Abstract
In a clinical and cytogenetic survey of 39 individuals with Prader-Labhart-Willi syndrome (PLWS) (23 males and 16 females ranging in age from 2 weeks to 39 years), an interstitial deletion of chromosome 15 (breakpoints q11 and q13) was identified in 21 cases and apparently normal chromosomes in the remainder. Studies of parental chromosome 15 variants showed that the del[15q] was paternal in origin, although chromosomes of both parents were normal. All chromosome deletions were de novo events. Possible causes for the chromosome deletion and the role of chromosome rearrangements in individuals with PLWS are discussed. Clinical characteristics of the deletion and nondeletion groups were recorded and compared with 124 individuals reported in the literature. Individuals with the chromosome deletion were found to have lighter hair, eye, and skin color, greater sun sensitivity, and higher intelligence scores than individuals with normal chromosomes. Correlation studies of metacarpophalangeal pattern profile variables and dermatoglyphic findings indicate apparent homogeneity of the deletion group and heterogeneity of individuals with PLWS and normal chromosomes.
Keywords: Prader-Labhart-Willi syndrome (PLWS), high-resolution chromosome analysis, 15q deletion, chromosome subgroup differences, parental origin studies
INTRODUCTION
The Prader-Labhart-Willi syndrome (PLWS) was first described in nine patients by Prader, Labhart, and Willi in 1956, and subsequently over 500 cases have been reported [Prader et al, 1956; Zellweger and Schneider, 1968; Zellweger, 1969; Pearson et al, 1971; Hall and Smith, 1972; Hamilton et al, 1972; Smith, 1976; Bergsma, 1979; Holm et al, 1981; Naselli et al, 1981; Ledbetter et al 1982; Bray et al, 1983; Matter et al, 1983; Cassidy et al, 1984]. Individuals with PLWS, generally sporadic in occurrence, have infantile hypotonia, early childhood obesity, mental deficiency (intelligence quotient range from 20 to 80 with an average of 60 [Hall and Smith, 1972], short stature, small hands and feet, and hypogonadism. Most of these findings are present in any one patient, but the syndrome is clinically variable. The incidence of PLWS has been estimated at one in 25,000 live births and accounts for about 1 % of all mentally retarded persons [Zellweger and Soper, 1979]. The cause of PLWS is not clear, although abnormal chromosome findings have been reported in individuals with PLWS. The first reported cytogenetically abnormal individual had a 47,XYY karyotype [Dunn et al, 1961]. Two years later Bühler et al [1963] reported the first case with a translocation of a D-group chromosome. Since that time several additional chromosome abnormalities have been described. In 1976, Hawkey and Smithies reviewed the literature and reported that ten of 61 patients with PLWS had a chromosome abnormality identified with standard banded or unbanded chromosome preparations. Some of the chromosome “abnormalities” included mosaic Klinefelter syndrome and familial markers of chromosomes 16, 22, and Y, which were also found in phenotypically normal relatives. Thus most individuals with PLWS had normal chromosomes.
In 1980, Ledbetter et al reported a chromosome 15 deletion in patients with PLWS. Using high-resolution chromosome banding, these investigators found a small deletion of the proximal long arm of chromosome 15 in four of five patients. One hundred thirty-one cases of PLWS have been analyzed with high-resolution chromosome procedures, and a chromosome 15 abnormality, usually a proximal q arm deletion, was identified in 82 individuals [Ledbetter et al, 1981; 1982; Wyandt et al, 1981; Bonucelli et al, 1982; Butler et al, 1982b; Kousseff, 1982; Charrow et al, 1983; Mattei et al, 1983; Winsor and Welch, 1983; Butler, 1984; Cassidy et al, 1984]. These findings are similar to the deletion of the short arm of chromosome 11 found in Wilms-aniridia syndrome [Francke et al, 1979; Riccardi et al, 1980].
We report on the clinical and cytogenetic findings of 39 patients with PLWS and studies of the parents of these individuals.
MATERIALS AND METHODS
Subjects and Clinical Evaluation
The criteria for inclusion of individuals with PLWS in this study were infantile hypotonia, hypogonadism, delayed psychomotor development and/or mental retardation, early childhood obesity, small hands and feet, and short stature. The sample comprised 23 males and 16 females ranging in age from 2 weeks to 38.5 years with a mean of 13.5 years. An additional eight individuals were examined but not included in the sample because of atypical findings.
Pregnancy history (including fetal activity, pregnancy duration, gestational problems, and possible fetal insults), family history, a general physical examination, and health history were obtained on each individual. The degree of hypotonia, muscle coordination, physical activity, intelligence, feeding difficulty, hair and eye color, skin complexion, and behavior pattern were noted. Dermatoglyphic prints of hands and feet were obtained and have been reported previously [Reed and Butler, 1984]. Hand radiographs and 28 anthropometric measurements of the subjects and first-degree relatives were obtained.
All individuals with PLWS in the study were white. There appears to be a paucity of blacks with this condition [Butler et al, 1982a; Golden et al, 1984]. Approximately 95% of individuals with this syndrome are white and of European descent [Holm et al, 1981].
High-Resolution Chromosome Studies
All subjects were studied by high-resolution chromosome procedures. Two high-resolution chromosome culturing techniques were used including the synchronization technique and/or actinomycin D pretreatment [Yunis, 1976; Yu et al, 1981; Rybak et al, 1982]. Chromosome 15 in patients and parents was evaluated using several other cytogenetic techniques including GTG, QFQ, RBG, and AgNOR.
Blind studies were used throughout for unbiased interpretation of results. Each patient was studied using coded slides, coded photographs, or both. Microscopic analysis included detailed study of at least 20 cells, karyotypes of three to five cells, and an additional ten to 15 cells specifically viewed for chromosome 15. Therefore, at least 30 cells were analyzed in order to rule out mosaicism of the chromosome deletion.
Chromosomes of individuals with PLWS were scored from photographs without knowledge of identity. Fifteen to thirty pictures (combination of controls and individuals with PLWS with a minimum of ten pictures from the patient) were coded and examined without knowledge of subject identity. All chromosomes were analyzed, but focused attention was given to chromosome number 15. The chromosomes were scored as either normal or deleted. The determination of a deletion of the q11 or q12 bands was made on the basis of absence of these bands of the proximal long arm and/ or a shorter distance between the centromere and q14 band in the deleted chromosome. The code was broken after scoring and the results compared with the interpretation of the chromosomes carried out at the microscope.
GTG, QFQ, and AgNOR staining procedures were used to identify the chromosome variants. GTG stained chromosomes were scored for satellite stalk and short arm length in 13 families. QFQ stained chromosomes were scored for satellite intensity in 13 families [Paris Conference Supplement, 1975]. AgNOR-stained chromosomes were scored for activity and size of the silver precipitate in ten families.
The variants were identified at high resolution by sequential staining with G-banding and silver of the nucleolar organizing region (NOR) or G- and Q-banding. Variants informative for chromosome identification were used for parental origin of the chromosome deletion.
RESULTS
Parental Population
The combined parental ages at conception of 38 children with PLWS (N = 20 with deletion and N = 18 with normal chromosomes) had a unimodel distribution. The mean maternal and paternal age at conception of the child with PLWS were 25 years 11 months and 29 years 2 months, respectively. The mean maternal and paternal age at conception of the child between the two chromosomal classes did not differ.
Diagnosis of Individuals With PLWS
The average age at the time of diagnosis of the 39 individuals with PLWS was 7 years 2 months with a range of 6 weeks to 32 years. The age of diagnosis in the deletion group was 9 years 4 months, and the age of diagnosis in the nondeletion group was 5 years 4 months. Twenty-five percent of all patients were diagnosed by 3 years of age and 11 % in adulthood.
Pregnancy, Birth, and Neonatal History
The mean gestational period for all children with PLWS in this study was 40.7 weeks, and 65% were premature. The duration of gestation did not differ between chromosomal classes.
The average birth length was 49.5 cm and the average birth weight was 2.92 kg for all individuals with PLWS. There was no difference between the two groups.
Reduced fetal activity was noted by 90% of mothers of individuals with the deletion and 81 % of mothers of individuals with normal chromosomes. There was no difference between the two groups.
Breech presentation was found in 35% of individuals with the chromosome 15 deletion and 24% of individuals with normal chromosomes. C-section delivery was required in 18% of all cases.
The most common neonatal complication found in infants with PLWS was an inadequate suck reflex, which necessitated switching from breast to a bottle with a large bore nipple, forced feedings with a spoon or dropper or gavage feedings in all cases. Thirty-eight percent of individuals with the chromosome 15 deletion and 39% of individuals with normal chromosomes in this study required intermittent gavage feedings, and a gastrostomy was required for one female with a chromosome deletion.
Hypotonia is a cardinal manifestation of PLWS and was found in all individuals in this study. Hypotonia improved gradually but was still present in several individuals at age 6 years. The average time of onset of improvement in muscle tone for the deletion group was 11.3 months and 11.6 months for the nondeletion group.
Psychomotor Development
Psychomotor development usually was delayed in individuals with PLWS compared to normal individuals (Fig. 1). Only one individual reached all three developmental stages at the appropriate time. The mean age for crawling was 15.8 months in the nondeletion group and 16.8 months in the deletion group. Forty-two percent of patients with normal chromosomes were crawling by 12 months compared to 33% of those individuals with the chromosome 15 deletion. Thirty-six percent of individuals did not crawl.
Fig. 1.

Age at achievement of developmental stages of individuals with PLWS with and without the chromosome 15 deletion.
The average age for walking alone was 27.6 months for the nondeletion group and 29.2 months in the deletion group. Sixty-nine percent of patients with normal chromosomes were walking alone by 24 months compared to 50% of those individuals with the chromosome 15 deletion.
Individuals with PLWS and normal chromosomes had a ten word vocabulary at an average age of 39.1 months. The average age for similar attainment in the deletion group was 38.5 months. Fifty-eight percent of individuals with deletions were talking by age 3 years compared with 65% of individuals with normal chromosomes. Speech appeared to be the most delayed of the developmental milestones. There were no differences in psychomotor development between the two chromosome groups.
Mental Status
The average intelligence quotient (IQ) obtained from medical records of 13 individuals with PLWS but lacking the deletion was 59.2 with a range from 42 to 84 compared to an IQ of 69.6 with a range from 50 to 90 in 16 individuals with the deletion. Several psychological tests were used in both subgroups. The Stanford-Binet test was used on 20 of the 29 individuals with PLWS. The average age of examination in the nondeletion group was 8 years compared with 9 years 2 months in the deletion group. The group IQ means differed as demonstrated by two-tailed t- test (P < 0.05).
Hair, Eye, and Skin
Hair color was judged as brown to black in 5% and blond to light brown in 95% of individuals with the deletion compared to 50% with brown to black and 50% with blond to light brown in individuals without the deletion. The hair color difference between the two chromosome groups is significant at P < 0.01 as demonstrated by χ2 test. Individuals with the deletion had lighter hair color than other relatives at a comparable age, whereas most individuals lacking the deletion had hair color similar to that of other relatives. Photographs of two males (one with and one without the deletion) at comparable ages are shown in Figure 2.
Fig. 2.

Facial phenotypes of a male with the deletion (top) and a male lacking the deletion (bottom) both with PLWS each shown from ages 1–10 years.
In both PLWS groups the predominant eye color was blue. All individuals with a deletion had blue eyes. Seventy-two percent of individuals without the deletion had blue eyes. Eye color was recorded as hazel/green to brown in 28% of individuals lacking the deletion and none of those individuals bearing the deletion. This difference was significant (P < 0.05).
Skin complexion and sun sensitivity were judged by observation and/or history. Skin complexion was judged as normal or fair and/or ligher than that of other relatives at a comparable age. Ninety-five percent of individuals having the deletion possessed fair complexion in comparison with 28% of individuals lacking the deletion. This difference was significant (P < 0.001).
Sun sensitivity is related to skin complexion. An individual was judged as sun sensitive if exposure was reported to produce a rash, extreme burn without tanning, or photophobia. Sixty-seven percent of individuals with the deletion were judged to have sun sensitivity compared to 28% of individuals without the deletion. This difference was signficant (P < 0.05).
Comparisons of the metacarpophalangeal pattern profile (MCPP) and dermatoglyphic findings have been reported previously [Butler and Meaney, 1985; Reed and Butler, 1984]. The mean hand profile of 38 individuals with PLWS was essentially flat; the profiles differed in the metacarpal area in the two chromosome groups. A discriminant analysis of individuals with PLWS vs control individuals produced a function of three MCPP variables and age. A deficiency of plantar dermatoglyphic pattern intensity was restricted only to individuals with chromosome 15 deletions and was characterized by a lack of plantar interdigital II–IV patterns with almost exclusively hallucal distal loops. Individuals with PLWS and the deletion were more similar than cases lacking the deletion with respect to dermatoglyphic patterns and MCPP variables.
Comparison of other clinical characteristics including neurological function, minor facial anomalies, sexual development, and limb, oral, metabolic, and musculoskeletal systems is shown in Table I. Comparison of clinical features in the present study with published surveys is shown in Table II.
TABLE I.
Clinical Manifestations of Prader-Labhart-Willi Syndrome Individuals
| Description | Chromosome subgroup
|
||
|---|---|---|---|
| Deletion (%) | Nondeletion (%) | P | |
| Gestation | |||
| Preterm delivery | 4/20 (20) | 2/17 (12) | NS |
| Postterm delivery | 7/20 (35) | 11/17 (65) | NS |
| Breech presentation | 7/20 (35) | 4/17 (24) | NS |
| Reduced fetal vigor | 17/19 (90) | 13/16 (81) | NS |
| Neonatal period and infancy | |||
| Birth weight (<2.27 kg) | 1/20 (5) | 2/18 (11) | NS |
| Birth length (<47.O cm) | 1/18 (6) | 3/17 (18) | NS |
| Hypotonia | 21/21 (100) | 18/18 (100) | NS |
| Neonatal feeding difficulty | 21/21 (100) | 18/18 (100) | NS |
| Delayed development | 21/21 (100) | 17/18 (94) | NS |
| CNS function and behavior | |||
| Mental impairment (IQ≤9O) | 19/19 (100) | 16/16 (100) | NS |
| Vision problems | 9/19 (47) | 5/16 (31) | NS |
| Seizures | 5/20 (25) | 4/18 (22) | NS |
| Sleepiness | 15/21 (71) | 10/18 (56) | NS |
| Hyperphagia | 17/21 (81) | 16/18 (89) | NS |
| Hyperactivity | 1/21 (5) | 1/18 (6) | NS |
| Personality problems | 15/21 (71) | 13/18 (72) | NS |
| Habit of picking sores | 16/21 (76) | 15/18 (83) | NS |
| Growth | |||
| Obesitya | 18/21 (86) | 15/17 (88) | NS |
| Short stature (<−2 SD) | 9/21 (43) | 9/18 (50) | NS |
| Face | |||
| Strabismus | 14/21 (67) | 12/18 (67) | NS |
| Bifrontal diameter (<−2 SD) | 15/21 (71) | 12/18 (67) | NS |
| Almond-shaped palpebral fissures | 16/21 (76) | 13/18 (72) | NS |
| Sexual development | |||
| Cryptorchidism (males) | 11/11 (100) | 12/12 (100) | NS |
| Hypogenitalism (males) | 11/11 (100) | 12/12 (100) | NS |
| Menstruation (females)b | 3/7 (43) | 1/4 (25) | NS |
| Precocious puberty | 0/21 (0) | 1/18 (6) | NS |
| Limbs | |||
| Small hands and feet (< −2 SD) | 5/21 (24) | 5/18 (28) | NS |
| Hair, eye, and skin | |||
| Hair color brown/black | 1/21 (5) | 9/18 (50) | <.01 |
| Hair color blond/light brown | 20/21 (95) | 9 18 (50) | <.01 |
| Eye color hazel/green-brown | 0/21 (0) | 5/18 (28) | <.05 |
| Eye color blue | 21/21 (100) | 13/18 (72) | <.05 |
| Sun sensitivity | 14/21 (67) | 5/18 (28) | <.05 |
| Fair complexion | 18/19 (95) | 5/18 (28) | <.001 |
| Normal complexion | 1/19 (5) | 13/18 (73) | <.001 |
| Mouth | |||
| Early dental caries | 8/20 (40) | 9/17 (53) | NS |
| Enamel hypoplasia | 7/20 (35) | 6/17 (35) | NS |
| Thick saliva | 15/20 (75) | 11/17 (65) | NS |
| Dental malocclusion | 3/21 (14) | 3/18 (17) | NS |
| Metabolic system | |||
| Thyroid gland abnormality | 1/21 (5) | 1/18 (6) | NS |
| Diabetic glucose tolerance | 5/21 (24) | 1/18 (6) | NS |
| Family history of obesity | 2/19 (10) | 5/18 (28) | NS |
| Family history of diabetes | 11/20 (56) | 10/18 (56) | NS |
| Musculoskeletal system | |||
| Scoliosis | 7/21 (33) | 8/18 (44) | NS |
| Skeletal defects (not scoliosis) | 8/21 (38) | 6/18 (33) | NS |
| Muscle biopsy | 4/21 (19) | 5/18 (28) | NS |
| Inguinal hernia repair | 1/21 (5) | 4/18 (22) | NS |
Triceps skinfold >85th percentile [Garn et al, 1975, 1980].
Menstruation noted in appropriately aged females.
TABLE II.
Frequency of Clinical Findings in Prader-Labhart-Willi Syndrome Individuals (%)
| Clinical features | Overall | [1]a | [2] | [3] | [4] | [5] | [6] | [7] | Present study (N = 39) |
|---|---|---|---|---|---|---|---|---|---|
| Gestation | |||||||||
| Reduction or absence of intrauterine fetal activity | 77 | NGb | 54 | NG | 74 | NG | 84 | NG | 86 |
| Breech delivery | 33 | 25 | 25 | NG | 40 | NG | 38 | NG | 30 |
| Neonatal period and infancy | |||||||||
| Low birth weight (< 2.27 kg) | 36 | 12 | 76c | 36c | 21 | 24 | 20 | NG | 17 |
| Neonatal feeding difficulty | 94 | 100 | 91 | 100 | 100 | 60 | 90 | 100 | 100 |
| Hypotonia | 100 | 100 | 100 | 100 | 100 | NG | 100 | 100 | 100 |
| Retarded psychomotor development | 98 | 100 | NG | 100 | 100 | NG | 90 | 92 | 100 |
| CNS function and behavior | |||||||||
| Mental impairment | 99 | 100 | 94d | 100 | 97 | 100 | 100 | 100 | 100 |
| Convulsions | 19 | NG | 12 | 0 | 16 | 39 | 20 | NG | 24 |
| Hyperphagia | 86 | NG | NG | 100 | NG | NG | 71 | 100 | 85 |
| Growth | |||||||||
| Obesity | 93 | 100 | 100 | 100 | 100 | 65 | 100 | 100 | 87f |
| Short stature (< −1 SD) | 78 | 63 | 100 | 57 | 94 | 50 | 90 | 83 | 71 |
| Facies | |||||||||
| Almond-shaped palpebral fissures | 55 | NG | NG | NG | [9 | NG | NG | 92 | 74 |
| Sexual Development | |||||||||
| Hypogenitalism | 98 | 88 | 100 | 100c | 100 | NG | 100 | 83 | 100 |
| Limbs | |||||||||
| Small hands and feet | 76 | NG | 100 | 86 | 79 | NG | 100 | 67 | 71 |
| Metabolic system | |||||||||
| Reduced glucose tolerance | 34 | NG | NG | 93 | 30 | NG | NG | NG | 15 |
1, Laurance [1967], N = 8; 2, Dunn [1968], N = 17; 3, Zellweger and Schneider [1968], N = 14; 4, Hall and Smith [1972], N = 32; 5, Mattel et al [1983], N = 20; 6, Bray et al [1983], N = 21; 7, Cassidy et al [1984], N = 12.
NG, not given.
3,000 g used as cut-off.
IQ 70 used as cut-off.
Males only.
Triceps skinfold >85th percentile.
There was no history of other relatives with PLWS among family members in this study except for one monozygotic twin member (a monochorionic placenta noted at birth) with PLWS and the other twin with questionable PLWS. The other twin had hypogonadism, seizures, and a similar facial appearance (by history). Unfortunately, this twin died at age 13 years and the diagnosis of PLWS was not confirmed. To date, three sets of monozyotic twins concordant for PLWS have been described [Naselli et al, 1981].
High-Resolution Chromosome Studies
In a clinical and cytogenetic survey of 39 individuals with PLWS (23 males and 16 females), an interstitial deletion of chromosome 15 (breakpoints q11 and q13) was identified in 21 patients (54%) and normal chromosomes in the remaining individuals. A prometaphase chromosome 15 idiogram showing the breakpoints at q11 and q13 and representative prometaphase chromosomes of normals and individuals with PLWS and the interstitial deletion is presented in Figure 3. No other chromosome aberration or mosaicism was identified in the 39 cases.
Fig. 3.

A prometaphase chromosome 15 idiogram (850 band level) showing the breakpoints at q11 and q13 and representative prometaphase chromosomes of a normal control individual and patients with the interstitial deletion. The 15q12 band is indicated by the arrow in each of the normal chromosomes.
Parental studies to establish the source of the chromosome deletion in 13 families utilized variants involving the short arm and satellite region of chromosome 15. Those regions at or near the centromere are useful for linkage analysis because of their position and constitutive heterchromatin composition both of which presumably preclude crossing over. Representative chromosome 15 pairs from two of the families are presented in Figures 4 and 5. In all 13 families the chromosome 15 donated by the father was recognized as the chromosome in which the deletion had occurred in the abnormal child (Table III). Both sets of parents’ chromosomes were normal; thus all chromosome deletions were de novo. The probability that the father would donate the chromosome resulting in the deletion in all 13 cases was less than one in 2,000 [Butler and Palmer, 1983].
Fig. 4.

Family 53166; 12-year-old white male with 46,XY,del(15)(q11q13) karyotype. Parental chromosomes were normal. The patient’s chromosome 15 (D), which carried the deletion, was identified with GTG and QFQ heteromorphisms. Chromosome D has pale satellite intensity with QFQ-banding and small satellite stalk length and normal short arm with GTG-banding. Chromosome D was identified in the father and recognized as the deletion chromosome in the child.
Fig. 5.
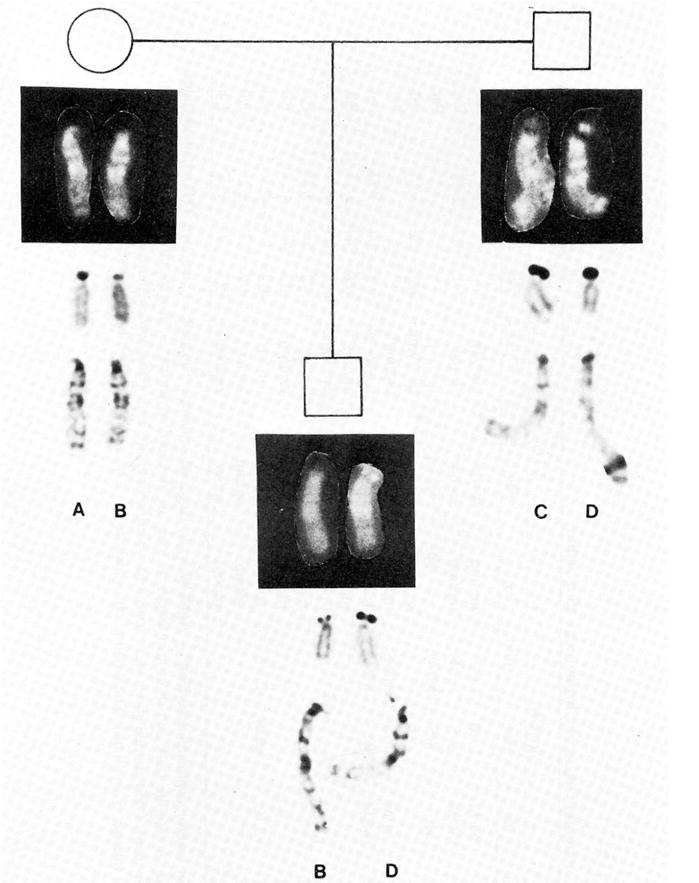
Family 52937; 8.7-year-old white male with 46,XY,del(15)(q11q13) karyotype. Parental chromosomes were normal. The patient’s chromosome 15 (D), which carried the deletion, was identified with GTG, AgNOR, and QFQ heteromorphisms. Chromosome D has intense satellite intensity with QFQ-banding, large silver precipitate with AgNOR staining and long satellite stalk length and normal short arm with GTG-banding. Chromosome D was identified in the father and recognized as the deletion chromosome in the child.
TABLE III.
Parental Origin of Chromosome 15 Deletion
| Patient
|
AgNOR
|
GTG
|
QFQ
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Sex | Pat | Mat | Child | Pat | Mat | Child | Pat | Mat | Child | Origin |
| 54783 | F | M/M | L/M | L/M* | mp/mp | sp/1p | mp*/1p | Pat | |||
| 26676 | F | M/M | L/S | L/M* | mp/mp | 1p/mp | 1p/mp* | 4/1 | 3/2 | 2/1* | Pat |
| 54917 | M | M/− | S/S | M*/S | −p+/sp | −p/−p | sp*/−p | Pat | |||
| 54101 | M | sp−/sp− | mp/mp− | sp−*/mp | 2/2 | 3/2 | 3/2* | Pat | |||
| 23276 | M | M/M | L/S | M*/S | mp/mp | 1p/mp | mp/mp* | Pat | |||
| 54573 | M | M/M | M/− | M*/− | 1p/mp | 1p/−p | mp*/−p | 3/3 | 2/1 | 3*/1 | Pat |
| 53706 | M | mp/mp | 1p/sp | 1p/mp* | 1/1 | 3/1 | 3/1* | Pat | |||
| 54220 | F | 1p/1p | −p+/mp | 1p*/mp | 4/2 | 1/1 | 4*/1 | Pat | |||
| 53166 | M | mp/sp | 1p/1p | 1p/sp* | 3/2 | 5/3 | 5/2* | Pat | |||
| 29583 | M | mp/mp | mp/mp | mp/mp* | 2/2 | 4/4 | 4/2* | Pat | |||
| 52937 | M | L/L | M/S | L*/S | 1p/1p | sp/sp | 1p*/sp | 4/2 | 1/1 | 4*/1 | Pat |
| 55274 | F | sp/mp | sp/mp | mp/sp* | 4/2 | 3/3 | 3/2* | Pat | |||
| 55276 | F | mp/mp | sp/sp | mp*/sp | 3/2 | 2/2 | 3*/2 | Pat | |||
The code for heteromorphisms described by AgNOR is: L, large; M, medium; S, small; —, inactive. GTG-stained slides were scored for satellite stalk and short arm length (I, long; m, medium; s, short; —, absent stalk; p +, long; p, normal; p−, absent p arm). QFQ-stained slides were scored for satellite intensity after Paris nomenclature (1, negative; 2, pale; 3, medium; 4, intense; 5, brilliant). The deletion chromosome in each case is identified by an asterisk.
DISCUSSION
Significant differences have been demonstrated in hair and eye color between patients with and without the deletion in this study. Individuals with the deletion frequently had lighter hair color than other relatives. Patients with the deletion were also judged to have lighter skin complexion and were more sun sensitive than individuals without the deletion. Based on these findings, it is possible that three subgroups of PLWS exist: 1) individuals with chromosome 15 deletion and hair color lighter than relatives, 2) individuals without the deleted 15 and dark hair and normal complexion, and 3) individuals without the deletion and lighter hair and/or complexion than other relatives. The possibility exists that the latter group have a submicro-scopic deletion not detectable with current cytogenetic techniques. These observations are preliminary, and quantitative colorimetric studies of both skin and hair of patients and their relatives as well as biochemical studies of hair should be undertaken.
The difference in hair color found in patients with PLWS and the chromosome deletion might be related to the tyrosinase enzyme involved in pigment development. Recently, a small infant with tyrosinemia and PLWS with a deletion of the proximal region of 15q was described [Fernhoff et al, 1984]. Hittner et al [1982] examined tyrosinase activity in nine individuals with PLWS and the del(l5q) and found a reduced level. They did not examine patients with PLWS and normal chromosomes. If reduced tyrosinase activity is present in patients with the deletion, the pathogenesis of the lighter hair and skin complexion might be identified. It is possible that a gene controlling tyrosinase activity is present on proximal l5q or the segment deleted in one-half of patients with PLWS. DNA hybridization studies using probes for the tyrosinase gene is an approach to consider in comparing those patients with and without chromosome deletion.
All individuals having the deletion in our study had blue eyes compared to 72 % of patients with normal chromosomes. Hittner et al [1982] identified oculoalbinoidism or reduced pigment in the iridies of nine individuals with PLWS and the del(15q) but did not evaluate those cases lacking the deletion.
The average IQ score of 29 patients in this study was 65, similar to IQ scores in published reports [Holm et al, 1981]. The Stanford-Binet test was the most frequently used psychological test. The average IQ for the group without the deletion was significantly lower than the group with the deletion. However, these results should be considered tentative pending study of larger samples and uniform testing procedures.
As previously noted, no differences have been demonstrated in this study between individuals with and without the deletion with respect to behavior, facial appearance, sexual development, and limb, oral, metabolic, and musculoskeletal systems. However, dermatoglyphic and metacarpophalangeal pattern profile differences have been identified previously [Butler et al, 1982b; Butler and Meaney, 1984; Reed and Butler, 1984].
PLWS is variable, and subgroups might be defined [Forssman and Hagberg, 1964; Laurance, 1967; Ledbetter et al, 1980]. Presently, we recognize patients with PLWS with normal chromosomes and those with a chromosome anomaly, most often a deletion of chromosome 15. The group of patients with the chromosome 15 deletion is more homogeneous with regard to dermatoglyphic findings [Reed and Butler, 1984], metacarpophalangeal pattern profile [Butler et al, 1982b; Butler and Meaney, 1984], hair and eye color, and skin complexion [Butler, 1984].
It might be possible to develop a clinical picture of the individual with PLWS and the chromosome 15 deletion. Consistent findings for the patient with the deletion include the classical PLWS manifestations (hypotonia, obesity, hypogonadism, mental impairment) and also light hair and eye color and fair complexion.
It is more difficult to differentiate those individuals clinically with PLWS and normal chromosomes. Clinically they appear to be more variable. Therefore, the original diagnostic criteria established by Prader et al [1956] might fit the clinical picture observed in patients with the deletion moreso than those patients with PLWS and apparently normal chromosomes.
Data from eight surveys of PLWS representing 163 PLWS individuals are shown in Table II. Because of the large number of individuals reported, it is now possible to determine the frequency of clinical manifestations. This information will be useful in the discussions with parents of children with PLWS and other relatives. With additional clinical and cytogenetic research it might be possible to determine the frequency of clinical findings in individuals with the deletion and those lacking the deletion. This additional information could be used more effectively in counseling parents of children with or without the chromosome 15 deletion.
In spite of the large number of cases of PLWS reported, the cause is not entirely understood. Recently, an interstitial deletion of chromosome 15 has been reported in approximately 50% of cases [Butler et al, 1982b; Ledbetter et al, 1982; Mattei et al, 1983; Butler, 1984]. In our study, 54 percent of the 39 individuals with the syndrome were identified as having an interstitial deletion of chromosome 15. Thus, in one-half of the individuals studied the cause seems to be related to a deletion of proximal l5q.
Initially, translocation l5q; l5q resulting in deletion of the short arms of chromosome 15 was thought to be causally related to the phenotype of PLWS [Ledbetter et al, 1981]. This hypothesis was challenged in subsequent reports of individuals with normal phenotypes and identical translocations or translocations involving one chromosome 15 and another autosome in patients with PLWS. These patients might have incurred deletions of proximal l5q in the translocation process. High-resolution analysis should be applied in all cases with the translocations specifically considering the presence or absence of 15q11 to 15q13.
Alternatively, rearrangements of proximal l5q might produce changes in gene expression as a result of position effect or submicroscopic deletions. Little evidence for either exists in patients with congenital abnormalities other than a few cases of de novo balanced translocations or inversions reported to have abnormal phenotypes [Funderburk et al, 1977; Tharapel et al, 1977; Ayme et al, 1979].
In the present study, normal chromosomes were identified in 46% of cases of PLWS studied with high-resolution methods. These findings affirm the heterogeneity of PLWS. Chromosomally abnormal cases might constitute one subtype. Studies are currently in progress with DNA probes of proximal l5q to establish whether or not a small part of this region is absent. Skin cultures of patients with normal chromosomes might resolve the question of mosaicism and help in identifying additional PLWS subtypes.
An autosomal recessive mode of inheritance has been suggested for PLWS and might account for some individuals with normal chromosomes [Hall and Smith, 1972; Mattei et al, 1983]. A recessive gene, if located on band l5q11, could express itself in the hemizygous state when the homologous gene is missing, as in deletions. A recessive gene could also express itself in the trisomic state as in cases of supernumerary chromosomes that have been reported [Kousseff, 1982; Ledbetter et al, 1982].
No previous studies of the parental origin of a chromosome deletion syndrome have been reported, although Chamberlin and Magenis [1980] suggested paternal involvement in various de novo chromosome rearrangements. The evidence presented in this study combined with the various de novo chromosome rearrangements reported by Chamberlin and Magenis [1980] indicates that paternal origin of de novo chromosome rearrangements is a general phenomenon.
The frequent paternal origin of deletion in PLWS can be explained by differences in male and female meiosis. DNA proliferation of spermatogonial cells is continuous in male gametogenesis. In females, meiosis is arrested at the dicytotene stage of meiosis I during fetal life, and the cells do not undergo the continued replication of DNA as in the male. Thus male gametogenesis might be more vulnerable to mutations and to environmental insult occurring through an effect on DNA replication than female gametogenesis. If chromosome 15 is sensitive to environmental effects, there might be a greater chance for chromosomal deletions to occur during the continuous DNA replication in male meiosis compared to a limited amount in female meiosis. Therefore, epidemiological investigations specifically of the male parent of patients with de novo deletions are suggested.
Acknowledgments
The authors acknowledge the use of the facilities of Computing Services, Indiana University-Purdue University at Indianapolis. The authors wish to thank Drs. Joe Christian, Andree Walczak, Rebecca Wappner, and David Weaver for their advice and assistance. This study was supported in part by PHS-5T32 GMO7468 to (M.G.B.).
References
- Ayme S, Mattei MG, Matti JR, Giraud F. Abnormal childhood phenotypes associated with the same balanced chromosome rearrangements as in the parents. Hum Genet. 1979;48:7–12. doi: 10.1007/BF00273267. [DOI] [PubMed] [Google Scholar]
- Bergsma D. Birth Defects Compendium. New York: Alan R. Liss, Inc; 1979. [Google Scholar]
- Bonucelli CM, Stetten G, Levitt RC, Levin LS, Pyeritz RE. Prader-Willi syndrome associated with an interstitial deletion of chromosome 15. Johns Hopkins Med J. 1982;15:237–242. [PubMed] [Google Scholar]
- Bray GA, Dahms WT, Swerdloff RS, Fiser RH, Atkinson RL, Carrel RE. The Prader-Willi syndrome: A study of 40 patients and a review of the literature. Medicine. 1983;62:59–80. [PubMed] [Google Scholar]
- Bühler EH, Rossier R, Bodis I, Vulliet V, Bühler UK, Stalder G. Chromosomal translocation in a mentally deficient child with cryptorchidism. Acta Paediatr Helv. 1963;52:177–182. doi: 10.1111/j.1651-2227.1963.tb03762.x. [DOI] [PubMed] [Google Scholar]
- Butler MG. PhD Thesis. Indiana University; 1984. Clinical and cytogenetic survey of the Prader-Willi syndrome. [Google Scholar]
- Butler MG, Kaler SG, Yu PL, Meaney FJ. Metacarpophalangeal pattern profile analysis in Prader-Willi syndrome. Clin Genet. 1982b;22:315–320. doi: 10.1111/j.1399-0004.1982.tb01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Meaney FJ. Metacarpophalangeal pattern profile analysis in Prader-Willi syndrome: A follow-up report on 38 cases. Clin Genet. 1985;28:27–30. doi: 10.1111/j.1399-0004.1985.tb01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Palmer CG. Parental origin of chromosome 15 deletions in Prader-Willi syndrome. Lancet. 1983;1:1285–1286. doi: 10.1016/s0140-6736(83)92745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MG, Weaver DD, Meaney FJ. Prader-Willi syndrome: Are there population differences? Clin Genet. 1982a;22:292–294. doi: 10.1111/j.1399-0004.1982.tb01449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy SB, Thuline HC, Holm VJ. Deletion of chromosome 15(q11–13) in a Prader-Labhart-Willi syndrome clinic population. Am J Med Genet. 1984;17:485–495. doi: 10.1002/ajmg.1320170211. [DOI] [PubMed] [Google Scholar]
- Chamberlin J, Magenis RE. Parental origin of de novo chromosome rearrangements. Hum Genet. 1980;53:343–347. doi: 10.1007/BF00287054. [DOI] [PubMed] [Google Scholar]
- Charrow J, Balkin N, Cohen MM. Translocations in Prader-Willi syndrome. Clin Genet. 1983;23:304–307. doi: 10.1111/j.1399-0004.1983.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Dunn HG. The Prader-Labhart-Willi syndrome: Review of the literature and the report of nine cases. Acta Pediatr Scand [Suppl] 1968;186:1–38. doi: 10.1111/j.1651-2227.1968.tb06038.x. [DOI] [PubMed] [Google Scholar]
- Dunn HG, Ford DK, Auersperg N, Miller JR. Benign congenital hypotonia with chromosomal anomaly. Pediatrics. 1961;28:578–591. [PubMed] [Google Scholar]
- Fernhoff PM, Brown A, Blake E, Elsas LJ. Newborn metabolic screening in Georgia: Five years experience. Paper presented at National Newborn Screening Symposium; Orlando, Florida. February 7–9 1984.1984. [Google Scholar]
- Forssman H, Hagberg B. Prader-Willi syndrome in a boy of ten with prediabetes. Acta Paediatr Scand. 1964;53:70–78. doi: 10.1111/j.1651-2227.1964.tb07208.x. [DOI] [PubMed] [Google Scholar]
- Francke U, Holmes LB, Atkins L, Riccardi VM. Aniridia-Wilms’ tumor association: Evidence for specific deletion of 11p13. Cytogenet Cell Genet. 1979;24:185–192. doi: 10.1159/000131375. [DOI] [PubMed] [Google Scholar]
- Funderburk SJ, Spence MA, Sparkes RS. Mental retardation associated with “balanced” chromosome rearrangements. Am J Hum Genet. 1977;29:136–141. [PMC free article] [PubMed] [Google Scholar]
- Garn SM, Bailey SM, Cole PE. Continuities and changes in fatness and obesity. In: Schemmel R, editor. Nutrition, Physiology and Obesity. Boca Raton, Florida: CRC Press; 1980. [Google Scholar]
- Garn SM, Clark DC, Guire KE. Growth, body composition and development of obese and lean children. Curr Concepts Nutr. 1975;3:23–46. [PubMed] [Google Scholar]
- Golden WL, Hanchett JM, Breslin N, Steele MW. Prader-Willi syndrome in black females. Clin Genet. 1984;26:161–163. doi: 10.1111/j.1399-0004.1984.tb00807.x. [DOI] [PubMed] [Google Scholar]
- Hall BD, Smith DW. Prader-Willi syndrome. J Pediatr. 1972;81:286–293. doi: 10.1016/s0022-3476(72)80297-x. [DOI] [PubMed] [Google Scholar]
- Hamilton CR, Scully RE, Kliman B. Hypogonadotropism in Prader-Willi syndrome. Am J Med. 1972;52:322–329. doi: 10.1016/0002-9343(72)90019-8. [DOI] [PubMed] [Google Scholar]
- Hawkey CJ, Smithies A. The Prader-Willi syndrome with a 15/15 translocation: Case report and review of the literature. J Med Genet. 1976;13:152–156. doi: 10.1136/jmg.13.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hittner HM, King RA, Riccardi VM, Ledbetter DH, Borda RP, Ferrell RE, Kretzer FL. Oculocutaneous albinoidism as a manifestation of reduced neural crest derivatives in the Prader-Willi syndrome. Am J Ophthalmol. 1982;94:328–337. doi: 10.1016/0002-9394(82)90358-0. [DOI] [PubMed] [Google Scholar]
- Holm VA, Sulzbacher S, Pipes PL. The Prader-Willi Syndrome. Baltimore: University Park Press; 1981. [Google Scholar]
- Kousseff BG. The cytogenetic controversy in the Prader-Labhart-Willi syndrome. Am J Med Genet. 1982;13:431–439. doi: 10.1002/ajmg.1320130412. [DOI] [PubMed] [Google Scholar]
- Laurance BM. Hypotonia, mental retardation, obesity and crytorchidism associated with dwarfism and diabetes in children. Arch Dis Child. 1967;42:126–139. doi: 10.1136/adc.42.222.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter DH, Mascarello JT, Riccardi VM, Harper VD, Airhart SD, Strobel RJ. Chromosome 15 abnormalities and the Prader-Willi syndrome: A follow-up report of 40 cases. Am J Hum Genet. 1982;34:278–285. [PMC free article] [PubMed] [Google Scholar]
- Ledbetter DH, Riccardi VM, Airhart SD, Strobel RJ, Keenan SB, Crawford JD. Deletions of chromosome 15 as a cause of the Prader-Willi syndrome. N Engl J Med. 1981;304:325–329. doi: 10.1056/NEJM198102053040604. [DOI] [PubMed] [Google Scholar]
- Ledbetter DH, Riccardi VM, Youngbloom SA, Strobel RJ, Keenan BS, Crawford JD, Louro JM. Deletion (l5q) as a cause of the Prader-Willi syndrome (PWS) Am J Hum Genet. 1980;32:77A. [Google Scholar]
- Mattei JF, Mattei MG, Giraud F. Prader-Willi syndrome and chromosome 15: A clinical discussion of 20 cases. Hum Genet. 1983;64:356–362. doi: 10.1007/BF00292367. [DOI] [PubMed] [Google Scholar]
- Naselli A, Vignolo M, DiBattista E, Aicardi B. The Prader-Labhart-Willi syndrome: Third recorded case occurring in monozygotic twins. Acta Med Auxol. 1981;13:5–24. [Google Scholar]
- Paris Conference Supplement. Standardization in human cytogenetics. New York: Alan R Liss, Inc for The National Foundation—March of Dimes; 1975. pp. 1–36. BD:OAS XI(9) [Google Scholar]
- Pearson KD, Steinbach HL, Bier DM. Roentgenographic manifestations of the Prader-Willi syndrome. Radiology. 1971;100:369. doi: 10.1148/100.2.369. [DOI] [PubMed] [Google Scholar]
- Prader A, Labhart A, Willi H. Ein syndrom von adipositas, klienwuchs, Kryptorchismus und Oligophrenie nach myatonieartigen Zustand im Neugeborenenalter. Schweiz Med Wochenschr. 1956;86:1260–1261. [Google Scholar]
- Reed T, Butler MG. Dermatoglyphic features in Prader-Willi syndrome with respect to chromosomal findings. Clin Genet. 1984;25:341–346. doi: 10.1111/j.1399-0004.1984.tb02001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi VM, Hittner HM, Francke U, Yunis JJ, Ledbetter D, Borges W. The Aniridia-Wilms’ tumor association: The critical role of chromosome band 11p13. Cancer Genet Cytogenet. 1980;2:131–137. [Google Scholar]
- Rybak J, Tharapel A, Robinett S, Garcia M, Mankinen C, Freeman M. A simple reproducible method for prometaphase chromosome analysis. Hum Genet. 1982;60:328–333. doi: 10.1007/BF00569213. [DOI] [PubMed] [Google Scholar]
- Smith DW. Recognizable Patterns of Human Malformations. Philadelphia: W.B. Saunders Co; 1976. [Google Scholar]
- Tharapel AT, Summitt RL, Wilroy RS, Martens P. Apparently balanced de novo translocations in patients with abnormal phenotypes: Report of 6 cases. Clin Genet. 1977;11:255–269. doi: 10.1111/j.1399-0004.1977.tb01310.x. [DOI] [PubMed] [Google Scholar]
- Winsor EJT, Welch JP. Prader-Willi syndrome associated with inversion of chromosome 15. Clin Genet. 1983;24:456–461. doi: 10.1111/j.1399-0004.1983.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Wyandt HE, Patil S, Shah HO, Hanson JW, Zellweger H, Kelly TE, Dolan LM, Wilson WG. Problems in the detection of 15q deletion in patients with Prader-Willi syndrome. Am J Hum Genet. 1981;33:127A. [Google Scholar]
- Yu RL, Aronson MM, Nichols WW. High resolution bands in human fibroblast chromosomes induced by Actinomycin D. Cytogenet Cell Genet. 1981;31:111–114. doi: 10.1159/000131634. [DOI] [PubMed] [Google Scholar]
- Yunis JJ. High resolution of human chromosomes. Science. 1976;191:1268–1270. doi: 10.1126/science.1257746. [DOI] [PubMed] [Google Scholar]
- Zellweger H. The HHHO or Prader-Willi syndrome. New York: Alan R Liss, Inc for The National Foundation—March of Dimes; 1969. pp. 15–17. BD:OAS V(2) [Google Scholar]
- Zellweger H, Schneider HJ. Syndrome of hypotonia-hypomentia-hypogonadism-obesity (HHHO) or Prader-Willi syndrome. Am J Dis Child. 1968;115:588–598. doi: 10.1001/archpedi.1968.02100010590009. [DOI] [PubMed] [Google Scholar]
- Zellweger H, Soper RT. The Prader-Willi syndrome. Med Hygiene. 1979;37:3338–3345. [Google Scholar]


