Abstract
Several cytokines have been detected in human milk but their relative concentrations differ among women and vary over time in the same person. The drivers of such differences have been only partially identified, while the effect of luminal cytokines in the fine-regulation of the intestinal immune system is increasingly appreciated. The aim of this study was to investigate the associations between obstetrical complications and human milk cytokine profiles in a cohort of Peruvian women giving birth to Low Birth Weight (LBW) infants.
Colostrum and mature human milk samples were collected from 301 Peruvian women bearing LBW infants. The concentration of twenty-three cytokines was measured using the Luminex platform.
Ninety-nine percent of women had at least one identified obstetrical complication leading to intra-uterine growth restriction and/or preterm birth. Median weight at birth was 1,420 grams; median gestational age 31 weeks.
A core of 12 cytokines, mainly involved in innate immunity and epithelial cell integrity, was detectable in most samples. Maternal age, maternal infection, hypertensive disorders, preterm labor, and premature rupture of membranes were associated with specific cytokine profiles both in colostrum and mature human milk. Mothers of Very LBW (VLBW) neonates had significantly higher concentrations of chemokines and growth factor cytokines both in their colostrum and mature milk compared with mothers of larger neonates. Thus, maternal conditions affecting pregnancy duration and in utero growth are also associated with specific human milk cytokine signatures.
Keywords: Human milk cytokines, Low-birth weight infants, Pregnancy complications
1. Introduction
Human milk is regarded as the premier source of nutrition for infants until at least 6 months of age (AAP, 2012; WHO, 2003). It contains the nutrients necessary to promote adequate growth and bioactive substances essential for maturation of the neonatal immune system and intestinal function (Agarwal et al., 2011). Animal and in vitro studies suggest that cytokines may have an important role in the proliferation and migration of intestinal epithelial cells and in modulation of the mucosal immune system (Nguyen et al., 2014; Parigi et al., 2015; Rautava et al., 2012). Their effect could be especially important in low birth weight (LBW) infants (birth weight equal to or below 2500 grams), a population at increased risk of mortality and complications like sepsis or necrotizing enterocolitis (Neu and Walker, 2011; Stoll et al., 2002). Among small infants, the intake of human milk is directly associated with decreased mortality and morbidity but the underlying mechanisms are unclear (Corpeleijn et al., 2012; Meinzen-Derr et al., 2009; Sisk et al., 2007).
This study is an exploratory analysis conducted on samples and data from a large randomized controlled trial evaluating supplemental lactoferrin for the prevention of late onset sepsis in neonates. The aim of this analysis was to characterize the cytokine profile of human milk in a cohort of mothers of LBW neonates from poor neighborhoods of Lima, Peru. These women are exposed to a high burden of infectious diseases throughout their lives and have a low intake of animal foods and high quality proteins, with potentially significant effects on their immune systems. As many obstetrical complications leading to intrauterine growth restriction and/or preterm birth may be associated with perturbations of the maternal immune system, we hypothesized that specific pregnancy disorders would be associated with distinctive human milk cytokine profiles and affect the newborn’s growth even after birth.
2 Methods
2.1 Study participants
Participants were recruited at Hospital Nacional Cayetano Heredia, Hospital Nacional Almenara, and Hospital Nacional Sabogal in Lima, Peru. Infants were eligible if they weighed between 500 and 2,000 grams and were admitted to the Neonatal Intensive Care Unit within the first 72 hours of life. Exclusion criteria included severe underlying problems preventing oral feeding, family history of cow milk allergy or chronic conditions potentially affecting growth (for example chromosomal abnormalities, anatomic abnormalities).
A study nurse gathered the demographic and clinical data from each mother and infant at the time of enrollment and at subsequent visits. As the primary study did not show a significant effect of lactoferrin on neonatal sepsis (Newburg et al., 2010) data from both arms were pooled.
This study was approved by the Institutional Review Boards (IRBs) at the Universidad Peruana Cayetano Heredia and the University of Texas Health Science Center at Houston. Written informed consent was obtained from all mothers.
2.2 Human milk sample collection and processing
While in the hospital, the mothers manually expressed 2–3 milliliters of human milk into a sterile container that was transported on ice to the laboratory. The samples were centrifuged at 13,000 rpm for 15 minutes, the fat layer was discarded and the aqueous component was stored at −70°C. Colostrum was defined as human milk samples collected on days zero to seven (median day 4, IQR 3–5) after birth and mature milk as human milk samples collected on days 25–37 (median day 29, IQR 27–32).
2.3 Cytokine measurement
A panel of 20 cytokines was analyzed using the BioPlex Pro human cytokine 20-plex immunoassay, and TGF-β1, TGF-β2, and TGF-β3 levels using BioPlex Pro TGF-β assay kits (Bio-Rad, Hercules, CA) based on Luminex xMAP technology according to the manufacturer’s protocols. For the 20-plex, the milk samples were diluted 4-fold with sample diluent containing 0.5% BSA prior to use. For the TGF-β assay, the milk samples were activated by adding 1 volume of 1N HCl to 5 volumes of sample and incubated at room temperature for 10 minutes. The samples were neutralized by adding the same volume of base (1.2N NaOH/0.5M HEPES buffer) and diluted 4-fold with sample diluent containing 0.5% BSA. All samples were analyzed in duplicates. Co-variance within and between assays was less than 10%.
2.4 Statistical analysis
Values less than the lower limit of detection were set as one-half of the lowest measurable concentration. Values higher than the upper limit of detection were set as twice the highest measureable concentration. Summary measures were reported as percentages or medians. Mann-Whitney U tests were used for analysis of continuous variables. Wilcoxon signed rank test was used for comparing cytokine levels in colostrum to mature milk. Spearman rank correlation was used to measure the association between cytokine levels and maternal age. As this was an exploratory, hypothesis-generating secondary analysis, sample sizes were not calculated and adjustment for multiple correlations was not made. Analyses were performed using STATA v12 (College Station, TX) and GraphPad Prism v6 (La Jolla, CA).
3 Results
3.1 Clinical and demographic information
Demographic and clinical data were available on 300 of the 301 mothers with milk samples available for cytokine analysis (Table 1). The median maternal age was 30 years (IQR 24, 34). The median monthly household income was 1,000 Peruvian Soles (approximately US $300), with a median of 4 people (IQR 3, 6) per household. Median gestational age, assessed either by obstetrical ultrasounds, Ballard clinical score or reliable date of last menstrual period was 31 weeks (IQR 29, 33). Only 4/300 (1.3%) mothers delivered at term.
Table 1.
Clinical and demographic information of the Peruvian mothers and neonates enrolled in the study
| Demographics (N=300) | ||
| Monthly Household Income (Peruvian Soles), median (IQR) | 1000 | (800; 1,750) |
| People living in the household, median (IQR) | 4 | (3, 6) |
| Pregnancy-Related Complications | ||
| (Pre) eclampsia, n. women (%) | 97 | (32) |
| Pregnancy-induced Hypertension, n. women (%) | 5 | (2) |
| HELLP syndrome, n. women (%) | 6 | (2) |
| Multiple pregnancy, n. women (%) | 48 | (16) |
| Peripartum complications | ||
| Premature Labor, n. women (%) | 134 | (45) |
| Premature rupture of membranes, n. women (%) | 93 | (31) |
| Peripartum Maternal Infection, n. women (%) | 77 | (26) |
| Urinary Tract Infection, n. women (%) | 49 | (16) |
| Hemorrhage, n. women (%) | 36 | (12) |
| Peripartum Fever, n. women (%) | 27 | (9) |
| Treatment | ||
| Antenatal Steroids, n. women (%) | 209 | (70) |
| C-Section, n. women (%) | 242 | (81) |
| Newborns | ||
| Birth Weight (g), median (IQR) | 1,420 | (1,140; 1,709) |
| Very Low Birth Weight infants, n. (%) | 173 | (58) |
| Preterm delivery, n (%) | 296 | (99) |
| Gestational age at birth (wks), median (IQR) | 31 | (29–33) |
Of the 300 mothers, 298 (99%) experienced obstetrical complications (Table 1). Women with peripartum infections were slightly younger than women without infections (median age 29 years versus 30, P=0.04). There were no other associations between maternal age and obstetrical complications.
3.2 Human milk cytokine levels
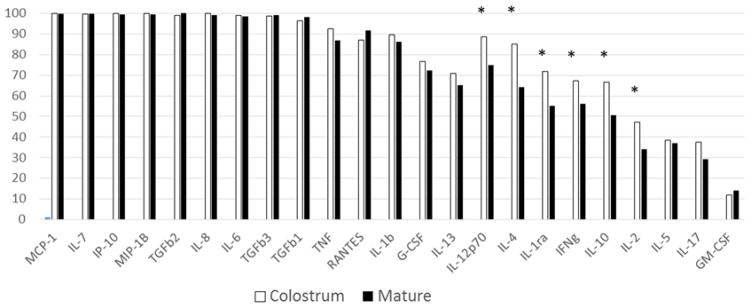
We analyzed 440 samples of human milk, including 223 colostrum samples and 217 mature milk samples. Of note, when colostrum samples were collected, 74/223 babies had received some formula, and when mature milk were collected, 76/217 babies had received some formula. The percentage of samples with detectable levels varied for each cytokine (Figure 1). Remarkably, cytokines produced by innate immune cells and chemokines were consistently expressed at detectable levels in colostrum and mature milk. The range of concentrations observed for each cytokine is reported in Table 2.
Figure 1. Percentage of samples with detectable levels of human milk cytokines.
The total number of samples tested was 440, including 223 colostrum and 217 mature milk samples. The frequency of detectability was significantly different between colostrum and mature milk for 6 cytokines: IL-12, P≤0.001; IL-4, P<0.001; IL-1ra, P<0.001; IFN-γ, P=0.01; IL-10, P=0.01; IL-2, P=0.02. Statistical significance was calculated using the Chi square test. * indicates P values <0.05.
Table 2.
Range of cytokine values measured in colostrum and mature milk samples with detectable levels.
| N. samples with detectable levels | Median | 25th | 75th | Min | Max | |
|---|---|---|---|---|---|---|
| Cytokines secreted by Innate Immune system cells | ||||||
| IL-1ra | 280 | 44 | 0.15 | 219 | 0.15 | 34,210 |
| IL-1β | 387 | 1.09 | 0.65 | 2.2 | 0.05 | 117 |
| IL-6 | 435 | 32 | 9.13 | 91 | 0.75 | 1,599 |
| IL-10 | 258 | 1.98 | 0.03 | 6.82 | 0 | 1,936 |
| IL-12p70 | 361 | 2.26 | 0.67 | 4.97 | 0.01 | 1,027 |
| TNF | 395 | 2.69 | 1.14 | 5.93 | 0.01 | 310 |
| IP-10 | 439 | 31,810 | 12,808 | 84,897 | 219 | 737,678 |
| Chemokines | ||||||
| MCP-1 | 440 | 919 | 285 | 2293 | 0.7 | 18,399 |
| MIP-1β | 439 | 219 | 96 | 527 | 1 | 22,668 |
| RANTES | 393 | 118 | 23 | 323 | 0.13 | 5,307 |
| IL-8 | 438 | 211 | 54 | 918 | 1 | 23,681 |
| Cytokines derived from adaptive immune system cells | ||||||
| Th17 lymphocytes | ||||||
| IL-17 | 147 | 4.74 | 0.1 | 10.22 | 0.1 | 35 |
| Th2 lymphocytes | ||||||
| IL-4 | 330 | 1 | 0 | 2.54 | 0 | 6.43 |
| IL-5 | 166 | 0.97 | 0.37 | 1.18 | 0.05 | 19.3 |
| IL-13 | 300 | 0.44 | 0.26 | 0.62 | 0 | 6.43 |
| Th1 lymphocytes | ||||||
| IFN-γ | 272 | 22 | 0.35 | 65 | 0.35 | 799 |
| IL-2 | 180 | 2.05 | 0.11 | 7.77 | 0.11 | 1,126 |
| Transforming growth factors | ||||||
| TGF-β1 | 428 | 1,342 | 649 | 2,436 | 4 | 19,321 |
| TGF-β2 | 439 | 1,001 | 420 | 1,766 | 1 | 45,220 |
| TGF-β3 | 435 | 91 | 51 | 165 | 2 | 1,229 |
| Hematopoietic Factors | ||||||
| IL-7 | 439 | 231 | 69 | 644 | 1 | 4,321 |
| GM-CSF | 57 | 4.17 | 0.11 | 9.73 | 0.11 | 41 |
| G-CSF | 328 | 2.15 | 0.01 | 6.18 | 0.01 | 44 |
The percentage of the samples with detectable levels out of the 440 samples are shown in Figure 1. All values are expressed as pg/mL.
Next, we compared the cytokine concentration between colostrum and mature milk in 139 sample pairs. Overall, 13 of 23 cytokines, predominantly cytokines produced by innate immune cells and chemokines, had significantly higher concentration in colostrum than in mature samples (Table 3).
Table 3.
Cytokine levels in paired colostrum and mature milk samples (N=139 participants).
| Colostrum | Mature Milk | ||||
|---|---|---|---|---|---|
|
| |||||
| Median | (25th, 75th) | Median | (25th,75th) | P* | |
| Cytokines derived from innate immune system cells | |||||
| IL-1ra | 8.46 | (1.45, 221.05) | 1.45 | (1.45, 38.85) | <0.001 |
| IL-1β | 1.37 | (0.73, 3.02) | 0.71 | (0.30, 1.27) | <0.001 |
| IL-6 | 52.33 | (24.42, 109.45) | 14.37 | (2.68, 50.71) | <0.001 |
| IL-10 | 0.005 | (0.005, 2.72) | 0.005 | (0.005, 2.14) | 0.006 |
| IL-12 | 2.71 | (0.34, 5.00) | 0.98 | (0.005, 2.46) | <0.001 |
| TNF | 3.89 | (1.93, 6.64) | 1.50 | (0.27, 3.07) | <0.001 |
| IP-10 | 23,196 | (9861, 45,693) | 54,224 | (18,947, 128,204) | <0.001 |
| Chemokines | |||||
| MCP-1 | 1,487 | (736, 3,263) | 416 | (134, 1,469) | <0.001 |
| MIP-1β | 374 | (226, 1,107) | 117 | (55, 224) | <0.001 |
| RANTES | 60.56 | (0.13, 157.00) | 170.42 | (5.49, 545.60) | <0.001 |
| IL-8 | 682 | (248, 2,008) | 63 | (223, 179) | <0.001 |
| Cytokines derived from adaptive immune system cells | |||||
| Th17 lymphocytes | |||||
| IL-17 | 0.10 | (0.10, 0.41) | 0.10 | (0.10, 0.10) | 0.01 |
| Th2 lymphocytes | |||||
| IL-4 | 0.93 | (0.15, 2.65) | 0.15 | (0.15, 0.83) | <0.001 |
| IL-5 | 0.05 | (0.05, 0.75) | 0.50 | (0.50, 0.82) | <0.001 |
| IL-13 | 0.31 | (0.05, 0.58) | 0.28 | (0.05, 0.49) | ns |
| Th1 lymphocytes | |||||
| IFN-γ | 0.72 | (0.35, 53.05) | 0.35 | (0.35, 17.76) | <0.001 |
| IL-2 | 0.11 | (0.15, 3.99) | 0.11 | (0.15, 0.11) | <0.001 |
| Transforming growth factors | |||||
| TGF-β1 | 1,276 | (575, 2,514) | 1,590 | (674, 2,765) | ns |
| TGF-β2 | 1,030 | (506, 1,786) | 865 | (272, 2,258) | ns |
| TGF-β3 | 114 | (56, 225) | 70 | (44, 117) | <0.001 |
| Hematopoietic factors | |||||
| IL-7 | 250 | (64, 673) | 272 | (84, 813) | ns |
| GM-CSF | 0.11 | (0.15, 0.11) | 0.11 | (0.11, 0.15) | ns |
| G-CSF | 0.3 | (0.01, 4.18) | 0.12 | (0.01, 4.27) | ns |
All values are reported as pg/mL. ns=non-significant: P value>0.05.
Wilcoxon matched-pairs signed-rank test
3.3 Human milk cytokines and maternal factors
We aimed to determine whether clinical conditions or other maternal characteristics were associated with human milk composition. First, we evaluated the impact of age. Older maternal age at delivery was correlated with higher levels of IL-6 (ρ=0.3, P=0.03) in colostrum and RANTES in mature milk (ρ=0.2, P=0.009).
3.3.1 Peripartum infections
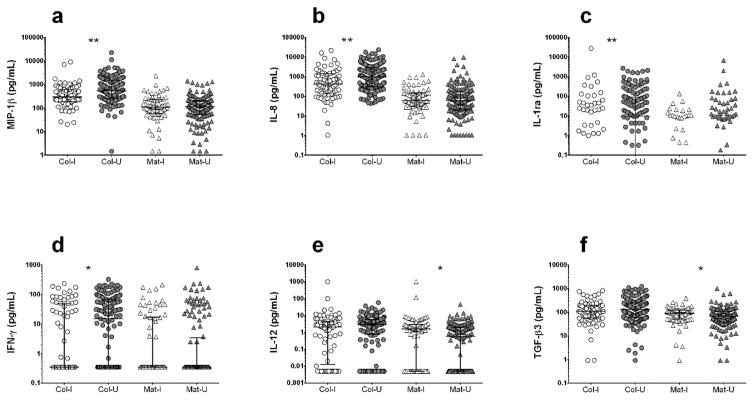
Colostrum of mothers with peripartum infections had significantly lower levels of three pro-inflammatory cytokines compared with women without infections: IL-8, a chemotactic factor for neutrophils; MIP-1β, a chemotactic factor for innate immune system; and IFN-γ, the canonical Th1 cytokine. The anti-inflammatory cytokine IL-1ra, which inhibits IL-1 signaling by acting as a decoy ligand for the IL-1 receptor, was also decreased (Figure 2). These differences were no longer detected in mature milk, although mature milk from women with peripartum infections had slightly higher levels of IL-12, TGF-β3 (Figure 2, Supplementary Table 1) and IL-1β (Supplementary Table 1). In sum, colostrum from women with peripartum infections had lower pro-inflammatory cytokine levels and mature milk had a small increase in pro-inflammatory mediators.
Figure 2. Comparison of colostrum and mature human milk cytokine levels between women with and without peripartum infections.
Women with documented peripartum infections or fever (n=97) were compared to women without infections (n=203). A set of 4 cytokines were decreased in colostrum of women with infections: (a) MIP-1β, (b) IL-8, (c) IL-1ra, (d) IFN-γ. Two cytokines (e) IL-12 and (f) TGF-β3, conversely, were slightly higher in mature milk samples. Col-I, colostrum samples from women with infections; Col-U, colostrum samples from women without infections; Mat-I, mature milk samples from women with infections; Mat-U, mature milk samples from women without infections. *P<0.05; **P<0.01. Statistical significance was calculated using the Mann-Whitney U test.
3.3.2 Hypertensive disorders
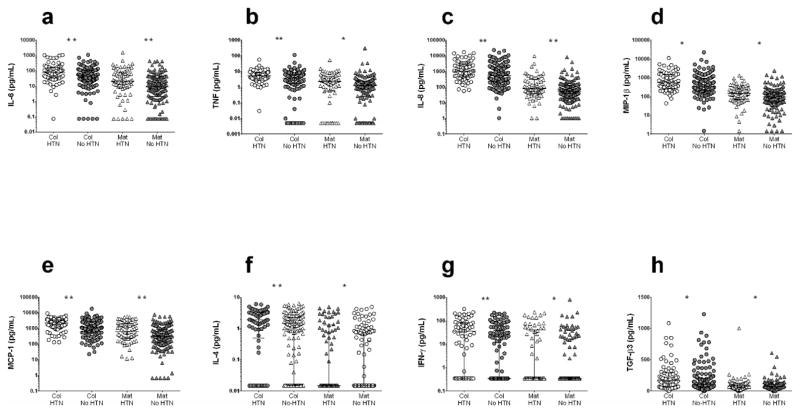
Mothers with any hypertensive disorder, including gestational hypertension, preeclampsia, eclampsia or HELLP syndrome, had higher levels of several cytokines in both colostrum and mature milk compared with women without hypertensive disorders, including pro-inflammatory innate system cytokines IL-6 and TNF, chemokines IL-8, MCP-1, and MIP-1β, adaptive immune system cytokines IFN-γ, and IL-4 and TGF-β3, involved in intestinal cell turnover (Figure 3, Supplementary Table 2). Colostrum, but not mature milk, from mothers with hypertensive disorders had higher concentrations of IL-1β (1.78 pg/mL versus 1.18 pg/mL, P=0.006) and IL-1ra (79.58 pg/mL versus 0.15 pg/mL, P=0.008) (Supplementary Table 2). Thus, women with hypertensive disorders secrete more pro-inflammatory cytokines.
Figure 3. Comparison of human milk cytokine levels between women with and without pregnancy-related hypertensive disorders.
Women with pregnancy-related hypertensive disorders (n=101) were compared to women without hypertensive disorders (n=199). Women with hypertensive disorders had higher levels of (a) IL-6, (b) TNF, (c) IL-8, (d) MIP-1β, (e) MCP-1, (f) IL-4, (g) IFN-γ, and (h) TGF-β3 in their colostrum and mature milk. Col-HTN, colostrum from hypertensive mothers; Col-No HTN, colostrum of mothers with no hypertension; Mat-HTN, mature milk from hypertensive mothers; Mat-No HTN, mature milk from mothers with no hypertension. *P<0.05; **P<0.01. Statistical significance was calculated using the Mann-Whitney U test
3.3.3 Preterm labor
Mothers who experienced spontaneous preterm labor had lower IL-8 (501.98 pg/mL versus 1005.17 pg/mL, P=0.01) and IL-1ra (0.15 pg/mL versus 38.4 pg/mL, P=0.05) levels in their colostrum compared with women with term or induced labor, but slightly higher levels of IL-13 (0.46 pg/mL versus 0.38 pg/mL, P=0.001), a cytokine with anti-inflammatory activity. Mature milk from mothers with spontaneous preterm labor also had lower IL-8 (46 pg/mL versus 74 pg/mL, P=0.03), IL-6 (10.35 pg/mL versus 17.32 pg/mL, P=0.03), MIP-1β (86 pg/mL versus 123 pg/mL, P=0.03) and IL-7 (202 pg/mL versus 336 pg/mL, P=0.003) levels, a cytokine produced by epithelial and stromal cells that can induce B, T and NK cell proliferation (Supplementary Table 3). Thus, women with spontaneous preterm labor tended to secrete less pro-inflammatory cytokines, chemokines and hematopoietic factors in their human milk for several weeks after delivery.
3.3.4 Mode of delivery, twin pregnancy and antenatal steroids
Delivery by C-section was not associated with alterations in colostrum cytokine composition, but mature milk IP-10 levels were significantly higher among mothers who delivered by C-section compared to vaginal delivery (52,959 pg/mL versus 30,382 pg/mL, P=0.03). Women with twin pregnancies only had lower RANTES concentration in colostrum samples (median 24.9 pg/mL versus 73.5 pg/mL, P=0.03), but no differences in mature milk. Notably, use of antenatal steroids was not associated with changes in colostrum or mature milk cytokine profiles. Thus, these factors had relatively little impact on human milk cytokine profiles.
3.4 Colostrum cytokine profile in mothers of VLBW and LBW infants
We compared human milk samples of mothers with Very LBW infants (VLBW; birth weight below 1500g) to samples of women with LBW babies. The median gestational age was 29 weeks (IQR 27, 31) in the VLBW group and 33 weeks (IQR 32, 34) in the LBW group. Prevalence of complications, type of delivery, use of antenatal steroids and maternal age were not different between the groups (data not shown).
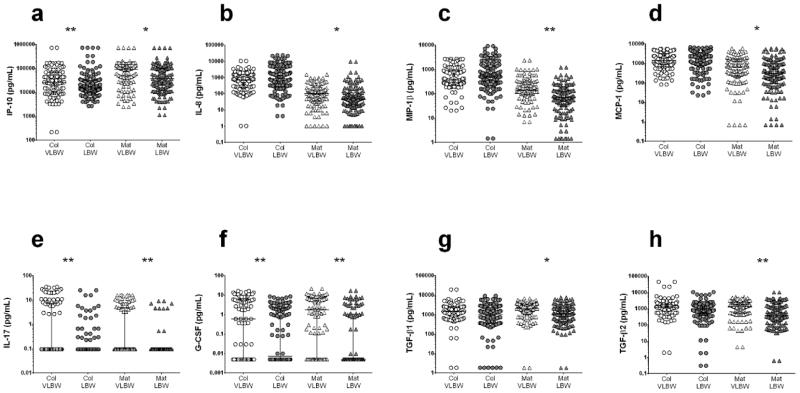
Mothers of VLBW infants had higher levels of cytokines during both stages of lactation compared to LBW infants. Their colostrum was enriched in innate immune system cytokines IP-10, IL-10 and G-CSF, and IL-17, which facilitates mucosal defense against invasive pathogens (Figure 4, Supplementary Table 4). Their mature milk still had higher levels of IP-10, IL-10, G-CSF and IL-17, and was also enriched in chemokines MIP-1β and IL-8, the immunomodulating factor TGF-β2 and pro-inflammatory IL-6 (Figure 4, Supplementary Table 4). The adaptive immune system cytokines IL-2, IL-4 and IFN-γ were only detectable at low levels and showed a tendency toward higher concentrations in the mature milk of mothers of VLBW infants compared to mothers of LBW babies; TGF-β1, TGF-β2 and TGF-β3 and chemotactic factor MCP-1 also tended to be higher in mature milk from mothers of VLBW infants. Thus, the milk of mothers of VLBW infants tends to contain higher concentrations of several cytokines and chemokines compared to LBW neonates.
Figure 4. Human milk concentration of selected cytokines among women delivering Very Low Birth Weight (VLBW) babies versus Low Birth Weight (LBW) babies.
Colostrum (Col) and mature (Mat) milk of mothers of VLBW newborns had higher levels of (a) IP-10, (e) IL-17 and (f) G-CSF, while (b) IL-8, (c) MIP-1β, (d) MCP-1, (g) TGF-β1 and (h) TGF-β2 were higher only in mature samples; *P<0.05; **P<0.01. Statistical significance was calculated using the Mann-Whitney U test.
4 Discussion
Despite the changes in human milk throughout lactation, the function of its immunological constituents, drivers of inter-individual differences and the implications for the neonate remain unclear. In this population of women living in a resource-limited setting who delivered LBW babies, we found that: 1) A core of 12 cytokines (TGF-β1-3, IL-1β, IL-6, IL-7 and TNF) and chemokines (MCP-1, MIP-1β, IP-10, IL-8, RANTES) was present in virtually all colostrum and mature human milk samples; 2) Colostrum had higher concentrations of most cytokines and chemokines compared to mature human milk; 3) Infection, hypertensive disorders and preterm labor were associated with shifts in colostrum and mature milk immune profiles; 4) Mothers who delivered VLBW neonates had higher concentrations of many cytokines and chemokines in their human milk compared with mothers of larger neonates. Together, our results indicate that maternal and gestational factors can influence both colostrum and mature milk cytokine profiles.
Consistent with other studies, we found that many cytokines are present in detectable levels in human milk and in higher concentrations in colostrum (Agarwal et al., 2011; Castellote et al., 2011; Chollet-Hinton et al., 2014; Hawkes et al., 2000; Trend et al., 2016). Chemokines associated with innate immunity (MCP-1, MIP-1β, IP-10, IL-8, RANTES) and cytokines that impact epithelial cell function (TGF-βs) or induce lymphocyte maturation (IL-6 and IL-7) were almost universally detectable. The concentrations we observed were comparable to reports from industrialized countries, except for higher IP-10 and MCP-1 levels and lower IL-5 and IL-13 levels. We also saw increased, rather than decreased, IP-10, RANTES and IL-7 in both colostrum and mature samples (Agarwal et al., 2011; Marcuzzi et al., 2013). Differing results could be attributed to methodology, including timing of sample collection, sample preservation and assays used, but also to biological dissimilarities depending on genetic, environmental or medical factors.
Few studies have examined the effect of maternal conditions other than allergy on human milk cytokine composition. We found that women with peripartum infections had lower colostrum levels of pro-inflammatory cytokines compared to women without infections, yet many acute infections are characterized by increased circulating levels of these mediators. Thus, alterations in human milk cytokine levels may reflect differential trafficking of immune cells into the mammary gland rather than mirror systemic concentrations. In contrast, women with pregnancy-related hypertension disorders showed a marked increase in cytokines involved in innate and Th1 activation. Indeed, women experiencing pre-eclampsia have higher circulating Th1 cytokine levels (Azizieh et al., 2005). Infections and hypertensive disorders induced a shift in opposite direction of some cytokines, suggesting the etiology of maternal inflammation can have differential effects on human milk composition.
We also observed that colostrum from mothers with VLBW neonates was richer in immunostimulatory proteins, such as IP-10, which recruits monocytes, macrophages, dendritic cells, T lymphocytes and NK cells; G-CSF, which stimulates granulocyte production; and IL-17, which induces secretion of antimicrobial peptides from epithelial cells and facilitates neutrophil recruitment (Levy, 2007). The difference was even more impressive in mature milk samples. These data expand the finding of earlier studies reporting elevated levels of IL-6, IL-8, TGF-β2, human defensins, and soluble CD14 in colostrum of women who delivered preterm babies (Castellote et al., 2011; Trend et al., 2015) and suggest that events that alter the course of pregnancy may influence human milk composition for several weeks after birth, with possible effects on the mucosal immunomodulation and clinical outcomes of the neonates.
Newborns have immature immune systems, with functionally impaired neutrophils, reduced antigen-presenting activity, attenuated responses to pattern-recognition receptor stimulation, and a skewing toward Th2/anti-inflammatory responses (Currie et al., 2011; Hartel et al., 2008; Levy, 2007; Strunk et al., 2007; Tatad et al., 2008). This skewing (Adkins, 2013; Dowling and Levy, 2014; Elahi et al., 2013) favors the establishment of the microbe-host homeostasis during early colonization of skin and mucosal surfaces (Lotz et al., 2006). The Th2 cytokines such as IL-4 and IL-13, detected in most of our samples and in higher concentration in colostrum, are anti-inflammatory, down-regulate Toll-like receptor activation in human intestinal epithelial cells (Mueller et al., 2006), and would maintain tolerance during initial bacterial colonization. Similarly, TGF-β1 and TGF-β2 would induce T regulatory cells in mesenteric lymph nodes (Renz et al., 2012) and reduce mucosal levels of inflammatory cytokines (Nguyen et al., 2014; Rautava et al., 2012).
The anti-inflammatory properties of human milk may explain why breastfed infants are protected against necrotizing enterocolitis (NEC), a disease apparently triggered by failure to downregulate the inflammatory response to colonizing intestinal bacteria (LeBouder et al., 2006; Levy, 2007). Human milk cytokines like TGF-β2 modulate LPS-induced secretion of pro-inflammatory cytokines (Nguyen et al., 2014) and proliferation and differentiation of lymphocytes, phagocytes, and dendritic cells (Agarwal et al., 2011; Chatterton et al., 2013). In fact, TGF-β family cytokines were detectable in all samples we tested, and at increased concentrations in the mature milk of women delivering VLBW infants, the population with the highest risk of NEC, suggesting highly conserved function and supporting the importance of human milk consumption for premature infants.
Human milk is also protective against neonatal sepsis (Corpeleijn et al., 2012), likely mediated by antimicrobial components and modulation of the neonatal immune system (Parigi et al., 2015). IP-10 and chemokines such as IL-8, MCP-1, MIP-1β and IL-17 from the mother’s colostrum may activate the innate immune system against invading pathogens and strengthen the intestinal barrier. In fact, in vitro experiments suggest that, at the levels found in human milk, IL-8 facilitates the maturation of intestinal epithelial cells (Maheshwari et al., 2002; Nguyen et al., 2014). Within the intestinal mucosa, the differentiation of naïve CD4 cells into Th17 cells, which help defend against extracellular pathogens, is modulated by TGFβs concentrations (Chatterton et al., 2013; Galvez, 2014). Thus, human milk may be selectively anti-inflammatory and antimicrobial. Fluctuations of cytokine levels, like those observed in our study, may compromise the epithelial mucosal barrier integrity and the capacity of mucosal immune cells to contain bacterial translocation, thus increasing the risk of sepsis.
Our study has some limitations, as this was a secondary analysis conducted on already collected samples and data. First, there is no control group and therefore no data on human milk cytokines from healthy Peruvian mothers delivering term babies for comparison. Second, human milk cells were not available to profile and clarify the etiology of differences in the relative abundance of cytokines. Third, maternal and neonatal blood samples are not available for analysis, limiting our ability to assess the degree to which human milk reflects the maternal immune system and influences the neonatal immune system. However, this is among the most comprehensive datasets on human milk cytokines reported, in terms of number of women enrolled, availability of samples across different stages of lactation, and number of cytokines examined.
5 Conclusion
Complications of pregnancy influence the concentration of cytokines and chemokines in human milk, potentially affecting the initial priming of the neonatal immune system. In depth understanding of the interplay between human milk immune components and the intestinal mucosa system could open the way for innovative approaches to neonatal health.
Supplementary Material
Highlights.
1) A core of 12 cytokines is detected in human milk across different lactation stages
2) Specific pregnancy complications are associated with distinct breast milk cytokine signatures
3) Mothers of very small neonates have higher concentrations of many cytokines in their breast milk
Acknowledgments
Funding: This work was funded by the Grand Challenges Explorations Grant from Bill & Melinda Gates Foundation, Grant OPP1015669 (T.J.O.), by the Public Health Service award R01-HD067694-01A1 from the National Institute of Child Health and Human Development (NICHD), USA (T.J.O.), and by the Center for Tropical Diseases-Institute for Human Infections and Immunity at the University of Texas Medical Branch at Galveston (N.S.U.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the Gates Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AAP. Breastfeeding and the use of human milk. Pediatrics. 2012:e827–841. [Google Scholar]
- Adkins B. Neonatal immunology: responses to pathogenic microorganisms and epigenetics reveal an “immunodiverse” developmental state. Immunologic research. 2013;57:246–257. doi: 10.1007/s12026-013-8439-2. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Karmaus W, Davis S, Gangur V. Immune markers in breast milk and fetal and maternal body fluids: a systematic review of perinatal concentrations. Journal of human lactation: official journal of International Lactation Consultant Association. 2011;27:171–186. doi: 10.1177/0890334410395761. [DOI] [PubMed] [Google Scholar]
- Azizieh F, Raghupathy R, Makhseed M. Maternal cytokine production patterns in women with pre-eclampsia. American journal of reproductive immunology. 2005;54:30–37. doi: 10.1111/j.1600-0897.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- Castellote C, Casillas R, Ramirez-Santana C, Perez-Cano FJ, Castell M, Moretones MG, Lopez-Sabater MC, Franch A. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. The Journal of nutrition. 2011;141:1181–1187. doi: 10.3945/jn.110.133652. [DOI] [PubMed] [Google Scholar]
- Chatterton DE, Nguyen DN, Bering SB, Sangild PT. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. The international journal of biochemistry & cell biology. 2013;45:1730–1747. doi: 10.1016/j.biocel.2013.04.028. [DOI] [PubMed] [Google Scholar]
- Chollet-Hinton LS, Stuebe AM, Casbas-Hernandez P, Chetwynd E, Troester MA. Temporal trends in the inflammatory cytokine profile of human breastmilk. Breastfeeding medicine: the official journal of the Academy of Breastfeeding Medicine. 2014;9:530–537. doi: 10.1089/bfm.2014.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpeleijn WE, Kouwenhoven SM, Paap MC, van Vliet I, Scheerder I, Muizer Y, Helder OK, van Goudoever JB, Vermeulen MJ. Intake of own mother’s milk during the first days of life is associated with decreased morbidity and mortality in very low birth weight infants during the first 60 days of life. Neonatology. 2012;102:276–281. doi: 10.1159/000341335. [DOI] [PubMed] [Google Scholar]
- Currie AJ, Curtis S, Strunk T, Riley K, Liyanage K, Prescott S, Doherty D, Simmer K, Richmond P, Burgner D. Preterm infants have deficient monocyte and lymphocyte cytokine responses to group B streptococcus. Infection and immunity. 2011;79:1588–1596. doi: 10.1128/IAI.00535-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling DJ, Levy O. Ontogeny of early life immunity. Trends in immunology. 2014;35:299–310. doi: 10.1016/j.it.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN inflammation. 2014;2014:928461. doi: 10.1155/2014/928461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartel C, Osthues I, Rupp J, Haase B, Roder K, Gopel W, Herting E, Schultz C. Characterisation of the host inflammatory response to Staphylococcus epidermidis in neonatal whole blood. Archives of disease in childhood Fetal and neonatal edition. 2008;93:F140–145. doi: 10.1136/adc.2007.124685. [DOI] [PubMed] [Google Scholar]
- Hawkes JS, Bryan DL, Gibson RA. Cytokine production by leukocytes from human milk. Advances in experimental medicine and biology. 2000;478:391–392. doi: 10.1007/0-306-46830-1_42. [DOI] [PubMed] [Google Scholar]
- LeBouder E, Rey-Nores JE, Raby AC, Affolter M, Vidal K, Thornton CA, Labeta MO. Modulation of neonatal microbial recognition: TLR-mediated innate immune responses are specifically and differentially modulated by human milk. Journal of immunology. 2006;176:3742–3752. doi: 10.4049/jimmunol.176.6.3742. [DOI] [PubMed] [Google Scholar]
- Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nature reviews Immunology. 2007;7:379–390. doi: 10.1038/nri2075. [DOI] [PubMed] [Google Scholar]
- Lotz M, Gutle D, Walther S, Menard S, Bogdan C, Hornef MW. Postnatal acquisition of endotoxin tolerance in intestinal epithelial cells. The Journal of experimental medicine. 2006;203:973–984. doi: 10.1084/jem.20050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari A, Lu W, Lacson A, Barleycorn AA, Nolan S, Christensen RD, Calhoun DA. Effects of interleukin-8 on the developing human intestine. Cytokine. 2002;20:256–267. doi: 10.1006/cyto.2002.1996. [DOI] [PubMed] [Google Scholar]
- Marcuzzi A, Vecchi Brumatti L, Caruso L, Copertino M, Davanzo R, Radillo O, Comar M, Monasta L. Presence of IL-9 in paired samples of human colostrum and transitional milk. Journal of human lactation: official journal of International Lactation Consultant Association. 2013;29:26–31. doi: 10.1177/0890334412466958. [DOI] [PubMed] [Google Scholar]
- Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants’ risk of necrotizing enterocolitis or death. Journal of perinatology: official journal of the California Perinatal Association. 2009;29:57–62. doi: 10.1038/jp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller T, Terada T, Rosenberg IM, Shibolet O, Podolsky DK. Th2 cytokines down-regulate TLR expression and function in human intestinal epithelial cells. Journal of immunology. 2006;176:5805–5814. doi: 10.4049/jimmunol.176.10.5805. [DOI] [PubMed] [Google Scholar]
- Neu J, Walker WA. Necrotizing enterocolitis. The New England journal of medicine. 2011;364:255–264. doi: 10.1056/NEJMra1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburg DS, Woo JG, Morrow AL. Characteristics and potential functions of human milk adiponectin. The Journal of pediatrics. 2010;156:S41–46. doi: 10.1016/j.jpeds.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DN, Sangild PT, Ostergaard MV, Bering SB, Chatterton DE. Transforming growth factor-beta2 and endotoxin interact to regulate homeostasis via interleukin-8 levels in the immature intestine. American journal of physiology Gastrointestinal and liver physiology. 2014;307:G689–699. doi: 10.1152/ajpgi.00193.2014. [DOI] [PubMed] [Google Scholar]
- Parigi SM, Eldh M, Larssen P, Gabrielsson S, Villablanca EJ. Breast Milk and Solid Food Shaping Intestinal Immunity. Frontiers in immunology. 2015;6:415. doi: 10.3389/fimmu.2015.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautava S, Lu L, Nanthakumar NN, Dubert-Ferrandon A, Walker WA. TGF-beta2 induces maturation of immature human intestinal epithelial cells and inhibits inflammatory cytokine responses induced via the NF-kappaB pathway. Journal of pediatric gastroenterology and nutrition. 2012;54:630–638. doi: 10.1097/MPG.0b013e31823e7c29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz H, Brandtzaeg P, Hornef M. The impact of perinatal immune development on mucosal homeostasis and chronic inflammation. Nature reviews Immunology. 2012;12:9–23. doi: 10.1038/nri3112. [DOI] [PubMed] [Google Scholar]
- Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O’Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. Journal of perinatology: official journal of the California Perinatal Association. 2007;27:428–433. doi: 10.1038/sj.jp.7211758. [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- Strunk T, Richmond P, Simmer K, Currie A, Levy O, Burgner D. Neonatal immune responses to coagulase-negative staphylococci. Current opinion in infectious diseases. 2007;20:370–375. doi: 10.1097/QCO.0b013e3281a7ec98. [DOI] [PubMed] [Google Scholar]
- Tatad AM, Nesin M, Peoples J, Cheung S, Lin H, Sison C, Perlman J, Cunningham-Rundles S. Cytokine expression in response to bacterial antigens in preterm and term infant cord blood monocytes. Neonatology. 2008;94:8–15. doi: 10.1159/000112541. [DOI] [PubMed] [Google Scholar]
- Trend S, Strunk T, Hibbert J, Kok CH, Zhang G, Doherty DA, Richmond P, Burgner D, Simmer K, Davidson DJ, Currie AJ. Antimicrobial protein and Peptide concentrations and activity in human breast milk consumed by preterm infants at risk of late-onset neonatal sepsis. PloS one. 2015;10:e0117038. doi: 10.1371/journal.pone.0117038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trend S, Strunk T, Lloyd ML, Kok CH, Metcalfe J, Geddes DT, Lai CT, Richmond P, Doherty DA, Simmer K, Currie A. Levels of innate immune factors in preterm and term mothers’ breast milk during the 1st month postpartum. The British journal of nutrition. 2016;115:1178–1193. doi: 10.1017/S0007114516000234. [DOI] [PubMed] [Google Scholar]
- WHO, W.H.O. Global strategy for infant and young child feeding. Geneva, Switzerland: 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.