Abstract
Therapeutic delivery of regeneration-promoting biological factors directly to the site of injury has demonstrated its efficacy in various injury models. Several reports describe improved tissue regeneration following local injection of tissue specific growth factors, cytokines and chemokines. Evidence exists that combined cytokine/growth factor treatment is superior for optimizing tissue repair by targeting different aspects of the regeneration response. The purpose of this study was to evaluate the therapeutic potential of the controlled delivery of stromal cell-derived factor-1alpha (SDF-1α) alone or in combination with insulin-like growth factor-I (SDF-1α/IGF-I) for the treatment of tourniquet-induced ischemia/reperfusion injury (TK-I/R) of skeletal muscle. We hypothesized that SDF-1α will promote sustained stem cell recruitment to the site of muscle injury, while IGF-I will induce progenitor cell differentiation to effectively restore muscle contractile function after TK-I/R injury while concurrently reducing apoptosis. Utilizing a novel poly-ethylene glycol PEGylated fibrin gel matrix (PEG-Fib), we incorporated SDF-1α alone (PEG-Fib/SDF-1α) or in combination with IGF-I (PEG-Fib/SDF-1α/IGF-I) for controlled release at the site of acute muscle injury. Despite enhanced cell recruitment and revascularization of the regenerating muscle after SDF-1α treatment, functional analysis showed no benefit from PEG-Fib/SDF-1α therapy, while dual delivery of PEG-Fib/SDF-1α/IGF-I resulted in IGF-I-mediated improvement of maximal force recovery and SDF-1α-driven in vivo neovasculogenesis. Histological data supported functional data, as well as highlighted the important differences in the regeneration process among treatment groups. This study provides evidence that while revascularization may be necessary for maximizing muscle force recovery, without modulation of other effects of inflammation it is insufficient.
Introduction
Skeletal muscle tissue has a remarkable ability to regenerate. Nevertheless, muscle regenerative capacity is reduced during ageing and can be greatly compromised following severe injuries.1 Functional deficits are commonly a consequence of impaired regenerative responses, leading to partial or complete loss of muscle function.2
In animal models cell-based therapies have been used successfully to enhance muscle regeneration.3–9 Transfers of myoblasts/satellite cells,10 mesenchymal cells,11 bone marrow-derived stem cells,12,13 peripheral blood-derived stem cells14 and other tissue resident stem cell populations3,8 with multi-lineage potential are tested with hopes to develop viable treatments for skeletal muscle injuries and muscle wasting disorders. In pre-clinical trials myoblast transplantation showed great promise for the treatment of localized muscular dystrophies as well as several conditions such as urinary and anal incontinence.7 Several serious challenges still preclude the widespread use of stem-cell based therapies in clinic: (1) the need for standardized in vitro culture systems to raise sufficient and homogeneous stem cell populations;6,15 and (2) the ability to control cell fate before and after transplantation to avoid undesirable transdifferentiation and potential for malignant transformation.16 Although, such issues as immune rejection, poor survival, limited trafficking and engraftment at the site of injury are existing limitations,7 several studies still show transient benefits from stem cell therapies due to the modulation of local inflammation through the release of anti-inflammatory mediators, as well as secondary effects on resident or locally recruited cells.12,13,17–21 Overall, with better characterization of microenvironmental components influencing the outcome of tissue regeneration, more combination therapies are likely to emerge including simultaneous delivery of several growth factors, cytokines and chemokines, co-transplantation of multiple cell populations and combinatorial treatments with both growth factors/cytokines/chemokines and cells. As such, co-transplantation studies using innate immune cells and human myoblasts were effective at stimulating myoblast proliferation and engraftment into mouse dystrophic muscle.22 Co-delivery of SDF-1α transgene and endothelial progenitors enhanced cell engraftment and subsequent angiogenesis of the ischemic muscle.23 Despite recent developments, the use of stem cell therapies is precluded by safety concerns. Therefore, identification of stem cell-trophic and regulatory factors and their subsequent incorporation into biodegradable matrices for the delivery into injured tissues represents a safer alternative to cell-based therapies.
Various synthetic scaffolds have been designed to deliver biomolecules to the site of acute injury.24–26 Polyethylene glycol (PEG) is a synthetic polymer. It has been used extensively for delivering covalently attached proteins in vivo. PEG-fibrinogen (PEG-Fib) results from coupling of PEG with fibrinogen molecules, enabling generation of a biodegradable PEG-Fib matrix after thrombin addition, while maintaining the capacity to covalently bind various protein factors. This biological scaffold is useful in several aspects: (1) it provides controlled release of conjugated protein factors; (2) it decreases the rate of protein clearance by the immune system; and (3) it localizes the delivery of protein factors to a particular site or tissue.27 Recently our group used PEG-Fib to conjugate and deliver IGF-I to improve functional recovery of skeletal muscle following TK-I/R injury.28 Zhang et al.29 successfully used PEG-Fib matrix-based delivery of SDF-1α to enhance myocardial remodeling. Delivery of SDF-1α as well as other cytokines, chemokines and growth factors directly to the site of acute injury has been successfully accomplished by other research groups.24–26
SDF-1α or CXCL12 is a small pro-inflammatory cytokine. Its expression is transiently induced after ischemic injury in several tissues including skeletal muscle.30,31 During inflammation and injury, SDF-1α was shown to be the most potent chemoattractive signal for CXCR4+ cells and is considered a major stem cell homing factor.32,33 CXCR4-expressing cells include hematopoietic stem cells,29 endothelial progenitor cells,23,32,34 mesenchymal stem cells,35 satellite cells36 as well as monocytes and lymphocytes.33 In the models of ischemic limb damage, SDF-1α was shown to restore perfusion and enhance regeneration via recruitment of a CXCR4+ cell fraction with pro-angiogenic properties.25,37 In contrast, in a model of kidney I/R injury SDF-1α was shown to have no effects on recruitment of stem cells to the kidney, however, disruption of SDF-α1 severely increased renal dysfunction and injury38,39 highlighting its requirement in locally mediated tissue repair. Injury models of myocardial regeneration provide substantial evidence that SDF-1α mediated therapies are beneficial due to improved survival of local and recruited progenitor cells as well as enhanced neovascularization.29,40 Overall, strong evidence exists for the requirement of SDF-1α-mediated signaling in orchestration of tissue regeneration, albeit the exact mechanisms of action may be tissue- and injury-specific.
IGF-I is a pro-regenerative,41 anti-inflammatory growth factor.42 Major effects of IGF-I include regulation of myoblast proliferation, differentiation and survival,41,43 modulation of inflammatory response,42 stimulation of anabolic pathways44–46 and atrophy prevention.47 Our group has previously shown major pro-regenerative effects of IGF-I following in vivo PEG-Fib/IGF-I delivery into the TK-I/R injured muscle.28
Motivated by our previous findings that PEG-Fib/IGF-I delivery significantly enhances muscle regeneration we wanted to address the therapeutic efficacy of combined PEG-Fib/SDF-1α/IGF-I and PEG-Fib/SDF-1α therapies on functional muscle regeneration following TK-I/R injury. We hypothesized that elevating and maintaining SDF-1α levels via PEG-Fib-mediated release at the site of I/R injury will promote the recruitment of resident, as well as circulating CXCR4-expressing progenitor cells, to the site of injury. In turn, combined incorporation of IGF-I into a biodegradable matrix should further stimulate stem cell proliferation and differentiation to subsequently improve functional regeneration of TK-I/R injured muscle.
Materials and methods
Animals
Male Sprague-Dawley rats (6–9 months; Charles River) were maintained on a 12-hour light/dark cycle and housed individually. Animals were allowed ad libitum access to food and water. All experimental procedures were approved and conducted in accordance with guidelines set by the University of Texas at Austin and the Institutional Animal Care and Use Committee.
Tourniquet application
The 2-hour tourniquet-induced ischemia/reperfusion (TK-I/R) model of skeletal muscle injury was induced as previously described.2 Briefly, randomly selected hind limb was elevated and a pneumatic tourniquet cuff (D.E. Hokanson, Inc.; Bellevue, WA) was placed proximal to the knee. The cuff was inflated to 250 mmHg using the Portable Tourniquet System (Delfi Medical Innovations Inc.; Vancouver, BC, Canada) for 2 hours. During the course of this procedure, rats were anesthetized with 2% to 2.5% isoflurane and body heat was maintained with the use of a heat lamp. The analgesic, carprofen, was administered prior to tourniquet application, 12- and 24-hours post-TK-I/R injury for pain management.
PEGylated fibrin preparation and delivery
Protein factor conjugated PEGylated fibrin gel was prepared as previously described.27,29 Briefly, human fibrinogen (Sigma-Aldrich Co.; St. Louis, MO) was reconstituted in Tris-buffered saline (40 mg mL−1, pH 7.8) and reacted with bifunctional SG-PEG-SG (NOF America Corp, Irvine, CA) in 5: 1 PEG: fibrinogen molar ratio with or without the addition of rat SDF-1α (PeproTech Inc.; Rocky Hill, NJ) and human IGF-I (Pepro Tech Inc.; Rocky Hill, NJ). Gel polymerization was induced by the addition of 25 U mL−1 of human thrombin (Sigma). The final concentration of fibrinogen was 10 mg mL−1, PEG 0.5 mg ml−1, SDF-1α 10 μg mL−1, IGF-I 25 μg ml−1. Twenty-four hours post TK-I/R injury, 0.25 mL of empty PEGylated fibrin gel (Peg-Fib; n = 6), SDF-1α conjugated PEGylated fibrin gel (Peg-Fib/SDF-1α; n = 6), SDF-1α and IGF-I conjugated PEGylated fibrin gel (Peg-Fib/SDF-1α/IGF-1; n = 6) was injected into the lateral gastrocnemius (LGAS) muscle of the TK-injured limb. PEG-Fib-containing treatments were injected in liquid form and polymerized in situ. Functional assessments were performed at 14 days of reperfusion.
Functional assessment
Following 14 days of TK-I/R injury, in situ evaluations of lateral gastrocnemius (LGAS) force production were performed on the tourniquet and contralateral leg (uninjured) as previously described.2 Briefly, animals were anesthetized with isoflurane, and the skin of the hindlimb was removed to expose the hamstring. LGAS muscle was isolated; innervation to the medial GAS was removed. The Achilles tendon was attached to the lever arm of a dual mode servomotor (Aurora Scientific Model 310B Inc.; Aurora, ON, Canada). The muscle was stimulated using a stimulator (A-M Systems, Carlsborg, WA, Model 2100) with electrodes applied to the tibial nerve. Optimal length (Lo) was determined by finding the length producing the maximal twitch force at 0.5 Hz at 5 V. Maximal peak tetanic tension (Po) was measured at 150 Hz and the minimal voltage required to elicit a maximal Po response. Each tetanic contraction was followed by 2 minutes of rest. Muscle temperature was maintained with a heat lamp and warm mineral oil. Data was collected and analyzed using LabView software. After the completion of the contractile measurements, the muscles were harvested, weighed, embedded in OCT compound, and frozen in liquid nitrogen-cooled isopentane. The muscles were stored in a −80°C freezer until histological analysis.
Histological analysis, immunofluorescent and immunohistochemical tissue staining
Frozen, OCT-embedded muscle samples following 14 days of recovery after I/R injury were sectioned on a cryostat (Leica CM1900; Leica Microsystems Inc.; Buffalo Grove, IL) and placed on a warm slide. Hematoxylin & eosin (H&E) s and Masson’s trichrome (Polyscience, Warrington, PA, USA) staining were performed as previously described,28 and slides were observed with a light microscope (Nikon Diaphot, Nikon Corp.; Tokyo, Japan) with the 20× objective lens. Images were taken using a mounted digital camera (Optronix Microfire; Optronix; Goleta, CA). Myofiber cross-sectional area (CSA) was measured using ImageJ software. Immunofluorescence protocols were previously described.48 Briefly, sectioned tissue was blocked with 5% normal donkey serum and 1% BSA in PBS, and stained with primary anti-CXCR4 antibody (1: 200; Novus Biologicals, Littleton, CO, USA, H00007852-M04). Primary antibody staining was detected with the donkey anti-mouse IgG-TRITC fluorescein (1: 100; Santa Cruz Biotechnology, Inc.; Santa Cruz, CA) and counterstained with DAPI (1: 1000; Molecular Probe, OR, USA, D1306). CXCR4+ cells were identified using Leica (DM IL) fluorescence microscope with the 20× objective lens, photographed using AxioCam MRm Microscope Camera and quantified using ImageJ Software. PEG-Fib group n = 6; PEG-Fib/SDF-1 group n = 5 control leg, n = 6 TK leg; PEG-Fib/SDF-1/IGF-I group n = 5.
To measure CD31+ cell density, sections were stained sections with the anti-rat CD31 (PECAM-1) antibody (1: 25; BD Pharmingen, San Jose, CA, USA, 550300) followed by incubation with the avidin-biotin enzyme kit (Vectastain ABC Kit; Vector Laboratories, Irvine, CA, USA) and Pierce DAB substrate kit (Thermo Scientific, Rockford, IL, USA). CD31+ cell density was determined from the number of CD31+ cell per muscle fiber using Image J software.
Western blotting
Western blotting was performed as previously described.49 Briefly, samples were prepared and boiled in 2× Laemmli’s sample buffer at a ratio of 1: 1 for 5 minutes, samples were loaded into each well of a 5% stacking/12.5% separating poly-acrylamide gel. Following SDS-PAGE, proteins were transferred to a PVDF membrane (Millipore) and blocked with 5% milk in 0.1% Tween-20 in TBS (TBST) for 1 h. Membrane was incubated in a 1: 1000 dilution of primary antibody to rabbit anti-rat SDF-1α (Peprotech) in 5% milk-TBST overnight at 4°C. Protein bands were detected using 1: 1000 dilution of goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (Pierce) in 5% milk-TBST for 2 h. Following detection of SDF-1α protein, membrane was washed, incubated in stripping buffer at 50°C for 50 min, blocked and re-blotted with primary rabbit anti-human IGF-I antibody (Peprotech) at 1: 1000 dilution overnight at 4°C. Secondary detection was performed as described above. Blots were imaged with the Chemidoc XRS system (Bio-Rad).
Statistical analysis
Functional values were analyzed using one-way ANOVA to compare groups, and the Tukey post-hoc test was used to compare between data sets (p < 0.05). The values are represented as the mean ± SEM, unless noted otherwise.
Results
Identification of SDF-1α and IGF-I by western blot
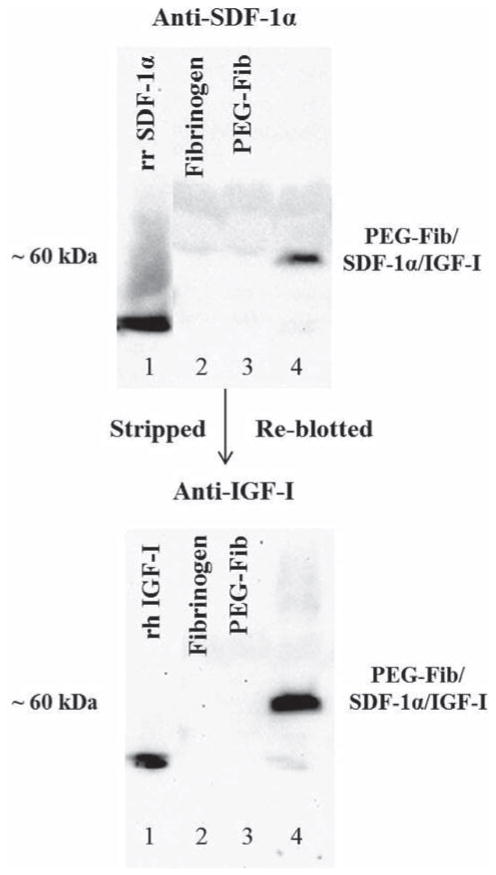
Our group has previously shown the successful conjugation of IGF-I growth factor to PEG-Fib matrix.28 In turn, the success of SDF-1α conjugation to PEG-Fib matrix was extensively characterized by Zhang et al.29 We utilized already established protocols to conjugate SDF-1α and IGF-I to the PEGylated fibrin matrix. By using Western Blot we confirmed the presence of SDF-1α and IGF-I in our gels (Fig. 1). Co-incubation of SDF-1α and IGF-I recombinant proteins with PEG-Fib matrix resulted in the formation of large PEG-Fib/SDF-1α/IGF-I complexes detected by SDF-1α and IGF-I specific antibodies (Fig. 1; Lane 4). Fibrinogen (α-chain 63.5 kDa, β-chain 56 kDa, γ-chain 47 kDa) binds PEG (3.4 kDa), SDF-1α (10 kDa) and IGF-I (7.5 kDa) to form SDF-1α/IGF-I-containing aggregates visualized at ≥60 kDa size. No staining was detected with fibrinogen only (Lane 2) and PEGylated fibrinogen (Lane 3). Success of the PEGylation procedure is reflected in the virtual absence of staining to unconjugated SDF-1α and IGF-I (Fig. 1; Lane 4). Our prior work has demonstrated progressive release of SDF-1α and IGF-I from PEG-Fib matrix in vitro.28,29
Fig. 1.
Western blots showing the binding of SDF-1α and IGF-I to conjugated poly(ethylene glycol) (PEG) fibrinogen (Fib). Lane 1: recombinant rat SDF-1α (20 μg ml−1) (top), recombinant human IGF-I (50 μg ml−1) (bottom); Lane 2: fibrinogen (10 mg ml−1); Lane 3: PEGylated fibrinogen; Lane 4: PEGylated fibrinogen/SDF-1α/IGF-I probed against SDF-1α (top) and IGF-I (bottom) ≥60 kDa in size. Final concentrations of SDF-1α and IGF-I following PEGylation were 10 μg ml−1 and 25 μg ml−1 respectively.
SDF-1α delivery promotes the persistence of CXCR4+ cells at the site of injury 14 days post-reperfusion
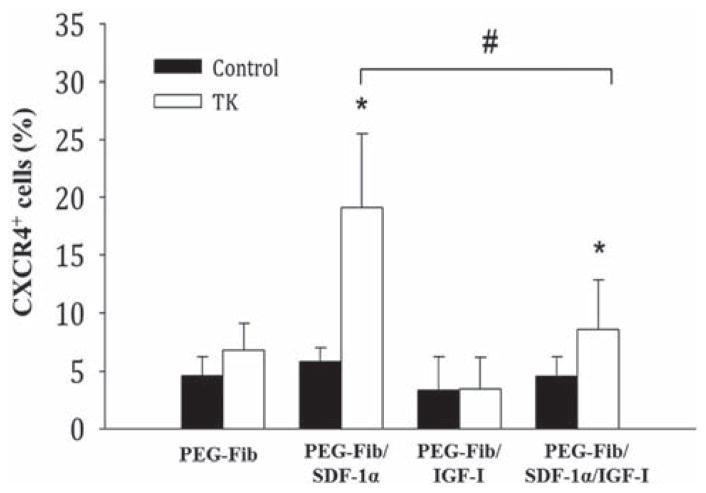
The aim of this experiment was to address whether PEG-Fib/SDF-1α delivery was effective in promoting the recruitment CXCR4+ cells to the site of injury. In vitro release kinetics showed that SDF-1α can be progressively released across 7 days.29 We hypothesized that progressive release of high concentrations of SDF-1α from PEG-Fib matrix in the presence of the inflammatory response will efficiently recruit high numbers of CXCR4+ cells to the site of I/R injury. To address this, we used fluorescence microscopy to identify CXCR4-expressing cells within TK-I/R injured skeletal muscle treated with PEG-Fib, PEG-Fib/SDF-1α, PEG-Fib/IGF-I and PEG-Fib/SDF-Iα/IGF-I at a late time point of 14 days post-reperfusion. As expected, we saw significantly higher CXCR4+ cells present within injured muscles treated with PEG-Fib/SDF-1α (19.12 ± 6.39% vs. 4.64 ± 1.52%) compared to other groups (Fig. 2). Interestingly, the presence of IGF-I was enough to suppress the effects of SDF-1α on the recruitment of CXCR4+ cells (Fig. 2).
Fig. 2.
Quantification of CXCR4+ cells within I/R injured skeletal muscle 14 days post-reperfusion. Animals were treated with PEGylated fibrin (PEG-Fib), PEGylated fibrin conjugated to SDF-1α (PEG-Fib/SDF-1α), PEGylated fibrin conjugated to IGF-I (PEG-Fib/IGF-1), and PEGylated fibrin conjugated to SDF-1α and IGF-I (PEG-Fib/SDF-1α/IGF-I) 24 h after TK-I/R injury and analyzed 14 days post-reperfusion. Contralateral control (n = 5, 3 fields of view per animal); TK-I/R (n = 6, 3 fields of view per animal). Values expressed as mean ± SEM, one-way ANOVA, Tukey post-hoc: *p < 0.05 versus PEG-Fib, #p < 0.05 PEG-Fib/SDF-1α versus PEG-Fib/SDF-1α/IGF-I.
SDF-1α treatment of injured skeletal muscle enhances tissue revascularization
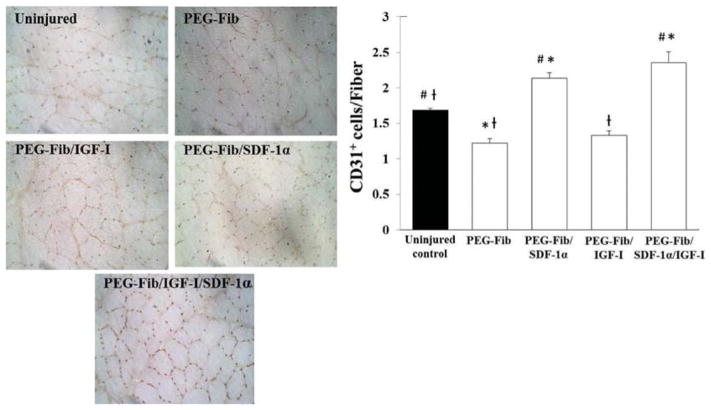
CXCR4 receptor is highly expressed on endothelial cells as well as endothelial cell progenitors rendering these cells sensitive to chemotactic gradients of SDF-1α.50–52 In vitro and in vivo models show strong effects from the SDF-1/CXCR-4 axis on tissue neoangiogenesis and neovascularization.50 We addressed whether PEG-Fib/SDF-1α treatment alone or in combination with IGF-I had an effect on muscle revascularization after I/R injury. Both treatment groups showed significantly higher number of CD31+ cells per muscle fiber (CD31+ cells/Fiber) when compared to uninjured control, PEG-Fib and PEG-Fib/IGF-I groups (Fig. 3). These findings support literature reported pro-angiogenic effects of SDF-1α as well as provide additional evidence for significant increases in revascularization of injured skeletal muscle tissue after PEG-Fib-mediated SDF-1α delivery.
Fig. 3.
Identification and quantification of CD31+ cells within I/R injured skeletal muscle 14 days post-reperfusion. Animals were treated with PEGylated fibrin (PEG-Fib), PEGylated fibrin conjugated to SDF-1α (PEG-Fib/SDF-1α), PEGylated fibrin conjugated to IGF-I (PEG-Fib/IGF-1), and PEGylated fibrin conjugated to SDF-1α and IGF-I (PEG-Fib/SDF-1α/IGF-I) 24 h after TK-I/R injury and analyzed 14 days post-reperfusion. Representative images of CD31+ staining (200×) and quantification of CD31+ cells/muscle fiber (n = 3, 3 fields of view per animal). Values expressed as mean ± SEM, oneway ANOVA, Tukey post-hoc: *p < 0.05 versus uninjured control, #p < 0.05 versus PEG-Fib group, †p < 0.05 versus PEG-Fib/SDF-1α group.
Improved functional regeneration after PEGylated fibrin delivery of IGF-I without therapeutically beneficial effects from SDF-1α
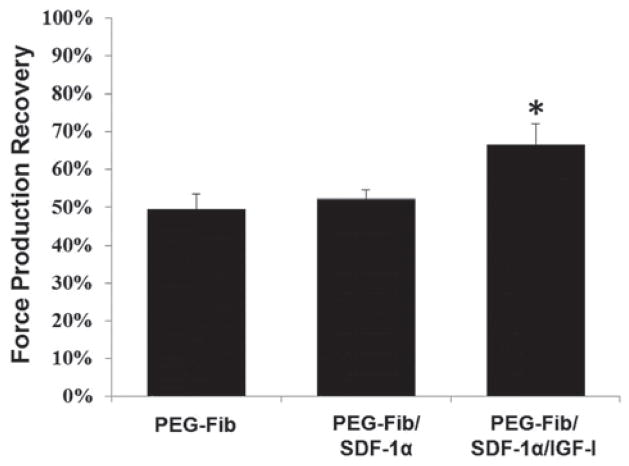
We aimed to determine the efficacy of PEG-Fib-mediated controlled release of SDF-1α and IGF-I directly at the site of I/R injury on functional recovery of skeletal muscle. To address this, we administered either empty PEG-Fib matrix, PEG-Fib matrix conjugated to SDF-1α or PEG-Fib matrix conjugated to SDF-1α/IGF-I i.m. into TK-I/R injured LGAS 24 h after TK release. Surprisingly, we observed no significant difference in maximum force production recovery with PEG-Fib/SDF-1α (52.05 ± 6.00%) compared to the PEG-Fib (49.48 ± 9.87%) treatment groups. As expected, PEG-Fib/SDF-1α/IGF-1 treatment resulted in improved recovery of force, compared to PEG-Fib treatment (66.50 ± 13.37% vs. 49.48 ± 9.87%; P < 0.05) as seen in Fig. 4. The tetanic force production in PEG-Fib/SDF-1α/IGF-I group was similar to maximum force production achieved following PEG-Fib/IGF-I treatment28 suggesting that positive effect from treatment was primarily due to the presence and bioactivity of IGF-I.
Fig. 4.
Percent maximum force production recovery among treatment groups 14 days after I/R injury. Maximum tetanic force production (P0) of the LGAS was measured in situ from the following groups: PEGylated fibrin (PEG-Fib), PEGylated fibrin conjugated to SDF-1α (PEG-Fib/SDF-1), and PEGylated fibrin conjugated to SDF-1α and IGF-I (PEG-Fib/SDF-1α/IGF-I). The P0 were compared to the contralateral leg that received no injury. Values expressed as mean ± SEM, one-way ANOVA, Tukey post-hoc: *p < 0.05 versus PEG-Fib, n = 6.
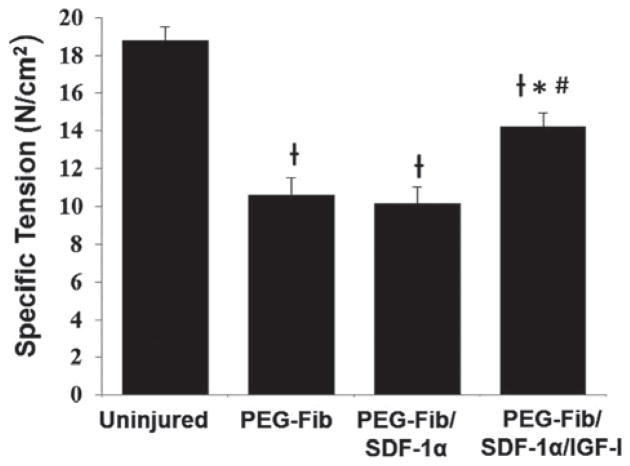
Specific tension values (SP0) were determined across groups in order to normalize tetanic forces to muscle cross sectional areas. Consistent with force recovery values, there were no significant differences between specific tensions for the PEG-Fib (10.59 ± 2.41 N cm−2) and PEG-Fib/SDF-1α (10.13 ± 2.21 N cm−2) groups. There was a significant increase in specific tension in PEG-Fib/SDF-1α/IGF-1 group (14.22 ± 1.79 N cm−2) compared to PEG-Fib and PEG-Fib/SDF-1α groups (p < 0.05) (Fig. 5). Previous delivery PEG-Fib and PEG-Fib/IGF-I into TK-I/R injured muscle generated SP0 values of 11.7 ± 1.0 N cm−2 and 14.8 ± 0.6 N cm−2 respectively28 supporting consistency of our data and highlighting the lack of SDF-1α effect in the PEG-Fib/SDF-1α/IGF-1 group. Muscle weights across groups were not significantly different (data not shown).
Fig. 5.
Functional recovery of tetanic tension among treatment groups 14 days after I/R injury. Specific tension (SP0) of the LGAS was measured in situ for the following groups: PEGylated fibrin (PEG-Fib), PEGylated fibrin conjugated to SDF-1α (PEG-Fib/SDF-1), and PEGylated fibrin conjugated to SDF-1α and IGF-I (PEG-Fib/SDF-1/IGF-I). Values expressed as mean ± SEM, one-way ANOVA, Tukey post-hoc: †p < 0.05 versus uninjured, *p < 0.05 versus PEG-Fib, #p < 0.05 versus PEG-Fib/SDF-1, n = 6.
These results suggest that SDF-1α delivery to TK-I/R injured muscle does not provide significant therapeutic benefit. The beneficial effect from dual PEG-Fib/SDF1α/IGF-I factor delivery is mediated primarily by IGF-I.
Histological evaluation of regenerating muscle tissue supports functional results, but points to differences in regeneration mechanisms among groups
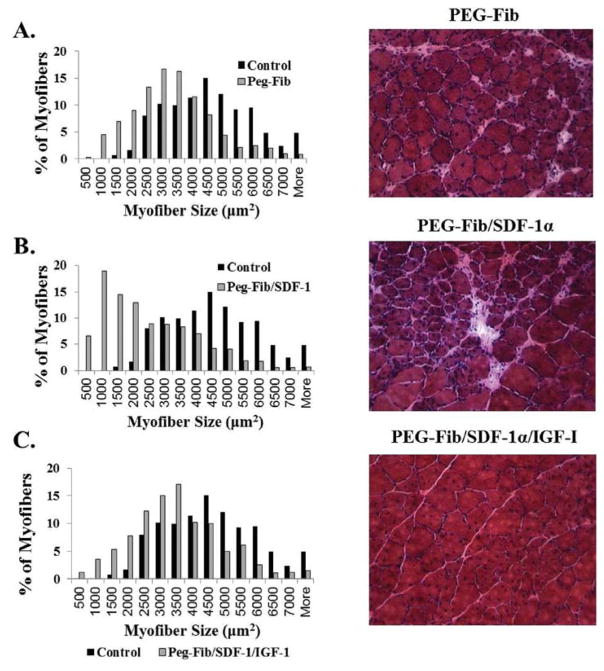
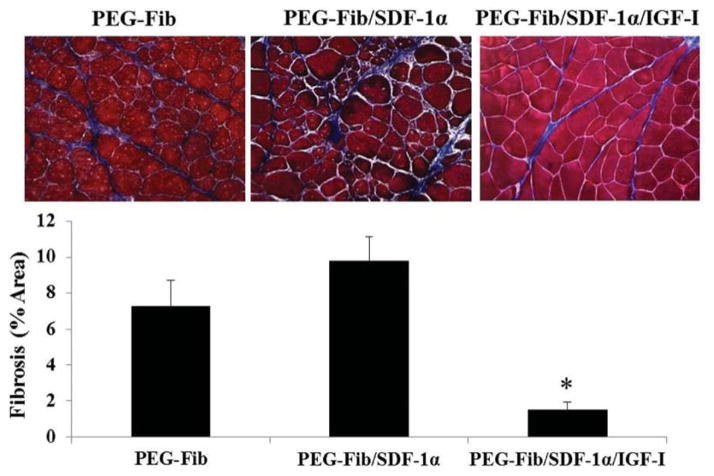
Histological evaluation of H&E stained muscle sections at 14 days post-reperfusion in general supports the functional data results described above, albeit, several important and interesting distinctions are apparent between groups. For example, the PEG-Fib/SDF-1α treatment group showed a much greater distribution of smaller myofibers than the PEG-Fib group despite similar contractile deficiencies (Fig. 6A and B). This may point to potential differences in the regeneration process that may have taken place subsequent to SDF-1α delivery. The presence of large myofibers of round morphology in PEG-Fib samples is most likely an indication of an ongoing regeneration process. Also, inflammatory exudate and fibrotic areas are evident during gross examination of muscle sections treated with PEG-Fib (Fig. 6A). Persistent inflammation within regenerating tissue has been associated with increased collagen deposition.53 In turn, increased fibrosis may lead to contractile dysfunction by decreasing myofiber occupancy. We evaluated collagen deposition in our tissues using Trichrome staining. As expected, we saw significantly higher fibrosis in PEG-Fib and PEG-Fib/SDF-1α treated muscles (Fig. 7).
Fig. 6.
Histological analysis of I/R injured skeletal muscle 14 days post-reperfusion. H&E stained sections were examined for fiber size distribution (200×). Data expressed as percent myofibers of a given area (μm2). Representative images are included: (A) PEGylated fibrin (PEG-Fib), (B) PEGylated fibrin conjugated to SDF-1α (PEG-Fib/SDF-1α), (C) PEGylated fibrin conjugated to SDF-1α and IGF-I (PEG-Fib/SDF-1α/IGF-I).
Fig. 7.
Quantification of collagen deposition in I/R injured skeletal muscle 14 days post-reperfusion. Animals were treated with PEGylated fibrin (PEG-Fib), PEGylated fibrin conjugated to SDF-1α (PEG-Fib/SDF-1α), and PEGylated fibrin conjugated to SDF-1α and IGF-I (PEG-Fib/SDF-1α/IGF-I) 24 h after TK-I/R injury and analyzed 14 days post-reperfusion. Representative images of trichrome staining (200×) where collagen staining is shown in blue (n = 3, 3 fields of view per animal). Values expressed as mean ± SEM, one-way ANOVA, Tukey post-hoc: *p < 0.05 versus PEG-Fib group.
Muscles treated with PEG-Fib/SDF-1α/IGF-I, as expected, showed almost no signs of injury induced pathology and minimal fibrosis, their myofiber size distribution was comparable to that of control muscle (Fig. 6C and 7). Histologically (H&E), muscles treated with PEG-Fib/SDF-1α/IGF-I look identical to muscle treated with PEG-Fib/IGF-I.28 Therefore, histological examination supports functional studies and provides further evidence that SDF-1α delivery via PEG-Fib does not enhance myofiber regeneration despite enhanced revascularization at 14 days after I/R injury. We believe that the beneficial effect from dual PEG-Fib/SDF-1α/IGF-I delivery is mainly due to the effect of IGF-I on muscle force recovery. Whether the lack of beneficial effects at 14 days in PEG-Fib/SDF-1α group indicates delayed resolution of inflammation during the acute stages of muscle regeneration was not determined.
In conclusion, we have shown that PEG-Fib mediated delivery of the chemokine, SDF-1α recruits CXCR4+ cells to the injured muscle, enhances muscle neovascularization, however, does not accelerate force recovery and myofiber regeneration in I/R injured skeletal muscle at 14 days. In contrast, dual PEG-Fib-mediated delivery of SDF-1α/IGF-I improves tissue revascularization, leads to increased myofiber size and decreased muscle tissue fibrosis. Most importantly, dual SDF-1α/IGF-I treatment enhances functional regeneration of skeletal muscle tissue after TK-I/R injury, although, positive effects on skeletal muscle force recovery appear to be primarily IGF-I-mediated.
Discussion
In this study we used a PEGylated fibrin-based matrix to deliver SDF-1α chemokine alone or in combination with IGF-I growth factor to the site of acute skeletal muscle TK-I/R injury. Motivated by our previous success in the delivery of PEG-Fib/IGF-I to enhance functional muscle regeneration after I/R injury28 we aimed to address the efficiency of a combined matrix-based SDF-1α/IGF-I therapeutic approach on restoring muscle function post TK-I/R injury. We found no added benefit on the restoration of muscle contractile function from combined PEG-Fib/SDF-1α/IGF-I therapy compared to PEG-Fib/IGF-I treatment at the 14 day time point. However, the presence of SDF-1α significantly enhanced muscle tissue revascularization after TK-I/R injury.
Taking into consideration multiple literature-reported beneficial effects of SDF-1α treatment on regeneration of ischemic tissues, including skeletal muscle,25 we were surprised to find no functional improvements following PEG-Fib/SDF-1α therapy. Albeit to our knowledge, we are the first group to evaluate the effect of SDF-1α treatment on functional regeneration of skeletal muscle after TK-I/R injury. In addition to functional results, histological data strengthened our conclusions and provided additional evidence of ongoing degenerative/regenerative cycling at the two-week time point after TK-I/R injury in the PEG-Fib/SDF-1α treatment group characterized by an abundance of smaller myofibers and increased fibrosis, despite persistence of CXCR4+ cells at the site of injury and enhanced tissue revascularization. Although not demonstrating a positive effect at this time point, our results were not completely unexpected.
SDF-1α is a chemokine, strongly induced in an inflammatory setting.54 It is known to be a powerful chemoattractant for CXCR4-expressing stem cell populations as well as bone marrow-derived immune cells.33,54,55 As such, SDF-1α was shown to be a potent chemoattractant of inflammatory monocytes in vivo, greater even than action of monocyte chemoattractant protein-1 (MCP-1).33 Various cancers use the SDF-1α/CXCR4 signaling axis to recruit inflammatory macrophages to the tissues.56,57 In a model of spinal cord injury, locally expressed SDF-1α in conjunction with matrix metalloproteinase-9 supports the migration of monocytes into the injured spinal cord.31 Another recent report provides compelling evidence that the CXCR7 receptor is induced during monocyte-to-macrophage transition and is expressed at higher levels on M1 macrophages. Therefore, in addition to promoting macrophage recruitment, SDF-1α signals via CXCR7 to enhance macrophage phagocytosis, contributing to pathogenesis of atherosclerosis.58 Myocardial CXCR4 overexpression led to the exacerbation of I/R injury in the heart by increasing inflammatory infiltrate,59 while transendocardial delivery of SDF-1α failed to improve myocardial perfusion and ventricular function. 60 These studies suggest that exaggerated signaling via SDF-1α-CXCR4/7 axis may lead to detrimental effects on tissue regeneration especially during early stages of muscle regeneration where efficient resolution of the inflammatory response is required for the timely onset of tissue repair.61,62
In our model of TK-I/R injury, tissue necrosis, vascular damage, severe inflammation and functional deficits are the hallmarks of I/R-induced muscle pathology.2,63 It is established that inflammatory monocytes/macrophages (M1) are recruited early in regeneration and, although, absolutely required for the clearance of necrotic debris at the site of injury, their persistence often exacerbates inflammation and delays regeneration.64–66 Muscle fiber necrosis following I/R injury is a potent pro-inflammatory activator of recruited monocytes.67 Recently, high mobility group box-1, a nuclear protein released by necrotic cells, was shown to form a heterocomplex with SDF-1α and act via CXCR4 to recruit inflammatory cells.68 I/R-induced muscle necrosis combined with progressive release of SDF-1α from PEG-Fib matrix in our injury model may have contributed to the recruitment of additional inflammatory CXCR4+ cells prolonging local inflammation and delaying onset of muscle regeneration. Immunofluorescence data showing increased numbers of CXCR4+ cells in muscles treated with PEG-Fib/SDF-1α late in regeneration response serve as evidence of either ongoing cell recruitment or local proliferation, both of which are characteristics of early phase regenerative events.69
In literature, the beneficial role of SDF-1α is associated with improved restoration of ischemic tissue perfusion and neovascularization. 40 Several reports mention SDF-1α-recruited CXCR4+CD11b+ cells as primary mediators of neovascularization. 37 Multiple solid tumors exploit the SDF-1α/CXCR4 axis for the recruitment of M1 macrophages to promote and support the establishment of the vascular supply for tumor survival. As such, in a highly inflammatory context, a proangiogenic environment leads to the formation of immature and fragile neovessels. The recruitment of CD34+ endothelial progenitor cells via the SDF-1α/CXCR4 signaling axis may contribute to inflammatory angiogenesis.23 In our model, both groups treated with SDF-1α showed significantly enhanced muscle revascularization/neovascularization, even when compared with uninjured control. However, administration of PEG-Fib/SDF-1α often resulted in leakage of blood throughout the muscle (data not shown), which can be a consequence of rupture, permeability and lack of stability of newly formed microvasculature in this group. The abundance of small myofibers and the persistence of CXCR4+ cells at the site of injury as late as two weeks post-reperfusion provide further evidence for the ongoing degeneration/regeneration sequence of events. Increased collagen deposition in PEG-Fib and PEG-Fib/SDF-1α treated muscles may be indicative of M2 macrophage activity in the inflammatory setting.70 These cells appear in the muscles as early as 3 days post-reperfusion71 and produce arginase-1 and TGF-β factors both of which contribute to extra-cellular matrix deposition.72 Overall, there appears to be no functional benefit from SDF-1α treatment up to 14 days post-TK-I/R injury compared to matrix delivery alone, despite apparent enhancement in neovascularization.
Interestingly, in the combined delivery of PEG-Fib/SDF-1α/IGF-I, IGF-I was able to complement the SDF-1α-mediated neovascularization effect. Muscles treated with PEG-Fib matrix containing both SDF-1α and IGF-I showed enhanced revascularization, increased myofiber distribution, decreased fibrosis and enhanced contractile function when compared to PEG-Fib matrix delivery alone. Our group has previously shown significant beneficial effects of PEG-Fib/IGF-I administration on muscle recovery following TK-I/R injury. It was apparent that functional improvements using PEG-Fib/SDF-1α/IGF-I therapy were very similar to functional recovery using PEG-Fib/IGF-I therapy of TK-I/R injured muscles.28 Therefore, we concluded that beneficial effects of PEG-Fib/SDF-1α/IGF-I therapy on restoration of muscle contractile function are primarily attributed to IGF-I activity, a potent anti-inflammatory, pro-regenerative, anti-apoptotic and hypertrophy-promoting growth factor.42 In order to better understand the effect of exogenous SDF-1α delivery on muscle regeneration, we need to perform additional functional testing to evaluate the benefit of enhanced vascularization on restoration of work capacity in PEG-Fib/SDF-1α/IGF-I treatment group, as well as characterize and quantify SDF-1α-mediated effects on inflammatory and precursor cells recruitment at the early stages of muscle regeneration.
The release kinetics of SDF-1α/IGF-1 from PEG-Fib matrix were previously evaluated by our group as well as Zhang et al.29 Sequence of factor release at the site of acute injury may be responsible for the therapeutic effect seen after dual PEG-Fib/SDF-1α/IGF-I delivery. The majority of IGF-I is released from the matrix within the first 24 hours and at physiologically relevant levels over a 4-day period, while slightly larger SDF-I is progressively released over 7 days. The powerful anti-inflammatory, pro-regenerative signal delivered via IGF-I may have inhibited SDF-1-dependent inflammatory cell recruitment at the later stages of muscle regeneration and/or facilitated earlier inflammatory resolution and onset of tissue repair. Future studies should address how changing the order and kinetics of SDF-1α/IGF-I release may impact functional tissue regeneration.
Conclusion
We did not observe functional improvements after PEG-Fib/SDF-1α treatment, despite treatment-induced increase in persistence of CXCR4+ cells and enhanced tissue revascularization at two weeks after initial injury. Functional analysis showed no significant difference in maximal force recovery between matrix alone treatment and addition of SDF-1α. As expected, combined PEG-Fib/SDF-1α/IGF-I delivery in addition to enhanced revascularization, significantly improved functional recovery following TK-I/R. However, the effect of combined PEG-Fib/SDF-1α/IGF-I therapy on recovery of muscle force appeared to be IGF-I mediated. Our data confirm the requirement for IGF-I in promoting muscle repair and pro-angiogenic effects of SDF-1α on tissue revascularization. We did not show beneficial effects of SDF-1α treatment on contractile force recovery at 14 days after TK-I/R injury. Nevertheless, combined growth factor therapy can offer multiple benefits provided that one can manipulate the release order, kinetics and gradients of delivered mediators in the microenvironment in spatio-temporal manner to promote efficient repair.
References
- 1.Vignaud A, Hourde C, Medja F, Agbulut O, Butler-Browne G, Ferry A. Impaired skeletal muscle repair after ischemia-reperfusion injury in mice. J Biomed Biotechnol. 2010;2010:724914. doi: 10.1155/2010/724914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammers DW, Merritt EK, Matheny RW, Jr, Adamo ML, Walters TJ, Estep JS, et al. Functional deficits and insulin-like growth factor-I gene expression following tourniquet-induced injury of skeletal muscle in young and old rats. J Appl Physiol. 2008;105:1274–1281. doi: 10.1152/japplphysiol.90418.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceafalan LC, Popescu BO, Hinescu ME. Cellular players in skeletal muscle regeneration. BioMed Res Int. 2014;2014:957014. doi: 10.1155/2014/957014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong J, Shin K, Lee SB, Lee DR, Kwon H. Patient-tailored application for Duchene muscular dystrophy on mdx mice based induced mesenchymal stem cells. Exp Mol Pathol. 2014;97:253–258. doi: 10.1016/j.yexmp.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Meregalli M, Farini A, Sitzia C, Torrente Y. Advancements in stem cells treatment of skeletal muscle wasting. Front Physiol. 2014;5:48. doi: 10.3389/fphys.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YX, Dumont NA, Rudnicki MA. Muscle stem cells at a glance. J Cell Sci. 2014;127:4543–4548. doi: 10.1242/jcs.151209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tedesco FS, Cossu G. Stem cell therapies for muscle disorders. Curr Opin Neurol. 2012;25:597–603. doi: 10.1097/WCO.0b013e328357f288. [DOI] [PubMed] [Google Scholar]
- 8.Usas A, Maciulaitis J, Maciulaitis R, Jakuboniene N, Milasius A, Huard J. Skeletal muscle-derived stem cells: implications for cell-mediated therapies. Medicina. 2011;47:469–479. [PubMed] [Google Scholar]
- 9.Meng J, Adkin CF, Arechavala-Gomeza V, Boldrin L, Muntoni F, Morgan JE. The contribution of human synovial stem cells to skeletal muscle regeneration. Neuromuscul Disord. 2010;20:6–15. doi: 10.1016/j.nmd.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Boldrin L, Morgan JE. Activating muscle stem cells: therapeutic potential in muscle diseases. Curr Opin Neurol. 2007;20:577–582. doi: 10.1097/WCO.0b013e3282ef5919. [DOI] [PubMed] [Google Scholar]
- 11.Liew A, O’Brien T. Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther. 2012;3:28. doi: 10.1186/scrt119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corona BT, Rathbone CR. Accelerated functional recovery after skeletal muscle ischemia-reperfusion injury using freshly isolated bone marrow cells. J Surg Res. 2014;188:100–109. doi: 10.1016/j.jss.2013.12.026. [DOI] [PubMed] [Google Scholar]
- 13.Corona BT, Wenke JC, Walters TJ, Rathbone CR. Intramuscular transplantation and survival of freshly isolated bone marrow cells following skeletal muscle ischemia-reperfusion injury. J Trauma Acute Care Surg. 2013;75:S142–S149. doi: 10.1097/TA.0b013e31829ac1fa. [DOI] [PubMed] [Google Scholar]
- 14.Quattrocelli M, Cassano M, Crippa S, Perini I, Sampaolesi M. Cell therapy strategies and improvements for muscular dystrophy. Cell Death Differ. 2010;17:1222–1229. doi: 10.1038/cdd.2009.160. [DOI] [PubMed] [Google Scholar]
- 15.Viswanathan S, Keating A, Deans R, Hematti P, Prockop D, Stroncek DF, et al. Soliciting strategies for developing cell-based reference materials to advance mesenchymal stromal cell research and clinical translation. Stem Cells Dev. 2014;23:1157–1167. doi: 10.1089/scd.2013.0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 17.Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. doi: 10.3389/fimmu.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20. doi: 10.1038/mt.2011.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen XK, Rathbone CR, Walters TJ. Treatment of tourniquet-induced ischemia reperfusion injury with muscle progenitor cells. J Surg Res. 2011;170:e65–e73. doi: 10.1016/j.jss.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 21.Ota S, Uehara K, Nozaki M, Kobayashi T, Terada S, Tobita K, et al. Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis. Am J Sports Med. 2011;39:1912–1922. doi: 10.1177/0363546511415239. [DOI] [PubMed] [Google Scholar]
- 22.Bencze M, Negroni E, Vallese D, Yacoub-Youssef H, Chaouch S, Wolff A, et al. Proinflammatory macrophages enhance the regenerative capacity of human myoblasts by modifying their kinetics of proliferation and differentiation. Mol Ther. 2012;20:2168–2179. doi: 10.1038/mt.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuliszewski MA, Kobulnik J, Lindner JR, Stewart DJ, Leong-Poi H. Vascular gene transfer of SDF-1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle. Mol Ther. 2011;19:895–902. doi: 10.1038/mt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, et al. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci U S A. 2010;107:3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuraitis D, Zhang P, Zhang Y, Padavan DT, McEwan K, Sofrenovic T, et al. A stromal cell-derived factor-1 releasing matrix enhances the progenitor cell response and blood vessel growth in ischaemic skeletal muscle. Eur Cells Mater. 2011;22:109–123. doi: 10.22203/ecm.v022a09. [DOI] [PubMed] [Google Scholar]
- 26.Thevenot PT, Nair AM, Shen J, Lotfi P, Ko CY, Tang L. The effect of incorporation of SDF-1alpha into PLGA scaffolds on stem cell recruitment and the inflammatory response. Biomaterials. 2010;31:3997–4008. doi: 10.1016/j.biomaterials.2010.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drinnan CT, Zhang G, Alexander MA, Pulido AS, Suggs LJ. Multimodal release of transforming growth factor-beta1 and the BB isoform of platelet derived growth factor from PEGylated fibrin gels. J Controlled Release. 2010;147:180–186. doi: 10.1016/j.jconrel.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Hammers DW, Sarathy A, Pham CB, Drinnan CT, Farrar RP, Suggs LJ. Controlled release of IGF-I from a biodegradable matrix improves functional recovery of skeletal muscle from ischemia/reperfusion. Biotechnol Bioeng. 2012;109:1051–1059. doi: 10.1002/bit.24382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G, Nakamura Y, Wang X, Hu Q, Suggs LJ, Zhang J. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007;13:2063–2071. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 30.Ho TK, Tsui J, Xu S, Leoni P, Abraham DJ, Baker DM. Angiogenic effects of stromal cell-derived factor-1 (SDF-1/CXCL12) variants in vitro and the in vivo expressions of CXCL12 variants and CXCR4 in human critical leg ischemia. J Vasc Surg. 2010;51:689–699. doi: 10.1016/j.jvs.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Trivedi A, Lee JU, Lohela M, Lee SM, Fandel TM, et al. Matrix metalloproteinase-9 and stromal cell-derived factor-1 act synergistically to support migration of blood-borne monocytes into the injured spinal cord. J Neurosci. 2011;31:15894–15903. doi: 10.1523/JNEUROSCI.3943-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamed S, Egozi D, Dawood H, Keren A, Kruchevsky D, Ben-Nun O, et al. The chemokine stromal cell-derived factor-1alpha promotes endothelial progenitor cell-mediated neovascularization of human transplanted fat tissue in diabetic immunocompromised mice. Plast Reconstr Surg. 2013;132:239e–250e. doi: 10.1097/PRS.0b013e31829587e9. [DOI] [PubMed] [Google Scholar]
- 35.Ziaei R, Ayatollahi M, Yaghobi R, Sahraeian Z, Zarghami N. Involvement of TNF-alpha in differential gene expression pattern of CXCR4 on human marrow-derived mesenchymal stem cells. Mol Biol Rep. 2014;41:1059–1066. doi: 10.1007/s11033-013-2951-2. [DOI] [PubMed] [Google Scholar]
- 36.Ratajczak MZ, Majka M, Kucia M, Drukala J, Pietrzkowski Z, Peiper S, et al. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363–371. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- 37.Wragg A, Mellad JA, Beltran LE, Konoplyannikov M, San H, Boozer S, et al. VEGFR1/CXCR4-positive progenitor cells modulate local inflammation and augment tissue perfusion by a SDF-1-dependent mechanism. J Mol Med. 2008;86:1221–1232. doi: 10.1007/s00109-008-0390-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stokman G, Stroo I, Claessen N, Teske GJ, Florquin S, Leemans JC. SDF-1 provides morphological and functional protection against renal ischaemia/reperfusion injury. Nephrol Dial Transplant. 2010;25:3852–3859. doi: 10.1093/ndt/gfq311. [DOI] [PubMed] [Google Scholar]
- 39.Stroo I, Stokman G, Teske GJ, Florquin S, Leemans JC. Haematopoietic stem cell migration to the ischemic damaged kidney is not altered by manipulating the SDF-1/CXCR4-axis. Nephrol Dial Transplant. 2009;24:2082–2088. doi: 10.1093/ndt/gfp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011;129:97–108. doi: 10.1016/j.pharmthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- 42.Pelosi L, Giacinti C, Nardis C, Borsellino G, Rizzuto E, Nicoletti C, et al. Local expression of IGF-1 accelerates muscle regeneration by rapidly modulating inflammatory cytokines and chemokines. FASEB J. 2007;21:1393–1402. doi: 10.1096/fj.06-7690com. [DOI] [PubMed] [Google Scholar]
- 43.Coleman ME, DeMayo F, Yin KC, Lee HM, Geske R, Montgomery C, et al. Myogenic vector expression of insulin-like growth factor I stimulates muscle cell differentiation and myofiber hypertrophy in transgenic mice. J Biol Chem. 1995;270:12109–12116. doi: 10.1074/jbc.270.20.12109. [DOI] [PubMed] [Google Scholar]
- 44.Adams GR, McCue SA. Localized infusion of IGF-I results in skeletal muscle hypertrophy in rats. J Appl Physiol. 1998;84:1716–1722. doi: 10.1152/jappl.1998.84.5.1716. [DOI] [PubMed] [Google Scholar]
- 45.Lee S, Barton ER, Sweeney HL, Farrar RP. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J Appl Physiol. 2004;96:1097–1104. doi: 10.1152/japplphysiol.00479.2003. [DOI] [PubMed] [Google Scholar]
- 46.Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, et al. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 47.Chakravarthy MV, Booth FW, Spangenburg EE. The molecular responses of skeletal muscle satellite cells to continuous expression of IGF-1: implications for the rescue of induced muscular atrophy in aged rats. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S44–S48. doi: 10.1123/ijsnem.11.s1.s44. [DOI] [PubMed] [Google Scholar]
- 48.Merritt EK, Hammers DW, Tierney M, Suggs LJ, Walters TJ, Farrar RP. Functional assessment of skeletal muscle regeneration utilizing homologous extra-cellular matrix as scaffolding. Tissue Eng, Part A. 2010;16:1395–1405. doi: 10.1089/ten.TEA.2009.0226. [DOI] [PubMed] [Google Scholar]
- 49.Hammers DW, Matheny RW, Jr, Sell C, Adamo ML, Walters TJ, Estep JS, et al. Impairment of IGF-I expression and anabolic signaling following ischemia/reperfusion in skeletal muscle of old mice. Exp Gerontol. 2011;46:265–272. doi: 10.1016/j.exger.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cencioni C, Capogrossi MC, Napolitano M. The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc Res. 2012;94:400–407. doi: 10.1093/cvr/cvs132. [DOI] [PubMed] [Google Scholar]
- 51.Mirshahi F, Pourtau J, Li H, Muraine M, Trochon V, Legrand E, et al. SDF-1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thromb Res. 2000;99:587–594. doi: 10.1016/s0049-3848(00)00292-9. [DOI] [PubMed] [Google Scholar]
- 52.Cavalera M, Frangogiannis NG. Targeting the chemokines in cardiac repair. Curr Pharm Des. 2014;20:1971–1979. doi: 10.2174/13816128113199990449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moyer AL, Wagner KR. Regeneration versus fibrosis in skeletal muscle. Curr Opin Rheumatol. 2011;23:568–573. doi: 10.1097/BOR.0b013e32834bac92. [DOI] [PubMed] [Google Scholar]
- 54.Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, et al. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- 55.Kucia M, Ratajczak J, Ratajczak MZ. Bone marrow as a source of circulating CXCR4+ tissue-committed stem cells. Biol Cell. 2005;97:133–146. doi: 10.1042/BC20040069. [DOI] [PubMed] [Google Scholar]
- 56.Schmid MC, Avraamides CJ, Foubert P, Shaked Y, Kang SW, Kerbel RS, et al. Combined blockade of integrin-alpha4beta1 plus cytokines SDF-1alpha or IL-1beta potently inhibits tumor inflammation and growth. Cancer Res. 2011;71:6965–6975. doi: 10.1158/0008-5472.CAN-11-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tseng D, Vasquez-Medrano DA, Brown JM. Targeting SDF-1/CXCR4 to inhibit tumour vasculature for treatment of glioblastomas. Br J Cancer. 2011;104:1805–1809. doi: 10.1038/bjc.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma W, Liu Y, Ellison N, Shen J. Induction of C-X-C chemokine receptor type 7 (CXCR7) switches stromal cell-derived factor-1 (SDF-1) signaling and phagocytic activity in macrophages linked to atherosclerosis. J Biol Chem. 2013;288:15481–15494. doi: 10.1074/jbc.M112.445510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, Chemaly E, Liang L, Kho C, Lee A, Park J, et al. Effects of CXCR4 gene transfer on cardiac function after ischemia-reperfusion injury. Am J Pathol. 2010;176:1705–1715. doi: 10.2353/ajpath.2010.090451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koch KC, Schaefer WM, Liehn EA, Rammos C, Mueller D, Schroeder J, et al. Effect of catheter-based transendocardial delivery of stromal cell-derived factor 1alpha on left ventricular function and perfusion in a porcine model of myocardial infarction. Basic Res Cardiol. 2006;101:69–77. doi: 10.1007/s00395-005-0570-3. [DOI] [PubMed] [Google Scholar]
- 61.Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Melton DW, Porter L, Sarwar ZU, McManus LM, Shireman PK. Altered macrophage phenotype transition impairs skeletal muscle regeneration. Am J Pathol. 2014;184:1167–1184. doi: 10.1016/j.ajpath.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10:620–630. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 64.Mounier R, Theret M, Arnold L, Cuvellier S, Bultot L, Goransson O, et al. AMPKalpha1 Regulates Macrophage Skewing at the Time of Resolution of Inflammation during Skeletal Muscle Regeneration. Cell Metab. 2013;18:251–264. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 65.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 66.Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc. 1995;27:1022–1032. doi: 10.1249/00005768-199507000-00011. [DOI] [PubMed] [Google Scholar]
- 67.Brechot N, Gomez E, Bignon M, Khallou-Laschet J, Dussiot M, Cazes A, et al. Modulation of macrophage activation state protects tissue from necrosis during critical limb ischemia in thrombospondin-1-deficient mice. PLoS One. 2008;3:e3950. doi: 10.1371/journal.pone.0003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, Venereau E, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–563. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y, Wehling-Henricks M, Samengo G, Tidball JG. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell. 2015;14:678–688. doi: 10.1111/acel.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hammers DW, Rybalko V, Merscham-Banda M, Hsieh PL, Suggs LJ, Farrar RP. Anti-inflammatory macrophages improve skeletal muscle recovery from ischemia/reperfusion. J Appl Physiol. 2015;118:1067–1074. doi: 10.1152/japplphysiol.00313.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukocyte Biol. 2013;93:875–881. doi: 10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]