Endocytosis pathways are used by eukaryotic cells for internalization of nutrients, signal transduction regulation, and modulation of plasma membrane composition. The cytoplasmic regions of plasma membrane “cargo” proteins harbor signals that are recognized by “cargo adaptor” proteins; this concentrates the cargo proteins at sites of budding for subsequent internalization via an endocytic vesicle. There are several endocytic pathways that can mediate the internalization of many different cargo proteins. The best-studied pathway is clathrin-dependent endocytosis (CDE), defined by a requirement for the protein clathrin, which is the major component of the endocytic vesicle coat. There are multiple clathrin-independent endocytosis (CIE) pathways that generally depend on cholesterol-rich membrane domains (i.e., rafts, which can also be encased by the protein caveolin to form membrane invaginations called caveolae).
Cargo internalization signals are short peptide motifs; yeast also uses posttranslationally conjugated ubiquitin (Ub) moieties (1). In this issue of PNAS, two studies show that Ub can act as an endocytic signal in mammalian cells; in the absence of clathrin, Ub can direct entry via a CIE pathway (2, 3). The idea that ubiquitylated cargo can travel via a CIE pathway was previously unrecognized, probably because the endocytic factors that can recognize and bind Ub (epsin and Eps15) were first shown to play key roles in the CDE pathway. Now, it seems that these endocytic factors may play multiple roles, depending on which signal the cargo protein presents, or which endocytic route is being used.
In recent years it has become clear that, in addition to its well known roles in proteasome-dependent protein degradation, Ub is also involved in protein trafficking and membrane protein internalization (4, 5). Ub not only modifies cargo proteins to act as an endocytic signal; components of the endocytic machinery are also reversibly ubiquitylated, possibly regulating their activities or interactions (6, 7). These trafficking roles for Ub require its Ile-44 residue, and are mediated by Ub-binding modules such as Ub interaction motifs (UIM) or Ub associated (UBA) domains, which are conserved features of the epsin and Eps15/R endocytic proteins (7–10). These proteins bind each other and to the cargo adaptor AP2; epsins and AP2 also directly bind clathrin.
Ub-mediated mechanisms seem to be particularly important for endocytosis and regulation of growth factor receptors (11); many studies focus on the EGF receptor (EGFR), a tyrosine kinase receptor. In the absence of the EGF ligand, >60% of EGFR is located at caveolae and noncaveolae rafts. Upon binding EGF, the EGFRs leave the rafts (12) and initiate a MAP kinase-signaling cascade before or during CDE (13). Depleting cells of cholesterol to reduce rafts (e.g., methyl-β cyclodextrin treatment) enhances MAP kinase signaling downstream of the activated EGFR that is internalized through CDE (14). Studies of the Ub-modified EGFR have focused primarily on the receptor's eventual delivery to the lysosome lumen where it is degraded, thus terminating the signaling cascade (reviewed in ref. 15); however, the role of ubiquitylation in determining the relationship between pools of EGFR found in rafts vs. clathrin-coated pits and in degradation vs. signaling has remained puzzling.
Ubiquitin can act as an endocytic signal in mammalian cells.
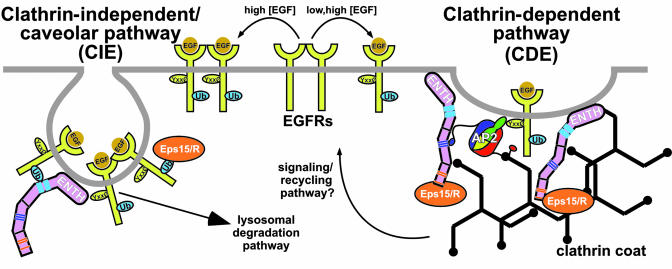
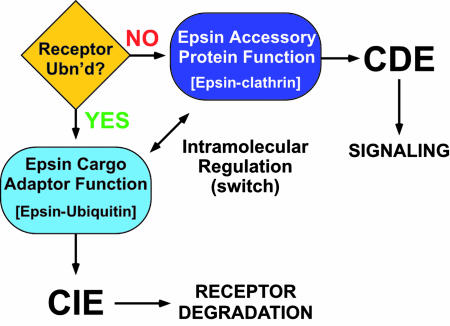
Sigismund et al. (3) show that EGFR follows different endocytic routes depending on the concentration of ligand delivered to the cells. In the presence of low [EGF], EGFR is exclusively internalized by CDE (Fig. 1), likely by its peptide signals binding AP2 (1). In contrast, the EGFR is ubiquitylated only when exposed to higher (physiological) [EGF]. Under these conditions, the EGFR is internalized via both CDE and CIE pathways; whether the EGFR traveling via the CDE pathway is Ub-modified is unclear. Chen and De Camilli (2) used a GFP–Ub fusion protein that associates with the plasma membrane via myristoylation to show that Ub is a powerful internalization signal; in cells depleted for clathrin, Ub can direct a CIE route. Both studies also demonstrate that Ub is a signal by using Ub-fusions with the critical Ile-44 residue mutated (thus preventing binding to Ub-binding proteins like epsin and eps15, and others). Furthermore, Sigismund et al. (3) suggest that Ub-dependent internalization is CIE-specific, occurring at caveolae. Importantly, Chen and De Camilli (2) show that the Ub-binding and the clathrin-binding capabilities of the epsins are mutually exclusive, suggestive of either intramolecular regulation of epsins acting as a switch or dual function that senses the Ub status of the cargo (Fig. 2).
Fig. 1.
CIE and CDE pathways. High [EGF] induces Ub modification of the EGFR, recognition by the UIMs of epsin and Eps15/R, and subsequent degradation. Low [EGF] promotes recognition by the classic AP2 cargo adaptor and internalization via clathrin-coated vesicles, leading to efficient signal transduction. Epsin and Eps15/R bind to each other and to other proteins to assist in the process. Binding motifs are color coded; ENTH, epsin N-terminal homology; Yxx0, tyrosine-based sorting signal.
Fig. 2.
Epsin as a switch that senses or is regulated by receptor ubiquitylation status.
These studies show how multiple endocytic pathways can internalize plasma membrane receptors, and the complex roles of the ever-ubiquitous Ub. It is tempting to suggest that, under certain basal conditions (e.g., low [ligand]), receptors are internalized via a default CDE pathway (Fig. 1). In this case, AP2 would recognize the cargo's peptide signals to promote cargo clustering in clathrin-coated pits. Epsin and Eps15 would act as accessory and scaffold proteins (e.g., recruiting other endocytosis proteins and clathrin, Fig. 1). This default CDE pathway may be instrumental for signal transduction and receptor recycling (16).
Under other physiological conditions (e.g., higher [ligand]), Ub modifications are more prevalent and a CIE component is added; this may end with Ub-modified EGFR degradation (3, 16). In CIE, epsin could act as a cargo adaptor by binding to receptor-attached Ub units; the findings of Chen and De Camilli predict that when the UIMs bind Ub, the clathrin-binding activity is occluded (or vice versa, epsin bound to clathrin may block UIM/Ub interactions). These mutually exclusive interactions may also regulate epsin, facilitating transit of EGFR-Ub via CDE.
How might a receptor choose different endocytic routes and fates based on ligand concentration-dependent differences? It is possible that in response to low vs. high [EGF], differential EGFR phosphorylation is induced, leading to recruitment of distinct signaling adaptors and activation of their downstream effectors (including Ub-ligases). Can epsins sense the presence of ubiquitylated receptors to act as either endocytic adaptors or accessory proteins, “switching” between the CDE (signaling) and the CIE (desensitizing) pathways (Fig. 2)? Because the ubiquitylation machinery is required for both internalization pathways, Ub may provide the receptor internalization signal in CIE; in CDE, the accessory proteins (e.g., epsins and Eps15/R) may be ubiquitylated to engage in intramolecular interactions (inhibiting Ub-receptor recognition) and enhance their clathrin-recruitment properties (6).
Endocytosis is interesting because it impacts many facets of cell physiology and homeostasis. These two studies present breakthroughs in two critical areas: Ub is an endocytic signal in mammalian cells, and epsin and Eps15 have previously unrecognized multifunctional properties. Now we need to understand how epsin and Eps15 protein activities are differentially regulated, and what are the regulatory roles contributed by Ub-modifications (of both cargo and endocytic machinery) within the two pathways. Previous studies may also benefit from reinterpretation in light of these findings.
References
- 1.Bonifacino, J. S. & Traub, L. M. (2003) Annu. Rev. Biochem. 72, 395–447. [DOI] [PubMed] [Google Scholar]
- 2.Chen, H. & De Camilli, P. (2005) Proc. Natl. Acad. Sci. USA 102, 2766–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigismund, S., Woelk, T., Puri, C., Maspero, E., Tacchetti, C., Transidico, P., Di Fiore, P. P. & Polo, S. (2005) Proc. Natl. Acad. Sci. USA 102, 2760–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aguilar, R. C. & Wendland, B. (2003) Curr. Opin. Cell Biol. 15, 184–190. [DOI] [PubMed] [Google Scholar]
- 5.Hicke, L. & Dunn, R. (2003) Annu. Rev. Cell Dev. Biol. 19, 141–172. [DOI] [PubMed] [Google Scholar]
- 6.Chen, H., Polo, S., Di Fiore, P. P. & De Camilli, P. V. (2003) Proc. Natl. Acad. Sci. USA 100, 14908–14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polo, S., Sigismund, S., Faretta, M., Guidi, M., Capua, M. R., Bossi, G., Chen, H., De Camilli, P. & Di Fiore, P. P. (2002) Nature 416, 451–455. [DOI] [PubMed] [Google Scholar]
- 8.Shih, S. C., Sloper-Mould, K. E. & Hicke, L. (2000) EMBO J. 19, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aguilar, R. C., Watson, H. A. & Wendland, B. (2003) J. Biol. Chem. 278, 10737–10743. [DOI] [PubMed] [Google Scholar]
- 10.Shih, S. C., Katzmann, D. J., Schnell, J. D., Sutanto, M., Emr, S. D. & Hicke, L. (2002) Nat. Cell Biol. 4, 389–393. [DOI] [PubMed] [Google Scholar]
- 11.Haglund, K., Di Fiore, P. P. & Dikic, I. (2003) Trends Biochem. Sci. 28, 598–603. [DOI] [PubMed] [Google Scholar]
- 12.Mineo, C., Gill, G. N. & Anderson, R. G. (1999) J. Biol. Chem. 274, 30636–30643. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter, G. (2000) BioEssays 22, 697–707. [DOI] [PubMed] [Google Scholar]
- 14.Takebayashi, M., Hayashi, T. & Su, T. P. (2004) Synapse 53, 90–103. [DOI] [PubMed] [Google Scholar]
- 15.Dikic, I. & Giordano, S. (2003) Curr. Opin. Cell Biol. 15, 128–135. [DOI] [PubMed] [Google Scholar]
- 16.Di Guglielmo, G. M., Le Roy, C., Goodfellow, A. F. & Wrana, J. L. (2003) Nat. Cell Biol. 5, 410–421. [DOI] [PubMed] [Google Scholar]