Abstract
Background
Overuse, the provision of health services for which harms outweigh the benefits, results in suboptimal patient care and may contribute to the rising costs of cancer care. We performed a systematic review of the evidence on overuse in oncology.
Methods
We searched Medline, EMBASE, the Cochrane Library, Web of Science, SCOPUS databases, and two grey literature sources, for articles published between December 1, 2011 and March 10, 2017. We included publications from December 2011 to evaluate the literature since the inception of the ABIM Foundation’s Choosing Wisely initiative in 2012. We included original research articles quantifying overuse of any medical service in patients with a cancer diagnosis when utilizing an acceptable standard to define care appropriateness, excluding studies of cancer screening. One of 4 investigator reviewed titles and abstracts and 2 of 4 reviewed each full-text article and extracted data. Methodology used PRISMA Guidelines.
Results
We identified 59 articles measuring overuse of 154 services related to imaging, procedures, and therapeutics in cancer management. The majority of studies addressed adult or geriatric patients (98%) and focused on US populations (76%); the most studied services were diagnostic imaging in low-risk prostate and breast cancer. Few studies evaluated active cancer therapeutics or interventions aimed at reducing overuse. Rates of overuse varied widely among services and among studies of the same service.
Conclusion
Despite recent attention to overuse in cancer, evidence identifying areas of overuse remains limited. Broader investigation, including assessment of active cancer treatment, is critical for identifying improvement targets to optimize value in cancer care.
Keywords: cancer care, health services research, health services, quality of care, utilization
Introduction
Despite a stable cancer incidence, the cost of cancer care is high and is rising more rapidly than costs in other medical sectors; in the US, the estimated total cost of cancer care was $125 billion in 2010 and is projected to increase to $173 billion US by 2020.(1) These escalating costs have led to concerns about the ability of the healthcare system to pay(2) and have led to removal of some drugs from coverage in the UK.(3) In the US, rising costs are also relevant to individual patients who are experiencing rising deductibles, increased cost shifting, and growing premiums.(1, 4) As a result, there is a growing emphasis on improving value in cancer management.(5, 6) One approach to improving value in cancer care is the identification and elimination of overuse.
In health care, overuse can be defined as the provision of medical care that has no benefit or for which harms outweigh potential benefits.(7) In 2012, the Institute of Medicine (IOM) estimated that in the US more than $750 billion a year, or nearly 30% of all medical expenses, resulted from unnecessary or inefficient services, contributing to thousands of unexpected deaths.(8) In response, there has been a call to action by national organizations to identify and eliminate overuse. In 2012, attention to overuse accelerated with the launch of the Choosing Wisely campaign from the ABIM Foundation, in which specialty societies identified services that patients and clinicians should question and reconsider.(9) The American Society of Clinical Oncology (ASCO) was an early supporter of Choosing Wisely.(10, 11)
Although there has been increased recognition by ASCO and others of the importance of reducing overuse to improve the value of cancer care, the scope of overuse in oncology has not been well described. A 2012 systemic review of overuse of all health care services in the United States included papers published from 1978–2011 and found few addressed overuse in cancer.(12) However, it is likely that additional studies have been undertaken in more recent years given the greater attention to overuse and value.(13, 14) To describe the current prevalence of overuse in cancer care and the state of the overuse literature in cancer, we performed a systematic review of published articles reporting rates of overuse of diagnostic tests, therapeutic procedures, and medications in the management of patients diagnosed with cancer. We chose to focus on patients with a cancer diagnosis and not on cancer screening since cancer care itself is particularly costly and since overuse of screening has been well discussed in the literature.(15–19)
Methods
Literature Search
This systematic review was conducted according to PRISMA guidelines.(20) We conducted systematic literature searches in five databases for references written in all languages with no specified sex or ages, limited to human-only research, and published from December 1, 2011 to March 10, 2017. We used controlled vocabulary and text words to search (1) MEDLINE (via PubMed), (2) EMBASE, (3) The Cochrane Library, (4) Web of Science, and (5) Scopus. The Web of Science and Scopus databases do not employ controlled vocabularies, so they were searched using only text words. We also conducted comprehensive searches in two grey literature sources: (a) Grey Literature Report provided by the New York Academy of Medicine and (b) Open Grey which is operated by the Institute of Scientific and Technical Information (INIST-CNRS) in Vandoeuvre-les-Nancy, France.
The search strategy included two major components that were linked together with the AND operator: (1) cancer terms including neoplasms, tumors, carcinomas, sarcomas, and malignancies; (2) health services overuse terms including laboratory testing, imaging, secondary screening/testing, overutilization, choosing wisely, overuse, and guideline adherence (see Figure, Supplemental Digital Content, for a complete list of MeSH and keyword terms used). After combining the concepts in all five databases, we added the following publication type filters to the search (where applicable): clinical trial, comparative study, controlled clinical trial, observational study, pragmatic clinical trial, review, systematic review, meta-analysis, technical report, and guidelines. We performed reference tracking by searching the references of all studies included for full-text review.
Figure. PRISMA Diagram: flow of articles in the systematic review.
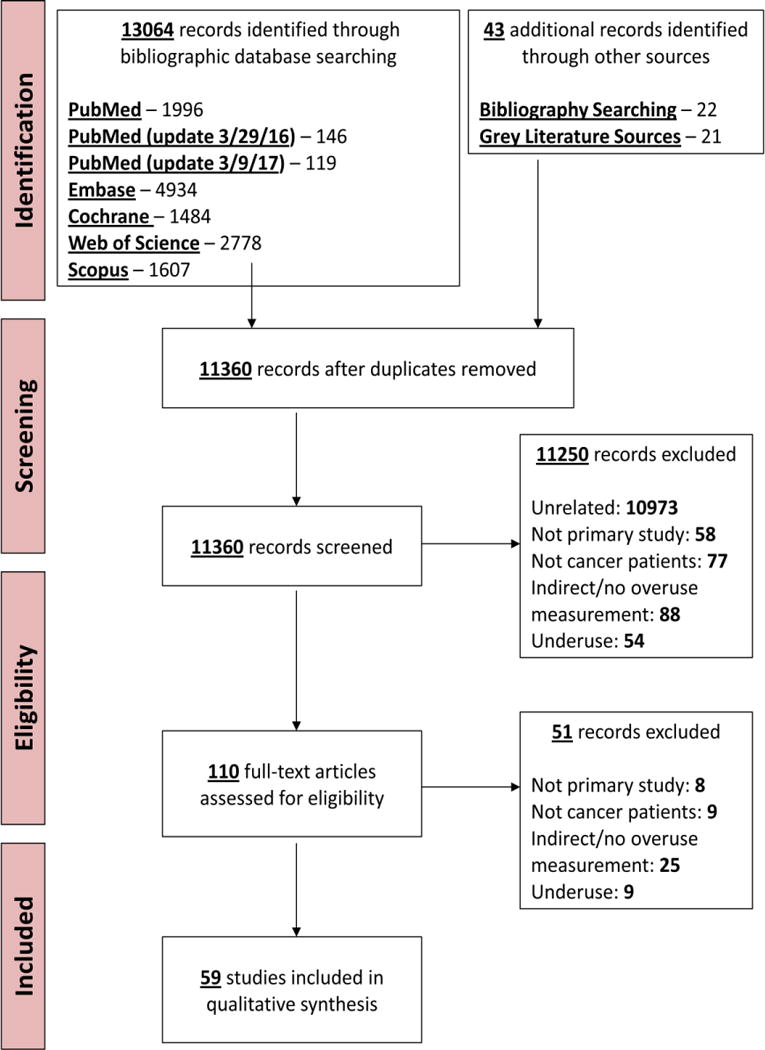
Flow of articles in the review
Study Selection
Each title and abstract was reviewed by one of four investigators (D.K., M.K., S.K., A.Y.) to determine inclusion for full-text review. Each full-text article, including those identified through reference tracking, was reviewed by a pair of investigators (D.K. and B.R., D.K. and M.K., S.B. and D.K., or S.B. and M.K.) to determine inclusion for qualitative synthesis. Disagreements were resolved by group consensus. We determined inter-rater reliability (Cohen κ) for each of the four pairs of full-text reviewers. The flow of article selection is presented in Figure.
Articles were eligible for inclusion if they were original research quantifying overuse of any medical service in patients with a cancer diagnosis and utilizing an acceptable standard that included: 1) a guideline from a governmental organization, 2) a guideline from a professional society, 3) a multidisciplinary panel consensus process (e.g. Rand Appropriateness Method) or 4) a Choosing Wisely recommendation. We excluded studies in patients without cancer including those evaluating cancer screening in the general population, and studies in which overuse rates were not presented or calculable.
Data Extraction
We developed a data extraction tool to collect information from each study in the review. Data extraction was performed by one reviewer (S.B., D.K., M.K., B.R.) and checked by a second reviewer (S.B. or D.K.) for accuracy. The following data were extracted from each study: general information about the publication (first author’s name, year of publication), study specifics (e.g. study design, data source, and sample size), cancers addressed, country of study, type of service (e.g. diagnostic vs. therapeutic), and where in the cancer continuum the service was provided. We categorized the cancer care continuum as diagnostic evaluation, active treatment, surveillance after active treatment, or end of life. We recorded specific service(s) evaluated, whether costs were reported with overuse and whether an intervention to reduce overuse was evaluated. We also noted whether overuse was presented as the percent of the population receiving a non-recommended service or as the percent of services provided inappropriately. We documented overuse of each individual service separately. When rates of overuse were not directly presented we calculated rates when possible and contacted study authors for rates of overuse or raw data when we were unable to calculate with information reported.
We assessed the quality of each study by assessing for potential bias in design. In all studies, we evaluated for bias in patient selection (e.g. one physician’s panel) and in the determination of the appropriateness of the service (e.g. determinations of appropriateness were subjective and non-reproducible). We categorized studies that used only claims-based data as having potential bias because the lack of detailed clinical information could lead investigators to incorrectly classify the appropriateness of particular services.
Data Analysis
Given the diversity of the literature, we did not believe that quantitative analysis was scientifically justified and conducted only qualitative data analysis. Inter-rater reliability for the decision to include the article in the review (Cohen’s kappa, 0.85, 0.66, 0.84, 0.82 for the four investigator pairs) was excellent.
We generated descriptive statistics to analyze studies included in the systematic review. We synthesized information for all services that were evaluated for overuse. We recorded overuse of either an aggregate of multiple services in a specific situation (e.g. any inappropriate surveillance imaging in breast cancer patients) and/or of an individual service (e.g. PET scan for surveillance in breast cancer patients) based on how the data was presented in the original article. We defined an individual service as a distinct test or treatment in a defined population based on the disease, specific test or treatment (e.g. bone scan versus CT), risk group or cancer stage (e.g. low risk prostate versus intermediate risk prostate), and year (e.g. bone scan in 1998 versus bone scan in 2006). If rates for both individual and aggregate services were available, we recorded both. For interventional studies, we defined overuse as the rate in the pre-intervention phase or control arm. To calculate descriptive statistics of services, we removed duplicates by discounting aggregate services if rates for individual services were also available (e.g. we discounted “any imaging [PET or CT]” if individual rates for PET and CT were available).
Results
Study Characteristics
Our primary search identified 13,064 articles, of which 59 met our inclusion criteria (Figure).(21–79) Characteristics of included studies are summarized in Table 1 and details of all studies are listed in Table 2. All studies were published in English, most were retrospective (92%), were completed in the US (76%), and addressed overuse in adult or geriatric cancer patients (98%). The National Cancer Institute’s linked Surveillance, Epidemiology, and End Results (SEER)-Medicare was the most commonly analyzed dataset, used in 37% of all studies; 14 studies (24%) were framed around a Choosing Wisely item. In terms of quality, 41 (69%) of studies had some form of bias, mostly due to use of claims-based data. Three studies (5%) evaluated an intervention to address overuse and 9 (15%) addressed financial costs associated with overuse. (Table 2)
Table 1.
Demographic and methodological characteristics of included studies (n=59)
| No. (%) | |
|---|---|
| Publication Year | |
| 2016 | 4 (7) |
| 2015 | 22 (37) |
| 2014 | 9 (15) |
| 2013 | 6 (10) |
| 2012 | 11 (19) |
| 2011 | 7 (12) |
| Country | |
| U.S | 45 (76) |
| Non-U.S. | 14 (24) |
| Study Type | |
| Retrospective | 54 (92) |
| Prospective | 5 (8) |
| Intervention evaluated | 3 (5) |
| SEER-Medicare | 22 (37) |
| Cooperative Group | 4 (7) |
| Choosing Wisely | 14 (24) |
| Bias present | 41 (69) |
| Patient populationa | |
| Adult | 34 (58) |
| Pediatric | 0 (0) |
| Adult & Pediatric | 1 (2) |
| Geriatric (≥65) | 24 (41) |
| Cost estimates presented |
9 (15) |
Abbreviations: U.S=United States; SEER=Surveillance, Epidemiology, and End Results Program
Percentages do not sum to 100 due to rounding
Table 2.
Study details from articles included in the review
| Author Year Journala |
Country, Patient population | Study design | Bias in study design (Reason for bias) |
Cancer type | Sample size | Phase of care | Services evaluated | Rate/range of overuse (%)b |
|---|---|---|---|---|---|---|---|---|
| Akalin 2015 |
Turkey, Adult | P | Yes (insufficient clinical detail) | Multiple | 36 | AT | Hospitalization in low-risk patients with fever and neutropenia | 53.0 |
| Backhus 2014 |
USA, Adult | R | Yes (insufficient clinical detail) | Lung | 3,808 | D | Bone scan if PET was already performed | 24.7 |
| Barni 2015 |
Italy, Adult | R | No | Lung | 153 | D | Diagnostic exploratory thoracotomy | 2.0 |
| Buhrkuhl 2012 |
New Zealand, Adult & pediatric | R | Yes (subjective determination of overuse) | Not specified | 63 | AT | Platelet transfusion | 28.0c |
| Choi 2011 |
USA, Geriatric | R | Yes (claims-based)d | Prostate | 6,444 | D | MRI, CT, Ultrasound, or bone scan after low-risk prostate cancer diagnosis | 36.2 |
| Cooper 2013 |
USA, Adult | R | No | Pancreas | 101 | D | PET, MRI, or CT after an index CT in patients with resectable pancreatic cancer | 94.0 |
| Crivello 2013 |
USA, Geriatric | R | Yes (claims-based)d | Breast | 67,874 | D | PET, MRI, CT, and bone scan in Stage I or II breast cancer | PET: 1.0 MRI: 0.7 CT 9.5 Bone scan: 13.9 |
| Daskivich 2011 |
USA, Adult | R | No | Prostate | Patients with high comorbidity score: 57 Patients aged >75 years: 44 |
AT | Radiation, brachytherapy, or radical prostatectomy in low-risk prostate cancer | High comorbidity score: 54.0 Aged> 75 years: 16.0 |
| Ellis 2015 |
USA, Geriatric | R | Yes (claims-based)d | Prostate | 12,943 | AT | Androgen deprivation therapy in low-risk prostate cancer | 18.5 |
| Erb 2016 |
USA, Geriatric | R | Yes (claims-based)d |
Lung | 9,321 | S | PET for surveillance | 2.7 – 24.8 (between years of 1998 and 2008) |
| Falchook 2015 |
USA, Geriatric | R | Yes (claims-based)d | Prostate | 41,817 | D | MRI, CT, or bone scan in early stage prostate cancer | Pelvic CT/MRI: 38.0 Bone scan: 43.0 |
| Falchook 2014 |
USA, Geriatric | R | Yes (claims-based, insufficient clinical detail)d | Prostate | 27,339 | D | Bone scan in low-risk prostate cancer | 41.1 |
| Feng 2015 |
Taiwan, Adult | R | Yes (subjective determination of overuse) | CRC, Gastric, H&N, Esophageal | 107 | AT, EOL | Parenteral nutrition | 14.1c |
| Geurts 2012 |
Netherlands, Adult | R | Yes (insufficient clinical detail) | Breast | 144 | S | Routine visits during surveillance | 31.0 – 76.0 (varies by year after treatment) |
| Goffredo 2015 |
USA, Adult | R | No | Thyroid | Papillary: 60,586 Medullary: 6,375 Anaplastic: 3,095 |
AT | Radioactive iodine after surgery for thyroid cancer | Papillary: 23.3 Medullary: 3.4 Anaplastic: 1.6 |
| Guy 2015 |
USA, Adult | R | No | Breast | 844 | AT | Adjuvant hormonal therapy, radiation, and chemotherapy in early stage breast cancer | 11.5 - 18.2 |
| Haas 2011 |
USA, Geriatric | R | No | Breast | 638 | AT | Trastuzumab in early stage breast cancer | 3.9 |
| Haddad 2013 |
USA, Adult | R | Yes (insufficient clinical detail) |
Melanoma | 546 | D | Preoperative PET, MRI, CT, x-ray, or bone scan | Chest x-ray: 70.0 PET, MRI, or CT: 14.0 |
| Hahn 2016 |
USA, Adult | R | No | Breast | 8,618 | S | CT, PET, bone scan and tumor markers for surveillance in early stage breast cancer | CT: 20.0c PET/Bone scan: 4.3c Tumor markers: 28c |
| Hahn 2015 |
USA, Adult | R | No | Breast | 10,010 | D | Pre-treatment PET, CT, or bone scan in early stage breast cancer | 15.0 |
| Hahn 2013 |
USA, Adult | R | No | Breast | 258 | S | Imaging (abdominal CT, breast MRI, chest CT, chest x-ray, or PET) and lab tests (CA15–3, CA125, CA27–29, or CEA) | Imaging: 55.0 Blood test: 80.0 |
| Han 2012 |
Canada, Adult | R | No | Breast | 231 | D | Ultrasound, x-ray, or bone scan in early stage breast cancer | 55.0 |
| Lavery 2011 |
USA, Adult | R | No | Prostate | 677 | AT | MRI, CT, or bone scan in low-risk prostate cancer | 48.0 |
| Linkugel 2015 |
USA, Adult | R | No | Breast | 3,291 | D | Pre-treatment PET, CT, or bone scan in early stage breast cancer | 27.0 |
| Lipitz-Snyderman 2016 |
USA, Geriatric | R | Yes (claims-based)d |
Breast | Imaging-D: 89,006 Imaging-S: 44.216 Radiation: 25,271 |
D, AT, S | Imaging-D: CT, PET, or bone scan for staging in early stage breast cancer Imaging-S: CT, PET, or bone scan for staging for surveillance in low-risk breast cancer Radiation: IMRT of breast in breast conserving therapy |
Imaging-D: 14.0 Imaging-S: 26.0 Radiation: 18.0 |
| Prostate | Imaging: 32,093 Radiation: 3,464 |
D, AT | Imaging: CT, PET, or bone scan for staging in early-stage prostate cancer Radiation: Extended fractionation for palliation of bone metastases |
Imaging: 41.0 Radiation: 35.0 |
||||
| Livingstone 2015 | Germany, Adult | P | Yes (patient selection) | Melanoma | 524 | S | S100 blood test or lymph node ultrasound in early stage melanoma cancer | 22.4 |
| Lochard-Lefrancois 2012 |
France, Adult | R | No | Multiple | 2,062 | AT | Non-recommended use of expensive drugs | 21.4c |
| Makarov 2016 |
US, Adult | R | No | Prostate | 30,029 | D | PET, CT, MRI, or bone scan at diagnosis in low-risk prostate cancer | 40.8 |
| Makarov 2015 |
USA, Geriatric | R | Yes (claims-based)d | Breast | 30,398 | D | PET, CT, or bone scan in low-risk breast cancer | 41.8 |
| Prostate | 9,219 | D | PET, CT, or bone scan in low-risk prostate cancer | 44.4 | ||||
| Makarov 2013 |
Sweden, Adult | R | Yes (insufficient clinical detail) |
Prostate | 99,879 | D | MRI, CT, or bone scan in low-risk prostate cancer | 13.0 |
| Makarov 2012 J Urol |
USA, Geriatric | R | Yes (claims-based)d | Prostate | 18,491 | D | MRI, CT, or bone scan in low-risk prostate cancer | 45.0 |
| Makarov 2012 Health Aff |
USA, Geriatric | R | Yes (claims-based)d | Prostate | 18,491 | D | MRI, CT, or bone scan in low-risk prostate cancer | 24.0 – 65.0 (varies across states) |
| Massarweh 2011 |
USA, Geriatric | R | Yes (claims-based)d | HCC | 3,696 | D | Diagnostic biopsy, MRI, CT, or ultrasound | Biopsy: 32.4 Imaging: 47.8 |
| Miller 2011 |
USA, Adult | P | Yes (insufficient clinical detail) |
Prostate | 375 | D | CT and bone scan in early stage prostate cancer | Bone scan: 10.0 CT: 14.0 |
| Palvolgyi 2011 |
USA, Adult | R | No | Prostate | 519 | D | Bone scan in low-risk prostate cancer | 25.0 |
| Panageas 2012 |
USA, Geriatric | R | Yes (claims-based)d | Breast | 25,555 | S | PET, MRI, CT, or bone scan in early stage breast cancer | 40.0 |
| Parmar 2013 |
USA, Geriatric | R | Yes (insufficient clinical detail) |
Breast | 8,598 | S | PET, MRI, CT, and bone scan in early stage breast cancer | Chest CT/MRI: 16.5 Head CT/MRI: 21.2 PET or PET/CT: 6.5 Bone scan: 16.4 |
| Paulson 2015 |
USA, Geriatric | R | Yes (claims-based)d | CRC | Colon: 23,990 Rectal: 5,665 |
S | CT in stage I CRC cancer | 26.0 |
| Peeraphatdit 2015 |
USA, Adult | R | No | HCC | 224 | D | Unnecessary biopsy | 34.0 |
| Porten 2014 |
USA, Adult | R | Yes (insufficient clinical detail) |
Prostate | 9,333 | D | MRI, CT, and bone scan in early stage prostate cancer | MRI: approximately 0e Bone scan: 12.0–15.0e CT: 11.0–28.0e |
| Prasad 2012 |
USA, Geriatric | R | Yes (claims-based)d | Prostate | 22,606 | D | CT or bone scan in low and intermediate-risk prostate cancer | 42.0 |
| Ramsey 2015 J Clin Onc |
USA, Geriatric | R | Yes (claims-based)d | Breast | 39,650 | S | Tumor markers (CEA, CA 15.3, CA27.29) in non-metastatic breast cancer | 42.0 |
| Ramsey 2015 J Onc Prac |
USA, Adult | R | Yes (insufficient clinical detail | Breast, Prostate, Lung | Varies by servicef | D, AT, S | Multiple servicesf | Varies by servicef |
| Ross 2015 |
USA, Adult | R | Yes (patient selection) | Prostate | 410 | D | CT and bone scan in low-risk prostate cancer | CT: 5.2 Bone scan: 3.7 |
| Sacks 2015 |
USA, Adult | R | Yes (insufficient clinical detail) |
Thyroid | Local study: 444 NCDB: 18,000 – 20,000 |
AT | Radioactive iodine in low-risk thyroid cancer | Local study: 27.7 NCDB: 50.3 |
| Salloum 2012 |
USA, Adult | R | Yes (insufficient clinical detail) |
Breast | 6,205 | S | Unnecessary metastatic evaluation after treatment for breast cancer | 65.0 |
| CRC | 2,297 | S | Unnecessary metastatic evaluation after treatment for CRC cancer | 73.0 | ||||
| Sammon 2015 | USA, Geriatric | R | Yes (claims-based)d | Prostate | 46,376 | AT | Androgen deprivation therapy in localized prostate cancer | 30.0 |
| Sawazaki 2014 | Japan, Adult | R | No | Prostate | 144 | D | Bone scan and CT in low-risk prostate cancer | Bone scan: 100 CT: 72.0 |
| Schroeck 2014 J Urol |
USA, Geriatric | R | Yes (claims-based)d | Prostate | 9,014 | D | Bone scan in low-risk prostate cancer | 34.1 |
| Schroeck 2014 Urology |
USA, Geriatric | R | Yes (claims-based)d | Prostate | 31,321 | D | Bone scan in low-risk prostate cancer | 30.0 |
| Segal 2014 |
USA, Geriatric | R | Yes (claims-based) | Breast | 1,451,142 | S | Tumor markers in women with history of breast cancer | 73.2 |
| Prostate | D | PET, CT, or bone scan in low-risk prostate cancer | 20.7 | |||||
| Shahinian 2015 | USA, Geriatric | R | Yes (claims-based)d | Prostate | 27,169 | AT | Androgen deprivation therapy | 31.1 – 46.6 |
| Shih 2014 |
USA, Geriatric | R | Yes (claims-based)d | Breast | 2,984 | AT | Trastuzumab without documentation of HER-2 status | 4.7c |
| Simonato 2012 |
Italy, Adult | P | No | Prostate | 1,063 | D | Preoperative CT, MRI, and bone scan in early stage prostate cancer | CT: 48.4 MRI 2.4 Bone scan: 71.2 |
| Simos 2015 CMAJ |
Canada, Adult | R | Yes (insufficient clinical detail) |
Breast | 26,547 | D | PET, MRI, CT, ultrasound, or x-ray in early stage breast cancer | 79.6 |
| Simos 2015 J Onc Prac |
Canada, Adult | R | Yes (insufficient clinical detail) |
Breast | 200 | D | CT, ultrasound, x-ray, or bone scan for distant metastases in early stage breast cancer | 82.6 |
| Swisher-McClure 2012 |
USA, Geriatric | R | Yes (claims-based)d | Prostate | 2,184 | AT | Androgen deprivation therapy in low-risk prostate cancer when undergoing external beam radiation therapy | 32.2 |
| Vazin 2015 |
Iran, Adult | P | No | Various hematologic cancers | 116 | AT | Antifungal use in bone marrow transplant patients with neutropenia | 19.0c |
| Weeks 2014 |
USA, Adult | R | Yes (insufficient clinical detail) | Breast, Lung, NHL | Varies by serviceg | D, AT, S | Multiple servicesg | Varies by serviceg |
Abbreviations: AT=Active treatment; CT=Computerized tomography; CEA=carcinoembryonic antigen; CRC=colorectal; D=Diagnostic; EOL=end of life; H&N=head and neck; HCC=Hepatocellular carcinoma; IMRT=intensity-modulated radiation therapy; MRI=Magnetic resonance imaging; NCDB=National Cancer Database; NHL= Non-Hodgkin’s Lymphoma; P=Prospective; PET=Positron emission tomography; R=Retrospective; S=Surveillance; USA=United States
Journal is reported for studies with the same first author and year
Reported as percentage of the population receiving the unnecessary service, unless otherwise noted
Reported as percentage of services being used inappropriately
used SEER-Medicare database
Data extracted from published graph; numbers not presented
Ramsey (JOP, 2015): PET/CT/bone scan in prostate staging: 10% (n=518); PET/CT/bone scan in breast staging: 22% (n=1,798); Tumor markers OR imaging in breast surveillance: 47% (n=629); white blood cell stimulating factors in low-risk patients: 30% (n=672)
Weeks (Ann Intern Med, 2014): Liver function test in breast surveillance: 62% (n=564); MRI in breast surveillance: 12% (n=928); chest x-ray in breast surveillance: 25% (n=928); chest CT in breast surveillance: 8% (n=928); PET in breast surveillance: 1% (n=928); hormonal therapy in breast: 74% (n=375); chemotherapy, post-menopausal breast patients: 36% (n=346); chemotherapy, pre-menopausal breast: 55% (n=266); diagnostic PET in stage III breast: 17% (n=598); diagnostic PET in stage I-II breast: 4% (n=6,827); diagnostic brain imaging in breast: 2% (n=6,827); radiation in breast: 4% (n=965); diagnostic brain imaging in lung: 37% (n=1,437); systemic therapy in lung: 40% (n=147); growth factors in Non-Hodgkin’s lymphoma: 23% (n=232)
Clinical services studied
Because many included studies reported overuse rates for multiple services, the 59 included studies assessed the overuse of 154 distinct services. The most common cancers addressed were breast (49% of services) and prostate (32% of services). (Table 3) In terms of phase of cancer care, studies were predominantly focused on diagnostic evaluation (56%) followed by post-treatment surveillance (23%), active treatment (19%) and end of life (1%). The most commonly evaluated service modality was imaging (71%) with a fair representation of numerous imaging modalities.
Table 3.
Classification of evaluated overused services by disease and service type (n=154)
| No. (%) | |
|---|---|
| Disease | |
| Breast | 76 (49) |
| Prostate | 50 (32) |
| Lung | 5 (3) |
| Non-CRC GI | 4 (3) |
| Colorectal | 2 (1) |
| Othera | 17 (11) |
| Phase | |
| Diagnostic | 87 (56) |
| Surveillance | 36 (23) |
| Active treatment | 30 (19) |
| Treatment and end of life | 1 (1) |
| Service | |
| Imaging | 109 (71)b |
| CT | 24 (16) |
| Bone Scan | 27 (18) |
| PET | 13 (8) |
| MRI | 9 (6) |
| X-ray | 7 (5) |
| Ultrasound | 6 (4) |
| Multiple imaging modalitiesc | 23 (15) |
| Radiation | 11 (7) |
| Lab | 10 (6) |
| Hormonal therapy | 7 (5) |
| Chemotherapy | 5 (3) |
| Targeted therapy | 2 (1) |
| Otherd | 10 (6) |
Abbreviations: CRC=colorectal; GI=gastrointestinal; CT=computed tomography; PET=positron emission tomography; MRI = magnetic resonance imaging
Includes services that were associated with another disease, multiple diseases, or an unspecified disease.
Percentages of imaging sub-services do not sum to imaging total due to rounding
Refers to services that evaluated more than one imaging modality
Includes: hospitalization, white-cell stimulating factors, antifungal use, thoracotomy, parenteral nutrition, routine visits during surveillance, prophylactic transfusion, biopsy
Multiple addressed services related to the overuse of imaging in early stage breast and prostate cancer. Overuse of imaging in the diagnostic evaluation of early prostate cancer was addressed 43 times with 20 (47%) of these evaluations relying on SEER-Medicare data. Similarly, 34 of the evaluated services related to diagnostic imaging for staging in early stage breast cancer, with 8 (24%) relying on SEER-Medicare data, most commonly assessing overuse of PET (n=7), CT (n=7), bone scan (n=7), or any advanced imaging (n=3). Overuse of radiographic surveillance following treatment for early stage breast cancer was also commonly addressed (n=22 evaluations).
Rates of overuse
The majority of studies (n=53, 90%) reported overuse as a percentage of the population receiving a non-recommended service and many (n=27, 46%) used administrative data to determine the prevalence of overuse. Three studies compared rates of overuse measured from administrative data to measurements for the same service using clinical data.(40, 41, 65) Studies of high-tech imaging at the time of diagnosis in early stage breast cancer found that administrative data over-reported clinically relevant imaging as overuse (prevalence 15% vs. 8% from clinical data).(40) In a second study, rates of overuse of post-treatment imaging for surveillance in early stage breast cancer were higher using administrative data from 8,618 patients compared to chart review from a subset of 110 patients from the larger dataset. The rates differed widely for CT (20% vs. 0.8%) and PET or Bone scan (4.3% vs. 0.8%). Interestingly, rates of overuse of tumor markers were similar from both data sources (28% vs. 28%).(41) The third study reported higher measured rates of overuse of radioactive iodine for low-risk thyroid from administrative (range 47–53%) versus clinical (range 20–32%) data (note that Table 2 reflects rates determined through administrative review).(65)
Rates of overuse varied widely between 0 and 100% across services. (Table 2) The most frequently studied services were bone scan (n=17 evaluations) and CT (n=11 evaluations) for staging of low and/or intermediate risk prostate cancer and tumor markers for surveillance in early stage breast cancer (n=9 evaluations); rates of overuse were 0.09–100%, 5–72%, and 5–77%, respectively across studies. Overuse of cancer-directed pharmacologic agents, including chemotherapy, targeted and hormonal therapies was measured in lung, breast and prostate cancer. Weeks and colleagues found that rates of overuse of chemotherapy were approximately 40% in patients with metastatic lung cancer and a poor performance status, 36% in post-menopausal women with limited metastatic breast cancer, and 55% in pre-menopausal women with limited metastatic breast cancer.(79) In the adjuvant setting, a study in rural Georgia reported 11.5% of women received overtreatment with hormonal therapy.(36) Targeted therapy was addressed in two studies evaluating the appropriate use of trastuzumab, the monoclonal antibody directed at the human epidermal receptor 2 (HER2). Overuse of trastuzumab was reported in 3.9% and 4.7% of patients due to a lack of documentation of HER2 testing.(37, 73) Three studies evaluated the overuse of anti-androgen therapy in low risk prostate cancer where it is not routinely recommended,(67, 72, 77) demonstrating a decline in rates of overuse over time(72) and high levels of geographic variation across the US.(77) Outside the US, a French study reported that approximately 21% of all chemotherapy administered for any cancer at two academic centers was administered against national guideline recommendations.(47)
Interventional studies
We identified three studies evaluating interventions; all aimed to reduce overuse of imaging in patients with newly diagnosed low- and intermediate-risk prostate cancer. In one study, Miller and colleagues evaluated guideline dissemination followed by utilization review and feedback through the Urological Surgery Quality Collaborative. They reported decreased rates of bone scans and CT scans from 31% to 21% and 28% to 13% (p<.01), respectively.(54) In a Swedish study, Makarov and colleagues reported decline in inappropriate diagnostic imaging over a 10-year period from 45% to 3% (p< .001) in patients with low-risk prostate cancer after national guideline dissemination. This appropriate decline was accompanied by a simultaneous unwanted decline in recommended imaging in high-risk patients from 63% to 47% (p<.001).(51) In the more recent MUSIC study, Ross and colleagues reported the results of a state-wide collaboration in Michigan to reduce diagnostic imaging in patients with low-risk prostate cancer. Rates of overuse of bone scans (3.7%) and CT scans (5.2%) were low at the start of the study and declined to 1.3% (p=.03) and 3.2% (p=.17), respectively.(64)
Discussion
Our review of overuse in cancer care delivery identified 59 articles published over the last 6 years evaluating 154 clinical services. The majority of studies focused on overuse of imaging in early stage breast cancer and low to intermediate risk prostate cancer, and despite concerns about the high cost of active cancer care only 29% of studies addressed services delivered during active treatment.(2) Rates of overuse varied widely among studies and among services addressed. Despite calls to reduce overuse, very few studies evaluated interventions and costs associated with overuse were rarely reported.
Overuse of imaging
There were multiple studies addressing imaging in breast and prostate cancers. However, the prevalence of overuse of these services remains difficult to define with rates of overuse of specific tests varying widely (though overuse of PET was consistently uncommon). Further, even in this well-studied clinical area, estimates of cost associated with overuse were rare. Despite this lack of clarity on the extent of the problem of overuse of diagnostic imaging in early prostate cancer, all three interventional studies in our review addressed methods to reduce it. Those interventions were generally successful, but the clinical and financial implications of that success are not clear and in one study, reductions in overuse were accompanied by unwanted reduction in recommended services.
Overuse of systemic therapy
Data is still lacking on some of the most concerning, and costly, areas of overuse in cancer. While new high-cost, cancer-directed therapies represent a significant driver of rising oncology care costs,(14, 80) few studies evaluated rates of overuse of cancer treatments, which can lead to financial harm even when used appropriately. We identified two studies evaluating overuse of newer, high-cost drugs, both of which focused on trastuzumab for patients with HER2-positive breast cancer; both reported relatively low rates of use in the absence of appropriate HER-2 testing.(37, 73) The remaining therapeutic studies evaluated chemotherapies more generically, but did not specifically address high-cost therapeutics.
Methodology of overuse research
Our review highlights important issues related to the research of overuse and informs possible strategies aimed at reducing inappropriate health care utilization in cancer patients. First, overuse can only be measured when a normative practice has been established. By definition, identifying overuse implies that there are established criteria for appropriate use of a service, available as a guideline or other standard. In cancer and many other diseases, there may be lack of consensus on optimal management in many clinical situations, so appropriate use cannot be determined. It may be that we identified numerous studies addressing imaging in early stage prostate and breast cancer because these were the services highlighted by the ASCO Choosing Wisely campaign in 2012. Further, studies of services for which appropriateness is more nuanced, such as chemotherapy use in patients with metastatic solid tumors and poor performance status, are challenging and therefore less likely to be performed, even if those services may be more important in terms of patient outcomes and cost.
In addition, even when appropriate care can be defined, its measurement can be difficult without detailed clinical information that often requires chart review. So while overuse is measurable in these situations, it is infrequently evaluated because doing so is time consuming and cumbersome. As a result, much of the cancer overuse literature focuses on issues where there are both clear recommendations and the opportunity to measure use through administrative datasets such as SEER-Medicare, mainly evaluating diagnostic imaging in early stage cancers and for post-treatment surveillance. Indeed, many (49%) studies we identified presented data from administrative datasets and over half (64%) of the services studied represented diagnostic and/or surveillance imaging. Over-representation of imaging and over-reliance on claims data for overuse research may bias both the topics of study and estimates of rates of overuse. Despite widespread concern about overuse at the end of life,(81, 82) we found only one study addressing overuse in this setting, likely because of the challenges of assessing appropriateness of this care. In addition, in the three studies we identified that used both clinical and administrative data to assess overuse, overuse rates derived from clinical data were much lower for most services than those identified through administrative data, suggesting that much of the literature may be overestimating the prevalence of overuse of imaging.(40, 41, 65) However, clinically documented indications in support of imaging might represent clinician efforts to secure imaging reimbursement in situations in which the clinician favors routine imaging; thus chart review may underestimate overuse. This phenomenon may be specific to evaluations of imaging, either because it requires insurance authorization or because it is done for a variety of clinical indications.(41) True rates of overuse of non-recommended imaging likely lie between the high rates derived from administrative data and the low rates derived from chart review.
Going forward, it will be critical both to focus inquiry on the areas of greatest clinical and/or financial importance and to generate reliable estimates of overuse informed by detailed clinical data. Priority areas for research will need to be defined, with participation from stakeholders including government, professional societies and patients, focusing on services with the most potential to harm patients or the health system. Choosing Wisely has become somewhat of a focal point since 2012, with 14 (24%) of included studies mentioning it. However, the emphasis in our study sample on relatively few clinical services suggests that we need to go further. Researchers must find creative ways to accurately measure overuse across populations while minimizing bias. Cancer cooperative groups that conduct clinical trials may provide opportunities to use relevant prospectively collected clinical data to measure overuse rates while enabling evaluations of interventions to reduce overuse.
Limitations
Our study has a number of limitations. First, standard MeSH terminology for overuse in MEDLINE was only introduced in 2016, so identifying articles reporting rates of overuse is challenging and we may have missed some. We addressed this by performing extensive reference tracking and by searching multiple databases, so it is unlikely we missed major publications. We excluded articles without a generally accepted standard for defining overuse. While this approach may have excluded some less rigorous but thematically relevant articles, our study provides an estimate of rates of true overuse to inform our understanding of the literature on overuse in cancer care delivery.
Conclusions
Despite recognition of the need to improve value in cancer care and the importance of avoiding overuse, our systematic review suggests gaps in our understanding of overuse in patients with cancer. While we found many studies evaluating diagnostic or surveillance imaging in breast and prostate cancer, there is a dearth of data on overuse in other clinical scenarios, particularly overuse of cancer therapeutics and at the end of life, and an emphasis on using administrative data. Given the enormity of the cost and potential harm associated with overuse in cancer care, there is a need to identify priority areas for investigation to expand the evidence base and inform future efforts to reduce overuse.
Supplementary Material
Acknowledgments
We want to thank Azalea Kim, MD, MBA, MPA, Duke University School of Medicine, for her contributions to this manuscript in reviewing titles and abstracts. Dr. Kim did not receive compensation for her contribution.
Funding: This work was supported by a Cancer Center Support Grant from the National Cancer Institute to Memorial Sloan Kettering Cancer Center (award number P30 CA008748). MK reported salary support from a grant from the National Cancer Institute career development grant (K07-CA187071). The authors report no potential conflicts of interest.
Footnotes
Conflict of Interest: No potential conflicts exist when that is the case
This work was previously presented as an abstract at ASCO National Conference in Chicago, Illinois in June 2016
Contributor Information
Shrujal S. Baxi, Head and Neck Oncology Service, Department of Medicine, Memorial Sloan Kettering Cancer Center, 300 East 66th Street, Rm 1459, New York, NY 10065, (646) 888-8236 (phone), (646) 888-8269 (fax).
Minal Kale, Department of Medicine, Mount Sinai Hospital, One Gustave Levy Place Box 1087, New York, NY 10029, USA, 212-824-7492 (phone), 212-824-2317 (fax).
Salomeh Keyhani, Department of Medicine, University of California San Francisco, 4150 Clement Street, San Francisco, CA 94121, USA, Phone: (415) 221-4810 ext. 25819.
Benjamin R Roman, Department of Surgery, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Rm C-1075, New York, NY 10065, 646-888-7508 (phone), 917-432-2311 (fax).
Annie Yang, Center for Health Policy and Outcomes, Memorial Sloan Kettering Cancer Center, 485 Lexington Avenue, New York, NY 10017, USA, 646-888-8202 (phone), 646-227-7102 (fax).
Antonio Derosa, Medical Library, Memorial Sloan Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, USA, 646-287-5845 (phone), 646-422-2316 (fax).
Deborah Korenstein, Center for Health Policy and Outcomes, Memorial Sloan Kettering Cancer Center, 485 Lexington Avenue, New York, NY 10017, USA, 646-888-8139 (phone), 929-321-1518 (fax).
References
- 1.Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meropol NJ, Schrag D, Smith TJ, et al. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27:3868–3874. doi: 10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]
- 3.Prasad V, Mailankody S. The UK cancer drugs fund experiment and the US cancer drug cost problem: bearing the cost of cancer drugs until it is unbearable. Mayo Clin Proc. 2016;91:707–712. doi: 10.1016/j.mayocp.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Bernard DS, Farr SL, Fang Z. National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008. J Clin Oncol. 2011;29:2821–2826. doi: 10.1200/JCO.2010.33.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan R, Peppercorn J, Sikora K, et al. Delivering affordable cancer care in high-income countries. Lancet Oncol. 2011;12:933–980. doi: 10.1016/S1470-2045(11)70141-3. [DOI] [PubMed] [Google Scholar]
- 6.Ramsey SD. How should we pay the piper when he’s calling the tune? On the long-term affordability of cancer care in the United States. J Clin Oncol. 2007;25:175–179. doi: 10.1200/JCO.2006.08.9805. [DOI] [PubMed] [Google Scholar]
- 7.Chassin MR, Galvin RW. The urgent need to improve health care quality. Institute of Medicine national roundtable on health care quality. JAMA. 1998;280:1000–1005. doi: 10.1001/jama.280.11.1000. [DOI] [PubMed] [Google Scholar]
- 8.IOM (Institute of Medicine) Best care at lower cost: the path to continuously learning health care in America. Washington, DC: The National Academies Press; 2013. [PubMed] [Google Scholar]
- 9.Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–1802. doi: 10.1001/jama.2012.476. [DOI] [PubMed] [Google Scholar]
- 10.Schnipper LE, Lyman GH, Blayney DW, et al. American Society of Clinical Oncology 2013 top five list in oncology. J Clin Oncol. 2013;31:4362–4370. doi: 10.1200/JCO.2013.53.3943. [DOI] [PubMed] [Google Scholar]
- 11.Schnipper LE, Smith TJ, Raghavan D, et al. American Society of Clinical Oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30:1715–1724. doi: 10.1200/JCO.2012.42.8375. [DOI] [PubMed] [Google Scholar]
- 12.Korenstein D, Falk R, Howell EA, et al. Overuse of health care services in the United States: an understudied problem. Arch Intern Med. 2012;172:171–178. doi: 10.1001/archinternmed.2011.772. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey S, Schickedanz A. How should we define value in cancer care? Oncologist. 2010;15:1–4. doi: 10.1634/theoncologist.2010-S1-1. [DOI] [PubMed] [Google Scholar]
- 14.American Society of Clinical Oncology. The state of cancer care in America, 2016: a report by the American Society of Clinical Oncology. J Oncol Pract. 2016;12:339–383. doi: 10.1200/JOP.2015.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruse GR, Khan SM, Zaslavsky AM, et al. Overuse of colonoscopy for colorectal cancer screening and surveillance. J Gen Intern Med. 2015;30:277–283. doi: 10.1007/s11606-014-3015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saini SD, Powell AA, Dominitz JA, et al. Developing and testing an electronic measure of screening colonoscopy overuse in a large integrated healthcare system. J Gen Intern Med. 2016;31:53–60. doi: 10.1007/s11606-015-3569-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan A, Kuo YF, Goodwin JS. Potential overuse of screening mammography and its association with access to primary care. Med Care. 2014;52:490–495. doi: 10.1097/MLR.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilt TJ, Harris RP, Qaseem A. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med. 2015;162:718–725. doi: 10.7326/M14-2326. [DOI] [PubMed] [Google Scholar]
- 19.Kepka D, Breen N, King JB, et al. Overuse of papanicolaou testing among older women and among women without a cervix. JAMA Intern Med. 2014;174:293–296. doi: 10.1001/jamainternmed.2013.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akalin E, Yaka E, Yilmaz S, et al. Compliance with the Infectious Diseases Society of America guidelines for emergency management of febrile neutropenia in cancer patients. Acta Medica Mediterranea. 2015;31:785–792. [Google Scholar]
- 22.Backhus LM, Farjah F, Varghese TK, et al. Appropriateness of imaging for lung cancer staging in a national cohort. J Clin Oncol. 2014;32:3428–3435. doi: 10.1200/JCO.2014.55.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barni S, Maiello E, Di Maio M, et al. Adherence to AIOM (Italian Association of Medical Oncology) lung cancer guidelines in Italian clinical practice: results from the RIGHT-3 (research for the identification of the most effective and highly accepted clinical guidelines for cancer treatment) study. Lung Cancer. 2015;90:234–242. doi: 10.1016/j.lungcan.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Buhrkuhl DC, Karlsson MKP, Carter JM. An audit of platelet transfusion within the Wellington Cancer Centre. Intern Med J. 2012;42:65–70. doi: 10.1111/j.1445-5994.2010.02358.x. [DOI] [PubMed] [Google Scholar]
- 25.Choi WW, Williams SB, Gu XM, et al. Overuse of imaging for staging in low risk prostate cancer. J Urol. 2011;185:1645–1649. doi: 10.1016/j.juro.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 26.Cooper M, Newman NA, Ibrahim AM, et al. Unnecessary tests and procedures in patients presenting with solid tumors of the pancreas. J Gastrointest Surg. 2013;17:1218–1223. doi: 10.1007/s11605-013-2213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crivello ML, Ruth K, Sigurdson ER, et al. Advanced imaging modalities in early stage breast cancer: preoperative use in the United States Medicare population. Ann Surg Oncol. 2013;20:102–110. doi: 10.1245/s10434-012-2571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daskivich TJ, Chamie K, Kwan L, et al. Overtreatment of men with low-risk prostate cancer and significant comorbidity. Cancer. 2011;117:2058–2066. doi: 10.1002/cncr.25751. [DOI] [PubMed] [Google Scholar]
- 29.Ellis SD, Nielsen ME, Carpenter WR, et al. Gonadotropin-releasing hormone agonist overuse: urologists’ response to reimbursement and characteristics associated with persistent overuse. Prostate Cancer Prostatic Dis. 2015;18:173–181. doi: 10.1038/pcan.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erb CT, Su KW, Soulos PR, et al. Surveillance practice patterns after curative intent therapy for stage I non-small-cell lung cancer in the Medicare population. Lung Cancer. 2016;99:200–207. doi: 10.1016/j.lungcan.2016.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falchook AD, Hendrix LH, Chen RC. Guideline-discordant use of imaging during work-up of newly diagnosed prostate cancer. J Oncol Pract. 2015;11:e239–e246. doi: 10.1200/JOP.2014.001818. [DOI] [PubMed] [Google Scholar]
- 32.Falchook AD, Salloum RG, Hendrix LH, et al. Use of bone scan during initial prostate cancer workup, downstream procedures, and associated medicare costs. Int J Radiat Oncol Biol Phys. 2014;89:243–248. doi: 10.1016/j.ijrobp.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feng YL, Lee CS, Chiu CC, et al. Appropriateness of parenteral nutrition usage in cancer patients. Nutr Cancer. 2015;67:1014–1017. doi: 10.1080/01635581.2015.1053501. [DOI] [PubMed] [Google Scholar]
- 34.Geurts SME, de Vegt F, Siesling S, et al. Pattern of follow-up care and early relapse detection in breast cancer patients. Breast Cancer Res Treat. 2012;136:859–868. doi: 10.1007/s10549-012-2297-9. [DOI] [PubMed] [Google Scholar]
- 35.Goffredo P, Thomas SM, Dinan MA, et al. Patterns of use and cost for inappropriate radioactive iodine treatment for thyroid cancer in the United States: use and misuse. JAMA Intern Med. 2015;175:638–640. doi: 10.1001/jamainternmed.2014.8020. [DOI] [PubMed] [Google Scholar]
- 36.Guy GP, Lipscomb J, Gillespie TW, et al. Variations in guideline-concordant breast cancer adjuvant therapy in rural Georgia. Health Serv Res. 2015;50:1088–1108. doi: 10.1111/1475-6773.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haas JS, Phillips KA, Liang SY, et al. Genomic testing and therapies for breast cancer in clinical practice. J Oncol Pract. 2011;7:e1s–e7s. doi: 10.1200/JOP.2011.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haddad D, Garvey EM, Mihalik L, et al. Preoperative imaging for early-stage cutaneous melanoma: predictors, usage, and utility at a single institution. Am J Surg. 2013;206:979–986. doi: 10.1016/j.amjsurg.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Hahn EE, Hays RD, Kahn KL, et al. Use of imaging and biomarker tests for posttreatment care of early-stage breast cancer survivors. Cancer. 2013;119:4316–4324. doi: 10.1002/cncr.28363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hahn EE, Tang T, Lee JS, et al. Use of imaging for staging of early-stage breast cancer in two integrated health care systems: adherence with a Choosing Wisely recommendation. J Oncol Pract. 2015;11:e320–e328. doi: 10.1200/JOP.2014.002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hahn EE, Tang T, Lee JS, et al. Use of posttreatment imaging and biomarkers in survivors of early-stage breast cancer: Inappropriate surveillance or necessary care? Cancer. 2016;122:908–916. doi: 10.1002/cncr.29811. [DOI] [PubMed] [Google Scholar]
- 42.Han D, Hogeveen S, Sweet Goldstein M, et al. Is knowledge translation adequate? A quality assurance study of staging investigations in early stage breast cancer patients. Breast Cancer Res Treat. 2012;132:1–7. doi: 10.1007/s10549-011-1786-6. [DOI] [PubMed] [Google Scholar]
- 43.Lavery HJ, Brajtbord JS, Levinson AW, et al. Unnecessary imaging for the staging of low-risk prostate cancer is common. Urology. 2011;77:274–278. doi: 10.1016/j.urology.2010.07.491. [DOI] [PubMed] [Google Scholar]
- 44.Linkugel A, Margenthaler J, Dull B, et al. Staging studies have limited utility for newly diagnosed stage I-II breast cancer. J Surg Res. 2015;196:33–38. doi: 10.1016/j.jss.2015.02.065. [DOI] [PubMed] [Google Scholar]
- 45.Lipitz-Snyderman A, Sima CS, Atoria CL, et al. Physician-driven variation in nonrecommended services among older adults diagnosed with cancer. JAMA Intern Med. 2016;176:1541–1548. doi: 10.1001/jamainternmed.2016.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Livingstone E, Krajewski C, Eigentler TK, et al. Prospective evaluation of follow-up in melanoma patients in Germany - results of a multicentre and longitudinal study. Eur J Cancer. 2015;51:653–667. doi: 10.1016/j.ejca.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Lochard-Lefrancois A, Blandin AC, Fagnoni P, et al. Appropriate cytotoxic drugs usages in solid tumors: Impact of appropriate use referentials. Le Pharmacien Hospitalier et Clinicien. 2012;47:e1–e7. [Google Scholar]
- 48.Makarov DV, Desai R, Yu JB, et al. Appropriate and inappropriate imaging rates for prostate cancer go hand in hand by region, as if set by thermostat. Health Aff (Millwood) 2012;31:730–740. doi: 10.1377/hlthaff.2011.0336. [DOI] [PubMed] [Google Scholar]
- 49.Makarov DV, Desai RA, Yu JB, et al. The population level prevalence and correlates of appropriate and inappropriate imaging to stage incident prostate cancer in the Medicare population. J Urol. 2012;187:97–102. doi: 10.1016/j.juro.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 50.Makarov DV, Hu EY, Walter D, et al. Appropriateness of prostate cancer imaging among veterans in a delivery system without incentives for overutilization. Health Serv Res. 2016;51:1021–1051. doi: 10.1111/1475-6773.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makarov DV, Loeb S, Ulmert D, et al. Prostate cancer imaging trends after a nationwide effort to discourage inappropriate prostate cancer imaging. J Natl Cancer Inst. 2013;105:1306–1313. doi: 10.1093/jnci/djt175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makarov DV, Soulos PR, Gold HT, et al. Regional-level correlations in inappropriate imaging rates for prostate and breast cancers: potential implications for the Choosing Wisely campaign. JAMA Oncol. 2015;1:185–194. doi: 10.1001/jamaoncol.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massarweh NN, Park JO, Bruix J, et al. Diagnostic imaging and biopsy use among elderly Medicare beneficiaries with hepatocellular carcinoma. J Oncol Pract. 2011;7:155–160. doi: 10.1200/JOP.2010.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller DC, Murtagh DS, Suh RS, et al. Regional collaboration to improve radiographic staging practices among men with early stage prostate cancer. J Urol. 2011;186:844–849. doi: 10.1016/j.juro.2011.04.078. [DOI] [PubMed] [Google Scholar]
- 55.Palvolgyi R, Daskivich TJ, Chamie K, et al. Bone scan overuse in staging of prostate cancer: an analysis of a Veterans Affairs cohort. Urology. 2011;77:1330–1336. doi: 10.1016/j.urology.2010.12.083. [DOI] [PubMed] [Google Scholar]
- 56.Panageas KS, Sima CS, Liberman L, et al. Use of high technology imaging for surveillance of early stage breast cancer. Breast Cancer Res Treat. 2012;131:663–670. doi: 10.1007/s10549-011-1773-y. [DOI] [PubMed] [Google Scholar]
- 57.Parmar AD, Sheffield KM, Vargas GM, et al. Quality of post-treatment surveillance of early stage breast cancer in Texas. Surgery. 2013;154:214–225. doi: 10.1016/j.surg.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paulson EC, Veenstra CM, Vachani A, et al. Trends in surveillance for resected colorectal cancer, 2001–2009. Cancer. 2015;121:3525–3533. doi: 10.1002/cncr.29469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peeraphatdit T, Naksuk N, Phatharacharukul P, et al. Adherence to diagnostic guidelines of hepatocellular carcinoma: 12-year experience in a veterans affairs medical center. Eur J Gastroenterol Hepatol. 2015;27:846–852. doi: 10.1097/MEG.0000000000000349. [DOI] [PubMed] [Google Scholar]
- 60.Porten SP, Smith A, Odisho AY, et al. Updated trends in imaging use in men diagnosed with prostate cancer. Prostate Cancer Prostatic Dis. 2014;17:246–251. doi: 10.1038/pcan.2014.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prasad SM, Gu XM, Lipsitz SR, et al. Inappropriate utilization of radiographic imaging in men with newly diagnosed prostate cancer in the United States. Cancer. 2012;118:1260–1267. doi: 10.1002/cncr.26416. [DOI] [PubMed] [Google Scholar]
- 62.Ramsey SD, Fedorenko C, Chauhan R, et al. Baseline estimates of adherence to American Society of Clinical Oncology/American Board of Internal Medicine Choosing Wisely initiative among patients with cancer enrolled with a large regional commercial health insurer. J Oncol Pract. 2015;11:338–343. doi: 10.1200/JOP.2014.002717. [DOI] [PubMed] [Google Scholar]
- 63.Ramsey SD, Henry NL, Gralow JR, et al. Tumor marker usage and medical care costs among older early-stage breast cancer survivors. J Clin Oncol. 2015;33:149–155. doi: 10.1200/JCO.2014.55.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ross I, Womble P, Ye J, et al. MUSIC: Patterns of care in the radiographic staging of men with newly diagnosed low risk prostate cancer. J Urol. 2015;193:1159–1162. doi: 10.1016/j.juro.2014.10.102. [DOI] [PubMed] [Google Scholar]
- 65.Sacks W, Wong RM, Bresee C, et al. Use of evidence-based guidelines reduces radioactive iodine treatment in patients with low-risk differentiated thyroid cancer. Thyroid. 2015;25:377–385. doi: 10.1089/thy.2014.0298. [DOI] [PubMed] [Google Scholar]
- 66.Salloum RG, Hornbrook MC, Fishman PA, et al. Adherence to surveillance care guidelines after breast and colorectal cancer treatment with curative intent. Cancer. 2012;118:5644–5651. doi: 10.1002/cncr.27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sammon JD, Abdollah F, Reznor G, et al. Patterns of declining use and the adverse effect of primary androgen deprivation on all-cause mortality in elderly men with prostate cancer. Eur Urol. 2015;68:32–39. doi: 10.1016/j.eururo.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 68.Sawazaki H, Sengiku A, Imamura M, et al. Feedback on baseline use of staging images is important to improve image overuse with newly diagnosed prostate cancer patients. Asian Pac J Cancer Prev. 2014;15:1707–1710. doi: 10.7314/apjcp.2014.15.4.1707. [DOI] [PubMed] [Google Scholar]
- 69.Schroeck FR, Kaufman SR, Jacobs BL, et al. Regional variation in quality of prostate cancer care. J Urol. 2014;191:957–962. doi: 10.1016/j.juro.2013.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schroeck FR, Kaufman SR, Jacobs BL, et al. Technology diffusion and prostate cancer quality of care. Urology. 2014;84:1066–1072. doi: 10.1016/j.urology.2014.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Segal JB, Bridges JF, Chang HY, et al. Identifying possible indicators of systematic overuse of health care procedures with claims data. Med Care. 2014;52:157–163. doi: 10.1097/MLR.0000000000000052. [DOI] [PubMed] [Google Scholar]
- 72.Shahinian VB, Kuo YF. Reimbursement cuts and changes in urologist use of androgen deprivation therapy for prostate cancer. BMC Urol. 2015;15 doi: 10.1186/s12894-015-0020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shih YC, Xu Y, Dong W, et al. First do no harm: population-based study shows non-evidence-based trastuzumab prescription may harm elderly women with breast cancer. Breast Cancer Res Treat. 2014;144:417–425. doi: 10.1007/s10549-014-2874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simonato A, Varca V, Gacci M, et al. Adherence to guidelines among Italian urologists on imaging preoperative staging of low-risk prostate cancer: Results from the MIRROR (Multicenter Italian Report on Radical prostatectomy Outcomes and Research) study. Adv Urol. 2012 doi: 10.1155/2012/651061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simos D, Catley C, Van Walraven C, et al. Imaging for distant metastases in women with early-stage breast cancer: A population-based cohort study. CMAJ. 2015;187:E387–E397. doi: 10.1503/cmaj.150003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simos D, Hutton B, Clemons M. Are physicians choosing wisely when imaging for distant metastases in women with operable breast cancer? J Oncol Pract. 2015;11:62–68. doi: 10.1200/JOP.2014.000125. [DOI] [PubMed] [Google Scholar]
- 77.Swisher-McClure S, Pollack CE, Christodouleas JP, et al. Variation in use of androgen suppression with external-beam radiotherapy for nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2012;83:8–15. doi: 10.1016/j.ijrobp.2011.06.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vazin A, Davarpanah MA, Ghalesoltani S. Antifungal agent utilization evaluation in hospitalized neutropenic cancer patients at a large teaching hospital. Drug Healthc Patient Saf. 2015;7:97–102. doi: 10.2147/DHPS.S80762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weeks JC, Uno H, Taback N, et al. Interinstitutional variation in management decisions for treatment of 4 common types of cancer: a multi-institutional cohort study. Ann Intern Med. 2014;161:20–30. doi: 10.7326/M13-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bergstrom R. Drivers of the cost of cancer care. Lancet Oncol. 2012;13:14–15. doi: 10.1016/S1470-2045(11)70370-9. [DOI] [PubMed] [Google Scholar]
- 81.Mack JW, Chen LH, Cannavale K, et al. End-of-life care intensity among adolescent and young adult patients with cancer in Kaiser Permanente southern California. JAMA Oncol. 2015;1:592–600. doi: 10.1001/jamaoncol.2015.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prigerson HG, Bao Y, Shah MA, et al. Chemotherapy use, performance status, and quality of life at the end of life. JAMA Oncol. 2015;1:778–784. doi: 10.1001/jamaoncol.2015.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


