Abstract
Background
Little is known about ways network-level factors that may influence the adoption of combination prevention behaviors among injection networks, or how network-oriented interventions might moderate this behavior change process.
Methods
A total of 232 unique injection risk networks in Philadelphia, PA, were randomized to a peer educator network-oriented intervention or standard of care control arm. Network-level aggregates reflecting the injection networks’ baseline substance use dynamics, social interactions, and the networks exposure to gender- and structural-related vulnerabilities were calculated and used to predict changes in the proportion of network members adopting safer injection practices at 6-month follow-up.
Results
At follow-up, safer injection practices were observed among 46.31% of a network’s members on average. In contrast, 25.7% of networks observed no change. Controlling for the effects of the intervention, significant network-level factors influencing network-level behavior change reflected larger sized injection networks (b=2.20, p=.013) with a greater proportion of members who shared needles (b=0.29, p<.001) and engaged in poly drug use at baseline (b=6.65, p=.021). Changes in a network’s safer injection practices were also observed for networks with fewer new network members (b=−0.31, p=.008), and for networks whose members were proportionally less likely to have experienced incarceration (b=−0.20, p=.012) or more likely to have been exposed to drug treatment (b=0.17, p=.034) in the 6-months prior to baseline. A significant interaction suggested the intervention uniquely facilitated change in safer injection practices among female-only networks (b=−0.32, p=.046).
Conclusions
Network-level factors offer insights into ways injection networks might be leveraged to promote combination prevention efforts.
Keywords: HIV, Combination prevention, Injection drug use, Social networks, Behavior change, Intervention
1. INTRODUCTION
Globally, approximately 1 in 5 persons who inject drugs (PWID) is living with HIV (Mathers et al., 2008), which warrants urgent action for targeted HIV prevention, treatment, and care (Mathers et al., 2010; Wolfe et al., 2010; Degenhardt et al., 2010; Degenhardt et al., 2014). As injection drug use is often a social process (Latkin et al., 2010), evidence suggests that successful implementation of combination prevention efforts is most effective when networks of PWID are viewed as partners in the HIV response, and empowered to deliver outreach, education, and service support to members of their own social and injection networks (Beyrer et al., 2010; World Health Organization, 2012).
While injection networks can be a source of support, they are often a source of health-related risks, with most HIV transmission behaviors occurring between close network members (Unger et al., 2006). The ways in which injection networks can be leveraged for HIV prevention is well demonstrated via interventions training PWID to become peer-educators and model safer injection behaviors to members of their personal injection and/or sexual risk networks. Such network-based approaches demonstrate an ability for PWID to outreach to and engage otherwise hard to reach populations of PWID across diverse injection communities worldwide, such as Vietnam (Go et al., 2013), Russia (Hoffman et al., 2013), the Ukraine (Booth et al., 2011), and the United States (Latkin, 1998; Weeks et al., 2009; Latkin et al., 2009a). These peer-educator delivered network-oriented approaches typically demonstrate significant intervention effects (Latkin, 1998; Weeks et al., 2009; Latkin et al., 2009a; Tobin et al., 2011) predicting reductions in injection risk behaviors at the individual-level; though, as with many behavioral interventions, risk reduction in both intervention and control arms is often observed. Understanding how networks, as micro-social environments, facilitate health behavior change could benefit future efforts to widely implement and effectively target combination prevention services.
Secondary analyses of one such peer-delivered network-oriented intervention, the HPTN 037 trial, offered insights into factors through which peer educators promote safer injection behaviors among individual members of their injection networks. These findings demonstrate that social norms regarding injection practices were unique to individual networks (Latkin et al., 2009b). Network indexes trained as peer-educators predicted significant improvements in safer injection norms among network members at follow-up; these changes in norms further predicted significant improvements in safer injection behaviors among individual participants at later follow-ups (Latkin et al., 2013). Much of what is known about network-oriented interventions reflects individual processes of change. Rarely has the injection network been the unit of analysis. We take advantage of the large number of whole networks enrolled in the HPTN 037 trial to investigate how network-related factors, such as drug use patterns, types of social interactions, or network composition, might be leveraged to influence network-level behavior change.
The available literature on injection networks have identified associations between individual network members’ injection risk, HIV testing, and HIV serostatus and network factors such as alcohol and poly-substance use (crack cocaine) patterns (Latkin et al., 1996), levels of social support (Latkin et al., 2011) and trust among network members (Cepeda et al., 2011), network size (Cepeda et al., 2011), connectivity between network members or proximity to the index (Li et al., 2012; Flaer et al., 2013; Shahesmaeili et al., 2014), and stability of network member composition (Hoffmann et al., 1997; Costenbader et al., 2006; Cepeda et al., 2011). To our knowledge, these network factors have not been studied as potential predictors of change in injection risk practices at the network-level.
To advance these efforts, we examine injection networks enrolled in the HPTN 037 trial, enabling assessments of network members’ actual behaviors over time (vs. participants’ perceptions of their network members). Controlling for exposure to the intervention, we hypothesized greater network-level changes towards safer injection practices at follow-up would be observed for injection networks that: 1.) are larger and exhibit more risky substance use patterns offering more opportunities for change, 2.) exhibit more established social interactions where new norms can be transferred, and 3.) experience less exposure to gender and/or structural vulnerabilities in their network composition. Ways these network-level factors may further facilitate or impede (moderate) network-oriented intervention efforts are explored.
2. MATERIALS and METHODS
2.1 Trial design
The HPTN 037 trial was a phase 3 randomized study to evaluate the efficacy of a network-oriented peer-education intervention, in reducing HIV transmission and related risk behaviors among PWID and their network members. Standardized study protocols were implemented at two sites (Philadelphia, USA and Chiang Mai, Thailand) and approved by the affiliated Institutional Review Boards in both countries. Protocols and oversight activities are described elsewhere (Latkin et al., 2009a). Significant individual-level intervention effects on reduced injection risk behaviors were observed in the Philadelphia site (Latkin et al., 2009a). No intervention effects were observed for the Chiang Mai site, where the Thai government’s war on drugs altered PWIDs’ social interactions, substantially contaminating the follow-up data (Simmons et al., 2015). The current analyses were restricted to participants from the Philadelphia study site that reported injecting drugs in the 3-months before baseline (N=651). Data from these participants’ baseline and 6-month follow-up were used, yielding a total of 232 unique injection risk networks.
2.2 Participants and Procedures
The trial enrolled networks comprised of an index and members of their HIV risk networks. Index participants were actively recruited by project staff targeting areas with high HIV and injection drug use prevalence. Eligible index participants were ≥18 years of age, reported injecting drugs at least 12 times in the past 3-months, tested HIV-negative, were not in medication-assisted treatment for opioid dependence (e.g., methadone) in the past 3-months, and recruited at least one member from their injection and/or sexual risk networks into the trial. Regardless of serostatus, eligible network members recruited by the index reported having injected drugs and/or having sex with the index in the past 3-months and were ≥18 years of age.
After providing written consent, participants were tested for HIV and administered a behavioral risk assessment by a trained interviewer. In addition, indexes were administered a Social Network Inventory at baseline to characterize the structure, and types of interactions indexes had with members of their injection and/or sexual risk networks (Latkin et al., 2003). Following baseline, network-level randomization occurred by randomizing index participants to intervention (training on how to be a peer educator) or control (no training). All participants received HIV testing and counseling and interviewer-delivered behavioral risk assessments once every 6-months for up to 30 months of follow-up. Lower than anticipated HIV incidence precludes analysis with a seroconversion endpoint. Hepatitis C status was not assessed in this trial. Study procedures are detailed elsewhere (Latkin et al., 2009a).
2.3 Interventions
In brief, indexes randomized to the intervention received a standardized, small-group, theory-based harm-reduction training, teaching them to help reduce injection- and sex-related HIV transmission risks (e.g., cleaning needles and works after injecting, using a male condom for vaginal sex) among network members via peer-education and behavioral modeling activities (Latkin et al., 2009a). These activities were designed to change HIV risk-related injunctive norms and behaviors among the index and network members (Latkin et al., 2013). In contrast, indexes randomized to the control arm received no formal HIV risk-reduction training above and beyond the HIV counseling and testing sessions delivered to all study participants at baseline and each 6-month follow-up.
2.4 Measures
For the present analyses, variable construction was limited to network members who reported injecting drugs in the past 3-months at baseline (active injectors), to define injection risk networks. This excluded network members only affiliated via sexual relations with the network index. Enrollment criteria ensured all index participants were active injectors. Predictor variables reflect network-level aggregates that included calculating the proportion of total network members (NTOAL = all active injectors in a network) who experienced the following characteristics ([N1…j/nTOAL]*100), or the mean level of a factor present within a network ([N1+…Nn]/NTOAL). Responses coded as ‘don’t know,’ or ‘not applicable’ were set to missing and are not included in the numerator or denominator. Specifically, we sought to characterize network-level factors that reflect the networks’ substance use dynamics, social interactions, and a networks’ exposure to gender and structural vulnerabilities.
2.4.1 Substance use dynamics
Network substance use variables reflected the total number of active injectors in a network (IDU Network Size), the proportion of network members at baseline, with whom the index had (1=Yes, 0=No) Shared Drugs, Shared Needles, or never discussed drug-related risk (No Risk Talk). Participants indicated (Yes=1, No=0) up to 6 types of non-injection drug use behaviors (range 0–6; smoking crack or amphetamines, sniffing cocaine or amphetamines, non-injected opiates or swallowing benzos/downers/sedatives). The mean number of non-injected drug types used by network members at baseline was assessed as an indicator of Poly Drug Use Involvement. The three-item AUDIT-C scoring criteria (range 0–12) was used to characterize participants’ baseline alcohol use as hazardous (1; men≥4, women≥3) or non-hazardous (0; men<4, women<3) (Bush et al., 1998). The proportion of network members with Hazardous Drinking patterns was then calculated.
2.4.2 Social interactions
How well established network social interactions were at baseline is reflected the proportion of New Members in the network who knew the index ≤6-months (Yes=1, No=0), as well as the proportion of network members who had Frequent Contact with the index (saw index≥1 per week; Yes=1, No=0). Types of social interactions were further characterized by assessing the Network Density reflecting the total number of members within an Index’s HIV risk network that were identified as being ‘friends’ with each other (range: Minimum = no network members are friends [0], Maximum = all network members are friends [number of ‘other’ network members (network size −1)* number of possible friendhips between two network members (network size÷2)]), and whether or not the index was trained to engage network members in risk reduction education and safer injection practices as an Intervention Network (1=Yes, intervention network, 0=No, control network). Due to the way the network data were gathered, network density reflects the degree of social interconnectedness within a network through which safer injection behaviors could be discussed or observed regardless of a network member’s injection drug use status.
2.4.3 Environmental exposures
Environmental vulnerabilities that may constrain individual autonomy over changing injection risk behaviors are reflected in the proportion of network members who were exposed to gender and/or structural risk and protective factors at baseline. The ways in which gendered-power dynamics can uniquely inhibit the autonomy of female injectors to reduce injection risk behaviors has been extensively documented in the literature (Auerbach and Smith, 2015; El-Bassel and Strathdee, 2015). Specifically, unequal distribution of economic, social, and sexual power increase the ability of male injection partners to control drug acquisition (e.g., male partner holds and controls distribution of drugs) and injection behaviors (e.g., male partner controls access to syringes and injection equipment, and may directly administer drug injections). Such limited autonomy may be reinforced through gender-based violence. In the absence of more direct measures, we conceptualize network exposure to these potential gender-related vulnerabilities as the proportion of network members who were Female-injectors (females who injected drugs in the past 3-months, 1=Yes, 0=No). Network exposure to structural-related vulnerabilities was indicated (1=Yes, 0=No) by the proportion of network members who had a history of Recent Incarceration (spending time in jail or prison in the past 6-months) or Recent Homelessness (living on the street, in a car, park or abandoned building in the past 6-months). Network exposure to structural-related protective factors was indicated (1=Yes, 0=No) by the proportion of network members with a history of Recent Drug Treatment (participating in any drug treatment program, drug counseling, or drug detoxification in the past 6-months).
2.4.4 Change in Network Injection-Related Risk
The network-level dependent variable reflected the proportion of network members who reduced their total number of injection-related HIV risk behaviors at 6-month follow-up.
The intervention targeted reductions across five distinct injection-related risk behaviors, which served as the primary individual-level behavioral outcomes of the HPTN 037 trial (Latkin et al., 2009a). At baseline and each follow-up, assessment participants recalled “in the last month how many times did you… 1) use rinse water that others had used, 2) used a cooker that others had used, 3) used cotton that others had used, 4) injected drugs that were frontloaded or backloaded into their syringe or needle, or 5) used a needle that others had discarded?” While these behaviors reflect drug preparation and injection practices, each is conceptualized as a unique behavioral target that may have been affected by the intervention to reduce, or possibly even increase, the potential risk of HIV transmission/acquisition among PWID. Responses to these five items were summed for each participant at baseline and at 6-month follow-up, then used to calculate a difference score (TotalBL−Total6M) reflecting a participant’s change in the total number of injection-related risk behaviors at 6-month follow-up. Negative change score values reflected participants who reduced their total number of risk behaviors at follow-up were coded as 1. Positive values reflect an increase in the total number of risk behaviors, and a value of zero reflected no change in risk behaviors at follow-up, both were coded as 0. To maintain the same denominator (i.e., injection network size) for calculating network-level aggregates at both time points, participants missing the 6-month follow-up (n=136) were coded as 0, assuming risk or no change in injection-related risk for that network member. Missing data on injection risk indicators at 6-month follow-up was not significantly (p >.05) associated with network membership or baseline demographic characteristics (gender, race, ethnicity, education, employment), suggesting the data were missing at random. A proportion was calculated for the number of network members who decreased their total number of injection-related risk behaviors in each network, serving as our primary network-level outcome for the current analysis.
2.5 Statistical Analysis
All analyses are performed at the network level (i.e., each network contributes one observation).
Bivariate relationships between the network-level predictors and change in the network’s collective risk behavior were examined using Kendall’s Tau-b (τ). Since we were interested in identifying predictors of network-level change in injection risk behaviors, and ways in which the intervention may have moderated these main effects, predictor variables with bivariate associations of p<.10 were retained, mean-centered, and an interaction term (variable*intervention Network [Yes=1, No=0]) was produced.
Next, significant predictor variables and their interaction terms were entered into an a priori block entry multiple linear regression model. Substance use predictors, expected to have the most proximal influence on changes in injection risk behaviors at follow-up, were entered into the model first (Block 1) followed by social interactions (Block 2), and network exposure vulnerable environments (Block 3). Potential interactions were explored to determine the direction and magnitude of slopes by networks random assignment to intervention vs. control treatment arm.
3. RESULTS
3.1 Baseline Network Characteristics
Of the 232 unique injection risk networks, network size ranged from 2 to 21 members, with an average of 6.89 (SD=3.05). On average, the network indexes reported sharing drugs with most members of their injection networks (90.09%, SD=20.69), but less frequently reported discussing injection-related risks (63.84%, SD=34.70), or sharing needles (48.85%, SD=29.23) with network members. Non-injection substance use patterns within networks suggest that network members who are actively injecting report poly drug use of ≥1 type of non-injection illicit drug(s) ( , SD=0.77). In addition, on average 40.23% of a network’s members report hazardous drinking patterns (SD=34.23).
Only 9.82% (SD=20.34) of a network’s active injectors joined their injection network in the past 6-months, with most network’s members (89.38%, SD=16.31) reporting frequent interactions with the network index (≥1 per week). Similarly, all or most network members were interconnected (i.e., network density) with an average of 12.63 network members having independent friendships with one another (SD=13.39). As would be expected, approximately half of the injection risk networks were randomized to the intervention arm (48.03%, SD=0.50).
A sizable minority of networks had injection risk network members characterized as being exposed to gender (25.62% female-injectors, SD=27.00) or structural vulnerabilities (18.90% recently incarcerated, SD=27.60; 24.21% recently homeless, SD=30.37). Similarly, on average, 25.38% (SD=28.38) of networks had members characterized as having accessed drug treatment in the previous 6-months.
3.2 Network-level Associations with Safer Injection Practices
On average, almost half of a network’s members reported safer network-level injection practices at 6-month follow-up (46.31%; SD=35.82, range 0–100%), whereas no change was observed among any network members in 26.70% of injection networks. Changes towards safer injection practices were significantly associated with having a higher proportion of a network’s members who shared needles (τ=0.176, p<.001), engaged in poly substance use with a greater range of non-injection illicit drug types (τ=0.111, p=.025), and having a greater proportion of network members who reported hazardous drinking patterns (τ=0.100, p=.053) at baseline (Table 1). Having a lower proportion of a network’s members who were new to the network (τ=−0.098, p=.072) was also associated with change toward safer injection practices, as was having a higher proportion of network members who were female-injectors (τ=0.103, p=.051), who were not incarcerated (τ=−0.116, p=.031), or who had accessed drug treatment in the 6-months prior to baseline (τ=0.159, p=.003).
Table 1.
Network-level factors associated with safer network-level injection practices (N = 232)
| Network Constructs | Network Descriptive Statistics | Bivariate Associations | |||
|---|---|---|---|---|---|
| Network Variables | Average % Network Members | value, (Range) | (SD) | τa | p-value |
| Baseline Substance Use Dynamics | |||||
| IDU Network Size | 6.89, (2–21) | (3.05) | 0.062 | .215 | |
| Shared Drugs | 90.09% | (20.69) | 0.050 | .360 | |
| Shared Needles | 48.85% | (29.23) | 0.176 | <.001*** | |
| No Risk Talk | 63.84% | (34.70) | 0.019 | .707 | |
| Poly Drug Use Involvement | 1.58, (0–4) | (0.77) | 0.111 | .025** | |
| Hazardous Drinking | 40.23% | (34.23) | 0.100 | .053* | |
| Baseline Social Interactions | |||||
| New Members | 9.82% | (20.34) | −0.098 | .072* | |
| Frequent Contact | 89.38% | (16.31) | −0.065 | .223 | |
| Network Density | 12.63, (0–99) | (13.39) | 0.035 | .478 | |
| Intervention Network | 48.03%† | (0.50) | −0.076 | .193 | |
| Baseline Environmental Exposures | |||||
| Female-injectors | 25.62% | (27.00) | 0.103 | .051* | |
| Recent Incarceration† | 18.90% | (27.60) | −0.116 | .031** | |
| Recent Homelessness† | 24.21% | (30.37) | 0.035 | .512 | |
| Recent Drug Treatment† | 25.38% | (28.53) | 0.159 | .003** | |
| 6-month Network Injection Risk | |||||
| Safer Injection Practices | 46.31% | (35.82) | – | – | |
Positive parameter estimates reflect an association with safer injection practices at follow-up, negative parameter estimates reflect an association with more risky or no change injection practices at follow-up. † Since randomization to study arm was done at the network-level, this value reflects the proportion of networks enrolled in the intervention, not the average proportion of network members enrolled in the intervention.
p≤ 0.10,
p≤ 0.05,
p≤ 0.001;
within a 6-month period prior to baseline
To test whether the relationship between a networks’ safer injection practices at follow-up and the proportion of a network’s members sharing needles reflected a regression to the mean, we stratified the outcome by the network’s median level of risk at baseline, where 50% of networks with a median level of risk or less, compared to 50% of networks with more than the median risk level. The observed effect holds for both groups (≤median risk: τ=0.148, p=.034, >median risk τ=0.17, p=.010), meaning the effect is not driven by high levels of risk at baseline.
3.3 Network-level Predictors of Safer Injection Practices
Despite non-significant bivariate associations, the size of the injection network (τ=0.062, p=.215) and random assignment to the intervention (τ=−0.076, p=.193) were retained in the multivariable linear regression model (Table 2) as theoretically important constructs influencing the likelihood network members would be exposed to safer/less-safe injection practices during follow-up.
Table 2.
Main and moderating effects of network-level changes towards safer injection practices (N=232)
| Network Predictors and Interaction Terms | b‡ | (SE) | t-statistic | p-value |
|---|---|---|---|---|
| Block 1: Substance Use Dynamics | ||||
| IDU Network Size‡ | 2.54 | 0.91 | 2.81 | .005 |
| Shared Needles | 0.36 | 0.08 | 4.35 | <.001 |
| Poly Drug Use Involvement | 5.48 | 2.94 | 1.87 | .063 |
| Block 2: Social Interactions | ||||
| IDU Network Size† | 2.39 | 0.90 | 2.67 | .008 |
| Shared Needles | 0.32 | 0.08 | 3.87 | <.001 |
| Poly Drug Use Involvement | 6.67 | 2.92 | 2.29 | .023 |
| New Members | −0.35 | 0.12 | −2.99 | .003 |
| New Members * Intervention Network | 0.45 | 0.24 | 1.90 | .059 |
| Intervention Network† | −6.27 | 4.47 | −1.40 | .162 |
| Block 3: Environmental Exposures | ||||
| IDU Network Size† | 2.20 | 0.88 | 2.50 | .013 |
| Shared Needles | 0.29 | 0.08 | 3.58 | <.001 |
| Poly Drug Use Involvement | 6.65 | 2.85 | 2.33 | .021 |
| New Members | −0.31 | 0.12 | −2.69 | .008 |
| New Members * Intervention Network | 0.43 | 0.23 | 1.88 | .062 |
| Intervention Network† | −3.80 | 4.41 | −0.86 | .390 |
| Female-injectors | 0.12 | 0.08 | 1.50 | .134 |
| Female-injectors * Intervention Network | −0.32 | 0.16 | −2.00 | .046 |
| Recent Incarceration | −0.20 | 0.08 | −2.53 | .012 |
| Recent Drug Treatment | 0.17 | 0.08 | 2.13 | .034 |
b-value = Δ in the proportion of network members reporting safer injection practices at follow up.
Retained in the regression analyses as theoretically important constructs.
Note: Step 1 R2= .102, Step 2 Δ R2= .042 (p= .013), Step 3 Δ R2= .063 (p= .002) F(10,221)= 5.778, p< .001.
Controlling for the effects of the intervention, a greater increase in safer injection practices was observed for networks in which the index had shared needles with larger proportion of its members (b=0.29, p<.001), for larger sized drug networks (b=2.20, p=.013) and networks with greater involvement in non-injection poly drug use (b=6.65, p=.021). Networks which had a lower proportion of new network members (b=−0.31, p=.008), fewer members who were recently incarcerated (b=−0.20, p=.012) or a higher proportion of members who accessed drug treatment (b=0.17, p=.034) just prior to baseline were also associated with safer injection practices. A significant interaction on safer injection practices was observed between the proportion of female-injectors (b=−0.32, p=.046) in a given network and treatment arm (intervention vs. control).
The final model significantly fit the observed data (F=5.778, p≤.001) accounting for 20% (R2=.207) of the variance in the proportion of network members engaging in safer injection practices at follow-up. Visual inspections of the distributions of residuals suggest the assumption of normality was met; as were assumptions of non-collinearity (Mean VIF ≤1, Tolerance:0.82–0.97) and independent errors (Durbin-Watson=1.026). Casewise diagnostics suggest no cases (individual networks) had undue influence on the model.
3.4 Peer-Education as a Moderator of Safer Injection Practices
For intervention networks, we observed a significant increase in the proportion of a network’s members reporting safer injection practices at follow-up as the proportion of females who inject drugs in the network increased (b=0.27, p=.018). However, for control networks, no significant change in safer injection practices was observed as the proportion of female-injectors in the network composition increased (b=−0.01, p=.907). A visual inspection of these relationships (Figure 1) shows that this observed difference by treatment arm mattered most when the network composition was 100% female-injectors, suggesting the intervention may have worked particularly well in facilitating safer injection practices for female-only networks.
Figure 1.
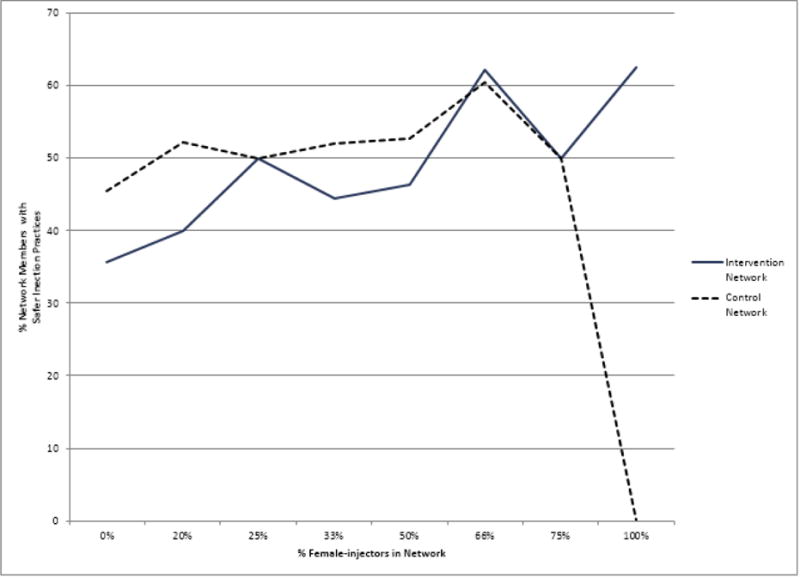
Intervention as a Moderator of the Association between the Proportion of Female Injectors in a Network and the Proportion of Network Members with Safer Injection Practices
4. DISCUSSION
In the current study, we found support for our hypothesis that a greater proportion of a network’s members adopt safer injection practices when they belong to larger more established injection networks with higher risk substance use patterns and fewer members exposed to structural vulnerabilities. In contrast, we did not find evidence that more frequent interactions or greater social connectedness between a network’s members influenced safer injection practices.
Our findings respond to previous cross-sectional work which found increased HIV risk was associated with larger sized drug networks (Cepeda et al., 2011) and more severe drug use patterns (Flaer et al., 2013), suggesting such substance use dynamics might afford greater opportunities for networks to introduce safer injection practices. Frequency of contact with the index or greater interconnectivity (e.g., friendships) between network members did not predict changes in a network’s injection practices, despite previous cross-sectional work reporting associations between these network factors and HIV risk behaviors (Li et al., 2012; Flaer et al., 2013; Shahesmaeili et al., 2014). This discrepancy may suggest a mediated network process where such social interaction factors indirectly promote changes in injection practices over time, as a mechanism through which new social norms lead to changes in injection practices (Latkin et al., 2013). Our findings support previous research that suggests instability of members within a network is associated with greater HIV risk (Costenbader et al., 2006; Cepeda et al., 2011), and that network instability is likely prompted by factors that disrupt a network’s access to resources (Hoffmann et al., 1997). To this end, our findings suggest structural vulnerabilities (incarceration) and protective factors (drug treatment exposure) may affect network-level changes in injection practices through their effects on the (in)stability of network members.
Of particular interest is that despite their low frequency, female-only injection networks were more likely to benefit from network-oriented peer-educator delivered interventions. Understanding that a network’s gender composition can only serve as an imperfect proxy for gender-based vulnerabilities, this finding suggests when women’s network-level exposure to gendered-power dynamics is limited (e.g., lower probabilities that injection practices are male dominated or tied to gender-based violence), the intervention facilitated greater reductions in injection risk; likely vis-à-vis greater autonomy over establishing safer injection norms and injection practices in female-only networks (Latkin et al., 2013; Auerbach and Smith, 2015). In contrast, even when exposure to gendered-power dynamics is limited, safer injection practices are not adopted among female-injectors in the absence of intervention. Injection-focused HIV prevention efforts like HPTN 037 have typically been developed to target risk reduction among PWID collectively, with limited attention to the role gender-based power dynamics have on limiting female-injectors’ autonomy to enact behavior change (Auerbach and Smith, 2015). We know of two HIV prevention efforts targeting the unique vulnerabilities of female-injectors: one in Russia (Wechsberg et al., 2012) and one in Mexico (Strathdee et al., 2013). Differences in study methods preclude direct comparisons. In combination, our findings suggest safer injection practices among female-injectors should benefit from gender-responsive interventions designed to strengthen ties (or form networks) among female-injectors facilitating autonomy over safer injection practices through female-only trainings and/or target ways to alter power dynamics in mixed-gender networks/injection dyads.
With few exceptions (Hoffmann et al., 1997; Costenbader et al., 2006; Li et al., 2012), the available literature examining network-level factors associated with HIV transmission risk, testing, and HIV-positive status among individual PWID has been cross-sectional and have therefore been unable to identify factors predictive of network-level behavior change. The current analyses demonstrate value-added in examining network-level predictors of behavior change with injection networks as the unit of analysis. However, these analyses are limited in their ability to address other injection risk contexts or ways in which such network-level factors may function over longer observation periods. As an initial attempt to characterize the change in network-level risk, our outcome measure was chosen to reflect the primary behavioral outcome of the larger HPTN 037 trial. As such this measure may not capture more nuanced changes in risks associated preparation and injection practices within a network (e.g., reductions in using discarded needles offset by an increase in sharing rinse water). To retain whole networks within the analyses, we assumed participants’ whose injection practices were not measured at follow-up to have not adopted safer injection practices. As such, some caution should be exercised when interpreting results, as this may have potentially lead to more narrow confidence intervals. Similarly, a network’s change in injection-related risks was assessed relative to that network’s risk at baseline, which does not capture changes in a network’s risk relative to other networks. Finally, changes in network-level risk cannot be examined in relation to subsequent HIV acquisition in this trial. Future work may seek to evaluate network-level predictors of change for specific injection risk behaviors and in relation to HIV seroconversion within networks. Having demonstrated the potential utility of such an approach, similar retrospective analyses of available network-oriented HIV prevention interventions are warranted to provide greater generalizability across injection network environments.
5. CONCLUSIONS
Despite these limitations, the current study lends support towards increasing access to and expanding the reach of combination HIV prevention efforts for PWID and their injection networks. Specifically, within the micro-social environment of injection networks, network-level factors highlight the ability to enact behavior change in larger more risky injection networks. These findings further emphasize that network-oriented combination prevention efforts may benefit from targeting structural factors influencing network stability (syringe access, policing and drug treatment practices) and maximizing female PWID’s autonomy to shape their personal injection networks or other environmental spaces (female-oriented harm reduction/drug treatment programming). Such efforts should be visible priorities integrated into current efforts to shift funding and policy efforts towards combination prevention for PWID. Collectively, such strategies may capitalize on predictors of network-level behavior change identified in the current study to maximize reductions in HIV incidence among injection communities.
Highlights.
Prospectively examines network-level mechanisms injection risk
Factors affecting instability of network structure affected HIV risk
Network gender composition moderated intervention effect
Acknowledgments
The authors wish to thank the participants, study site staff, and HPTN Scholars Program (Leadership Team: Erica Hamilton and Sam Griffith; Co-Chairs: Dr. Darrell Wheeler and Dr. Sten Vermund) who made the current analyses possible. We further extend our gratitude to Dr. Deborah Donnell for facilitating access to the HPTN 037 data and Dr. Eileen Pitpitan whose comments on the proposed analysis plan enhanced the strength and rigor of our findings.
The research reported in this work was supported by the HIV Prevention Trials Network (HPTN) Scholars Program through the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (UM1A0168619), Career Development Award through the National Institute of Drug Abuse (K01 DA039767) and the National Institute on Minority Health and Health Disparities (L60 MD009353).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statements
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributions
LRS is responsible for the conceptual development and analysis of the current study, under the mentorship of SAS and CL. Both CL and DM were responsible for the implementation of the study. All authors (LRS, SAS, DM, CL) contributed to the interpretation of the study results. All authors approved of the final manuscript before submission.
Conflict of Interest
No conflict declared.
References
- Auerbach JD, Smith LR. Theoretical foundations of research focused on HIV prevention among substance-involved women: A review of observational and intervention studies. J Acquir Immune Defic Syndr. 2015;69(Suppl 1):S146–54. doi: 10.1097/QAI.0000000000000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrer C, Malinowska-Sempruch K, Kamarulzaman A, Kazatchkine M, Sidibe M, Strathdee SA. Time to act: A call for comprehensive responses to HIV in people who use drugs. Lancet. 2010;376:551–563. doi: 10.1016/S0140-6736(10)60928-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth RE, Lehman W, Latkin C, Dvoryak S, Brewster John T, Royer MS, Sinistyna L. Individual and network interventions with injection drug users in 5 Ukraine cities. Am J Public Health. 2011;101:336–343. doi: 10.2105/AJPH.2009.172304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- Cepeda JA, Odinokova VA, Heimer R, Grau LE, Lyubimova A, Safiullina L, Levina OS, Niccolai LM. Drug network characteristics and HIV risk among injection drug users in Russia: The roles of trust, size, and stability. AIDS Behav. 2011;15:1003–1010. doi: 10.1007/s10461-010-9816-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costenbader EC, Astone NM, Latkin CA. The dynamics of injection drug users’ personal networks and HIV risk behaviors. Addiction. 2006;101:1003–1013. doi: 10.1111/j.1360-0443.2006.01431.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Mathers BM, Wirtz AL, Wolfe D, Kamarulzaman A, Carrieri MP, Strathdee SA, Malinowska-Sempruch K, Kazatchkine M, Beyrer C. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Policy. 2014;25:53–60. doi: 10.1016/j.drugpo.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet. 2010;376:285–301. doi: 10.1016/S0140-6736(10)60742-8. [DOI] [PubMed] [Google Scholar]
- El-Bassel N, Strathdee SA. Women who use or inject drugs: An action agenda for women-specific, multilevel, and combination HIV prevention and research. J Acquir Immune Defic Syndr. 2015;69(Suppl 2):S182–90. doi: 10.1097/QAI.0000000000000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaer PJ, Cistone PJ, Younis MZ, Parkash J. A connectivity model for assessment of HIV transmission risk in injection drug users (IDUs) Eval Program Plann. 2013;39:23–27. doi: 10.1016/j.evalprogplan.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Go VF, Frangakis C, Le Minh N, Latkin CA, Ha TV, Mo TT, Sripaipan T, Davis W, Zelaya C, Vu PT. Effects of an HIV peer prevention intervention on sexual and injecting risk behaviors among injecting drug users and their risk partners in Thai Nguyen, Vietnam: A randomized controlled trial. Soc Sci Med. 2013;96:154–164. doi: 10.1016/j.socscimed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman IF, Latkin CA, Kukhareva PV, Malov SV, Batluk JV, Shaboltas AV, Skochilov RV, Sokolov NV, Verevochkin SV, Hudgens MG. A peer-educator network HIV prevention intervention among injection drug users: Results of a randomized controlled trial in St. Petersburg, Russia AIDS Behav. 2013;17:2510–2520. doi: 10.1007/s10461-013-0563-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann JP, Su SS, Pach A. Changes in network characteristics and HIV risk behavior among injection drug users. Drug Alcohol Depend. 1997;46:41–51. doi: 10.1016/s0376-8716(97)00038-0. [DOI] [PubMed] [Google Scholar]
- Latkin CA, Donnell D, Metzger D, Sherman S, Aramrattna A, Davis-Vogel A, Quan VM, Gandham S, Vongchak T, Perdue T. The efficacy of a network intervention to reduce HIV risk behaviors among drug users and risk partners in Chiang Mai, Thailand and Philadelphia, USA. Soc Sci Med. 2009a;68:740–748. doi: 10.1016/j.socscimed.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkin CA, Mandell W, Vlahov D. The relationship between risk networks’ patterns of crack cocaine and alcohol consumption and HIV-related sexual behaviors among adult injection drug users: a prospective study. Drug Alcohol Depend. 1996;42:175–181. doi: 10.1016/s0376-8716(96)01279-3. [DOI] [PubMed] [Google Scholar]
- Latkin CA, Sherman S, Knowlton A. HIV prevention among drug users: Outcome of a network-oriented peer outreach intervention. Health Psychol. 2003;22:332. doi: 10.1037/0278-6133.22.4.332. [DOI] [PubMed] [Google Scholar]
- Latkin C, Donnell D, Celentano DD, Aramrattna A, Liu T, Vongchak T, Wiboonnatakul K, Davis-Vogel A, Metzger D. Relationships between social norms, social network characteristics, and HIV risk behaviors in Thailand and the United States. Health Psychol. 2009b;28:323. doi: 10.1037/a0014707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkin C, Donnell D, Liu T, Davey-Rothwell M, Celentano D, Metzger D. The dynamic relationship between social norms and behaviors: the results of an HIV prevention network intervention for injection drug users. Addiction. 2013;108:934–943. doi: 10.1111/add.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkin C, Weeks MR, Glasman L, Galletly C, Albarracin D. A dynamic social systems model for considering structural factors in HIV prevention and detection. AIDS Behav. 2010;14:222–238. doi: 10.1007/s10461-010-9804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkin C, Yang C, Srikrishnan AK, Solomon S, Mehta SH, Celentano DD, Kumar MS, Knowlton A, Solomon SS. The relationship between social network factors, HIV, and Hepatitis C among injection drug users in Chennai, India. Drug Alcohol Depend. 2011;117:50–54. doi: 10.1016/j.drugalcdep.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latkin CA. Outreach in natural settings: the use of peer leaders for HIV prevention among injecting drug users’ networks. Public Health Rep. 1998;113(Suppl 1):151–159. [PMC free article] [PubMed] [Google Scholar]
- Li J, Weeks MR, Borgatti SP, Clair S, Dickson-Gomez J. A social network approach to demonstrate the diffusion and change process of intervention from peer health advocates to the drug using community. Subst Use Misuse. 2012;47:474–490. doi: 10.3109/10826084.2012.644097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Ali H, Wiessing L, Hickman M, Mattick RP, Myers B, Ambekar A, Strathdee SA. HIV prevention, treatment, and care services for people who inject drugs: A systematic review of global, regional, and national coverage. Lancet. 2010;375:1014–1028. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Toufik A. Global epidemiology of injecting drug use and HIV among people who inject drugs: A systematic review. Lancet. 2008;372:1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- Shahesmaeili A, Haghdoost AA, Soori H. Network location and risk of human immunodeficiency virus transmission among injecting drug users: Results of multiple membership multilevel modeling of social networks. Addict Health. 2014;7 [PMC free article] [PubMed] [Google Scholar]
- Simmons N, Donnell D, Celentano DD, Aramrattana A, Davis-Vogel A, Metzger D, Latkin C. Assessment of contamination and misclassification biases in a randomized controlled trial of a social network peer education intervention to reduce HIV risk behaviors among drug users and risk partners in Philadelphia, PA and Chiang Mai, Thailand. AIDS Behav. 2015:1–10. doi: 10.1007/s10461-015-1073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Abramovitz D, Lozada R, Martinez G, Rangel MG, Vera A, Staines H, Magis-Rodriguez C, Patterson TL. Reductions in HIV/STI incidence and sharing of injection equipment among female sex workers who inject drugs: Results from a randomized controlled trial. PLoS One. 2013;8:e65812. doi: 10.1371/journal.pone.0065812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin KE, Kuramoto SJ, Davey-Rothwell MA, Latkin CA. The STEP into Action study: a peer-based, personal risk network-focused HIV prevention intervention with injection drug users in Baltimore, Maryland. Addiction. 2011;106:366–375. doi: 10.1111/j.1360-0443.2010.03146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger JB, Kipke MD, De Rosa CJ, Hyde J, Ritt-Olson A, Montgomery S. Needle-sharing among young IV drug users and their social network members: The influence of the injection partner’s characteristics on HIV risk behavior. Addict Behav. 2006;31:1607–1618. doi: 10.1016/j.addbeh.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Wechsberg WM, Krupitsky E, Romanova T, Zvartau E, Kline TL, Browne FA, Ellerson RM, Bobashev G, Zule WA, Jones HE. Double jeopardy—drug and sex risks among Russian women who inject drugs: initial feasibility and efficacy results of a small randomized controlled trial. Subst Abuse Treat Prev Policy. 2012;7 doi: 10.1186/1747-597X-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks MR, Li J, Dickson-Gomez J, Convey M, Martinez M, Radda K, Clair S. Outcomes of a peer HIV prevention program with injection drug and crack users: The risk avoidance partnership. Subst Use Misuse. 2009;44:253–281. doi: 10.1080/10826080802347677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: a review of barriers and ways forward. Lancet. 2010;376:355–366. doi: 10.1016/S0140-6736(10)60832-X. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO, UNODC, UNAIDS technical guide for countries to set targets for universal access to HIV prevention, treatment and care for injecting drug users–2012 revision. 2012. [Google Scholar]


