Abstract
Peripheral vascular disease (PVD) is one of the most prevalent vascular diseases in the U.S. afflicting an estimated 8 million people. Obstruction of peripheral arteries leads to insufficient nutrients and oxygen supply to extremities, which, if not treated properly, can potentially give rise to a severe condition called critical limb ischemia (CLI). CLI is associated with extremely high morbidities and mortalities. Conventional treatments such as angioplasty, atherectomy, stent implantation and bypass surgery have achieved some success in treating localized macrovascular disease but are limited by their invasiveness. An emerging alternative is the use of growth factor (delivered as genes or proteins) and cell therapy for PVD treatment. By delivering growth factors or cells to the ischemic tissue, one can stimulate the regeneration of functional vasculature network locally, re-perfuse the ischemic tissue, and thus salvage the limb. Here we review recent advance in nanomaterials, and discuss how their application can improve and facilitate growth factor or cell therapies. Specifically, nanoparticles (NPs) can serve as drug carrier and target to ischemic tissues and achieve localized and sustained release of pro-angiogenic proteins. As nonviral vectors, NPs can greatly enhance the transfection of target cells with pro-angiogenic genes with relatively fewer safety concern. Further, NPs may also be used in combination with cell therapy to enhance cell retention, cell survival and secretion of angiogenic factors. Lastly, nano/micro fibrous vascular grafts can be engineered to better mimic the structure and composition of native vessels, and hopefully overcome many complications/limitations associated with conventional synthetic grafts.
Keywords: peripheral vascular disease, nanomaterials, nanoparticles, growth factor, gene delivery, cell therapy, stem cells, tissue engineering, nanomedicine, critical limb ischemia, therapeutic angiogenesis, vascular graft
Graphical Abstract
Peripheral Vascular Disease (PVD): Current Treatments and Challenges
Peripheral vascular disease (PVD) is one of the most prevalent vascular disorders in the U.S., afflicting over 8 million people. The prevalence of PVD increases with age and affects 12–20% of the American population age 65 and over.1 In general, PVD refers to the obstruction or narrowing of the nonmyocardial arteries, most commonly the lower extremities but including the vasculature of kidney and other vascularized organs. The resulting lack of blood flow gradually deprives the tissue of oxygen and nutrients causing symptoms such as claudication, sores, ulcers and skin color change of affected limbs. There is a considerable risk of limb loss if effective interventions are not administered in time, giving rise to a condition known as critical limb ischemia (CLI).2 The annual incidence of CLI is estimated between 5 and 10 new cases per 10 000 in both the U.S. and Europe, with type-2 diabetes as one of the most important risk factors.3 Symptoms associated with CLI include skin lesions (ulcers or gangrene) and rest pain, both of which can significantly compromise a patient’s quality of life. CLI is associated with extremely high mortalities and morbidities. Studies have shown that 30% of patients not eligible for surgical revascularization will undergo major amputation and 25% of these patients will die within one year of the onset of CLI.4
To date, numerous strategies have been devised to restore blood perfusion in ischemic tissues and thus relieve rest pain, heal ulcers and prevent limb loss. Current treatments, such as angioplasty, atherectomy, stent implantation and bypass surgery can be effective in cases of localized macrovascular disease. Unfortunately, these conventional treatments are limited in several ways. First, the invasiveness of mechanical revascularization often renders them inapplicable to a subset of PVD patients who are physically unsuitable to undergo major surgeries.5 Second, for bypass surgeries, the use of autologous vascular grafts is limited by the physical condition of the patient, while using conventional synthetic grafts is associated with risks such as thrombosis, infection and hyperplasia.6 Lastly, for CLI patients, it is more important to achieve fast and short-term results to avoid limb loss, whereas targeting macrovascular disease may not bring immediate benefits.
Significant progress has been made to avoid such surgical interventions, by administrating pro-angiogenic growth factors (delivered either as proteins or as genes that encode them)7 and/or cell therapy (e.g., endothelial progenitor cells or other angiogenic stem cells)8 to stimulate and promote angiogenesis in ischemic tissues. For instance, clinical studies showed that intramuscular injection of hepatocyte growth factor (HGF) plasmids improved blood perfusion (measured by increase in ankle-brachial index (ABI) from 0.46 to 0.59) and reduced ischemic ulcer area by >25% in CLI patients.9 Clinical improvements in CLI patient symptoms were also reported upon intramuscular injection of both autologous bone marrow mononuclear cells (BM-MNCs) and vascular endothelial growth factor (VEGF) plasmids, leading to improved perfusion (ABI increased from 0.26 to 0.49) and reduction in rest pain.10
Despite these promising initial results, some recent phase II and phase III clinical trials of angiogenic gene therapy did not generate consistent benefits as expected.11 The strategy of direct injection of naked plasmids carrying angiogenic genes is ineffective due to low cellular transfection efficiency. Using viral vectors to deliver the genes can overcome the low transfection efficiency but raises safety concerns due to random insertion into the host cell genome. Additionally, integration of genes that lead to persistent expression of angiogenic factors raises the concern for developing pathological angiogenesis or tumorigenesis.11 Alternatively, using recombinant proteins is less likely to cause such long-term safety issues. However, growth factors generally have short circulation half-lives, requiring multiple injections to achieve sufficient and sustained growth factor levels at the ischemic site. Multiple injections of angiogenic growth factors may cause adverse effects such as hypotension,12 vascular leakage13 and tissue edema.14 Current cell therapy methods are also facing some potential challenges such as low cell retention, low viability post-transplantation and limited integration into host tissue.15
Opportunities and Promises: Nanoscale Strategies for PVD
The rapid development of nanotechnology in the past two decades has brought about enormous opportunities to the field of biomedical studies and applications. In particular, nanomedicine, referring to the medical application of nanotechnologies, is attracting intense activity. Nanoscale strategies offer new capabilities that are otherwise impossible to achieve. In the context of nanomedicine, two types of nanomaterials are most extensively used: nanoparticles and nanofibers. Nanoparticles (NPs) of a size from tens to several hundred nanometers can be easily endocytosed and have been serving as drug carriers for targeted delivery and/or imaging contrast agents for diagnostic and therapeutic purposes. The versatility of polymer-based NPs allows one to tailor physical and chemical properties to design multifunctional NPs, exhibiting the desired pharmacokinetic profile.16–18 Due to the high surface area to volume ratio, NPs are ideal for surface coating and modification to accommodate various therapeutic needs. For example, antibodies can be conjugated to NPs for targeted delivery and weaken off-target effects.19–21 Generally, NPs are used to protect their cargo (e.g., proteins or siRNAs) from undesired degradation, prolonging the half-life of drugs and making oral delivery of siRNAs and proteins feasible;22–26 however, NPs can be tuned to respond to specific physical/chemical cues such as pH27 and oxidative stress28 in order to release their cargo. Alternatively, nanofibers have been broadly studied, especially in the field of tissue engineering. Electrospun nanofibers are attractive because they can closely mimic the native, nanofibrous extracellular matrix (ECM) environment in which cells reside. The natural nanotopographies of ECM, just like biological or chemical cues, are crucial for the maintenance of cell phenotype and cell growth. To construct these nano-features in vitro, fabrication conditions can be tuned to obtain nanofibrous scaffolds of different fiber diameters, porosity, mechanical properties and orientations.29 In addition, nanofibers have large specific surface area that allows them to efficiently load various biomolecules (e.g., VEGF and PDGF) to encourage cell attachment and enhance angiogenic effects.
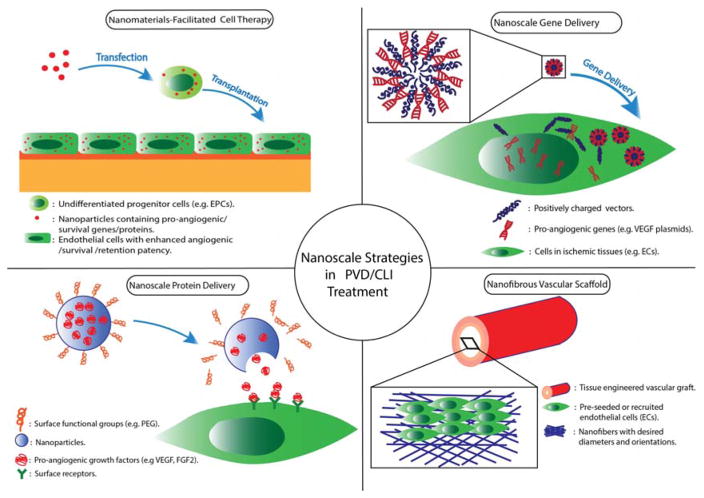
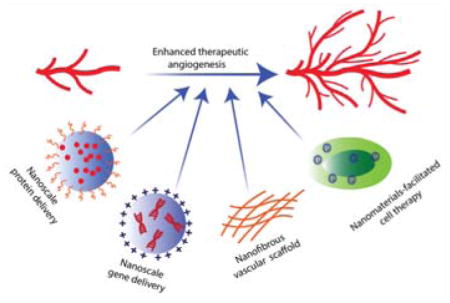
In this manuscript, we will focus on recent progress in nanomedicine for treating PVD, focusing on therapies for CLI. Specifically, we will review four nanoscale strategies (outlined in Figure 1). (1) As protein carriers, NPs can deliver angiogenic growth factors into ischemic tissue, reducing undesirable degradation and off-target effects, increasing the effective drug concentration and lowering the dosage requirements. (2) As gene carriers, NPs can effectively overcome the cell membrane barrier and release their cargo inside cells with relatively fewer safety concerns as compared to viral vectors. (3) Nanomaterials can be used to facilitate existing cell therapy strategies by preprogramming cells to increase their angiogenic or survival capabilities. (4) Tissue engineered nanofibrous vascular scaffolds are biocompatible and can be potentially produced on a large scale making them a promising alternative to synthetic grafts.
Figure 1.
Schematic illustration of nanoscale strategies in the treatment of peripheral vascular disease or critical limb ischemia.
Nanoscale Protein Delivery
Over the past decade, research and clinical studies have focused on using pro-angiogenic growth factors or genes to promote angiogenesis of ischemic tissues. Bolus injection of growth factors rarely generates satisfying clinical outcomes in part because of their short circulation half-life (in the order of several minutes).30 Yet, high sustained levels of angiogenic signals are essential for the development of stable neovascularization,31 requiring intramuscular or intra-arterial delivery of large growth factor dosages.32 This drawback of protein-based therapy can be overcome by the use of nanoscale devices. Encapsulating growth factors in nanocarriers protects them from undesired degradation, allows targeted delivery to the ischemic tissue, and enables their release in a controlled manner, leading to stronger therapeutic effects, potentially at lower dosages.
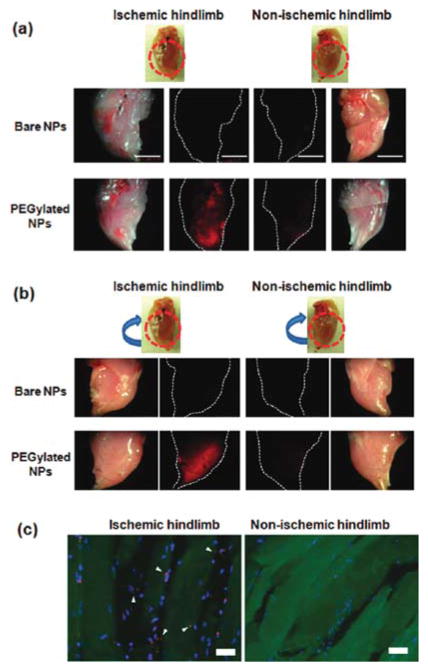
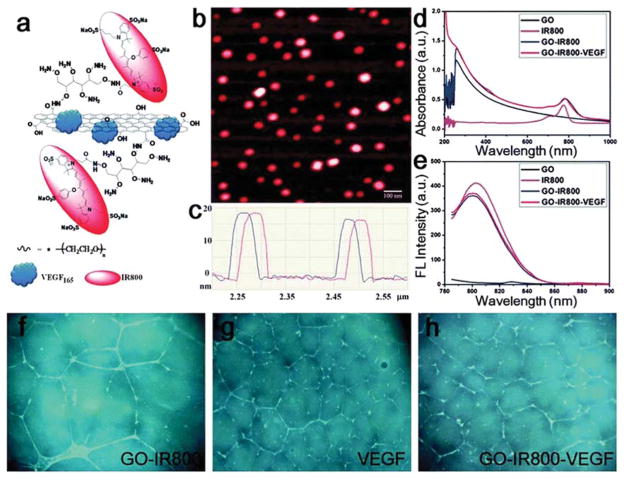
To date, a variety of nanomaterials have been employed to deliver growth factors to ischemic tissues, including poly(lactic-co-glycolic acid) (PLGA),33 PLGA: poloxamer blend NPs,34 gold NPs35 and graphene oxide36 (summarized in Table 1). Even without incorporation of specific antibodies or targeting molecules, it has been shown that upon intravenous injections to mouse models, NPs (<200 nm) would preferentially accumulate in ischemic limb than in healthy limb (Figure 2).35,36 Ischemic tissues, including tumor tissues, tend to secrete angiogenic factors which increase blood vessel permeability, leading to preferential NP accumulation. This well-known phenomenon is called the enhanced permeability and retention (EPR) effect.37 More interestingly, VEGF-coated graphene oxide NPs showed significantly higher targeting efficiency than empty NPs, implying that VEGF coated on the surface of the particles may not only act as a therapeutic reagent, but also serve as a targeting moiety,36 presumably due to overexpression of VEGF receptors on the cell surface comprising ischemic regions (Figure 3). With this intrinsic targeting capability, NPs carrying growth factors can be administered via intravenous injection, a less invasive and more convenient approach compared to intramuscular injection.
TABLE 1.
NPs for Growth Factor Delivery
| materials | cargo | size (nm) | delivery route | study model | dosage | outcome | source |
|---|---|---|---|---|---|---|---|
| Gold NPs | VEGF165 | 124 | Tail vein injection | Mouse hindlimb ischemia model | 3 μg | 1.7-fold increase in blood perfusion compared to the control mice | 35 |
| Graphene oxide (GO) NPs | VEGF165 | 20–50 | Tail vein injection | Mouse hindlimb ischemia model | 3 μg | 1.5-fold increase in blood perfusion compared to the control mice | 36 |
| PLGA NPs | VEGF165 | 200–600 | Thigh adductor muscle injection | Mouse hindlimb ischemia model | 420 ng | 2-fold increase in blood vessel connectivity and >3-fold increase in vessel volume compared to saline control | 33 |
| Dextran-co-gelatin NPs | VEGF165 | 130 | Thigh muscle injection | Rabbits hindlimb ischemia model | 1 mg | VEGF NPs restored blood perfusion to 85% of the healthy limb compared to 60% of free VEGF | 38 |
| Proteoliposomes | Syndecan-4 + FGF-2 | 400 | Intra-arterial delivery with osmotic pump | Rats hindlimb ischemia model | 5 μg of FGF-2 | Co-delivery of syndecan-4 proteoliposomes with free FGF-2 restored blood perfusion to almost 100% | 41 |
| PLGA: Poloxamer blend NPs | PDGF-BB or FGF-2 | 150 | NA | In vitro cell study | NA | Sustained release in vitro, increased the viability of bovine endothelial cells by 3-fold at 48 h | 34 |
Figure 2.
PEGylated Cy5.5-labeled silica nanoparticles (Cy-SiNPs) preferentially accumulated in ischemic tissue upon intravenous injection in mouse, as evidenced by fluorescent imaging on the front side (a) and the opposite side (b) of limb tissue as well as the fluorescent images of cryosectioned tissue (c), where NPs were red and nuclei were stained blue. Notably, same targeting effects were not observed with bare NPs. Reprinted with permission from ref 35. Copyright 2011 American Chemical Society.
Figure 3.
Graphene oxide nanoparticles (GO) loaded with VEGF by physical adsorption and conjugated to IR800, a commonly used near-infrared fluorescent dye, for VEGF delivery and imaging. (a) Schematic structure of IR800-VEGF-GO; (b) atomic force microscopy (AFM) images and (c) the height profile of IR800-GO (without VEGF); (d) absorption and (e) emission spectrum of various GO NPs. In vitro tube formation assay with (f) GO-IR800, (g) free VEGF, and (h) GO-IR800-VEGF, using human umbilical vascular endothelial cells (HUVECs). Reprinted from ref 36 with permission. Copyright 2013 Royal Society of Chemistry.
The versatility of NPs allows one to tailor their physical and chemical properties to design a specific release kinetic profile. A zero-order release kinetics profile is preferred to achieve a sustained growth factor concentration at the ischemic site without the need for multiple injections and to allow for the stabilization of newly formed vessels. Dextran-co-gelatin NPs are able to achieve near zero-order release, with 69% VEGF released over 10 days in vitro.38 Mesoporous silica NPs released basic fibroblast growth factor (bFGF) steadily for over 20 days in vitro, with 50% released in the first 8 days.39 In contrast, PLGA NPs showed a burst release profile, with 70% of encapsulated VEGF released within 2 days in vitro.33 However, the in vitro release profile may not precisely recapitulate the in vivo release kinetics40 given the biological and chemical complexity of the in vivo environment. Monitoring growth factors concentration in blood samples taken from the ischemic site should provide greater insight into the pharmacokinetics. Notably, growth factors may not require NP encapsulation to be functional; instead they can be conjugated onto the NP surface. Conjugating VEGFs covalently to gold NPs via gold–thiol bonds has demonstrated maintenance of VEGF bioactivity and stimulated endothelial cell growth to form new blood vessels.35 In this way, the undesired degradation of growth factors can be reduced, increasing the effective concentration at ischemic site.
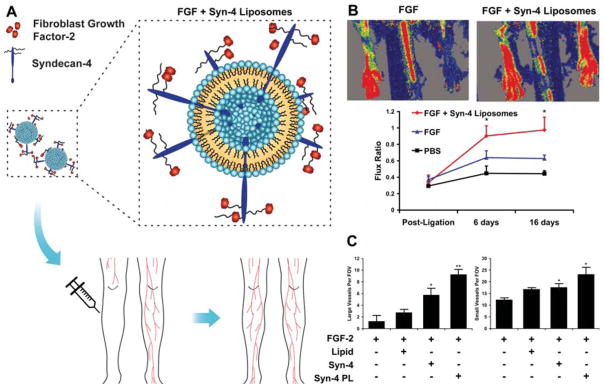
NP-based delivery of growth factors has yielded promising results in animal models. Intramuscular injection of dextran-co-gelatin NPs encapsulating 1 mg VEGF in total restored blood perfusion in ischemic tissue to 85% of the healthy tissue in a rabbit hind limb ischemia model.38 Intravenous injection of only 3 μg of VEGF conjugated on gold NPs improved blood perfusion of the ischemic limb by 1.7-fold, reaching over 90% blood perfusion of normal tissue whereas bolus injection of the same amount of free VEGF did not result in any significant improvements.35 Similarly, delivery of VEGF (3 μg) by graphene oxide NPs increased blood perfusion by 1.5-fold as measured by Doppler scanning.36 Liposomal codelivery of FGF-2 with syndecan-4, which is an important regulator of FGF-2 signaling, strengthened the cellular signaling responses to FGF-2, leading to increased FGF-2 uptake, and an 80% improvement in blood flow compared to delivery of FGF-2 alone (Figure 4).41 Particularly, this strategy not only increased the density of small vessels, it also significantly increased the number of large vessels of ischemic muscle. Co-morbidities including diabetes and hyperlipidemia can reduce the effectiveness of growth factor therapy.42 Moreover, codelivery of syndecan-4 proteoliposomes with FGF-2 increased the effectiveness of FGF-2 in diabetic mice to partially overcome this growth factor resistance.42
Figure 4.
(A) Schematic diagram of syndecan-4 and FGF-2 co-delivery using liposomes into ischemic tissue for effective revascularization. (B) Laser Doppler images of the rat ischemic hind limbs 14 days after induction of ischemia through femoral artery ligation. The quantification of blood flow at days 0, 7, and 14 are shown below. *Statistically different from all other groups (P < 0.05). (C) Quantification of large vessel number per field of view. Quantification of capillary number per field of view. *Statistically different from FGF group (P < 0.05). **Statistically different from all other groups (P < 0.05). Reprinted from ref 41 with permission. Copyright 2012 National Academy of Sciences of the United States of America.
Despite encouraging preclinical results, more rigorous studies are still required to fully understand the utility and limitations of nanoscale protein delivery. Most studies demonstrated the efficacy in healthy animal models of acute ischemia and neovascularization. On one hand, there is no doubt that rapid angiogenesis is critical for CLI patients. On the other hand, however, researchers have rarely, if ever, evaluated the long-term patency of the newly regenerated vasculature. Specifically, it is possible that as the supply of exogenous growth factors is depleted, neovasculature could potentially regress over time.43 Additionally, given that angiogenesis is a complex physiological process involving the coordination of several cell types, current methods are relatively simplistic, delivering one or two factors at most. In the future, multiplexed NPs carrying multiple enzymes and growth factors with optimum stoichiometry and sequential release may be developed to further enhance the angiogenic effects.44
Nanoscale Gene Delivery
One advantage of protein delivery over gene delivery is that proteins are readily bioactive and can directly act on their target cell membrane receptors, whereas genes require a complex process of delivery inside the cells and translocation into the nucleus to be transcribed and translated for expression. On the other hand, the delivered proteins will gradually be consumed, whereas genes can constitutively produce large amount of proteins so that sustained levels of growth factors can potentially be obtained without the need for multiple injections. Thus, a proper vector that can escort genes into the cells is crucial for the success of any gene therapy. Recently, the CRISPER/Cas9 system has revolutionized the field of gene delivery and gene editing, exploiting the cell’s immune system to activate or block specific genes. Yet, this systems requires adenovirus to deliver the Cas9 protein and associated RNA or synthetic transcription factors into the cell.45 Viral or adenoviral vectors are commonly used for their high transfection efficiency in research but they are associated with safety issues such as eliciting immune responses and/or causing insertional mutagenesis of host cells,46–48 which have been the major bottleneck of translating this technology into clinical settings.
Alternatively, nonviral vectors such as polymers and lipids are attractive owing to their relatively low toxicity and design flexibility. However, they are also generally less effective than viral vectors. To achieve efficient and effective gene delivery, numerous barriers, both intracellular and extracellular ones, need to be overcome.49 The nanocarrier needs to navigate through the bloodstream, protect the DNA from degradation by serum DNases, avoid being taken up by phagocytic cells or the reticuloendothelial system (RES), target to the specific cell type at the specific site, enter the target cell through internalization, escape from the endosome into the cytoplasm, and eventually translocate into nucleus and release the cargo. Additionally, other design targets also need to be met, including inexpensive synthesis, low toxicity and ease of manufacturing.49
To date, a wide variety of materials have been used for gene delivery, including polymeric materials (synthetic or natural) such as polyamidoamine dendrimers (PAMAM),50,51 polyethylenimine (PEI),52–54 carbohydrate-based polymers55,56 and peptides,57,58 inorganic materials such as gold NPs,59–61 silica NPs62,63 and carbon nanotubes,64 as well as numerous cationic lipid materials65–67 (summarized in Table 2). In general, different nanomaterials offer various features and advantages, but no single nanomaterial or design readily satisfies all the design targets mentioned above. Thus, it is critical to systematically compare and select appropriate nanomaterials for specific applications. For instance, PEI is a commonly used costeffective gene transfection reagent. It forms polyplexes with DNA via electrostatic interactions and has reasonably high transfection efficiency in vitro, partly due to “proton sponge” effects.68,69 However, PEI (especially high molecular weight PEI) has known cytotoxicity70 and poor biodistribution, with about 50% of the injected dosages accumulated in liver,69 making it less ideal for clinical use. Cationic lipid NPs are highly versatile in composition, architecture and fabrication methods. Cationic lipids consist of a covalently bound, positively charged, hydrophilic headgroup with a hydrophobic tail domain. By combining different lipids, a large library of agents with varying transfection activities and cytotoxicity are possible.71,72 Folate modified lipoplexes demonstrated greater resistance to serum DNase and showed efficient gene delivery both in vitro and in vivo.73,74 However, significant cytotoxic effects have been associated with cationic lipids, in part due to their positively charged hydrophilic head groups (e.g., quaternary and tertiary ammoniums).75 Gold NPs have also emerged as effective gene delivery vectors.59 The attractive properties of gold NPs include surface plasmon resonance, controllable reactivity with thiolgroups and ease of synthesis. Ghosh et al. reported that amino acids functionalized onto cationic gold NPs can complex with DNA and effectively enter cells, respond to intracellular glutathione level and subsequently release the DNA.61
TABLE 2.
NPs for Plasmid Delivery
| materials | cargo | size (nm) | delivery route | study model | dosage | outcome | source |
|---|---|---|---|---|---|---|---|
| Magnetic DNA-gelatin nanospheres | pDNA (VEGF) | 5–20 | Iliac artery injection | Rabbit hindlimb ischemia model | 200 μg plasmids | 60% increase in vessel density compared to untreated group | 76 |
| PLGA NPs | pDNA (VEGF) | 120–260 | Skeletal muscle injection | Mouse limb ischemia model | 8 μg plasmids | 30% higher in vivo VEGF expression and 60% higher capillary density than PEI-pVEGF treated group | 77 |
| PEG liposomes | pDNA (bFGF) | <200 | Skeletal muscle injection | Mouse hindlimb ischemia model | 10 μg plasmids | 70% blood flow increase, 60% increase in capillary density compared to naked DNA | 79 |
| PEG liposomes containing DOTAP | pDNA (bFGF) | 532 | Tail vein injection | Mouse hindlimb ischemia model | 50 μg plasmids | 70% increase in blood flow rates compared to untreated group | 78 |
| Peptides-DNA NPs | pDNA (HIF-1α) | 43–204 | Dermal application (NPs encapsulated in 3brin matrix) | Mouse wound model | 10 μg plasmids | 100% increase in number of CD31+vasculature structures compared to empty 3brin gels. | 57 |
| Heparinized Chitosan/poly(γ-glutamic acid) NPs | pDNA (bFGF) | 256 | NA | In vitro tube formation study | NA | pH responsive release; In vitro tube formation increased 1.5-fold compared to naked DNA | 58 |
Nanoscale gene delivery strategies, aimed to treat peripheral ischemia, have shown encouraging pre-clinical results. Intra-arterially injected, magnetic, gelatin nanospheres complexed with VEGF plasmid (5–20 nm) were magnetically guided to the ischemic site in a rabbit hindlimb ischemia model, resulting in 50% increase in blood vessel density compared to empty nanospheres.76 Similarly, intramuscular injection of PLGA NPs encapsulating VEGF plasmid increased capillary density by 2.6-fold compared to the untreated group in a mouse hind limb ischemia model, whereas PEI-DNA NPs only lead to a 1.4-fold increase in capillary density.77 This difference could partially be explained by the higher transfection efficiency of PLGA NPs than PEI as evidenced by VEGF expression in mouse limb. Notably, PEI was cytotoxic, causing a 4-fold increase in cell apoptosis than PLGA NPs.77 Another promising methodology is to combine ultrasound and nanocarriers.78,79 Utilizing NPs formulated to engulf gas bubbles, ultrasound can be employed for imaging and tracking the NPs as well as augmenting the intracellular delivery of materials. PEG-liposomes (200 nm) entrapping perfluoropropane gas as echo-contrast for the delivery of bFGF gene leads to 65% increase in blood flow in mouse ischemic limb compared to <40% with naked bFGF plasmid.79 Similarly, intravenous injection of PEGylated-perfluoropropane gas liposomes loaded with miRNA-126 (a negative regulator of VEGF inhibitors) leads to 30% increase in blood flow in mouse ischemic limb.80
Nanomaterial Facilitated Cell therapy
Cell therapy is an attractive alternative approach to achieve therapeutic angiogenesis with several unique advantages over growth factor therapy or gene therapy. First, transplanted cells may serve as a lasting source of multiple angiogenic growth factors so that stable neovasculature can be formed, whereas directly injected growth factors are subject to quick degradation. Second, transplanted cells can release growth factors and cytokines in a more balanced and physiologically relevant manner than gene therapy. Third, the implanted cells can differentiate and provide the mechanical and structural support for angiogenesis.81 Lastly, using autologous cells can circumvent the host immune response to gene therapy which can be lethal when administering viral vectors.82
Adult stem cell transplantation, using bone marrow mononuclear cells (BMMNCs)8 and mesenchymal stem cells (MSCs),83 have been most frequently employed to clinically treat CLI patients. BMMNCs are a mixture of cells containing around 1% CD34+ endothelial progenitor cells (EPCs).84 MSCs are multipotent cells that can be found in bone marrow, adipose tissue, umbilical cord blood and placenta,85 able to differentiate into chondrocytes,86 osteoblasts,87 adipocytes,87 cardiomyocytes88 and endothelial cells89 when guided with proper chemical/biological/mechanical cues. Using autologous BMMNCs or MSCs can minimize the risks of host immune responses. Cell therapies with BMMNCs and MSCs have achieved some encouraging clinical outcomes in the treatment of CLI. Intramuscular injection of 5.8 × 107 BMMNCs (9.8 × 106 CD34+ cells) to CLI patients resulted in significant improvements by increasing mean ABI from 0.26 to 0.41, mean transcutaneous oxygen pressure (TcpO2) from 28 to 52 mmHg and by reducing ulcer healing time over the period of 6 months.90 In a phase I study, intra-arterial injection of MSCs to CLI patients significantly increased ABI from 0.56 to 0.67 and TcpO2 from 13 to 38 mmHg.91 The therapeutic effects of adult stem cell therapy are not fully understood. In general, two mechanisms have been proposed. One possible explanation is that the observed therapeutic effects are due to paracrine and immunomodulatory effects. This is supported by a study showing that MSC-conditioned medium facilitated angiogenesis in a diabetic rat mode.92 A second proposed mechanism is cell replacement and engraftment. In this case, progenitors cell are believed to differentiate into endothelial cells and directly contribute to the formation of new blood vessels.93 Depending on disease model and cell type, the therapeutic mechanisms of cell therapy may be different.94
In spite of the encouraging preclinical/clinical outcomes, cell therapy is not without challenges. For instance, the BMMNCs utilized in clinical trials are generally a mixture of several cell populations that lack complete characterization,95 making it especially difficult to study or understand the underlying mechanisms and also raising concerns regarding the quality control of these cells. Using more differentiated cells is limited by the lack of in vitro expansion capacity, whereas less differentiated stem or progenitor cells are more proliferative but need to be properly guided to the desired differentiation pathway. Additionally, cell therapy strategies face several universal challenges, including insuffcient expression of desired growth factors, low cell viability and low engraftment in host tissue.
The application of nanomaterials can help overcome some of the aforementioned obstacles and markedly augment the benefits of cell therapy. Specifically, nanomaterials can serve as nonviral transfection vectors and program cells for enhanced viability, higher expression of angiogenic factors, as well as better cell retention. Yang et al. employed biodegradable poly(β-aminoester) (PBAE) NPs to deliver VEGF plasmid into MSCs. Intramuscular injection of these high VEGF expressing cells into a mouse hindlimb ischemia model led to a 2- to 4-fold increase in vessel densities and markedly decreased muscle degeneration and tissue fibrosis compared to injection of nontransfected cells.96 Similarly, myoblast cell sheets that were genetically engineered with PBAE-VEGF plasmid NPs successfully protected 5 out of 7 mice from limb loss and prevented the development of necrosis in a hindlimb ischemia model.97 Alternatively, miRNA delivery has been used to enhance the angiogenic effects of cell therapy. Gomes et al. formulated multilayered NPs that can be used simultaneously for miRNA delivery and cell tracking.98 The core consisted of PLGA and perfluoro-1,5 crown ether (PFCE) for MRI tracking and the surface was coated with protamine sulfate (PS), a cationic peptide for miRNA adsorption.98 These multifunctional NPs showed effective delivery (50%–90% transfection efficiency) of pro-survival/angiogenic miRNAs (miR132 and miR424) into endothelial cells (ECs). Endothelial cells engineered with NP-miRNAs exhibited a 3-fold increase in cell survival and 3.5-fold increase in blood perfusion of ischemic mouse limb relative to limbs treated with cells alone. Nanomaterials can also be used to guide the injected cells to the ischemic site. Intravenously injected EPCs magnetized with polystyrene-copolymer NPs containing iron oxide could target to ischemic site and augment blood perfusion by 40% under external magnetic forces.99 Similarly, an 80% improvement in in vivo homing of EPCs to ischemic site was reported upon transfection with magnetic NPs (isolated from Magnetospirillum magneticum strain AMB-1).100 The increased EPC homing resulted in a 25% improvement in blood perfusion compared to untreated cells. It should be noted that the homing of cells (e.g., EPCs and MSCs) to sites of ischemia (or tumorigenesis) is a naturally occurring process facilitated by chemokines (e.g., SDF-1) and growth factors (e.g., VEGF and HGF) released from ischemic tissue.101 Therefore, to some degree, the injected therapeutic cells may preferentially target to the ischemic limb than the healthy limb even in absence of active targeting. In the future, it would be interesting to investigate the efficacy of natural recruiting by chemical cues in comparison to active targeting by physical cues and the potential synergistic effects between them.
The increased understanding of various cell surface receptors, their corresponding ligands, and downstream signaling networks, allows researchers to employ nanoscale strategies to manipulate cell surface composition/structures for enhanced cell viability, improved homing to the target tissue and stronger therapeutic effects upon transplantation (as previously reviewed).102 Recently, Cheng et al.103 developed bifunctional iron based NPs coated with carboxylated dextran. The dextran coating allowed conjugation of two antibodies: one to target the ischemic heart tissue (myosin light chain) and one to target exogenous/endogenous MSCs (CD45). Thus, the iron NPs had several roles: first, to guide implanted cells to the injury site, second, to link these cells to injured tissue and third, to allow imaging of implanted cells. The enhanced therapeutic effect of increasing the number of implanted cells at the injury site was demonstrated in a myocardial infract rat model but can be easily applied toward CLI treatment by replacing the myosin light chain antibody, with SDF-1 for example. In this study NPs and MSCs were injected separately at different time points to demonstrate homing of circulating MSCs. However, it would be interesting to examine the therapeutic effect that primed MSCs, i.e. preconjugating MSCs with iron NPs, would have. Homing of MSCs was also controlled by converting the CD44 glycoform on their cell surface to E-selectin/L-selectin104 or by conjugating new receptors such as CXC chemokine receptor 4 (CXCR4), the receptor for SDF-1.105 In the latter, modified MSCs migrated toward an SDF-1 gradient, offering a new opportunity for targeting cell therapy to ischemic tissue. Alternatively, transfection of ischemic tissue with SDF-1 containing liposomes increased homing of CXCR4 expressing progenitor endothelial cells, resulting in improved neovascularization in a chronic ischemic hindlimb model.106 By developing cell-NP hybrids, cell viability and function following implantation can potentially be enhanced. Conjugating chitosan NPs to cells prior implantation can scavenge reactive oxygen species (ROS) generated during ischemia and thus improve the viability of transplanted cells as was shown for chitosan hydrogels.107 Coupling of cytokines containing NPs to the surface of implanted cells can elicit autocrine effects as was recently shown for maleimide–thiol conjugated liposome–T cells, exhibiting enhanced antitumor activity.108 Overall, cell surface engineering is a very versatile methodology that can exercise greater precision in cell targeting and cell homing processes (e.g., ligand–antigen binding, adhesion and migration) with relatively fewer long-term safety risks compared to genetic cellular engineering.
In terms of clinical application, comprehensive studies should be performed to compare the relative safety and efficacy of available methods. The safety of liposome conjugation (100–300 nm) to the surface of T cells was thoroughly evaluated by Mathias et al.108 both in vivo and in vitro. Yet, in the majority of the aforementioned studies, the effect of NPs was only evaluated in regard to their cytotoxicity in vitro. The potential in vivo side effects of NPs as well as long-term effects on cellular behavior (such as effect on stem cell ability to normally differentiate and function) need to be carefully evaluated (as further discussed in the From Bench to Bedside: A Long-Lasting Endeavor section).
Nanoscale Strategies in Tissue Engineered Vascular Grafts
Unlike angiogenic therapies that are intended to regenerate microvasculature at ischemic sites, a vascular graft aims to directly replace the diseased/blocked arteries. Conventional synthetic vascular grafts have been used for decades.109 Some success has been achieved with synthetic materials such as expanded polytetrafluoroethylene (ePFTE) and polyester (Dacron) as bypass conduits for relatively large vessels (>6 mm diameter). However, as small vessel replacements, synthetic grafts have poor outcomes with the primary causes being anastomotic intimal hyperplasia and thrombogenicity.6 A possible cause for development of hyperplasia can be perturbations in blood flow as a result of mechanical properties mismatch between the vascular graft and the native vessel. Additionally, since synthetic grafts do not have an intact layer of endothelial cell coverage, the lumenal surfaces are directly exposed to blood circulation. Thus, serum proteins such as albumin and fibrinogen tend to adsorb onto these synthetic materials and initiate blood coagulation cascades and immune responses. The thrombosis and inflammation can further disrupt the blood flow and contribute to the development of hyperplasia, leading to the ultimate occlusion of these small synthetic vascular grafts. Further, synthetic grafts (both large and small ones) are subject to the risk of infection,110 in part due to the fact that protein deposition on surfaces can promote bacteria adhesion and growth.
Vascular tissue engineering is aimed to meet the demand for biocompatible vascular grafts that can seamlessly integrate with native vasculature.111 In contrast to acellular synthetic grafts, tissue engineered vascular grafts (TEVGs) are either preseeded with autologous cells to form an intact layer of endothelium or actively recruit native tissue to grow in after implantation. Covered by endothelium composed of autologous ECs, TEVGs can avoid direct exposure of foreign materials and thus avoid serum protein deposition/biofouling. Therefore, TEVGs are expected to be less prone to induce blood coagulation, less likely to initiate host immune responses, and less susceptible to bacterial infection.
The ideal TEVG should mimic both the structure and composition of native blood vessels. In native blood vessels, ECs are aligned along the direction of blood flow to form an intact, interconnected layer of endothelium supported by basement membrane. The endothelial basement membrane provides mechanical support and also serves as reservoir of growth factors which are released to modulate ECs during basement membrane remodeling.112 Major components of basement membrane such as collagen fibers and polysaccharides have nanosize features. Aortic heart valve basement membrane exhibited sub-100 nm range features (e.g., fiber diameter and pore size).113 Furthermore, it has been shown that EC behavior, specifically cell morphology, cell adhesion and cell proliferation, is sensitive to small changes in nanotopographies. Polystyrene (PS) and poly(4-bromostyrene) (PBrS) demixed nano islands of different heights (13, 35, and 95 nm) significantly increased ECs cell spreading and number of arcuate-shaped cells compared to flat surfaces of the same chemistry, and the 13 nm islands resulted in significantly larger cell spreading than 35 or 95 nm islands.114 Human umbilical cord vein endothelial cells (HUVECs) grown on titanium surfaces with nanocleaves showed higher cell adhesion than other nanofeatures (e.g., nanorods, nanoporous) or control polished surfaces.115 Vascular smooth muscle cells (vSMCs) are the major functional cells present in the tunica media of arteries. Like ECs, vSMCs are also surrounded and regulated by nanoscale topologies. The interlamellar matrix of the tunica media contains microfibrils ranging from 100 to 500 nm.116 Collagen fibrils within media exhibited variable diameters depending on locations: from 37 nm near intima to 46 nm near the adventitia.116 Poly(methyl methacrylate) (PMMA) and poly(dimethylsiloxane) (PDMS) with nanopatterned gratings on surfaces significantly enhanced vSMCs cell alignment and reduced cell proliferation.117 In addition, microtopographical cues can alter both the differentiation of vSMCs and their inflammatory state.118
Researchers have shown great interest in employing nanoscale strategies to recapitulate the nano/micro scale interactions between cells and ECM. In general, three fabrication methods have been employed to produce nanofibers. Self-assembly, phase separation and electrospinning. Self-assembly refers to the spontaneous organization of individual components into ordered structure driven by noncovalent interactions (e.g., hydrophobic interactions and hydrogen bonding). This is a common naturally occurring process in cells.119 Peptide-amphiphiles (PAs) are a class of materials that combine the bioactivity of natural peptides and the chemical structures of surfactants. Because of this unique feature, PAs are commonly employed for production of self-assembled nanofibers.120 PAs containing Cardin-Weintraub sequences can bind to heparin, a biopolymer known to capture angiogenic growth factors such as VEGF and FGF-2.121 Heparin binding PAs (HBPAs) can be triggered by heparin addition and form a nanofiber-heparin gel, which was shown to promote angiogenesis in a rat corneal assay.122 Self-assembly can generate fibers on the lowest scales of ECM, between 5 and 8 nm; however, the process is relatively difficult to control and the yield is relatively low; consequently, the applicability of this method is limited.123
Phase separation is another way to create nanofibrous scaffolds, generally via five steps as described by Ma et al., including dissolution of polymer, phase separation (e.g., by thermal induction) and gelation, solvent exchange with water, freezing and lyophilization.124 One can obtain scaffolds with the desired chemical composition and morphology by varying parameters such as polymer concentration, gelation temperature and freezing temperature. For instance, high gelation temperature resulted into platelet-like structures whereas under low gelation temperature nanofibrous structures were formed.124 A major advantage of the phase separation method is that the pore size and interconnectivity of the scaffold can be precisely tailored through the addition of various porogens like inorganic salts.119 However, the phase separation method also suffers from relatively low yields and is thus unsuitable for large-scale industrial production.123
Currently, the most extensively used approach to produce nano/micro fibers is electrospinning. Electro-spinning is a highly tunable, versatile and cost-effective process. By varying parameters such as applied voltage, solution viscosity, volumetric charge density, distance to collector and collection method, one can precisely control the resulting structures and topographies such as fiber diameter, pore size and fiber orientation.125–128 In contrast to self-assembly or phase separation methods, which are limited to relatively few polymers, a wide variety of materials have been electrospun to produce fibrous matrices with nanoscale features, examples including synthetic materials such as poly-ε-caprolactone (PCL),129 poly(d-lactide) (PDLA),130 poly(l-lactide) (PLA)131 and polydioxane (PDO),132 and also natural polymers such as gelatins,133 collagens,134 fibrins135 and elastins.136 Additionally, electrospinning can be employed in both laboratory and industrial settings.123
Electrospun nanofibrous scaffolds have distinct advantages as nanomedicines. Specifically, electrospun fibers can be tailored to achieve desired topographical and structural features. These nanoscale features have been shown to promote cell attachment, cell growth or cell infiltration. ECs cultured on aligned electrospun PCL/collagen fibers of 100 or 300 nm diameter showed better cell alignment, elongated cell morphology, more cell–cell adhesions (measured by VE-cadherin staining) as well as more focal adhesions (measured by vinculin staining) compared to ECs grown on fibers with random orientations or larger diameters (1200 nm).137 Cell infiltration is also an important step for constructing a bioactive scaffold. One can increase cell penetration into the scaffold by increasing fiber dimension and pore size at the potential cost of reduced cell attachment or cell spreading.138 Besides, electrospun nanofibrous scaffolds can be tailored to match the mechanical properties of native arteries and thus reducing compliance mismatch and reducing the risk of hyperplasia. For instance, hybrid elastin/polycaprolactone scaffolds were shown to have burst pressures equivalent to internal mammary artery (around 2000 mmHg).139 In addition, due to the high surface-to-volume ratio, electrospun nanofibers can be efficiently loaded with factors to enhance the performance of the scaffold. Zhang et al. fabricated double-layered membrane by coaxial electrospinning and encapsulated VEGF/PDGF in the inner/outer layers, respectively. The release of VEGF/PDGF promoted the proliferation of vascular ECs and SMCs (Figure 5).140 Finally, electrospun nanofibers can be surface coated/modified to improve biocompatibility and thus enhance cell attachment. Li et al.141 modified the surface of electrospun PCL mats with two macromolecules: a zwitterionic poly(carboxybetaine methacrylate) (PCBMA) to reduce protein deposition and thus increase biocompatibility and a peptide selected by phage-display that can specifically capture circulating endothelial progenitor cells (EPCs) from blood circulation.
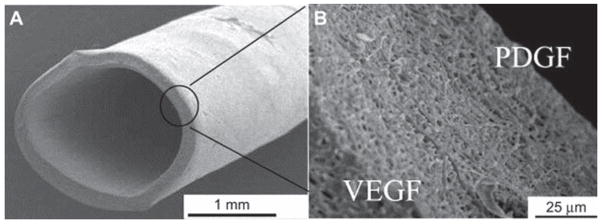
Figure 5.
Small diameter (2.2 mm) vascular graft with coaxial-electrospun double-layered membrane encapsulating VEGF/PDGF on the inner/outer layer respectively to enhance the growth of vascular endothelial cells (vECs) and vascular smooth muscle cells (vSMCs). SEM images of the vascular graft (A) and the cross section of the nanofibrous membrane (B). Reprinted from ref 140 with permission. Copyright 2013 Elsevier Ltd.
From Bench to Bedside: A Long-Lasting Endeavor
Translating a new drug/medical device from a laboratory to clinical setting is a challenging process. One of the main challenges is centered on safety evaluation. Safety considerations include genotoxicity, immunotoxicity, developmental toxicity and carcinogenicity. The nanoscale dimensions of NPs can be a double-edged sword when considering their biomedical applications. On one hand, the small size renders NPs ideal for surface coating/modification and eases their uptake by cells. On the other hand, since nanomaterials are of similar dimensions to natural macromolecules, the interactions between NPs and biomacromolecules and cells become more sophisticated. Additionally, NPs shape can affect uptake efficiency and internalization mechanism. Endothelial cells will uptake nearly double more discs than rod shaped NPs.142 Inflammatory and nephrotoxic effects triggered by NPs administration are size-dependent. Ten nm gold NPs increased white blood cells (WBCs) count whereas 5 and 30 nm NPs caused a drop in both WBCs and red blood cells (RBCs) count.143
To translate nanotechnologies into clinical settings, rigorous preclinical safety studies should be performed both in vivo and in vitro as has been previously reviewed.144,145 For in vitro assessment of cytotoxicity, DNA synthesis assays and DNA damage assays are critical to assess the risk of tumorigenesis. DNA damage can be evaluated by comet assay (single-strand breakage) or γ-H2AX immunostaining (double strand breakage).146 Oxidative stress is another commonly observed toxic effect associated with NPs, especially with metal oxide NPs and carbon nanotubes.147 NPs can induce the generation of reactive oxygen species (ROS) by either directly catalyzing ROS production148 or activating immune cells that would initiate an inflammatory response.149 ROS production and the resulting oxidative stress are believed to be responsible for various pathological events including inflammation, fibrosis and even carcinogenesis.147 ROS or oxidative stress can be assessed using oxidizable fluorescent probes such as dihydroethidium (DHE), carboxyl derivatives and other fluorescein derivatives. Additionally, proliferative assays (e.g., MTT assay), necrosis assays (e.g., propidium iodide staining) and apoptosis assays (e.g., annexin-V assay) are also commonly used, but these assays provide only limited insights into the molecular mechanisms underlying the nanotoxicity.
For in vivo assessment, biodistribution and drug clearance are usually examined. Particle properties such as composition, surface modification, size and charge can significantly affect biodistribution and clearance.150 Ideally, therapeutic NPs should be able to preferentially accumulate in target tissues (e.g., tumor or ischemic tissues). Protein adsorption onto NPs is a major concern because it promotes opsonization leading to rapid clearance of NPs by the reticuloendothelial system (RES).151 Nondegradable NPs usually accumulate in the liver and spleen. Biodistribution can be studied on both live animals or fixed tissues by detecting the fluorescent/radioactive tags152 or by using HPLC.153 Hematology analysis is a more convenient and clinically relevant method to examine in vivo toxicity. Significant changes in blood chemistry or cell composition may reffect signs of toxicity.145 Useful hematological parameters to monitor may include white blood cell (WBC) count, red blood cell (RBC) count, albumin concentration, alkaline phosphatase (ALP), creatinine, alanine transaminase, aspartate transaminase and hemoglobin.143,154 For instance, a rise in WBCs is typically a sign of an elevated inflammatory response, an increase in creatinine level signifies impairment of kidney function, and a low albumin level is a sign of liver or kidney disease. Histologic and pathologic analysis of fixed tissues can also provide useful insights into NP toxicity. One can perform basic H&E staining to examine any visible changes in cell and tissue morphology or, immunostain for specific cells/biomarkers that are indicative of pathological changes in organs/tissues (mostly liver, kidney, spleen, and thymus).
In spite of all currently existing methods to evaluate the safety of NPs, numerous challenges still remain. First, NPs are generally comprised of the nanocarrier, therapeutic payload and often surface functional groups. This multicomponent nature inevitably increases the difficulty in predicting the long-term behaviors of NPs, such as interactions with cells/biomolecules and degradation. Our current understanding of nanotoxicity mechanisms and NPs behavior in vivo is still incomplete. Minor variations in NP composition, architecture and surface modification can significantly influence the in vivo outcome.155 Therefore, it is difficult to generalize a set of desirable physiochemical characteristics that can be applied to different NP systems.155 As a result, safety and efficacy studies are required on a case-by-case basis and cannot be predicted from similar formulations of NPs. Finally, for a nanomaterial formulated in lab to be translated for clinical use, challenges remain in optimizing the manufacturing of nanomaterials in terms of process scale up, economics and quality control. As nanomaterials have been used in primarily early stage development studies, these critical considerations have not been addressed.
In addition to safety issues, efficacy is also a challenge. To justify the use of nanomaterials in the treatment of PVD, one needs to demonstrate that nanomaterials based therapies can achieve clinically satisfying outcomes which can hardly be accomplished with existing gene therapies or cell therapies. To date, to our knowledge, there has been very few, if any, clinical studies using NPs to treat PVD, specifically CLI. TEVGs have achieved promising clinical results for various applications such as treatment of congenital heart diseases156 and peripheral revascularization.157 Despite encouraging clinical outcomes, one limitation is that some of these most successful TEVGs rely on in vitro expansion of patients derived autologous cells, a procedure that can range from weeks to months, thus making them less suitable for CLI patients who need effective therapies in a short time.
CONCLUDING REMARKS
Nanomedicine is a relatively new but thriving field with significant potential. Nanoscale strategies show promise for applications that will revolutionize the therapeutic methodologies for various diseases from cancer to cardiovascular diseases. In the context of treating peripheral vascular diseases, numerous preclinical studies have demonstrated the advantages of using nanomaterials as either protein/gene carriers or as tissue engineered vascular grafts. As protein carriers, NPs generated sustained release of angiogenic factors in the localized microenvironment and enhanced angiogenesis efficacy, reducing the required dosages and thus lowering the cost of therapy and unwanted side effects. As gene carriers, NPs increase the transfection efficiency compared to naked DNAs and have relatively high safety profile compared to viral vectors. As tissue engineered vascular grafts, nanofibrous grafts with specific topographies, structures and surface functional groups can facilitate cell retention, cell survival, cell growth and secretion of angiogenic factors and may also possess the desired mechanical properties similar to native blood vessels.
However, there is a long way to go before nanoscale therapeutic strategies can be widely used in treating PVD and CLI patients in clinical settings, mostly because many of these have been shown to be safe but not efficacious. Currently, growth factor therapy, gene therapy and cell therapy are under clinical trials. Using nanomaterials with formulations that are not yet approved in these therapies will inevitably add another layer of complexity to safety evaluations. Additionally, promising preclinical results from animal models may not necessarily translate to successful human trials. One reason is that animal models are intrinsically limited in terms of precisely modeling human disease. Regarding the modeling of limb ischemia, one should note that rodents have distinct blood flow rates from humans. Additionally, most preclinical studies were performed within a very short time frame (~2 weeks). For therapeutic angiogenesis, the key is to generate stable neovasculature that can persistently supply blood to the ischemic tissues. However, many preclinical studies failed to demonstrate that the newly formed blood vessels did not regress as the therapy stops. Lastly, angiogenesis is a complex process that requires the coordination of multiple cell types such as endothelial cells, smooth muscle cells and pericytes with complex signaling regulations. However, existing nanoscale strategies are overly simplistic, mostly delivering just one or two growth factor/genes, which do not recapitulate the natural angiogenesis process.
In the future, more rigorous preclinical and clinical studies on the safety and efficacy of nanomaterial-based therapy are needed; specifically, extending the time frame of animal studies to confirm the long-term (e.g., several months) patency of the regenerated micro/macro vessels. Additionally, multiplexed nanomaterials should be designed to carry multiple proangiogenic factors, which, ideally, can be released by active control (e.g., infrared irradiation or ultrasound activation) or passively but sequentially released to better mimic the concentration gradients of growth factors involved in the angiogenesis and neovascularization processes. Ultimately, successful treatment of PVD or CLI may rely on more than one nanoscale strategy. For instance, a nanoscale protein/gene delivery may needed for immediate treatment for ischemic disease to avoid amputation while the replacement of the diseased arteries with tissue engineered nanofibrous scaffold may be needed to achieve long-term recovery. Looking at the rapid growth and numerous achievements in the field of nanomedicine, it is clear that nanoscale strategies will play a pivotal role in the future therapy of PVD diseases.
VOCABULARY
- Peripheral vascular disease
refers to the obstruction or narrowing of the nonmyocardial arteries, most commonly the lower extremities but including the vasculature of kidney and other organs
- Critical limb ischemia
is a significant blockage of the arteries of the lower limb resulting in skin lesions (ulcers or gangrene) and rest pain, both of which can significantly compromise a patient’s quality of life
- Nanomedicine
is the application of nanotechnology to problems in medicine
- Nonviral gene delivery
is the delivery of nucleic acid cargo into the nucleus of a cell through any means other than a viral vector includingelectroporation, microinjection, gene gun, hydrostatic pressure, continuous infusion, sonication, lipofection or polyplexes
- Electrospinning
is the application of a high voltage across a polymer solution droplet to draw the material into a continuous jet. This material can then be collected as a nanoscale fiber
Footnotes
Conflict of Interest: The authors declare no competing financial interest.
REFERENCES AND NOTES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, et al. Heart Disease and Stroke Statistics—2011 Update: A Report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouriel K. Peripheral Arterial Disease. Lancet. 2001;358:1257–1264. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 3.Minar E. Critical Limb Ischaemia. Hamostaseologie. 2009;29:102–9. [PubMed] [Google Scholar]
- 4.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (Tasc II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 5.Deveza L, Choi J, Yang F. Therapeutic Angiogenesis for Treating Cardiovascular Diseases. Theranostics. 2012;2:801–814. doi: 10.7150/thno.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zilla P, Bezuidenhout D, Human P. Prosthetic Vascular Grafts: Wrong Models, Wrong Questions and No Healing. Biomaterials. 2007;28:5009–5027. doi: 10.1016/j.biomaterials.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 7.Shimamura M, Nakagami H, Taniyama Y, Morishita R. Gene Therapy for Peripheral Arterial Disease. Expert Opin Biol Ther. 2014;14:1175–1184. doi: 10.1517/14712598.2014.912272. [DOI] [PubMed] [Google Scholar]
- 8.Raval Z, Losordo DW. Cell Therapy of Peripheral Arterial Disease: From Experimental Findings to Clinical Trials. Circ Res. 2013;112:1288–1302. doi: 10.1161/CIRCRESAHA.113.300565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morishita R, Makino H, Aoki M, Hashiya N, Yamasaki K, Azuma J, Taniyama Y, Sawa Y, Kaneda Y, Ogihara T. Phase I/Iia Clinical Trial of Therapeutic Angiogenesis Using Hepatocyte Growth Factor Gene Transfer to Treat Critical Limb Ischemia. Arterioscl Throm Vas. 2011;31:713–U507. doi: 10.1161/ATVBAHA.110.219550. [DOI] [PubMed] [Google Scholar]
- 10.Skora J, Barc P, Pupka A, Dawiskiba T, Korta K, Albert M, Szyber P. Transplantation of Autologous Bone Marrow Mononuclear Cells with VEGF Gene Improves Diabetic Critical Limb Ischaemia. Endokrynol Pol. 2013;64:129–138. [PubMed] [Google Scholar]
- 11.Gupta R, Tongers J, Losordo DW. Human Studies of Angiogenic Gene Therapy. Circ Res. 2009;105:724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuevas P, Carceller F, Ortega S, Zazo M, Nieto I, Gimenezgallego G. Hypotensive Activity of Fibroblast Growth-Factor. Science. 1991;254:1208–1210. doi: 10.1126/science.1957172. [DOI] [PubMed] [Google Scholar]
- 13.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-Resistant Blood Vessels in Mice Transgenically Overexpressing Angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 14.Rissanen TT, Markkanen JE, Arve K, Rutanen J, Kettunen MI, Vajanto I, Jauhiainen S, Cashion L, Gruchala M, Narvanen O, et al. Fibroblast Growth Factor-4 Induces Vascular Permeability, Angiogenesis, and Arteriogenesis in a Rabbit Hind Limb Ischemia Model. FASEB J. 2002;16:100. doi: 10.1096/fj.02-0377fje. [DOI] [PubMed] [Google Scholar]
- 15.Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, Eckey T, Henze E, Zeiher AM, Dimmeler S. Assessment of the Tissue Distribution of Transplanted Human Endothelial Progenitor Cells by Radioactive Labeling. Circulation. 2003;107:2134–2139. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 16.Li SD, Huang L. Pharmacokinetics and Biodistribution of Nanoparticles. Mol Pharmaceutics. 2008;5:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 17.De Jong WH, Borm PJA. Drug Delivery and Nano-particles: Applications and Hazards. Int J Nanomedicine. 2008;3:133–149. doi: 10.2147/ijn.s596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arvizo RR, Miranda OR, Moyano DF, Walden CA, Giri K, Bhattacharya R, Robertson JD, Rotello VM, Reid JM, Mukherjee P. Modulating Pharmacokinetics, Tumor Uptake and Biodistribution by Engineered Nano-particles. PLoS One. 2011;6:e24374. doi: 10.1371/journal.pone.0024374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Sayed IH, Huang X, El-Sayed MA. Surface Plasmon Resonance Scattering and Absorption of Anti-EGFR Antibody Conjugated Gold Nanoparticles in Cancer Diagnostics: Applications in Oral Cancer. Nano Lett. 2005;5:829–34. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 20.Dinauer N, Balthasar S, Weber C, Kreuter J, Langer K, von Briesen H. Selective Targeting of Antibody-Conjugated Nanoparticles to Leukemic Cells and Primary T-Lymphocytes. Biomaterials. 2005;26:5898–906. doi: 10.1016/j.biomaterials.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 21.Begent RHJ, Verhaar MJ, Chester KA, Casey JL, Green AJ, Napier MP, HopeStone LD, Cushen N, Keep PA, Johnson CJ, et al. Clinical Evidence of Efficient Tumor Targeting Based on Single-Chain Fv Antibody Selected from a Combinatorial Library. Nat Med. 1996;2:979–984. doi: 10.1038/nm0996-979. [DOI] [PubMed] [Google Scholar]
- 22.Robert S, Gysemans C, Takiishi T, Korf H, Spagnuolo I, Sebastiani G, Van Huynegem K, Steidler L, Caluwaerts S, Demetter P, et al. Oral Delivery of Glutamic Acid Decarboxylase (Gad)-65 and Il10 by Lactococcus Lactis Reverses Diabetes in Recent-Onset Nod Mice. Diabetes. 2014;63:2876–2887. doi: 10.2337/db13-1236. [DOI] [PubMed] [Google Scholar]
- 23.Lin YH, Liang HF, Chung CK, Chen MC, Sung HW. Physically Crosslinked Alginate/N,O-Carboxymethyl Chitosan Hydrogels with Calcium for Oral Delivery of Protein Drugs. Biomaterials. 2005;26:2105–2113. doi: 10.1016/j.biomaterials.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Rekha MR, Sharma CP. Oral Delivery of Therapeutic Protein/Peptide for Diabetes—Future Perspectives. Int J Pharm. 2013;440:48–62. doi: 10.1016/j.ijpharm.2012.03.056. [DOI] [PubMed] [Google Scholar]
- 25.Han L, Tang C, Yin CH. Oral Delivery of shRNA and siRNA via Multifunctional Polymeric Nanoparticles for Synergistic Cancer Therapy. Biomaterials. 2014;35:4589–4600. doi: 10.1016/j.biomaterials.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Akhtar S. Oral Delivery of siRNA and Antisense Oligonucleotides. J Drug Target. 2009;17:491–495. doi: 10.1080/10611860903057674. [DOI] [PubMed] [Google Scholar]
- 27.Gao WW, Chan JM, Farokhzad OC. PH-Responsive Nanoparticles for Drug Delivery. Mol Pharmaceutics. 2010;7:1913–1920. doi: 10.1021/mp100253e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lux CD, Joshi-Barr S, Nguyen T, Mahmoud E, Schopf E, Fomina N, Almutairi A. Biocompatible Polymeric Nanoparticles Degrade and Release Cargo in Response to Biologically Relevant Levels of Hydrogen Peroxide. J Am Chem Soc. 2013;134:15758–15764. doi: 10.1021/ja303372u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasan A, Memic A, Annabi N, Hossain M, Paul A, Dokmeci MR, Dehghani F, Khademhosseini A. Electrospun Scaffolds for Tissue Engineering of Vascular Grafts. Acta Biomater. 2014;10:11–25. doi: 10.1016/j.actbio.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazarous DF, Shou M, Scheinowitz M, Hodge E, Thirumurti V, Kitsiou AN, Stiber JA, Lobo AD, Hunsberger S, Guetta E, et al. Comparative Effects of Basic Fibroblast Growth Factor and Vascular Endothelial Growth Factor on Coronary Collateral Development and the Arterial Response to Injury. Circulation. 1996;94:1074–1082. doi: 10.1161/01.cir.94.5.1074. [DOI] [PubMed] [Google Scholar]
- 31.Marui A, Tabata Y, Kojima S, Yamamoto M, Tambara K, Nishina T, Saji Y, Inui KI, Hashida T, Yokoyama S, et al. Novel Approach to Therapeutic Angiogenesis for Patients with Critical Limb Ischemia by Sustained Release of Basic Fibroblast Growth Factor Using Biodegradable Gelatin Hydrogel—An Initial Report of the Phase I-Iia Study. Circ J. 2007;71:1181–1186. doi: 10.1253/circj.71.1181. [DOI] [PubMed] [Google Scholar]
- 32.Lazarous DF, Unger EF, Epstein SE, Stine A, Arevalo JL, Chew EY, Quyyumi AA. Basic Fibroblast Growth Factor in Patients with Intermittent Claudication: Results of a Phase I Trial. J Am Coll Cardiol. 2000;36:1239–1244. doi: 10.1016/s0735-1097(00)00882-2. [DOI] [PubMed] [Google Scholar]
- 33.Golub JS, Kim YT, Duvall CL, Bellamkonda RV, Gupta D, Lin AS, Weiss D, Taylor WR, Guldberg RE. Sustained VEGF Delivery via PLGA Nanoparticles Promotes Vascular Growth. Am J Physiol: Heart Circ Physiol. 2010;298:H1959–H1965. doi: 10.1152/ajpheart.00199.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.d’Angelo I, Garcia-Fuentes M, Parajo Y, Welle A, Vantus T, Horvath A, Bokonyi G, Keri G, Alonso MJ. Nanoparticles Based on PLGA:Poloxamer Blends for the Delivery of Proangiogenic Growth Factors. Mol Pharmaceutics. 2010;7:1724–1733. doi: 10.1021/mp1001262. [DOI] [PubMed] [Google Scholar]
- 35.Kim J, Cao L, Shvartsman D, Silva EA, Mooney DJ. Targeted Delivery of Nanoparticles to Ischemic Muscle for Imaging and Therapeutic Angiogenesis. Nano Lett. 2011;11:694–700. doi: 10.1021/nl103812a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun ZC, Huang P, Tong G, Lin J, Jin A, Rong PF, Zhu L, Nie LM, Niu G, Cao F, et al. VEGF-Loaded Graphene Oxide as Theranostics for Multi-Modality Imaging-Monitored Targeting Therapeutic Angiogenesis of Ischemic Muscle. Nanoscale. 2013;5:6857–6866. doi: 10.1039/c3nr01573d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peer D, Karp JM, Hong S, FarokHzad OC, Margalit R, Langer R. Nanocarriers as an Emerging Platform for Cancer Therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 38.Xie J, Wang H, Wang Y, Ren F, Yi W, Zhao K, Li Z, Zhao Q, Liu Z, Wu H, et al. Induction of Angiogenesis by Controlled Delivery of Vascular Endothelial Growth Factor Using Nanoparticles. Cardiovasc Ther. 2013;31:e12–18. doi: 10.1111/j.1755-5922.2012.00317.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Postovit LM, Wang DS, Gardiner RB, Harris R, Abdul MM, Thomas AA. In Situ Loading of Basic Fibroblast Growth Factor within Porous Silica Nanoparticles for a Prolonged Release. Nanoscale Res Lett. 2009;4:1297–1302. doi: 10.1007/s11671-009-9395-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramchandani M, Robinson D. In Vitro and in Vivo Release of Ciprofloxacin from PLGA 50:50 Implants. J Controlled Release. 1998;54:167–175. doi: 10.1016/s0168-3659(97)00113-2. [DOI] [PubMed] [Google Scholar]
- 41.Jang E, Albadawi H, Watkins MT, Edelman ER, Baker AB. Syndecan-4 Proteoliposomes Enhance Fibroblast Growth Factor-2 (FGF-2)-Induced Proliferation, Migration, and Neovascularization of Ischemic Muscle. Proc Natl Acad Sci US A. 2012;109:1679–1684. doi: 10.1073/pnas.1117885109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Das S, Singh G, Baker AB. Overcoming Disease-Induced Growth Factor Resistance in Therapeutic Angiogenesis Using Recombinant Co-Receptors Delivered by a Liposomal System. Biomaterials. 2014;35:196–205. doi: 10.1016/j.biomaterials.2013.09.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baluk P, Lee CG, Link H, Ator E, Haskell A, Elias JA, McDonald DM. Regulated Angiogenesis and Vascular Regression in Mice Overexpressing Vascular Endothelial Growth Factor in Airways. Am J Pathol. 2004;165:1071–1085. doi: 10.1016/S0002-9440(10)63369-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung E, Ricles LM, Stowers RS, Nam SY, Emelianov SY, Suggs LJ. Multifunctional Nanoscale Strategies for Enhancing and Monitoring Blood Vessel Regeneration. Nano Today. 2012;7:514–531. doi: 10.1016/j.nantod.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maggio I, Holkers M, Liu J, Janssen JM, Chen X, Goncalves MA. Adenoviral Vector Delivery of RNA-Guided CRISPR/Cas9 Nuclease Complexes Induces Targeted Mutagenesis in a Diverse Array of Human Cells. Sci Rep. 2014;4:5105. doi: 10.1038/srep05105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McTiernan CF, Mathier MA, Zhu X, Xiao X, Klein E, Swan CH, Mehdi H, Gibson G, Trichel AM, Glorioso JC, et al. Myocarditis Following Adeno-Associated Viral Gene Expression of Human Soluble Tnf Receptor (Tnfrii-Fc) in Baboon Hearts. Gene Ther. 2007;14:1613–1622. doi: 10.1038/sj.gt.3303020. [DOI] [PubMed] [Google Scholar]
- 47.Yockman JW, Kastenmeier A, Erickson HM, Brumbach JG, Whitten MG, Albanil A, Li DY, Kim SW, Bull DA. Novel Polymer Carriers and Gene Constructs for Treatment of Myocardial Ischemia and Infarction. J Controlled Release. 2008;132:260–266. doi: 10.1016/j.jconrel.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomas CE, Ehrhardt A, Kay MA. Progress and Problems with the Use of Viral Vectors for Gene Therapy. Nat Rev Genet. 2003;4:346–58. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 49.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and Development of Polymers for Gene Delivery. Nat Rev Drug Discovery. 2005;4:581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 50.Zhang XQ, Wang XL, Huang SW, Zhuo RX, Liu ZL, Mao HQ, Leong KW. In Vitro Gene Delivery Using Polyamidoamine Dendrimers with a Trimesyl Core. Biomacromolecules. 2005;6:341–350. doi: 10.1021/bm040060n. [DOI] [PubMed] [Google Scholar]
- 51.Cheng YY, Zhao LB, Li YW, Xu TW. Design of Biocompatible Dendrimers for Cancer Diagnosis and Therapy: Current Status and Future Perspectives. Chem Soc Rev. 2011;40:2673–2703. doi: 10.1039/c0cs00097c. [DOI] [PubMed] [Google Scholar]
- 52.Godbey WT, Wu KK, Mikos AG. Poly(Ethylenimine) and Its Role in Gene Delivery. J Controlled Release. 1999;60:149–160. doi: 10.1016/s0168-3659(99)00090-5. [DOI] [PubMed] [Google Scholar]
- 53.Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/Transferrin-PEI Complexes: Reduced Interaction with Blood Components, Extended Circulation in Blood and Potential for Systemic Gene Delivery. Gene Ther. 1999;6:595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 54.Zhang LF, Chen ZZ, Li YF. Dual-Degradable Disulfide-Containing PEI-Pluronic/DNA Polyplexes: Transfection Efficiency and Balancing Protection and DNA Release. Int J Nanomed. 2013;8:3689–3701. doi: 10.2147/IJN.S49595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Song YY, Lou B, Zhao P, Lin C. Multifunctional Disulfide-Based Cationic Dextran Conjugates for Intravenous Gene Delivery Targeting Ovarian Cancer Cells. Mol Pharmaceutics. 2014;11:2250–2261. doi: 10.1021/mp4006672. [DOI] [PubMed] [Google Scholar]
- 56.Tang Q, Cao B, Lei X, Sun BB, Zhang YQ, Cheng G. Dextran-Peptide Hybrid for Efficient Gene Delivery. Langmuir. 2014;30:5202–5208. doi: 10.1021/la500905z. [DOI] [PubMed] [Google Scholar]
- 57.Trentin D, Hall H, Wechsler S, Hubbell JA. Peptide-Matrix-Mediated Gene Transfer of an Oxygen-Insensitive Hypoxia-Inducible Factor-1alpha Variant for Local Induction of Angiogenesis. Proc Natl Acad Sci US A. 2006;103:2506–2511. doi: 10.1073/pnas.0505964102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang DW, Yu SH, Ho YC, Mi FL, Kuo PL, Sung HW. Heparinized Chitosan/Poly(Gamma-Glutamic Acid) Nanoparticles for Multi-Functional Delivery of Fibroblast Growth Factor and Heparin. Biomaterials. 2010;31:9320–9332. doi: 10.1016/j.biomaterials.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 59.Pissuwan D, Niidome T, Cortie MB. The Forthcoming Applications of Gold Nanoparticles in Drug and Gene Delivery Systems. J Controlled Release. 2011;149:65–71. doi: 10.1016/j.jconrel.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 60.Guo S, Huang Y, Jiang Q, Sun Y, Deng L, Liang Z, Du Q, Xing J, Zhao Y, Wang PC, et al. Enhanced Gene Delivery and siRNA Silencing by Gold Nanoparticles Coated with Charge-Reversal Polyelectrolyte. ACS Nano. 2010;4:5505–5511. doi: 10.1021/nn101638u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghosh PS, Kim CK, Han G, Forbes NS, Rotello VM. Efficient Gene Delivery Vectors by Tuning the Surface Charge Density of Amino Acid-Functionalized Gold Nanoparticles. ACS Nano. 2008;2:2213–2218. doi: 10.1021/nn800507t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim MH, Na HK, Kim YK, Ryoo SR, Cho HS, Lee KE, Jeon H, Ryoo R, Min DH. Facile Synthesis of Monodispersed Mesoporous Silica Nanoparticles with Ultralarge Pores and Their Application in Gene Delivery. ACS Nano. 2011;5:3568–3576. doi: 10.1021/nn103130q. [DOI] [PubMed] [Google Scholar]
- 63.Slowing II, Vivero-Escoto JL, Wu CW, Lin VSY. Mesoporous Silica Nanoparticles as Controlled Release Drug Delivery and Gene Transfection Carriers. Adv Drug Delivery Rev. 2008;60:1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 64.Pantarotto D, Singh R, McCarthy D, Erhardt M, Briand JP, Prato M, Kostarelos K, Bianco A. Functionalized Carbon Nanotubes for Plasmid DNA Gene Delivery. Angew Chem Int Ed. 2004;43:5242–5246. doi: 10.1002/anie.200460437. [DOI] [PubMed] [Google Scholar]
- 65.Zhi DF, Zhang SB, Cui SH, Zhao YA, Wang YH, Zhao DF. The Headgroup Evolution of Cationic Lipids for Gene Delivery. Bioconjugate Chem. 2013;24:487–519. doi: 10.1021/bc300381s. [DOI] [PubMed] [Google Scholar]
- 66.Fichert T, Regelin A, Massing U. Synthesis and Transfection Properties of Novel Non-Toxic Monocationic Lipids. Variation of Lipid Anchor, Spacer and Head Group Structure. Bioorg Med Chem Lett. 2000;10:787–791. doi: 10.1016/s0960-894x(00)00099-8. [DOI] [PubMed] [Google Scholar]
- 67.Bajaj A, Kondiah P, Bhattacharya S. Design, Synthesis, and in Vitro Gene Delivery Efficacies of Novel Cholesterol-Based Gemini Cationic Lipids and Their Serum Compatibility: A Structure-Activity Investigation. J Med Chem. 2007;50:2432–2442. doi: 10.1021/jm0611253. [DOI] [PubMed] [Google Scholar]
- 68.Zhu K, Guo CF, Lai H, Yang WL, Xia Y, Zhao D, Wang CS. Novel Hyperbranched Polyamidoamine Nanoparticles for Transfecting Skeletal Myoblasts with Vascular Endothelial Growth Factor Gene for Cardiac Repair. J Mater Sci: Mater Med. 2011;22:2477–2485. doi: 10.1007/s10856-011-4424-2. [DOI] [PubMed] [Google Scholar]
- 69.Kunath K, von Harpe A, Fischer D, Peterson H, Bickel U, Voigt K, Kissel T. Low-Molecular-Weight Polyethylenimine as a Non-Viral Vector for DNA Delivery: Comparison of Physicochemical Properties, Transfection Efficiency and in Vivo Distribution with High-Molecular-Weight Polyethylenimine. J Controlled Release. 2003;89:113–125. doi: 10.1016/s0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 70.Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A. A Two-Stage Poly(Ethylenimine)-Mediated Cytotoxicity: Implications for Gene Transfer/Therapy. Mol Ther. 2005;11:990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 71.Zhi D, Zhang S, Cui S, Zhao Y, Wang Y, Zhao D. The Headgroup Evolution of Cationic Lipids for Gene Delivery. Bioconjugate Chem. 2013;24:487–519. doi: 10.1021/bc300381s. [DOI] [PubMed] [Google Scholar]
- 72.Zhi D, Zhang S, Wang B, Zhao Y, Yang B, Yu S. Transfection Efficiency of Cationic Lipids with Different Hydrophobic Domains in Gene Delivery. Bioconjugate Chem. 2010;21:563–77. doi: 10.1021/bc900393r. [DOI] [PubMed] [Google Scholar]
- 73.Duarte S, Faneca H, Lima MC. Folate-Associated Lipoplexes Mediate Efficient Gene Delivery and Potent Antitumoral Activity in Vitro and in Vivo. Int J Pharm. 2012;423:365–377. doi: 10.1016/j.ijpharm.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 74.Duarte S, Faneca H, de Lima MC. Non-Covalent Association of Folate to Lipoplexes: A Promising Strategy to Improve Gene Delivery in the Presence of Serum. J Controlled Release. 2011;149:264–272. doi: 10.1016/j.jconrel.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 75.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of Cationic Lipids and Cationic Polymers in Gene Delivery. J Controlled Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 76.Jiang H, Zhang T, Sun X. Vascular Endothelial Growth Factor Gene Delivery by Magnetic DNA Nanospheres Ameliorates Limb Ischemia in Rabbits. J Surg Res. 2005;126:48–54. doi: 10.1016/j.jss.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Kang SW, Lim HW, Seo SW, Jeon O, Lee M, Kim BS. Nanosphere-Mediated Delivery of Vascular Endothelial Growth Factor Gene for Therapeutic Angiogenesis in Mouse Ischemic Limbs. Biomaterials. 2008;29:1109–1117. doi: 10.1016/j.biomaterials.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Negishi Y, Endo-Takahashi Y, Matsuki Y, Kato Y, Takagi N, Suzuki R, Maruyama K, Aramaki Y. Systemic Delivery Systems of Angiogenic Gene by Novel Bubble Liposomes Containing Cationic Lipid and Ultrasound Exposure. Mol Pharmaceutics. 2012;9:1834–1840. doi: 10.1021/mp200554c. [DOI] [PubMed] [Google Scholar]
- 79.Negishi Y, Matsuo K, Endo-Takahashi Y, Suzuki K, Matsuki Y, Takagi N, Suzuki R, Maruyama K, Aramaki Y. Delivery of an Angiogenic Gene into Ischemic Muscle by Novel Bubble Liposomes Followed by Ultrasound Exposure. Pharm Res. 2011;28:712–719. doi: 10.1007/s11095-010-0286-4. [DOI] [PubMed] [Google Scholar]
- 80.Endo-Takahashi Y, Negishi Y, Nakamura A, Ukai S, Ooaku K, Oda Y, Sugimoto K, Moriyasu F, Takagi N, Suzuki R, et al. Systemic Delivery of miR-126 by miRNA-Loaded Bubble Liposomes for the Treatment of Hindlimb Ischemia. Sci Rep. 2014;4:3883. doi: 10.1038/srep03883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Botham CM, Bennett WL, Cooke JP. Clinical Trials of Adult Stem Cell Therapy for Peripheral Artery Disease. Methodist Debakey Cardiovasc J. 2013;9:201–205. doi: 10.14797/mdcj-9-4-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sibbald B. Death but One Unintended Consequence of Gene-Therapy Trial. CMAJ. 2001;164:1612. [PMC free article] [PubMed] [Google Scholar]
- 83.Liew A, O’Brien T. Therapeutic Potential for Mesenchymal Stem Cell Transplantation in Critical Limb Ischemia. Stem Cell Res Ther. 2012;3:28. doi: 10.1186/scrt119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of Putative Progenitor Endothelial Cells for Angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 85.Barry FP, Murphy JM. Mesenchymal Stem Cells: Clinical Applications and Biological Characterization. Int J Biochem Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 86.Tscheudschilsuren G, Bosserhoff AK, Schlegel J, Vollmer D, Anton A, Alt V, Schnettler R, Brandt J, Proetzel G. Regulation of Mesenchymal Stem Cell and Chondrocyte Differentiation by MIA. Exp Cell Res. 2006;312:63–72. doi: 10.1016/j.yexcr.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 87.Chiellini C, Cochet O, Negroni L, Samson M, Poggi M, Ailhaud G, Alessi MC, Dani C, Amri EZ. Characterization of Human Mesenchymal Stem Cell Secretome at Early Steps of Adipocyte and Osteoblast Differentiation. BMC Mol Biol. 2008;9:26. doi: 10.1186/1471-2199-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao Q, Guo M, Jiang X, Hu X, Wang Y, Fan Y. A Cocktail Method for Promoting Cardiomyocyte Differentiation from Bone Marrow-Derived Mesenchymal Stem Cells. Stem Cells Int. 2014;2014:162024. doi: 10.1155/2014/162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Friedman MS, Long MW. Stem Cell Plasticity: CD34+Hematopoietic Cells and Mesenchymal Stem Cells Undergo Regulated Differentiation into Endothelial Cells. Blood. 2001;98:549a–550a. [Google Scholar]
- 90.Motukuru V, Suresh KR, Vivekanand V, Raj S, Girija KR. Therapeutic Angiogenesis in Buerger’s Disease (Thromboangiitis Obliterans) Patients with Critical Limb Ischemia by Autologous Transplantation of Bone Marrow Mononuclear Cells. J Vasc Surg. 2008;48:53S–60S. doi: 10.1016/j.jvs.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 91.Das AK, Bin Abdullah BJ, Dhillon SS, Vijanari A, Anoop CH, Gupta PK. Intra-Arterial Allogeneic Mesenchymal Stem Cells for Critical Limb Ischemia Are Safe and Efficacious: Report of a Phase I Study. World J Surg. 2013;37:915–922. doi: 10.1007/s00268-012-1892-6. [DOI] [PubMed] [Google Scholar]
- 92.Wang CY, Yang HB, Hsu HS, Chen LL, Tsai CC, Tsai KS, Yew TL, Kao YH, Hung SC. Mesenchymal Stem Cell-Conditioned Medium Facilitates Angiogenesis and Fracture Healing in Diabetic Rats. J Tissue Eng Regen Med. 2012;6:559–569. doi: 10.1002/term.461. [DOI] [PubMed] [Google Scholar]
- 93.Wang Y, Chen X, Cao W, Shi Y. Plasticity of Mesenchymal Stem Cells in Immunomodulation: Pathological and Therapeutic Implications. Nat Immunol. 2014;15:1009–1016. doi: 10.1038/ni.3002. [DOI] [PubMed] [Google Scholar]
- 94.Otsuru S, Gordon PL, Shimono K, Jethva R, Marino R, Phillips CL, Hofmann TJ, Veronesi E, Dominici M, Iwamoto M, et al. Transplanted Bone Marrow Mononuclear Cells and MSCs Impart Clinical Benefit to Children with Osteogenesis Imperfecta through Different Mechanisms. Blood. 2012;120:1933–1941. doi: 10.1182/blood-2011-12-400085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Barber CL, Iruela-Arispe ML. The Ever-Elusive Endothelial Progenitor Cell: Identities, Functions and Clinical Implications. Pediatr Res. 2006;59:722–722. doi: 10.1203/01.pdr.0000203553.46471.18. [DOI] [PubMed] [Google Scholar]
- 96.Yang F, Cho SW, Son SM, Bogatyrev SR, Singh D, Green JJ, Mei Y, Park S, Bhang SH, Kim BS, et al. Genetic Engineering of Human Stem Cells for Enhanced Angiogenesis Using Biodegradable Polymeric Nanoparticles. Proc Natl Acad Sci US A. 2010;107:3317–3322. doi: 10.1073/pnas.0905432106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee J, Jun I, Park HJ, Kang TJ, Shin H, Cho SW. Genetically Engineered Myoblast Sheet for Therapeutic Angiogenesis. Biomacromolecules. 2014;15:361–372. doi: 10.1021/bm401605f. [DOI] [PubMed] [Google Scholar]
- 98.Gomes RS, das Neves RP, Cochlin L, Lima A, Carvalho R, Korpisalo P, Dragneva G, Turunen M, Liimatainen T, Clarke K, et al. Efficient Pro-Survival/Angiogenic miRNA Delivery by an MRI-Detectable Nanomaterial. ACS Nano. 2013;7:3362–3372. doi: 10.1021/nn400171w. [DOI] [PubMed] [Google Scholar]
- 99.Koiwaya H, Sasaki K, Ueno T, Yokoyama S, Toyama Y, Ohtsuka M, Nakayoshi T, Mitsutake Y, Imaizumi T. Augmented Neovascularization with Magnetized Endothelial Progenitor Cells in Rats with Hind-Limb Ischemia. J Mol Cell Cardiol. 2011;51:33–40. doi: 10.1016/j.yjmcc.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 100.Kang HJ, Kim JY, Lee HJ, Kim KH, Kim TY, Lee CS, Lee HC, Park TH, Kim HS, Park YB. Magnetic Bionanoparticle Enhances Homing of Endothelial Progenitor Cells in Mouse Hindlimb Ischemia. Korean Circ J. 2012;42:390–396. doi: 10.4070/kcj.2012.42.6.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chavakis E, Urbich C, Dimmeler S. Homing and Engraftment of Progenitor Cells: A Prerequisite for Cell Therapy. J Mol Cell Cardiol. 2008;45:514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 102.Stephan MT, Irvine DJ. Enhancing Cell Therapies from the Outside In: Cell Surface Engineering Using Synthetic Nanomaterials. Nano Today. 2011;6:309–325. doi: 10.1016/j.nantod.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng K, Shen D, Hensley MT, Middleton R, Sun B, Liu W, De Couto G, Marban E. Magnetic Antibody-Linked Nanomatchmakers for Therapeutic Cell Targeting. Nat Commun. 2014;5:4880. doi: 10.1038/ncomms5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex Vivo Glycan Engineering of CD44 Programs Human Multipotent Mesenchymal Stromal Cell Trafficking to Bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- 105.Won YW, Patel AN, Bull DA. Cell Surface Engineering to Enhance Mesenchymal Stem Cell Migration toward an SDF-1 Gradient. Biomaterials. 2014;35:5627–5635. doi: 10.1016/j.biomaterials.2014.03.070. [DOI] [PubMed] [Google Scholar]
- 106.Stachel G, Trenkwalder T, Gotz F, El Aouni C, Muenchmeier N, Pfosser A, Nussbaum C, Sperandio M, Hatzopoulos AK, Hinkel R, et al. SDF-1 Fused to a Fractalkine Stalk and a GPI Anchor Enables Functional Neovascularization. Stem Cells. 2013;31:1795–1805. doi: 10.1002/stem.1439. [DOI] [PubMed] [Google Scholar]
- 107.Liu Z, Wang H, Wang Y, Lin Q, Yao A, Cao F, Li D, Zhou J, Duan C, Du Z, et al. The Influence of Chitosan Hydrogel on Stem Cell Engraftment, Survival and Homing in the Ischemic Myocardial Microenvironment. Biomaterials. 2012;33:3093–3106. doi: 10.1016/j.biomaterials.2011.12.044. [DOI] [PubMed] [Google Scholar]
- 108.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic Cell Engineering with Surface-Conjugated Synthetic Nanoparticles. Nat Med. 2010;16:1035–41. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Szilagyi DE, Smith RF, Elliott JP, Allen HM. Long-Term Behavior of a Dacron Arterial Substitute: Clinical, Roentgenologic and Histologic Correlations. Ann Surg. 1965;162:453–477. doi: 10.1097/00000658-196509000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seeger JM. Management of Patients with Prosthetic Vascular Graft Infection. Am Surg. 2000;66:166–177. [PubMed] [Google Scholar]
- 111.Weinberg CB, Bell E. A Blood Vessel Model Constructed from Collagen and Cultured Vascular Cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 112.LeBleu VS, Macdonald B, Kalluri R. Structure and Function of Basement Membranes. Exp Biol Med. 2007;232:1121–1129. doi: 10.3181/0703-MR-72. [DOI] [PubMed] [Google Scholar]
- 113.Brody S, Anilkumar T, Liliensiek S, Last JA, Murphy CJ, Pandit A. Characterizing Nanoscale Topography of the Aortic Heart Valve Basement Membrane for Tissue Engineering Heart Valve Scaffold Design. Tissue Eng. 2006;12:413–421. doi: 10.1089/ten.2006.12.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dalby MJ, Riehle MO, Johnstone H, Affrossman S, Curtis AS. In Vitro Reaction of Endothelial Cells to Polymer Demixed Nanotopography. Biomaterials. 2002;23:2945–2954. doi: 10.1016/s0142-9612(01)00424-0. [DOI] [PubMed] [Google Scholar]
- 115.Mohan CC, Sreerekha PR, Divyarani VV, Nair S, Chennazhi K, Menon D. Influence of Titania Nanotopography on Human Vascular Cell Functionality and Its Proliferation in Vitro. J Mater Chem. 2012;22:1326–1340. [Google Scholar]
- 116.Dingemans KP, Teeling P, Lagendijk JH, Becker AE. Extracellular Matrix of the Human Aortic Media: An Ultrastructural Histochemical and Immunohistochemical Study of the Adult Aortic Media. Anat Rec. 2000;258:1–14. doi: 10.1002/(SICI)1097-0185(20000101)258:1<1::AID-AR1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 117.Yim EK, Reano RM, Pang SW, Yee AF, Chen CS, Leong KW. Nanopattern-Induced Changes in Morphology and Motility of Smooth Muscle Cells. Biomaterials. 2005;26:5405–5413. doi: 10.1016/j.biomaterials.2005.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chaterji S, Kim P, Choe SH, Tsui JH, Lam CH, Ho DS, Baker AB, Kim DH. Synergistic Effects of Matrix Nanotopography and Stiffness on Vascular Smooth Muscle Cell Function. Tissue Eng Part A. 2014;20:2115–2126. doi: 10.1089/ten.tea.2013.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Barnes CP, Sell SA, Boland ED, Simpson DG, Bowlin GL. Nanofiber Technology: Designing the Next Generation of Tissue Engineering Scaffolds. Adv Drug Delivery Rev. 2007;59:1413–33. doi: 10.1016/j.addr.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 120.Cui H, Webber MJ, Stupp SI. Self-Assembly of Peptide Amphiphiles: From Molecules to Nanostructures to Biomaterials. Biopolymers. 2010;94:1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rajangam K, Arnold MS, Rocco MA, Stupp SI. Peptide Amphiphile Nanostructure-Heparin Interactions and Their Relationship to Bioactivity. Biomaterials. 2008;29:3298–3305. doi: 10.1016/j.biomaterials.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rajangam K, Behanna HA, Hui MJ, Han X, Hulvat JF, Lomasney JW, Stupp SI. Heparin Binding Nanostructures to Promote Growth of Blood Vessels. Nano Lett. 2006;6:2086–2090. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 123.Jayaraman K, Kotaki M, Zhang Y, Mo X, Ramakrishna S. Recent Advances in Polymer Nanofibers. J Nanosci Nanotechnol. 2004;4:52–65. [PubMed] [Google Scholar]
- 124.Ma PX, Zhang R. Synthetic Nano-Scale Fibrous Extracellular Matrix. J Biomed Mater Res. 1999;46:60–72. doi: 10.1002/(sici)1097-4636(199907)46:1<60::aid-jbm7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 125.Thompson CJ, Chase GG, Yarin AL, Reneker DH. Effects of Parameters on Nanofiber Diameter Determined from Electrospinning Model. Polymer. 2007;48:6913–6922. [Google Scholar]
- 126.Yordem OS, Papila M, Menceloglu YZ. Effects of Electrospinning Parameters on Polyacrylonitrile Nanofiber Diameter: An Investigation by Response Surface Methodology. Mater Design. 2008;29:34–44. [Google Scholar]
- 127.Rnjak-Kovacina J, Weiss AS. Increasing the Pore Size of Electrospun Scaffolds. Tissue Eng, Part B Rev. 2011;17:365–372. doi: 10.1089/ten.teb.2011.0235. [DOI] [PubMed] [Google Scholar]
- 128.Arras MML, Grasl C, Bergmeister H, Schima H. Electrospinning of Aligned Fibers with Adjustable Orientation Using Auxiliary Electrodes. Sci Technol Adv Mater. 2012;13:035008. doi: 10.1088/1468-6996/13/3/035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu H, Fan J, Chu CC, Wu J. Electrospinning of Small Diameter 3-D Nanofibrous Tubular Scaffolds with Controllable Nanofiber Orientations for Vascular Grafts. J Mater Sci Mater Med. 2010;21:3207–3215. doi: 10.1007/s10856-010-4164-8. [DOI] [PubMed] [Google Scholar]
- 130.Tsuji H, Nakano M, Hashimoto M, Takashima K, Katsura S, Mizuno A. Electrospinning of Poly(Lactic Acid) Stereocomplex Nanofibers. Biomacromolecules. 2006;7:3316–3320. doi: 10.1021/bm060786e. [DOI] [PubMed] [Google Scholar]
- 131.Liu W, Wei J, Chen Y, Huo P, Wei Y. Electrospinning of Poly(L-Lactide) Nanofibers Encapsulated with Water-Soluble Fullerenes for Bioimaging Application. ACS Appl Mater Interfaces. 2013;5:680–685. doi: 10.1021/am400037s. [DOI] [PubMed] [Google Scholar]
- 132.Boland ED, Coleman BD, Barnes CP, Simpson DG, Wnek GE, Bowlin GL. Electrospinning Polydioxanone for Biomedical Applications. Acta Biomater. 2005;1:115–123. doi: 10.1016/j.actbio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 133.Li MY, Guo Y, Wei Y, MacDiarmid AG, Lelkes PI. Electrospinning Polyaniline-Contained Gelatin Nano-fibers for Tissue Engineering Applications. Biomaterials. 2006;27:2705–2715. doi: 10.1016/j.biomaterials.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 134.Buttafoco L, Kolkman NG, Engbers-Buijtenhuijs P, Poot AA, Dijkstra PJ, Vermes I, Feijen J. Electrospinning of Collagen and Elastin for Tissue Engineering Applications. Biomaterials. 2006;27:724–734. doi: 10.1016/j.biomaterials.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 135.Perumcherry SR, Chennazhi KP, Nair SV, Menon D, Afeesh R. A Novel Method for the Fabrication of Fibrin-Based Electrospun Nanofibrous Scaffold for Tissue-Engineering Applications. Tissue Eng Part C Methods. 2011;17:1121–1130. doi: 10.1089/ten.TEC.2010.0734. [DOI] [PubMed] [Google Scholar]
- 136.Buttafoco L, Kolkman NG, Poot AA, Dijkstra PJ, Vermes I, Feijen J. Electrospinning Collagen and Elastin for Tissue Engineering Small Diameter Blood Vessels. J Controlled Release. 2005;101:322–324. [PubMed] [Google Scholar]
- 137.Whited BM, Rylander MN. The Influence of Electrospun Scaffold Topography on Endothelial Cell Morphology, Alignment, and Adhesion in Response to Fluid Flow. Biotechnol Bioeng. 2014;111:184–195. doi: 10.1002/bit.24995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pham QP, Sharma U, Mikos AG. Electrospun Poly-(Epsilon-Caprolactone) Microfiber and Multilayer Nanofiber/Microfiber Scaffolds: Characterization of Scaffolds and Measurement of Cellular Infiltration. Biomacromolecules. 2006;7:2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 139.Wise SG, Byrom MJ, Waterhouse A, Bannon PG, Weiss AS, Ng MK. A Multilayered Synthetic Human Elastin/Polycaprolactone Hybrid Vascular Graft with Tailored Mechanical Properties. Acta Biomater. 2011;7:295–303. doi: 10.1016/j.actbio.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 140.Zhang H, Jia X, Han F, Zhao J, Zhao Y, Fan Y, Yuan X. Dual-Delivery of VEGF and PDGF by Double-Layered Electrospun Membranes for Blood Vessel Regeneration. Biomaterials. 2013;34:2202–2212. doi: 10.1016/j.biomaterials.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 141.Li Q, Wang Z, Zhang S, Zheng W, Zhao Q, Zhang J, Wang L, Wang S, Kong D. Functionalization of the Surface of Electrospun Poly(Epsilon-Caprolactone) Mats Using Zwitterionic Poly(Carboxybetaine Methacrylate) and Cell-Specific Peptide for Endothelial Progenitor Cells Capture. Mater Sci Eng, C. 2013;33:1646–53. doi: 10.1016/j.msec.2012.12.074. [DOI] [PubMed] [Google Scholar]
- 142.Agarwal R, Singh V, Jurney P, Shi L, Sreenivasan SV, Roy K. Mammalian Cells Preferentially Internalize Hydrogel Nanodiscs over Nanorods and Use Shape-Specific Uptake Mechanisms. Proc Natl Acad Sci US A. 2013;110:17247–17252. doi: 10.1073/pnas.1305000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang XD, Wu D, Shen X, Liu PX, Yang N, Zhao B, Zhang H, Sun YM, Zhang LA, Fan FY. Size-Dependent in Vivo Toxicity of PEG-Coated Gold Nanoparticles. Int J Nanomed. 2011;6:2071–2081. doi: 10.2147/IJN.S21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Love SA, Maurer-Jones MA, Thompson JW, Lin YS, Haynes CL. Assessing Nanoparticle Toxicity. Annu Rev Anal Chem. 2012;5:181–205. doi: 10.1146/annurev-anchem-062011-143134. [DOI] [PubMed] [Google Scholar]
- 145.Marquis BJ, Love SA, Braun KL, Haynes CL. Analytical Methods to Assess Nanoparticle Toxicity. Analyst. 2009;134:425–439. doi: 10.1039/b818082b. [DOI] [PubMed] [Google Scholar]
- 146.Kuo LJ, Yang LX. Gamma-H2AX - a Novel Biomarker for DNA Double-Strand Breaks. In Vivo. 2008;22:305–309. [PubMed] [Google Scholar]
- 147.Manke A, Wang L, Rojanasakul Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. Biomed Res Int. 2013;2013:942916. doi: 10.1155/2013/942916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shvedova AA, Pietroiusti A, Fadeel B, Kagan VE. Mechanisms of Carbon Nanotube-Induced Toxicity: Focus on Oxidative Stress. Toxicol Appl Pharmacol. 2012;261:121–133. doi: 10.1016/j.taap.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kennedy IM, Wilson D, Barakat AI. Uptake and Inflammatory Effects of Nanoparticles in a Human Vascular Endothelial Cell Line. Res Rep — Health Eff Inst. 2009:3–32. [PubMed] [Google Scholar]
- 150.Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors Affecting the Clearance and Biodistribution of Polymeric Nanoparticles. Mol Pharmaceutics. 2008;5:505–515. doi: 10.1021/mp800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Frank MM, Fries LF. The Role of Complement in Inflammation and Phagocytosis. Immunol Today. 1991;12:322–326. doi: 10.1016/0167-5699(91)90009-I. [DOI] [PubMed] [Google Scholar]
- 152.Vasquez KO, Casavant C, Peterson JD. Quantitative Whole Body Biodistribution of Fluorescent-Labeled Agents by Non-Invasive Tomographic Imaging. PLoS One. 2011;6:e20594. doi: 10.1371/journal.pone.0020594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Wan Kim S, Seo MH. In Vivo Evaluation of Polymeric Micellar Paclitaxel Formulation: Toxicity and Efficacy. J Controlled Release. 2001;72:191–202. doi: 10.1016/s0168-3659(01)00275-9. [DOI] [PubMed] [Google Scholar]
- 154.Li J, Li Q, Xu J, Cai X, Liu R, Li Y, Ma J, Li W. Comparative Study on the Acute Pulmonary Toxicity Induced by 3 and 20 nm TiO2 Primary Particles in Mice. Environ Toxicol Pharmacol. 2007;24:239–244. doi: 10.1016/j.etap.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 155.Desai N. Challenges in Development of Nanoparticle-Based Therapeutics. AAPS J. 2012;14:282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Patterson JT, Gilliland T, Maxfield MW, Church S, Naito Y, Shinoka T, Breuer CK. Tissue-Engineered Vascular Grafts for Use in the Treatment of Congenital Heart Disease: From the Bench to the Clinic and Back Again. Regen Med. 2012;7:409–419. doi: 10.2217/rme.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.L’Heureux N, McAllister TN, de la Fuente LM. Tissue-Engineered Blood Vessel for Adult Arterial Revascularization. N Engl J Med. 2007;357:1451–1453. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]