comparison of arterial blood pressure in different strains of mice. Am J Hypertens. 2001;14:405–408. doi: 10.1016/s0895-7061(00)01285-1. [DOI] [PubMed] [Google Scholar]
- 17.Kramer K, van Acker SA, Voss HP, Grimbergen JA, van der Vijgh WJ, Bast A. Use of telemetry to record electrocardiogram and heart rate in freely moving mice. J Pharmacol Toxicol Methods. 1993;30:209–215. doi: 10.1016/1056-8719(93)90019-b. [DOI] [PubMed] [Google Scholar]
- 18.Otsuka M, Marks SA, Winnica DE, Amoscato AA, Pearce LL, Peterson J. Covalent modifications of hemoglobin by nitrite anion: formation kinetics and properties of nitrihemoglobin. Chem Res Toxicol. 2010;23:1786–1795. doi: 10.1021/tx100242w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashwa F, Pfennig N. The reductive enzymatic cleavage of thiosulfate. Methods and appliction. Arch Mikrobiol. 1972;81:36–44. doi: 10.1007/BF00715022. [DOI] [PubMed] [Google Scholar]
- 20.International Committee for Standardization in Haematology. Recommendations for reference method for haemoglobinometry in human blood (ICSH standard EP 6/2: 1977) and specifications for international haemiglobincyanide reference preparation (ICSH standard EP 6/3: 1977) J Clin Pathol. 1978;31:139–143. doi: 10.1136/jcp.31.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: etiology, pharmacology, and clinical management. Ann Emerg Med. 1999;34:646–656. doi: 10.1016/s0196-0644(99)70167-8. [DOI] [PubMed] [Google Scholar]
- 22.Borgese N, Pietrini G, Gaetani S. Concentration of NADH-cytochrome b5 reductase in erythrocytes of normal and methemoglobinemic individuals measured with a quantitative radioimmunoblotting assay. J Clin Invest. 1987;80:1296–1302. doi: 10.1172/JCI113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young JD, Dyar O, Xiong L, Howell S. Methaemoglobin production in normal adults inhaling low concentrations of nitric oxide. Intensive Care Med. 1994;20:581–584. doi: 10.1007/BF01705726. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls P. The effect of sulphide on cytochrome aa3. Isosteric and allosteric shifts of the reduced alpha-peak. Biochim Biophys Acta. 1975;396:24–35. doi: 10.1016/0005-2728(75)90186-3. [DOI] [PubMed] [Google Scholar]
- 25.Nicholls P. Inhibition of cytochrome c oxidase by sulphide. Biochem Soc Trans. 1975;3:316–319. doi: 10.1042/bst0030316. [DOI] [PubMed] [Google Scholar]
- 26.Prabhakaran K, Li L, Borowitz JL, Isom GE. Inducible nitric oxide synthase up-regulation and mitochondrial glutathione depletion mediate cyanide-induced necrosis in mesencephalic cells. J Neurosci Res. 2006;84:1003–1011. doi: 10.1002/jnr.20998. [DOI] [PubMed] [Google Scholar]
- 27.Way JL. Cyanide intoxication and its mechanism of antagonism. Annu Rev Pharmacol Toxicol. 1984;24:451–481. doi: 10.1146/annurev.pa.24.040184.002315. [DOI] [PubMed] [Google Scholar]
- 28.Isom GE, Burrows GE, Way JL. Effect of oxygen on the antagonism of cyanide intoxication--cytochrome oxidase, in vivo. Toxicol Appl Pharmacol. 1982;65:250–256. doi: 10.1016/0041-008x(82)90007-2. [DOI] [PubMed] [Google Scholar]
- 29.Pettersen JC, Cohen SD. The effects of cyanide on brain mitochondrial cytochrome oxidase and respiratory activities. J Appl Toxicol. 1993;13:9–14. doi: 10.1002/jat.2550130104. [DOI] [PubMed] [Google Scholar]
- 30.Way JL, Leung P, Cannon E, Morgan R, Tamulinas C, Leong-Way J, Baxter L, Nagi A, Chui C. The mechanism of cyanide intoxication and its antagonism. Ciba Found Symp. 1988;140:232–243. doi: 10.1002/9780470513712.ch14. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Li L, Prabhakaran K, Zhang L, Leavesley HB, Borowitz JL, Isom GE. Uncoupling protein-2 up-regulation and enhanced cyanide toxicity are mediated by PPARalpha activation and oxidative stress. Toxicol Appl Pharmacol. 2007;223:10–19. doi: 10.1016/j.taap.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alcorta R. Smoke inhalation & acute cyanide poisoning. Hydrogen cyanide poisoning proves increasingly common in smoke-inhalation victims. JEMS. 2004;29(suppl):6–15. quiz suppl 16–17. [PubMed] [Google Scholar]
- 33.Labianca DA. On the nature of cyanide poisoning. J Chem Educ. 1979;56:788–791. [Google Scholar]
- 34.Borak J. Pharmacologic mechanism of antidotes in cyanide and nitrile poisoning. J Occup Environ Med. 1995;37:793–794. doi: 10.1097/00043764-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Way JL, Sylvester D, Morgan RL, Isom GE, Burrows GE, Tamulinas CB. Recent perspectives on the toxicodynamic basis of cyanide antagonism. Fundam Appl Toxicol. 1984;4:S231–239. doi: 10.1016/0272-0590(84)90157-x. [DOI] [PubMed] [Google Scholar]
- 36.Borak J. Pharmacologic mechanism of antidotes in cyanide and nitrile poisoning. J Occup Environ Med. 1995;37:793–794. doi: 10.1097/00043764-199507000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Jensen MS, Nyborg NC, Thomsen ES. Various nitric oxide donors protect chick embryonic neurons from cyanide-induced apoptosis. Toxicol Sci. 2000;58:127–134. doi: 10.1093/toxsci/58.1.127. [DOI] [PubMed] [Google Scholar]
- 38.Leavesley HB, Li L, Prabhakaran K, Borowitz JL, Isom GE. Interaction of cyanide and nitric oxide with cytochrome c oxidase: implications for acute cyanide toxicity. Toxicol Sci. 2008;101:101–111. doi: 10.1093/toxsci/kfm254. [DOI] [PubMed] [Google Scholar]
- 39.Odunuga OO, Adenuga GA. Sodium nitrite alone protects the brain microsomal Ca(2+)-ATPase against potassium cyanide-induced neurotoxicity in rats. Biosci Rep. 1997;17:543–546. doi: 10.1023/a:1027312324077. [DOI] [PubMed] [Google Scholar]
- 40.Leavesley HB, Li L, Mukhopadhyay S, Borowitz JL, Isom GE. Nitrite-mediated antagonism of cyanide inhibition of cytochrome c oxidase in dopamine neurons. Toxicol Sci. 2010;115:569–576. doi: 10.1093/toxsci/kfq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, 3rd, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 42.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR, Jr, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundberg JO, Weitzberg E. NO generation from inorganic nitrate and nitrite: Role in physiology, nutrition and therapeutics. Arch Pharm Res. 2009;32:1119–1126. doi: 10.1007/s12272-009-1803-z. [DOI] [PubMed] [Google Scholar]
- 44.Lundberg JO, Weitzberg E. The biological role of nitrate and nitrite: the times they are a-changin’. Nitric Oxide. 2010;22:61–63. doi: 10.1016/j.niox.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 45.Alef MJ, Vallabhaneni R, Carchman E, Morris SM, Jr, Shiva S, Wang Y, Kelley EE, Tarpey MM, Gladwin MT, Tzeng E, Zuckerbraun BS. Nitrite-generated NO circumvents dysregulated arginine/NOS signaling to protect against intimal hyperplasia in Sprague-Dawley rats. J Clin Invest. 2011;121:1646–1656. doi: 10.1172/JCI44079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu P, Liu F, Yao Z, Wang CY, Chen DD, Tian Y, Zhang JH, Wu YH. Nitrite-derived nitric oxide by xanthine oxidoreductase protects the liver against ischemia-reperfusion injury. Hepatobiliary Pancreat Dis Int. 2005;4:350–355. [PubMed] [Google Scholar]
- 47.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 48.Liu S, Ju H. Nitrite reduction and detection at a carbon paste electrode containing hemoglobin and colloidal gold. Analyst. 2003;128:1420–1424. doi: 10.1039/b310100b. [DOI] [PubMed] [Google Scholar]
- 49.Massey V, Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J Biol Chem. 1970;245:6595–6598. [PubMed] [Google Scholar]
- 50.Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40:533–539. doi: 10.1007/s10863-008-9166-6. [DOI] [PubMed] [Google Scholar]
- 51.Jones MG, Bickar D, Wilson MT, Brunori M, Colosimo A, Sarti P. A reexamination of the reactions of cyanide with cytochrome c oxidase. Biochem J. 1984;220:57–66. doi: 10.1042/bj2200057. [DOI] [PMC free article] [PubMed] [Google Scholar]