Abstract
Background
Epigenetic mechanisms such as DNA methylation play an important role in regulating the pathophysiology of alcoholism. Chronic alcohol exposure leads to behavioral changes as well as decreased expression of genes associated with synaptic plasticity. In the liver, it has been documented that chronic alcohol exposure impairs methionine synthase (Ms) activity leading to a decrease in SAM/SAH ratio which results in DNA hypomethylation; however it is not known whether similar alterations of S-adenosyl methionine (SAM) and S-adenosyl homocysteine (SAH) levels are also produced in brain.
Method
Male adult Sprague Dawley rats were fed chronically with Leiber-DeCarli ethanol (9% v/v) or control diet. The ethanol diet-fed rats were withdrawn for 0 and 24 hrs. The cerebellum (CB) and liver tissues were dissected and used to investigate changes in one-carbon metabolism, SAM and SAH levels.
Results
We found that chronic ethanol exposure decreased SAM levels, SAM/SAH ratio, MS, methylene tetrahydrofolate reductase (Mthfr) and betaine homocysteine methyltransferase (Bhmt) expression and increased methionine adenosyltransferase-2b (Mat2b) but not Mat2a expression in the liver. In contrast, chronic ethanol exposure decreased SAH levels, increased SAM/SAH ratio and the expression of Mat2a and S-adenosyl homocysteine hydrolase (Ahyc), while the levels of SAM or Bhmt expression in CB remained unaltered. However, in both liver and CB, chronic ethanol exposure decreased the expression of Ms and increased Mat2b expression. All chronic ethanol-induced changes of one-carbon metabolism in cerebellum, but not liver, returned to near-normal levels during ethanol withdrawal.
Conclusion
These results indicate a decreased “methylation index” in liver and an increased “methylation index” in CB. The opposing changes of the “methylation index” suggest altered DNA methylation in liver and CB; thus implicating one-carbon metabolism in the pathophysiology of alcoholism.
Keywords: Alcohol Dependence, DNA Methylation, Liver, Brain, Methionine Metabolism
Introduction
The cerebellum is not only the coordination center for motor learning and control, but also plays a significant role in cognitive processing and sensory discrimination (Martin et al., 1996, Middleton and Strick 2001). In addition, cerebellar involvement in a number of higher-order cognitive functions such as language, visuospatial, executive and working memory processes has also been demonstrated (Stoodley 2012). This brain region is an important target for the toxicity and effects of ethanol. In fact, ethanol-induced cerebellar ataxia is one of the most consistent physical manifestations of acute alcohol consumption and appears to underlie an important brain circuit for regulating the consequences of alcohol intoxication (Dar 2015). Evidence also suggests that chronic alcohol-induced cerebellar deficits are persistent even after withdrawal from alcohol exposure (Sullivan 2000; Sullivan et al., 2005).
Research findings from several laboratories suggest that variation in cerebellar structure and processing, and cerebellar-dependent behavioral sensitivity to ethanol are heritable and associated with susceptibility to alcohol use disorders (AUDs)(Cservenka, 2016, Herting et al., 2011; Hill et al., 2007; Schuckit and Smith 1996; Schuckit, 1985). In addition, cerebellar-related genetic risk has been demonstrated in studies with a low-level of response (LLR) to ethanol phenotype (defined as requiring a higher dose of ethanol to attain a given effect) which is also associated with risk for developing AUD (Schuckit, 2009). Furthermore, cerebellar LLR phenotype is common in young men and women with a family history of alcohol use disorder (Eng et al., 2005; Schuckit, 1985) and can also predict the risk of developing AUD (Schuckit and Smith, 1996), while in rodents this phenotype is common in strains with high ethanol consumption (Gallaher et al., 1996). In fact, low cerebellar sensitivity to ethanol has been suggested as a risk factor to alcoholism (Kaplan et al., 2013). Although the molecular mechanism underlying ethanol-induced cerebellar ataxia and alcoholism has been linked to the transcriptional regulation of cAMP-response-element binding-protein (CREB) (Acquaah-Mensah et al., 2006), the cellular, biochemical and molecular mechanisms underlying ethanol-induced cerebellar ataxia and toxicity remain unclear.
Epigenetic mechanisms such as DNA methylation play an important role in regulating the alterations of synaptic plasticity associated with alcoholism and alcohol-drinking behaviors (Krishnan et al., 2014). Several laboratories have demonstrated that ethanol-induced acute and chronic effects are partly due to its regulatory actions on local and global DNA methylation patterns (Barbier et al., 2015; Wong et al., 2011). Furthermore, it is believed that the effects of ethanol on DNA methylation are mediated by its interference with the availability of S-adenosyl methionine (SAM), the principal biological methyl donor for all transmethylation reactions (Varela-Rey M et al., 2013). These effects have been well studied in liver tissue where chronic ethanol exposure impairs methionine synthase (Ms) activity leading to decreased conversion of S-adenosylmethionine (SAM) into S-adenosylhomocysteine (SAH) and a decrease of the SAM/SAH ratio in liver (Ji et al., 2008; Song et al., 2003). Perturbation of the SAM/SAH ratio brings about alterations of DNA-methyltransferase (Dnmt1, 3a, 3b) activity and DNA methylation which are catalyzed by specific SAM-dependent methyltransferases. These alterations of SAM metabolism lead to a reduction in hepatic DNA methylation capacity which results in the characteristic hallmark features that have been associated with alcohol-induced liver injury (Varela-Rey et al., 2013).
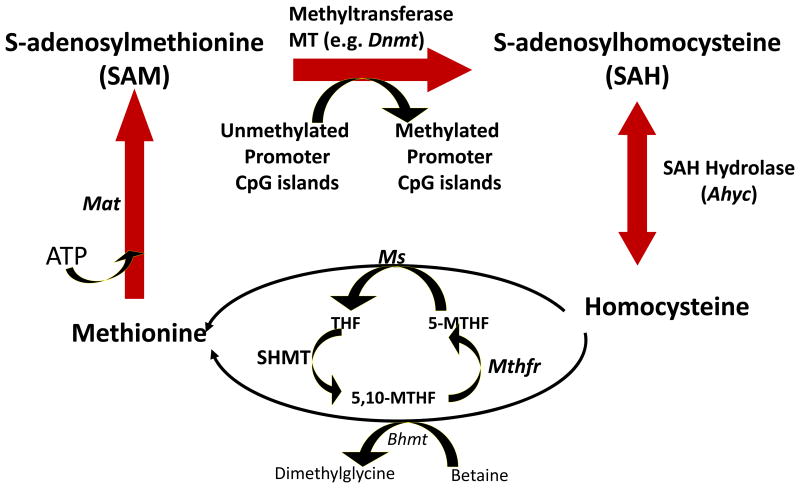
Ethanol-mediated alterations of methionine biosynthesis and metabolism (Fig. 1) are mediated by the inhibition of Ms activity or the activity of other key enzymes of the one-carbon metabolism such as methionine adenosyltransferase (Mat) catalytic (2a) and regulatory (2b) subunits, S-adenosyl homocysteine hydrolase (Ahyc), betaine homocysteine methyltransferase (Bhmt) and methylene tetrahydrofolate reductase (Mthfr)(Ji and Kaplowitz 2003; Lu et al., 2000; Villanueva and Halsted, 2004; Villanueva et al., 2007). The mechanism(s) through which chronic ethanol exposure alters DNA methylation pathways in the brain, and particularly the cerebellum, are still unknown. In addition, it is not known whether the perturbations of methionine metabolism that have been reported in liver tissue following chronic ethanol exposure are also present in brain structures. To the best of our knowledge, there are no studies linking changes in the levels of SAM and SAH or the SAM/SAH ratio in the liver with alterations in DNA methylation patterns in the cerebellum. Thus, we studied the effects of chronic ethanol exposure and its withdrawal on SAM and SAH levels and the expression of some key enzymes involved in one-carbon metabolism in rat cerebellum and compared the observed effects with those occurring in liver tissues of the same animals. We found that chronic ethanol exposure resulted in a decrease in SAM level and the SAM/SAH ratio with a slight increase in SAH levels in liver, whereas in the cerebellum chronic ethanol elicited an increase in SAM/SAH ratio by decreasing SAH levels with no effect on SAM levels. To the best of our knowledge, this is the first study comparing the effects of chronic ethanol exposure on the regulation of SAM and SAH availability and the SAM/SAH ratio in liver and cerebellum.
Fig.1.
Schematic representation of enzymes involve in the regulation of one-carbon metabolism. Ms= Methionine synthase, Mat= Methionine adenosyltransferase, SAH= S-adenosylhomocysteine, Ahyc= Sah hydrolase, THF= Tetrahydrofolate, 5-MTHF= Methylene-THF, Mthfr=Methylenetetrahyfolate reductase, Bhmt= Betaine homocysteine transferase, Dnmt= DNA-methyltransferase, SAM= S-adenosylmethionine.
Methods
Chronic Ethanol Treatment Paradigm
Adult male Sprague Dawley rats (Harlan, Indianapolis) were received and after one week of acclimatization they were housed individually and maintained on a 12/12-h light-dark cycle with standard food and water made available ad libitum. All experiments were carried out in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and approved by the animal committee at the University of Illinois at Chicago.
Rats were randomly assigned to three treatment groups: a) control-diet fed, b) ethanol-diet fed (0 hr withdrawal) and c) ethanol-diet fed (24 hr withdrawal) referred to as the ethanol-withdrawn group. The rats were either fed with Lieber-DeCarli control or ethanol liquid diet as previously described (Pandey et al., 2008). In brief, all three groups of rats were exposed to 80 ml/day of nutritionally complete Lieber-DeCarli liquid control diet (Bio-Serv, Frenchtown, USA) for 3 days. The control group continued with control diet for 16 days, while ethanol-treated groups were gradually introduced to ethanol and then maintained on 9% v/v ethanol diet for 15-16 days. Ethanol-diet fed rats were withdrawn for 0 or 24 hr. Rats were pair-fed and their liquid diet intake and body weight was closely monitored. We and other investigators have used this alcohol treatment paradigm and reported blood ethanol levels in the range of 172-198 mg/100 ml for ethanol-diet fed (0 hr withdrawn) rats, whereas the blood ethanol level after 24 h of ethanol withdrawal was 0 mg/100 ml (Hunter et al., 1975; Baldwin et al., 1991; Pandey et al., 1992, 2008a,b; You et al., 2014). It is important to point out that we have used the above animal model to establish brain molecular mechanisms of alcohol dependence (Pandey et al., 2008a,b; You et al., 201), and other investigators have also used a similar model to investigate mechanisms related to hepatic injury and one-carbon metabolism (Kharbanda et al., 2014). All rats were euthanized using isoflurane and cerebellum and liver tissue was dissected and frozen at -80oC until used for RT-qPCR and measurement of SAM and SAH levels.
RNA extraction
Total RNA was isolated from cerebellum and liver tissues using the TRIzol reagent (Life Technologies 15596-026; Life Technologies Corporation, USA) and further purified using the Qiagen RNeasy Kit (Qiagen, Valencia, CA, USA).
Real-time polymerase chain reaction (PCR) quantification
Total RNA was converted to cDNA using the Applied Biosystems (USA) High Capacity Archive Kit (4368813). Relative quantitative real-time polymerase chain reaction (qPCR) was performed with the Applied Biosynthesis Real-Time PCR system using Fermentas Maxima SYBR Green/ROX qPCR Master Mix (K0222; Fermentas International Inc., Canada). PCR mixtures were run on a Stratagene (USA) Mx3005P QPCR System. Primers were designed to cross over one intron to amplify cDNA and yield an amplicon between 124-299 base pairs (primer sequences are provided in Table 1). Dissociation curves were conducted to establish the presence of a single amplicon at the predicted melting temperature and a lack of primer-dimer formation. A comparative threshold cycle (CT) validation experiment was done to determine target and reference primer efficiency. For normalization of mRNA expression, Gapdh was used as internal control. CT value was used for relative quantification of target gene expression and normalized to Gapdh and the relative expression levels were calculated as CT (Livak and Schmittgen, 2001).
Table 1. Primer Sequences used for real-time PCR.
| Gene | Forward | Reverse |
| Mat2a | 5′-CTTCCATGAGGCGTTCATCGA | 5′-TCAATGGCAGCTCTAGATGTAATTTC |
| Mat2b | 5′-CATGCCTGAGATGCCGGAG | 5′-GTCTTGCTCTGCGAAAGCCA |
| Ms | 5′-GATCTCGTGAGCCAGTTCGTCA | 5′- CCGAAGAATGGCTTCAATCTCATCC |
| Mthfr | 5′-TCGTGGCCCCAGCTTCA | 5′- ACAGAAAGTCCCACGCAGC |
| Ahyc | 5′-ATGATGGCCAATGGGATACTGAAG | 5′- GTCAATCTCGGTGATGATGACTCG |
| Bhmt | 5′-TAGCGGCTACCATGTGCATC | 5′-ATCCCATCTGGTGGCAACTC |
| Dnmt1 | 5′-AAGCCAGCTATGCGACTTGGAAAC | 5′-ACA ACC GTTGGCTTTCTGAGTGAG |
| Dnmt3a | 5′-CACCTACAACAAGCAGCCCATGTA | 5′-AGCCTTGCCAGTGTCACTTTCATC |
| Dnmt3b | 5′-TGTGCAGAGTCCATTGCTGTAGGA | 5′-GCTTCCGCCAATCACCAAGTCAAA |
| Gapdh | 5′-ACCAGGGCTGCCTTCTCT | 5′-ATCTCGCTCCTGGAAGATGGT |
Measurement of SAM and SAH Levels by HPLC
Rat cerebellum or liver tissues were weighed and homogenized in 2.5 (100μl/40mg wet tissue) or 0.5 (2 ml/mg wet tissue weight) volumes of 0.4 M HClO4 respectively, then centrifuged at 10,000 × g for 5 min. Supernatants were filtered through 0.22 μm Millipore membranes and 100 μl (cerebellum) or 25 μl (liver) aliquots were injected into a reverse-phase high-pressure liquid chromatography column (Symmetry C18 4.6 × 250 nm; Waters, Milford, Massachusetts, USA). The mobile phase consisted of three solvents: solvent A, 40 mM NaH2PO4 and 8 mM 1-octanesulfonic acid sodium salt adjusted to pH 3.0 with H3PO4; solvent B, 40% methanol in solvent A; and solvent C, 100% methanol. The column was equilibrated with solvent A at a flow rate of 1 ml/min; after sample injection (100 or 25 μl) separation was obtained using a multistep gradient: 45 min at the equilibration conditions, up to 50% solvent B in 1 min and maintained for 8 min, up to 100% solvent B in 30 sec and maintained for 16.5 min, then down to 50% solvent B and 50% solvent C in 1 min and maintained for 10 min to wash and return to equilibration condition for a minimum of 30 min before subsequent injections.
Standards
SAM chloride dihydrochloride and SAH obtained from Sigma-Aldrich (St. Louis, MO, USA) were dissolved in water and diluted with 0.4 M HClO4 to the final concentration used for HPLC analysis. Aliquots (100 μl) of the standard solutions were injected onto the HPLC column and retention times for SAH and SAM were 17.2 min and 21.1 min respectively. Five point calibration curves for SAM and SAH were linear in the range of 25-800 ng of the respective standards.
Statistical analysis
Data were analyzed using one-way ANOVA and group comparisons were performed using the Duncan-Multiple range test. Significance was set at p<0.05 for all experiments.
Results
Effects of chronic ethanol exposure and withdrawal on various key steps of the one-carbon metabolism in the cerebellum
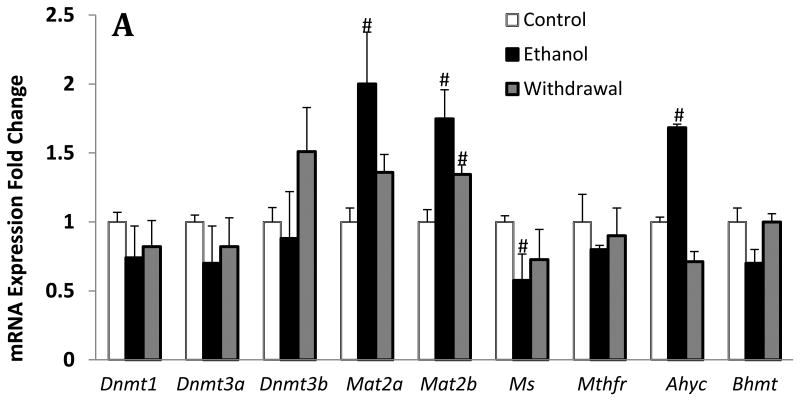
To examine the effects of chronic ethanol exposure and its withdrawal on the expression levels of key enzymes of one-carbon metabolism (Fig.1), we measured relative mRNA expression for Mat2a & 2b, Ms, Ahyc, Mthfr, and Bhmt. We found that chronic ethanol exposure resulted in a 45% decrease of Ms, 70% increase of Ahyc, and almost two-fold increase of Mat2b and Mat2b mRNA expression in the cerebellum, whereas the expression levels for Mthfr and Bhmt were not significantly changed (Fig 2A). While the changes in Ms, Ahyc and Mat2a returned to normal levels during ethanol withdrawal, the changes in Mat2b remained significantly elevated in this group. In addition, we show that the expression levels for the DNA transmethylation enzymes Dnmt1, Dnmt3a and Dnmt3b were not changed by chronic ethanol exposure and its withdrawal (Fig 2A).
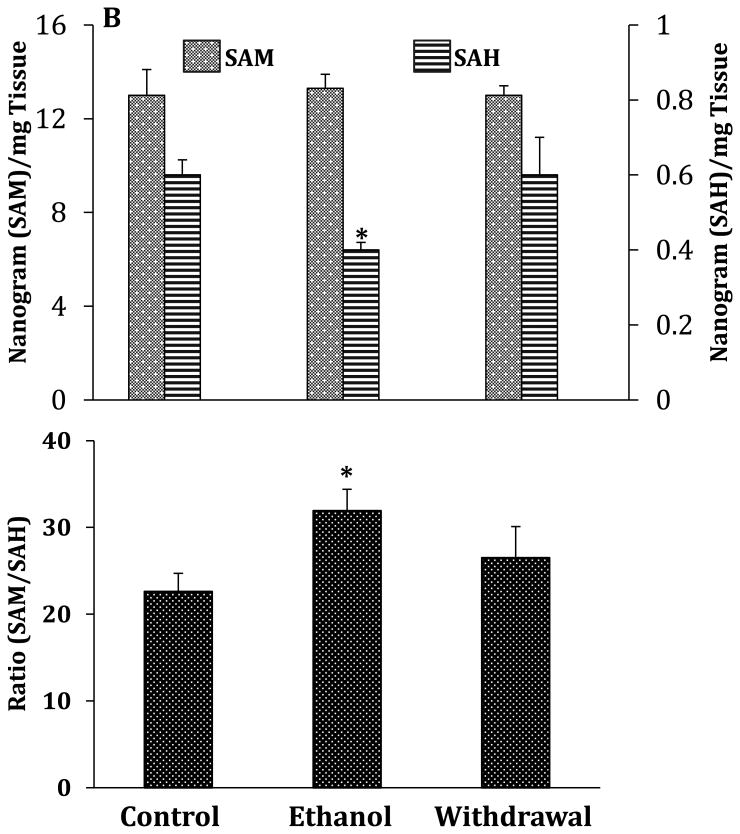
Fig.2.
Effects of chronic ethanol exposure and its withdrawal on various key steps of one-carbon metabolism in the cerebellum. (A) mRNA expression of key enzymes; (B) levels of SAM and SAH and SAM/SAH ratio. Values are represented as mean ± SEM of n=6 rats per group; p<0.05 one way ANOVA followed by Duncan-Multiple Range test.
We also examined whether there was any association between the alteration of the enzymes of one-carbon metabolism with SAM and SAH levels in cerebellum. The results of this measurement show that the levels of SAM are approximately 22-fold higher than those of SAH. In addition, chronic ethanol exposure resulted in a 35% decrease in SAH levels and a significant increase in the SAM/SAH ratio with no changes in SAM levels (Fig.2B). Additionally, the changes in SAH levels and the SAM/SAH ratio returned to near-normal levels during ethanol withdrawal.
Effects of chronic ethanol exposure and withdrawal on various key steps of one-carbon metabolism in liver
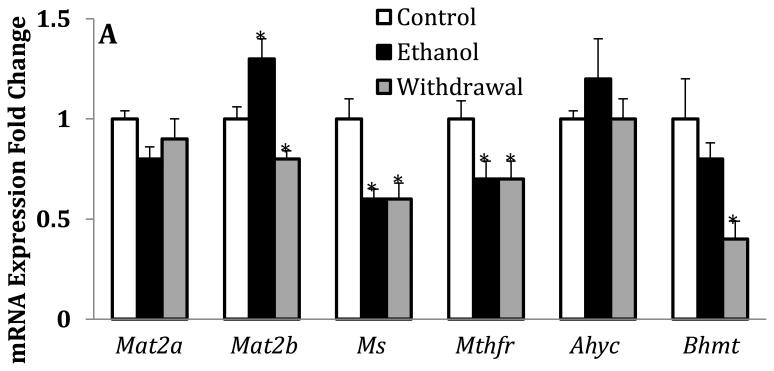
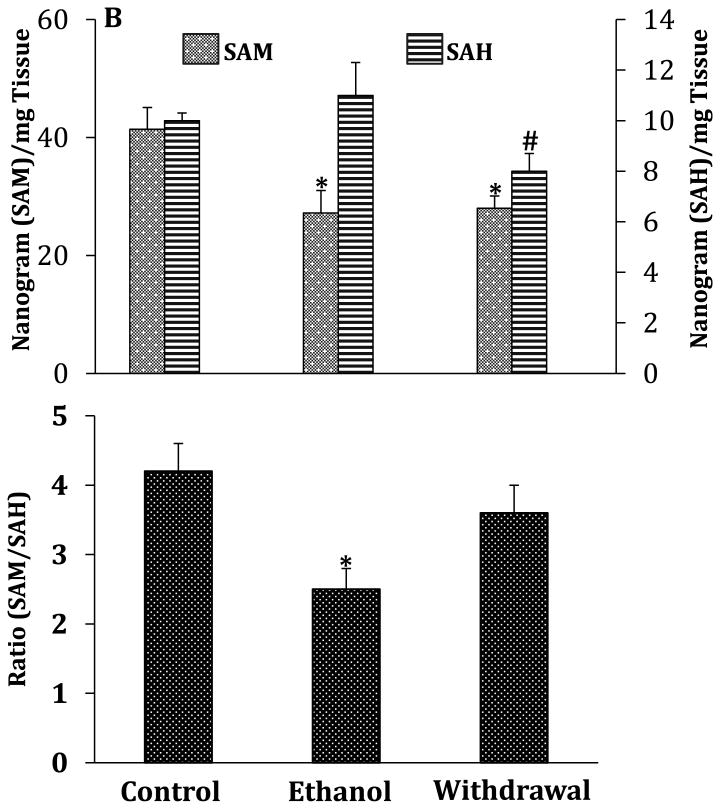
To examine whether the chronic ethanol exposure paradigm used here elicits similar changes in the liver that have previously been reported (Tsukamoto and Lu, 2001)), we also measured the levels of SAM and SAH, determined SAM/SAH ratio and the relative mRNA expression of Mat2a & 2b, Ms, Mthfr, Ahyc and Bhmt in liver. We show here that chronic ethanol exposure significantly increased Mat2b expression without changing the expression of Mat2a (Fig.3A). In addition, and as observed in cerebellum, this chronic ethanol exposure paradigm also produced a significant decrease of Ms expression but did not change the expression of Ahyc which was increased by 70% in cerebellum. In contrast to the changes observed in the cerebellum, chronic ethanol-feeding elicited a 30% and 20% decrease in the mRNA expression levels of Mthfr and Bhmt respectively. In contrast to the cerebellum, all the perturbations of the one-carbon metabolism elicited by chronic ethanol exposure were sustained during withdrawal (Fig.3A versus Fig. 2A). As previously reported (Halsted et al., 1996; Tsukamato and Lu, 2001; Erman et al., 2004; Purohit et al., 2007), we also confirmed here that this chronic ethanol exposure paradigm results in a 35% decrease in the levels of SAM and a 40% decrease in the SAM/SAH ratio (Fig.3B). In addition, the levels of SAM are approximately 4-fold higher than those of SAH. Compared to the cerebellum, the levels of SAM are 2-3 fold higher while the levels of SAH are about 15-17 fold higher in the liver. Whereas chronic ethanol exposure only produced a 10% increase in the levels of SAH, the levels decreased by 20% during withdrawal without a significant change in the SAM/SAH ratio, contrasting with the changes found in cerebellum.
Fig.3.
Effects of chronic ethanol exposure and its withdrawal on various key steps of one-carbon metabolism in the liver. (A) mRNA expression of key enzymes; (B) levels of SAM and SAH and SAM/SAH ratio. Values are represented as mean ± SEM of n=6 rats per group; p<0.05 one way ANOVA followed by Duncan-Multiple Range test.
Discussion
The main objective of our study was to examine the effects of long-term ethanol exposure and withdrawal on SAM and SAH levels, the resulting SAM/SAH ratio, and the expression of key enzymes that regulate the availability of SAM (Fig. 1) in the cerebellum and compare these changes to one-carbon metabolism in the liver. We not only confirmed that, in liver, chronic ethanol exposure decreased Ms expression, SAM levels and the SAM/SAH ratio but also extended these findings and observed that chronic ethanol exposure decreased Mthfr and Bhmt expression and increased Mat2b expression in the liver. Interestingly, chronic ethanol exposure decreased the levels of SAH and increased the SAM/SAH ratio and the expression of Mat2a and Ahyc without changing the levels of SAM and Bhmt expression in the cerebellum. However, in both liver and cerebellum, chronic ethanol exposure decreased Ms expression and increased Mat2b expression. Furthermore, with the exception of liver Bhmt expression, all these changes returned to near-normal levels during ethanol withdrawal.
Outcomes of human and animal studies suggest that differing degrees of chronic alcohol-induced liver injury are associated with impairment of Ms activity which leads to decreased hepatic concentrations of SAM and folate but increased plasma homocysteine concentrations and hepatic SAH levels (Cravo et al., 1996; Halsted et al., 1996; de la Vega et al. 2001, Ji and Kaplowitz, 2003; Barak et al., 2003). Moreover, SAM or betaine supplementation has been shown to prevent alcoholic liver injury in animal studies (Ji and Kaplowitz, 2003, Song et al., 2003, Ji et al., 2008). It has been demonstrated that SAM and betaine exert protective action against chronic alcohol-induced liver injury by several mechanisms including the down-regulation of tumor necrosis factor, the up-regulation of interleukin-10 synthesis, attenuation of oxidative stress by restoring glutathione (GSH) concentrations, and increasing SAM/SAH ratio (Fernandez-Checa et al., 2002, Ishii et al., 2003, Song et al., Yang et al., 2004, Barve et al., 2006; Villanueva et al., 2007; Ji et al., 2008; Jung et al., 2013). Thus, one can infer that chronic alcohol-induced liver injury is partly mediated by perturbation of key steps of the one-carbon metabolism (Fig. 1). For the first time, our data demonstrates that chronic alcohol exposure also modifies one-carbon metabolism in the cerebellum, suggesting that one-carbon metabolism in both the liver and brain may contribute to the pathophysiology of alcoholism.
While chronic ethanol exposure resulted in a significant decrease of Ms expression in both brain and cerebellum, Bhmt and Mthfr expression were significantly decreased in the liver but remain unchanged in cerebellum. It has been suggested that under normal conditions, alcohol-induced reduction of Ms activity leads to a reduction of SAM levels and enhances the production of SAH and homocysteine in hepatocytes. To compensate for the reduction of Ms activity, Bhmt activity is induced after alcohol consumption. However, after extended periods of alcohol exposure, this compensatory mechanism is not maintained (Variela-Ray et al., 2013), consequently leading to a reduction of SAM levels, an increase levels of homocysteine and SAH, and a reduced SAM/SAH ratio (Varela-Rey et al. 2013). These alterations of one-carbon metabolism in the liver may contribute to the reduced hepatic SAM levels observed in patients hospitalized for alcohol related hepatitis (Lee et al., 2004). The results of our study suggest that the normal compensatory mechanism that is triggered by a reduction of Ms activity in the liver is non-functional after chronic alcohol exposure whereas in the cerebellum, this compensatory pathway might still be intact. In addition, our finding of chronic ethanol-induced decreases in Ahyc mRNA expression (Fig.3A) is agreement with the published finding of Villanueva and Halsted (2003) and is a plausible explanation for the significant decreases of SAH and SAM levels in liver but not cerebellum. In contrast to the liver, the significant increase of Ahyc mRNA expression level in cerebellum (Fig.2A) correlates with the decreased SAH level in cerebellum (Fig. 2B) and may account for the increased SAM/SAH ratio in cerebellum. This is a reasonable inference because in the one-carbon cycle, Ahyc reversibly hydrolyzes SAH and releases homocysteine and adenosine as end products (Fig.1). Under normal physiological condition, homocysteine is utilized by Ms for the biosynthesis of methionine and, in this case, Ahyc-mediated hydrolysis favors the rapid metabolism of SAH into homocysteine and adenosine. However, chronic ethanol-induced accumulation of homocysteine and adenosine (Villanueva and Halsted, 2004) drives the reversible synthesis of SAH (Fig.1). Although in the present study we did not include measurement of Mat1a mRNA expression, chronic ethanol-induced decreases in transcript levels of Mat1a in the liver has been reported previously (Villanueva and Halsted, 2004; Lu et al., 2000) and may also account for the significantly decreased SAM level in the liver (Fig. 3B). All together, these results allow us to speculate that chronic ethanol-induced alterations of one-carbon metabolism and the levels of SAM and SAH in liver and cerebellum might differentially regulate DNA methylation mechanisms during alcoholism.
As depicted in Fig. 1, methionine is converted to SAM in a reaction catalyzed by Mat (1a & b are predominantly expressed in the liver while 2a & 2b are ubiquitously expressed). Thus, the availability of SAM for transmethylation reactions is regulated by one-carbon metabolism. DNA N-methyltransferases (Dnmts), glycine N-methyltransferase (Gnmt) and other transmethylation enzymes transfer a methyl group from SAM to a large variety of acceptor substrates (DNA, RNA, enzymes, proteins, etc) and release SAH as a by-product. SAH in turn acts as potent competitive allosteric inhibitor of Mat (1a, 1b, 2a and 2b) and other transmethylation reactions, and the activity of methyltransferases depends not only on the availability of SAM but also on the cellular concentrations of SAH. Furthermore, SAH cellular concentration depends on the activity of Ahyc which reversibly hydrolyzes SAH into homocysteine plus adenosine (Fig. 1). Ultimately, the cellular methylation capacity or potential depends on the SAM/SAH ratio (also known as “methylation index”). Interestingly, it is well established that the Mat2b (regulatory) subunit regulates the activity of the Mat2a (catalytic) subunit by reducing its Km for L-methionine and also rendering it more susceptible to feed-back inhibition by SAH (LeGros et al., 2001). Hence, the findings presented here suggest that a decrease of the SAM/SAH ratio or SAM levels in the liver might lead to decreased transmethylation reactions or DNA hypomethylation (Tsukamato and Lu, 2001). In fact, it has been hypothesized that the reduction of SAM availability or the decrease in the SAM/SAH ratio leads to a reduction of hepatic methylation capacity resulting to the characteristic hallmark features that have been associated with chronic alcohol-induced liver injury (Varela-Rey et al., 2013). In contrast, we can infer that the increase of the SAM/SAH ratio or decrease in SAH found in the cerebellum of chronic ethanol-exposed animals may lead to increased local DNA methylation patterns (hypermethylation) and other transmethylation reactions particularly in cerebellar Purkinje cells, granular cells and other cellular structures. These changes in cellular methylation capacity might serve as a protective mechanism to preserve these cellular components from chronic alcohol-induced oxidative stress, mitochondrial damage and inhibition of DNA repair processes (Seitz and Stickel, 2007).
In a recent study that examined the expression of Mat2b in human postmortem brain, Ponomarev et al (2012) reported a downregulation of Mat2b mRNA in postmortem cortex and the central and basolateral amygdala of human alcoholics. Although the levels of SAM and SAH were not measured in this study, based on the results of Blasco et al (2005) the authors concluded that the downregulation of Mat2b may be responsible for the decreased SAM levels seen in human postmortem tissue. This inference was based on the premise that Mat2b changes the kinetic properties of Mat2a, thereby rendering it more susceptible to product inhibition by SAM. If this was the case, decreased expression of Mat2b would render Mat2a less susceptible to product inhibition by SAM and thus lead to increased SAM levels. In contrast, we show here that in liver tissue of rats exposed to chronic alcohol, increased expression of Mat2b is associated with decreased levels of SAM and increased levels of SAH. Perhaps the increased expression of Mat2b reduces the Km of Mat2a for methionine and renders it more susceptible to feed-back inhibition by SAH (LeGros et al., 2001; Mato et al., 2002; Varela-Rey et al., 2013; Fu et al., 2000; De Cabo et al., 1994). However, it will be important to establish in future studies whether the differential changes in the “methylation index” we observed in brain, specifically the cerebellum, correlate with differences in the level of DNA methylation of target genes in the same tissue. In addition, we will also investigate whether the effects of chronic ethanol exposure on one-carbon metabolism and the “methylation index” in the cerebellum are also present in other brain areas or are specific to the cerebellum.
In conclusion, we demonstrated here that while chronic ethanol exposure decreased SAM levels and SAM/SAH ratio in the liver, it decreased SAH levels and increased SAM/SAH ratio in the cerebellum. These results indicate a decrease and an increase of “methylation index” in the liver and cerebellum, respectively. The tissue-specific effects of chronic alcohol exposure on the “methylation index” suggest a decrease and an increase in DNA methylation (hypomethylation and hypermethylation) in the liver and cerebellum, respectively. Taken together, our findings clearly implicate impaired methylation mechanisms due to altered one-carbon metabolism in the pathophysiology of alcoholism.
Acknowledgments
This study was supported by NIAAA, P50 AA022538 to SCP and AG, senior VA Research career award to SCP, 1RO1MH101043 and 1RO1MH093348 to AG.
Footnotes
Conflict of Interest: None
Reference List
- Acquaah-Mensah GK, Misra V, Biswal S. Ethanol sensitivity: a central role for CREB transcriptional regulation in the cerebellum. BMC Genomics. 2006;7:308–93. doi: 10.1186/1471-2164-7-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HA, Rassnick S, River J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103:227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- Barak AJ, Beckenhauer HC, Mailliard ME, Kharbanda KK, Tuma DJ. Betaine lowers elevated S-adenosylhomocysteine levels in hepatocytes from ethanol-fed rats. J Nutrition. 2003;133(9):2845–2848. doi: 10.1093/jn/133.9.2845. [DOI] [PubMed] [Google Scholar]
- Barbier E, Tapocik JD, Juergens N, Pitcairn C, Borich A, Schank JR, Sun H, Schuebel K, Zhou Z, Yuan Q, Vendruscolo LF, Goldman D, Heilig M. DNA methylation in the medial prefrontal cortex regulates alcohol-induced behavioral plasticity. J Neurosci. 2015;35(15):6153–6164. doi: 10.1523/JNEUROSCI.4571-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barve S, Joshi-Barve S, Song Z, Hill D, Hote P, Deaciuc I, McClain C. Interactions of cytokines, S-adenosylmethionine and S-adenosylhomocysteine in alcohol-induced liver disease and immune suppression. J Gastroenterol Hepatol Suppl. 2006;3:S34–42. doi: 10.1111/j.1440-1746.2006.04590.x. [DOI] [PubMed] [Google Scholar]
- Blasco C, Caballeri J, Deulofeu R, Lliogona A, Pares A, Lluis JM, Gual A, Rodes J. Prevalence and mechanism of hyperhomocysteinemia in chronic alcoholics. Alcohol Clin Exp Res. 2005;29:1044–1048. doi: 10.1097/01.alc.0000169265.36440.ee. [DOI] [PubMed] [Google Scholar]
- Cravo ML, Gloria LM, Selhib J, Nadeau MR, Camilo ME, Resende MP, Cardoso JN, Leitao CN, Mira FC. Hyperhomocysteinemia in chronic alcoholism: correlation with folate, vitamin B-12 and vitamin B-6. Am J Clin Nutr. 1996;63(2):220–224. doi: 10.1093/ajcn/63.2.220. [DOI] [PubMed] [Google Scholar]
- Cservenka A. Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend. 2016;158:8–21. doi: 10.1016/j.drugalcdep.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar MS. Ethanol-induced cerebella ataxia: cellular and molecular mechanisms. Cerebellum. 2015;14:447–465. doi: 10.1007/s12311-014-0638-4. [DOI] [PubMed] [Google Scholar]
- De Cabo SF, Hazen MJ, Molero ML, Fernandez-Piequeras J. S-adenosyl-L-homocysteine: a non-cytotoxic hypomethylating agent. Experientia. 1994;50(7):658–9. doi: 10.1007/BF01952867. [DOI] [PubMed] [Google Scholar]
- de la Vega MJ, Santolaria F, Gonzalez-Reimers E, Alema MR, Milena A, Martinez-Riera A, Gonzalez-Garcia High prevalence of hyperhomocysteinemia in chronic alcoholism: the importance of the thermolabile form of the enzyme methylenetetrahydrofolate reductase (Mthfr) Alcohol. 2001;25(2):59–67. doi: 10.1016/s0741-8329(01)00167-7. [DOI] [PubMed] [Google Scholar]
- Eng MY, Schuckit MA, Smith IL. The level of response to alcohol in daughters of alcoholics and controls. Drug and Alcohol Depend. 2005;79(1):83–93. doi: 10.1016/j.drugalcdep.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Erman F, Balkan J, Cevikbas U. Betaine or taurine administration prevents fibrosis and lipid peroxidation induced by rat liver by ethanol plus carbon tetrachloride intoxication. Amino Acids. 2004;27:199–205. doi: 10.1007/s00726-004-0105-5. [DOI] [PubMed] [Google Scholar]
- Fernandez-Checa JC, Colell A, Garcia-Ruiz C. S-adenosyl-L-methionine and mitochondria reduced glutathione depletion in alcoholic liver disease. Alcohol. 2002;27(3):179–83. doi: 10.1016/s0741-8329(02)00229-x. [DOI] [PubMed] [Google Scholar]
- Fu Wm Dudman NPB, Perry MA, Young K, Wang XL. Interrelations between plasma homocysteine and intracellular S-adenosylhomocysteine. Biochem Biophys Res Commun. 2000;271(1):47–53. doi: 10.1006/bbrc.2000.2587. [DOI] [PubMed] [Google Scholar]
- Gallaher EJ, Jones GE, Belknap JK, Crabbe JC. Identification of genetic markers for initial sensitivity and rapid tolerance to ethanol-induced ataxia using quantitative trait locus analysis in BXD recombinant inbred mice. J Pharmacol Exp Ther. 1996;277(2):604–612. [PubMed] [Google Scholar]
- Halsted CH, Villaneuva J, Chandler CJ, Stabler SP, Allen RH, Muskhelishvilli L, James SJ, Poirier L. Ethanol feeding of micropigs alters methionine metabolism and increases hepatocellular apoptosis and proliferation. Hepatology. 1996;23(3):497–505. doi: 10.1002/hep.510230314. [DOI] [PubMed] [Google Scholar]
- Herting MM, Fair D, Nagel BJ. Altered fronto-cerebral connectivity in alcohol naïve youth with a family history of alcoholism. Neuroimage. 2011;54:2582–2589. doi: 10.1016/j.neuroimage.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Kostelnik B, Holmes B, Goradia D, McDermott M, Diwadkar V, Keshavan M. FMRI bold response to the eyes task in offspring from multiplex alcohol dependence families. Alcohol Clin Exp Res. 2001;31:2028–2035. doi: 10.1111/j.1530-0277.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter BE, Riley JN, Walker DW. Ethanol dependence in the rat: a parametric analysis. Pharmacol Biochem Behav. 1975;3:619–629. doi: 10.1016/0091-3057(75)90183-5. [DOI] [PubMed] [Google Scholar]
- Ishii H, Adachi M, Fernandez-Checa JC, Cederbaum AI, Deaciuc IV, Nanji AA. Role of apoptosis in alcoholic liver injury. Alcohol Clin Exp Res. 2003;27:1207–1212. [PubMed] [Google Scholar]
- Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress and liver injury in alcohol-fed mice. Gastroenterology. 2003;12:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- Ji C, Shinohara M, Vance D, Than TA, Ookhtens M, Chan C, Kaplowitz Effects of transgenic extrahepatic expression of betaine-homocysteine methyltransferase on alcohol or homocysteine-induced fatty liver. Alcoholism: Clin Exp Res. 2008;32(6):1049–1058. doi: 10.1111/j.1530-0277.2008.00666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YS, Kim SJ, Kwon DY, Ahn CW, Kim YS, Choi DW, Kim YC. Alleviation of alcoholic liver injury by betaine involves an enhancement of antioxidant defense via regulation of sulfur amino acid metabolism. Food Chem Toxicol. 2013;62:292–298. doi: 10.1016/j.fct.2013.08.049. [DOI] [PubMed] [Google Scholar]
- Kaplan JS, Mohr C, Rossi DJ. Opposite action of alcohol on tonic GABA (A) receptor currents mediated by nNOS and PKC activity. Nat Neurosci. 2013;16:1783–93. doi: 10.1038/nn.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda KK. Alcoholic liver disease and methionine metabolism. Semin Liver Dis. 2009;29:155–165. doi: 10.1055/s-0029-1214371. [DOI] [PubMed] [Google Scholar]
- Kharbanda KK, Todero SL, Thomas PG, Orlicky DJ, Osna NA, French SW, Tuma DJ. Increased methylation demand exacerbates ethanol-induced liver injury. Exp Mol Pathol. 2014;97:49–56. doi: 10.1016/j.yexmp.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Lee TD, Sadd ME, Mendler MH, Bottiglier T, Kanel G, Mato JM, Lu SC. Abnormal hepatic methionine and glutathione metabolism in patients with alcoholic hepatitis. Alcoholism: Clin Exp Res. 2004;28(1):173–181. doi: 10.1097/01.ALC.0000108654.77178.03. [DOI] [PubMed] [Google Scholar]
- LeGros L, Halim AB, Chamberlin ME, Geller A, Kotb M. Regulation of human Mat2b gene encoding the regulatory beta subunit of methionine adenosyltransferase, MATII. J Biol Chem. 2001;275:24918–24924. doi: 10.1074/jbc.M102816200. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu SC, Huang Z, Yang H, Mato JM, Avila MA, Tsukamato H. Changes in methionine adenosyltransferase and S-adenosylmethionine homeostasis in alcoholic rat liver. Am J Physiol Gastro Liv Phusiol. 2000;279:G178–G185. doi: 10.1152/ajpgi.2000.279.1.G178. [DOI] [PubMed] [Google Scholar]
- Mato JM, Corrales FJ, Lu SC, Avila MA. S-adenosylmethionine: a control switch that regulates liver function. FASEB J. 2002;16:15–26. doi: 10.1096/fj.01-0401rev. [DOI] [PubMed] [Google Scholar]
- Martin TA, keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms.I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119:1183–98. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J. Neurosci. 2001;21(2):700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Piano MR, Schwartz DW, David JM, Pandey GN. Effect of ethanol administration and withdrawal on serotonin receptor subtypes and receptor-mediated phosphoinositide hydrolysis in rat brain. Alcohol Clin Exp Res. 1992;16:1110–1116. doi: 10.1111/j.1530-0277.1992.tb00706.x. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008a;28(10):2589–2600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ugale R, Prakash A, Xu T, Misra K. Effector immediate-early gene arc in the amygdala plays a critical role in alcoholism. J Neurosci. 2008b;28(10):2589–2600. doi: 10.1523/JNEUROSCI.4752-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield D. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci. 2012;32(5):1884–1897. doi: 10.1523/JNEUROSCI.3136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V, Abdelmalek MF, Barve S, Benevenga NJ, Halsted CH, Kaplowitz N, Kharbanda KK, Liu Qy Lu SC, McClain CJ, Swanson C, Zakhari S. Role of S-adenosylmethionine, folate and betaine in the treatment of alcoholic liver disease: summary of a symposium. Am J Clin Nutr. 2007;86:14–24. doi: 10.1093/ajcn/86.1.14. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Ethanol-induced changes in body sway in men at high alcoholism risk. Arch. Gen. Psychiatry. 1985;42:375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psych. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373(9662):492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nature Rev Cancer. 2007;7(8):599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- Song Z, Zhou Z, Chen T, Hill D, Kang J, Barve S, McClain C. S-adenosylmethionine (SAMe) protects against acute alcohol-induced hepatotoxicity in mice small star, filled. J Nutr Biochem. 2003;14:591–597. doi: 10.1016/s0955-2863(03)00116-5. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ. The Cerebellum and cognition: evidence from functional imaging studies. Cerebellum. 2012;1(2):352–365. doi: 10.1007/s12311-011-0260-7. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Human vulnerability to alcoholism: evidence from neuroimaging studies. In: Noronha A, Eckhardt M, Warren MK, editors. Review of NIAAA's neuroscience and behavioral research portfolio. NIAAA Research Monograph No.34 National Institutes of Health; Bethesda MD: 2000. pp. 473–508. [Google Scholar]
- Sullivan EV, Deshmukh A, De Rosa E, Rosenbloom MJ, Pfefferbaum A. Striatal and forebrain nuclei volumes: contribution to motor function and working memory deficits in alcoholism. Biol Psych. 2005;57(7):768–776. doi: 10.1016/j.biopsych.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Tsukamato H, Lu SC. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 2001;15:1335–1349. doi: 10.1096/fj.00-0650rev. [DOI] [PubMed] [Google Scholar]
- Varela-Rey M, Woodho A, Martinez-Chanter M, Mato JM, LU SC. Alcohol, DNA methylation and cancer. Alcohol Res. 2013;35(1):25–35. doi: 10.35946/arcr.v35.1.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva JA, Halsted CH. Hepatic transmethylation reactions in micropigs with alcoholic liver disease. Hepatology. 2004;39:1303–1310. doi: 10.1002/hep.20168. [DOI] [PubMed] [Google Scholar]
- Villanueva JA, Esfandiari F, White ME, Devaraj S, French SW, Halsted CH. S-adenosylmethionine attenuates oxidative liver injury in micropigs fed ethanol with a folate-deficient diet. Alcohol Clin Exp Res. 2007;31(11):1934–1943. doi: 10.1111/j.1530-0277.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- Wong CC, Mill J, Fernandes C. Drugs and addiction: an introduction to epigenetics. Addiction. 2011:106, 480–489. doi: 10.1111/j.1360-0443.2010.03321.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Sadda MR, Li M, Zeng Y, Chen L, Bae W, Ou X, Runnegar MT, Mato JM, Lu SC. S-adenosylmethionine and its metabolite induce apoptosis in HepG2 cells: role of protein phosphatase 1 and Bcl-x(S) Hepatology. 2004;40:221–231. doi: 10.1002/hep.20274. [DOI] [PubMed] [Google Scholar]
- You C, Zhang H, Sakharkar AJ, Teppen TL, Pandey SC. Reversal of deficits in dendritic spines, BDNF, and Arc expression in the amygdala during alcohol dependence by HDAC inhibitor treatment. Int. Journal of Neuropsychopharm. 2014;17:313–322. doi: 10.1017/S1461145713001144. [DOI] [PMC free article] [PubMed] [Google Scholar]