Abstract
Protein tyrosine phosphatases (PTPs) are a family of enzymes essential for numerous cellular processes, and several PTPs have been validated as therapeutic targets for human diseases. Historically, development of drugs targeting PTPs has been highly challenging, leading to stigmatization of these enzymes as undruggable targets. Despite these difficulties, efforts to drug PTPs have persisted, and recent years have seen an influx of new probes, providing opportunities for biological examination of old and new PTP targets. Here we will discuss progress towards drugging PTPs, with special emphasis on development of selective probes with biological activity. We will describe development of new small-molecule orthosteric, allosteric and oligomerization PTP inhibitors, and discuss new studies targeting the receptor PTP subfamily with biologics.
Keywords: Protein tyrosine phosphatase, drug target, inhibitor, small-molecule, allosteric, biologic
Protein tyrosine phosphatases
Tyrosine phosphorylation of intracellular proteins is a post-translational modification used to control cell signaling in nearly every biological context[1]. Tyrosine phosphorylation is controlled by the opposing actions of protein tyrosine kinases (PTKs, see Glossary), which catalyze phosphorylation of proteins on tyrosine residues, and protein tyrosine phosphatases (PTPs), which remove the phosphate[1]. This dynamic regulates a range of cellular processes including survival, growth, migration, differentiation and energy metabolism; consequently, anomalous tyrosine phosphorylation is implicated in numerous human diseases[2,3]. Agents targeting PTKs and PTPs have been heavily pursued for therapeutic interventions, and although several drugs targeting PTKs are in clinical use[3], PTP-targeted drugs are not yet available.
More than 100 PTPs are encoded in the human genome, and are organized into three major classes (Box 1)[2,4,5]. As shown in Table 1, all three classes are represented among PTPs under consideration as drug targets[6].
Box 1. The classification of protein tyrosine phosphatases.
The protein tyrosine phosphatases (PTPs) are characterized by a conserved amino acid sequence (V/H)CX5R, called the “PTP signature motif”. This motif contains a catalytic Cys residue, which acts as a nucleophile during catalysis, and an Arg residue, which assists in substrate binding[2,4,5].
Class I is the largest and includes the “classical” phosphotyrosine-specific PTPs and the classical and atypical dual-specific PTPs (DUSPs).
Class II consists of only the low molecular weight PTP (LMPTP or LMW-PTP).
Class III contains the 3 isoforms of cell division cycle 25 (CDC25) PTPs.
Table 1.
PTPs discussed in this review and the indications for which they are being explored.
| Refs. | |
|---|---|
| Class I Receptor PTPs | |
| VE-PTP. Encoded by the PTPRB gene, VE-PTP is a transmembrane PTP expressed in endothelial cells. VE-PTP dephosphorylates and inhibits the activation of Tie2, a receptor PTK that suppresses vascular leakage. VE-PTP is being targeted as a therapeutic for retinal and choroidal vascular disease, particularly diabetic macular edema. | [19,20,71] |
| CD45. Encoded by the PTPRC gene, CD45 is a transmembrane PTP expressed on the surface of nearly all hematopoietic cells. CD45 is the target of radioimmunotherapy strategies to deliver radiation to immune cells and tissues in patients with leukemias, lymphomas, or myelodysplasias. Since mutations in PTPRC associate with autoimmune diseases and CD45 is critical for signaling in immune cells by dephosphorylation of SFKs, this enzyme has also been explored as a target for immunosuppression. CD45 has also been proposed as a target for Ebola and anthax infections. | [44,59,72,73] |
| RPTPσ. Encoded by the PTPRS gene, RPTPσ is a transmembrane PTP expressed in the nervous system and stromal cells that acts as a receptor for extracellular matrix proteoglycans through its N-terminal immunoglobulin-like domains. RPTPσ dephosphorylates the cytoskeletal-associated protein ezrin. RPTPσ is being considered a target for axon regrowth/regeneration following spinal cord injury or spinal root avulsion injury, for reversing cardiac sympathetic denervation caused by myocardial infarction, and for non-immunosuppressive therapy for rheumatoid arthritis. | [65–67,70] |
| Class I Non-Receptor PTPs | |
| PTP1B. Encoded by the PTPN1 gene, PTP1B was the first PTP identified and the first validated PTP therapeutic target. PTP1B is ubiquitously expressed and contains an N-terminal PTP domain and a C-terminal regulatory region. PTP1B acts as an inhibitor of insulin and leptin signaling. PTP1B has been sought as a drug target for type 2 diabetes, obesity and cancer and was recently proposed as a target for Rett syndrome and stress-induced anxiety. | [6,9,74] |
| STEP. Encoded by the PTPN5 gene, STEP is expressed as 2 major isoforms (STEP46 and STEP61) in the brain. STEP contains KIM region N-terminal to the PTP domain that allows STEP to interact with its MAPK substrates ERK and p38. STEP acts as an inhibitor of synaptic strengthening. High STEP expression was observed in the prefrontal cortex in human postmortem Alzheimer’s disease patients and mouse models. STEP is being considered as a target for neurological disorders such as Alzheimer’s disease and schizophrenia. | [33,75,76] |
| SHP-2. Encoded by the PTPN11 gene, SHP-2 is ubiquitously expressed. SHP-2 contains 2 SH2 domains N-terminal to the catalytic domain, and undergoes an intramolecular autoregulation mechanism in which the SH2 domains bind to the catalytic domain and block its activity. PTPN11 is a proto-oncogene; gain-of-function mutations in SHP-2 can cause Noonan Syndrome, Leopard syndrome and cancers. SHP-2 has long been considered a drug target for cancer, and recently is being explored as a target for rheumatoid arthritis. | [25,77] |
| PTPN22. Encoded by the PTPN22 gene, PTPN22 is expressed in hematopoietic cells. PTPN22 contains an N-terminal PTP domain, an interdomain region, and a C-terminal domain with 4 proline-rich motifs. PTPN22 acts as a negative regulator of early mediators of TCR signaling. A single nucleotide polymorphism (C1858T) in PTPN22 is associated with autoimmunity, thus PTPN22 is being considered as a target for autoimmune diseases such as rheumatoid arthritis and type 1 diabetes. | [12] |
| Class I Dual-Specific PTPs | |
| DUSP6. Encoded by the DUSP6 gene, DUSP6 is a widely expressed classical DSP that dephosphorylates and inhibits the MAPK ERK. DUSP6 is activated by ERK substrate binding, which induces a conformational change that positions Asp262 to serve as an acid during catalysis. DUSP6 has been suggested as a potential target for elimination of pre-B acute lymphoblastic leukemia cells. | [41,43] |
| PRL-1/2/3. Encoded by the PTP4A1/2/3 genes, PRL enzymes are prenylated DSPs. PRL-1 and PRL-2 are nearly ubiquitous, while PRL-3 expression is restricted to the heart, skeletal muscle, vasculature and brain. PRLs contain a PTP domain and a C-terminal prenylation motif that recruits them to the plasma membrane. PRL-1 homotrimerizes in the crystalline state; trimerization is essential for its growth and migration-promoting functions in human epithelial kidney 293 cells. PRL enzymes are being explored as therapeutic targets for cancers, including melanoma and leukemias. | [56,57] |
| Class II PTPs | |
| LMPTP. Encoded by the ACP1 gene, LMPTP is ubiquitously expressed as 2 isoforms, LMPTP-A and LMPTP-B. LMPTP inhibits insulin signaling by IR dephosphorylation. LMPTP is being considered as a target for type 2 diabetes and heart failure. | [16,78] |
| Class III PTPs | |
| CDC25A/B/C. Encoded by the CDC25A/B/C genes, CDC25 enzymes are expressed in most tissues and dephosphorylate pTyr and pThr residues. CDC25 enzymes regulate cell cycle progression by dephosphorylation and activation of cyclin-dependent kinases within their ATP-binding loops. CDC25A and B are overexpressed in a number of human cancers and are sometimes associated with poor prognosis. Inhibition of all 3 CDC25 isoforms is considered a therapeutic strategy for cancer. | [35] |
| Bacterial PTPs | |
| mPTPA. mPTPA from Mycobacterium tuberculosis is a low-molecular weight PTP virulence factor secreted by Mtb into host macrophages, and thus is considered a potential drug target for TB infection. | [79] |
KIM, kinase interaction motif; MAPK, mitogen-activated protein kinase; Refs., references; SFK, SRC family kinase
During the last 15 years, extensive data validating protein tyrosine phosphatase 1B (PTP1B) – an inhibitor of insulin signaling and SH2 domain-containing PTP 2 (SHP-2) –an oncogene and promoter of growth factor signaling- as therapeutic targets for type 2 diabetes/obesity and cancer sparked considerable excitement –and use of resources- in drugging these enzymes[2,6]. Major programs in industry and academic laboratories were dedicated to development of small-molecule PTP inhibitors. These programs largely focused on orthosteric inhibitors; however, efforts were frustrated by the highly charged and highly conserved nature of the PTP active-site. While the charged active-site allows for high affinity accommodation of negatively charged pTyr residues, potent orthosteric PTP inhibitors tend also to be highly charged, which can limit their cell-permeability, bioavailability, and potential for drug development. The high level of active-site conservation among PTPs adds another layer of difficulty, as potent inhibitors often target multiple PTPs. Ultimately, the generation of potent, selective, bioavailable PTP inhibitors suitable for therapeutic use was largely unsuccessful, and PTPs acquired a reputation as “challenging”, “intractable” and “undruggable” targets[2,6].
Despite these setbacks, efforts to drug PTPs continued, and recent years experienced a resurgent global interest in these enzymes. In addition to noteworthy progress in competitive, orthosteric PTP inhibitor development, an influx of new strategies to attack these enzymes has occurred. Moreover, an increasing number of PTPs are being proposed as clinically relevant targets. Here we will describe recent progress towards drugging PTPs, calling particular attention to approaches –orthosteric, allosteric and oligomerization-inhibiting small-molecule, and biologic-(Figure 1, Key Figure) yielding selective agents with biological activity.
Figure 1. Key Figure. Recent approaches for developing PTP-targeting drugs.
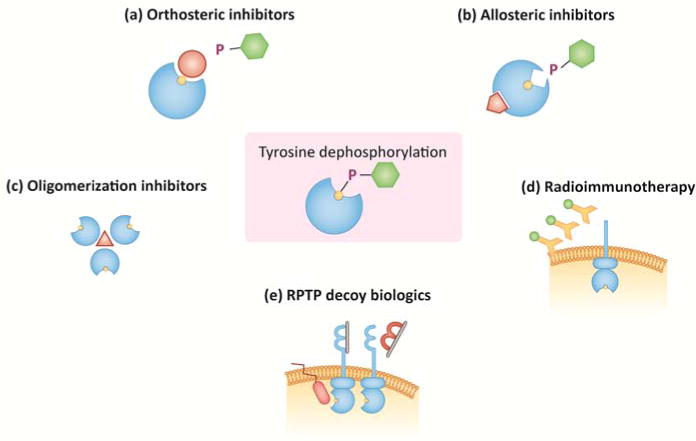
Tyrosine phosphorylation occurs when PTPs hydrolytically remove phosphate (P) from Tyr amino acids (depicted as turquoise hexagon). The reaction involves transient covalent interaction with the PTP active-site nucleophile (Cys in Class I, II and III PTPs; Asp in aspartate-based PTPs; depicted in yellow) (a–c) Approaches in small-molecule PTP inhibitor development. (a) Orthosteric inhibitors bind to the enzyme active-site, and typically compete with substrate for binding. (b) Allosteric inhibitors bind outside of the enzyme active-site, inducing or stabilizing a catalytically unfavorable enzyme conformation. (c) Oligomerization inhibitors are being used to disrupt trimerization of PRL proteins. (d–e) Approaches in RPTP-targeted biologics development. (d) The RPTP CD45 has been the object of radioimmunotherapy strategies, which involve conjugation of an antibody to a radioactive agent for specific delivery of radiation to hematopoietic cells and tissues. (e) RPTPσ is being targeted with decoy biologics that mimic regions of the protein. A cell-penetrating wedge peptide mimetic targets the RPTPσ intracellular region. A decoy protein of the extracellular RPTPσ Ig1&2 domains targets RPTPσ by disrupting interactions with extracellular matrix proteoglycans (depicted as gray bar).
Trends in small-molecule PTP inhibitor development
Orthosteric small-molecule inhibitors
While an orthosteric, or active-site, small-molecule approach (Figure 1a) must face the difficulties of the PTP active-site head-on, this area of inhibitor development has seen tremendous persistence. Remarkably, the traditional approach of reversible competitive inhibition is still being sought and yielding some excellent probes. Additionally, alternative methods, such as uncompetitive inhibition and irreversible inhibition are also being explored. As a result of these efforts, high-quality orthosteric inhibitors are emerging (Table 2), providing new opportunities for biological examination of old and new targets.
Table 2.
Features of recent orthosteric small-molecule PTP inhibitors
| Chemical structure | Special features | Potency & selectivity | Discovery | MOA | Refs. | |
|---|---|---|---|---|---|---|
| CPT-157633: PTP1B inhibitor |
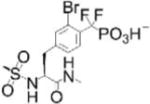
|
Potent; Improved symptoms in Rett syndrome mouse model (5 mg/kg i.p. or s.c. daily) | PTP1B Ki=40 nM; ~2-fold selective vs TCPTP; greater vs SHP-2, LAR, RPTPα & RPTPμ; no inhibition of PEZ, JSP1 or PTEN | Developed by Ceptyr, Inc. | Reversible, competitive | [9] |
| LTV-1: PTPN22 inhibitor |
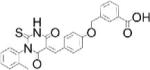
|
Reduced frequency of autoreactive B cells in a mouse model of central B cell tolerance (0.15 or 0.75 mg twice daily i.p. for 1 week) | PTPN22 IC50=0.51 μM; 3-fold vs TCPTP & PTP1B; 46-fold vs SHP-1; 59-fold vs CD45; >200-fold vs PTP-PEST | 50,000-compound screen using KM [OMFP substrate]; counterscreen against HePTP and VHR | Reversible, competitive | [13] |
| Compound 28: LMPTP inhibitor |
|
Highly selective, induced fit mechanism, increased insulin signaling in human HepG2 hepatocytes at [low-μM] | LMPTP IC50=2.1 μM; >50-fold selectivity vs panel of 24 PTPs | Screen of SPAA pharmacophore-based library, followed by structure-guided medicinal chemistry | Reversible, competitive, generates change in conformation of active-site signature motif | [15] |
| L335-M34: mPTPA inhibitor |
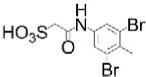
|
Orally bioavailable; reduced TB infection in guinea pig model (50 mg/kg daily for 2 weeks orally) in combination with HRZ | mPTPA IC50=160 nM; >20-fold selectivity vs panel of 18 PTPs, including mPTPB and human LMPTP | Fragment-based optimization of SPAA pharmacophore | Reversible, competitive | [17] |
| AKB-9778: VE-PTP inhibitor |
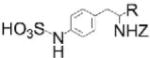
|
Remarkable potency; currently in clinical trials for diabetic macular edema | VE-PTP IC50=17 pM; DEP-1 IC50=36 pM; RPTPγ IC50 =100 pM; >45-fold selectivity vs PTP1B, greater selectivity vs other PTPs | Screen of Proctor and Gamble Pharmaceutical’s corporate repository, followed by structure-guided medicinal chemistry | Reversible, competitive | [19,21,22] |
| 11a-1: SHP-2 inhibitor |
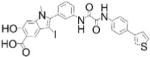
|
Daily i.p. administration reduced disease in mouse melanoma (15 mg/kg) and rheumatoid arthritis (7.5 mg/kg) models | SHP-2 IC50 =200 nM; >5-fold selectivity over panel of 21 PTPs | Structure-guided combinatorial library based off previous SHP-2 inhibitor II-B08 | Reversible, non- competitive; binds SHP-2 active-site and groove formed by β5–β6 E-loop | [25,27,28] |
| Compd. 23: LMPTP inhibitor |
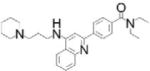
|
Highly selective, uncompetitive mechanism, orally bioavailable; reversed high-fat diet induced diabetes in mice (0.05% food admixture) | LMPTP IC50 =800 nM; no inhibition of panel of 15 PTPs | 364,168-compound screen from NIH MLPCN using high [OMFP substrate], followed by medicinal chemistry | Reversible, uncompetitive | [30] |
| TC-2153:STEP inhibitor |
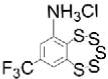
|
Potent; improved cognition and motor function in mouse models of AD and schizophrenia (10 mg/kg) | STEP61 IC50=93 nM; STEP46 IC50=57 nM; HePTP IC50=364 nM; PTP- SL IC50=221 nM; greater selectivity vs other PTPs | Elemental sulfur contamination in library used for STEP inhibitor screen; TC-2153 chosen due to similar chemical structure | Irreversible; likely covalent interaction with STEP Cys472 | [31,80] |
| IRC-083864: CDC25 inhibitor |
|
Potent, orally bioavailable, reduced prostate (70 mg/kg p.o. cycles) and pancreatic (10 mg/kg i.v. qwk × 4) carcinoma tumor growth | CDC25 IC50~20–50 nM; selective vs alkaline phosphatase and the CD45, LAR, PTP1B, PTP-PEST, VHR and VHX | Developed based on irreversible quinone CDC25 inhibitor BN82685 | Irreversible | [32,35] |
JSP1, c-JUN N-terminal kinase (JNK) stimulatory phosphatase 1; PEZ, phosphatase with ezrin domain; PTEN, phosphatase and tensin homolog; VHX, Vaccinia virus-VH1-related MKP X
Reversible competitive inhibitors
Reversible inhibitors bind to enzymes, typically through noncovalent interactions, with rapid association and dissociation rates[7]. Competitive inhibitors bind to an enzyme at the site of substrate binding, hence competing with substrate for binding to the enzyme. In competitive inhibition, binding of either the substrate or inhibitor to the enzyme is mutually exclusive[8]. Here we will discuss the selective, reversible competitive inhibitors that are being used to validate several PTPs as drug targets.
CPT-157633, a difluoromethylphosphonic acid PTP1B inhibitor, was used to explore Rett syndrome (RTT) as a new indication for PTP1B [9]. RTT is an X-linked neurodevelopmental disorder often caused by mutations in the transcriptional regulator methyl CpG-binding protein 2 (MECP2)[10]. This study showed that PTP1B expression was suppressed by MECP2, and that PTP1B was upregulated in RTT patients and in the heterozygous Mecp2-null RTT mouse model. CPT-157633 is a potent active-site inhibitor with selectivity over several PTPs, albeit moderate for the structurally similar T cell protein tyrosine phosphatase (TCPTP). Daily administration of 5 mg/kg CPT-157633 intraperitoneally (i.p.) or subcutaneously (s.c.) improved behavior and motor skills in Mecp2−/+ female mice and increased survival in Mecp2−/y male mice. PTP1B was previously proposed to inhibit tyrosine phosphorylation of the tropomyosin receptor kinase B (TRKB) -the receptor for brain derived neurotrophic factor-, which is downregulated during RTT[11]. CPT-157633 administration increased brain TRKB Tyr phosphorylation in wild-type (WT) and Mecp2−/+ female mice, and a substrate-trapping PTP1B mutant precipitated TRKB from mouse brain lysates, suggesting TRKB is a PTP1B substrate in the brain and that augmenting this pathway through PTP1B inhibition could be an RTT therapeutic strategy[9].
Multiple lines of evidence suggest inhibition of PTPN22 –encoded by a major autoimmunity gene- as a strategy for eliminating autoreactive lymphocytes in carriers of an autoimmune-predisposing PTPN22 variant (C1858T)[12]. PTPN22 inhibitor LTV-1 was identified by screening with small-molecule substrate 3-O-methylfluorescein phosphate (OMFP)[13]. Molecular docking and structure-activity relationship (SAR) studies suggested that LTV-1 interacts with the active-site phosphate-binding loop and a nearby hydrophobic pocket when PTPN22 is in an open conformation. LTV-1 shows moderate selectivity for PTPN22 over PTP1B and TCPTP; however, it is highly selective over other PTPs, including the closely-related PTP-PEST. Since PTPN22 is an inhibitor of T cell receptor (TCR) signaling, LTV-1 activity in cells was confirmed in PTPN22-dependent TCR-signaling assays in the Jurkat-TAg T cell line and in primary human T cells. LTV-1 was used in NOD-scid-common γ chain knockout (NSG) mice engrafted with human hematopoietic stem cells (HSCs) to examine the role of PTPN22 in central B cell tolerance[14]. Engraftment of HSCs from PTPN22-C1858T carriers led to increased development of autoreactive B cells compared to engraftment of cells from non-carriers; this phenotype was significantly reduced by treatment of engrafted mice with LTV-1. A similar reduction in autoreactive B cell frequency was obtained when HSCs from PTPN22-C1858T carriers subjected to PTPN22 knockdown were engrafted. These findings suggest potential for PTPN22 inhibition in resetting impaired B cell tolerance in autoimmune patients carrying the PTPN22-C1858T allele.
The α-sulfophenylacetic amide (SPAA) pharmacophore from Cefsulodin, a β-lactam antibiotic that inhibits several PTPs, inspired generation of selective competitive inhibitors of low-molecular weight PTP (LMPTP or LMW-PTP)[15], an inhibitor of insulin receptor (IR) phosphorylation[16]. Compound 28 was identified from a library generated by reacting α-sulfophenylacetic acid with varying amines, followed by structure-guided medicinal chemistry[15]. X-ray co-crystallization revealed an “induced-fit mechanism”, in which Compound 28 induces an active-site conformational change, generating a hydrophobic cavity that accommodates the inhibitor α-phenyl ring. The extremely high selectivity for LMPTP over other PTPs is likely explained by differences in the active-site signature motif residues between LMPTP and Class I PTPs, which contribute to the active-site electrostatic charge and shape. Additionally, low-μM concentrations of Compound 28 increased insulin-induced protein kinase B (PKB/AKT) phosphorylation in human HepG2 hepatocytes[15].
Fragment-based optimization of SPAA also led to discovery of L335-M34, a selective inhibitor of Mycobacterium tuberculosis (Mtb) LMPTP (mPTPA), an Mtb virulence factor[17]. L335-M34 does not inhibit human LMPTP, and decreased bacterial load in Mtb-infected macrophages (IC90=1.38 μM), without displaying in vitro anti-Mtb activity[17]. Importantly, L335-M34 displayed oral bioavailability when administered to guinea pigs without causing weight loss or overt toxicity. When administered to guinea pigs orally together with the anti-tuberculosis combination treatment isoniazid, rifampin, pyrazinamide (HRZ) 4 weeks after Mtb infection, lung inflammation was substantially lower compared to guinea pigs treated with HRZ alone after 6 weeks. Additionally, treatment with HRZ+L335-M34 combined with the non-competitive inhibitor of mPTPB –also an Mtb virulence factor- L01-Z08[18] for 2 weeks significantly reduced lung bacillary burden compared to HRZ treatment alone. These findings suggest targeting mPTPA or mPTPA+mPTPB as a potential combination therapy strategy for tuberculosis infection.
An exciting development in the PTP field is the progression of orthosteric small-molecule vascular endothelial PTP (VE-PTP) inhibitor AKB-9778 into clinical trials for diabetic macular edema (DME)[19,20] (Box 2). In vivo VE-PTP inhibition is hypothesized to activate the VE-PTP substrate angiopoietin 1 receptor 2 (Tie2), leading to reduced vascular leakage and ocular neovascularization[21,22]. AKB-9778 was developed from a phenylsulfamic acid core identified in Proctor and Gamble Pharmaceutical’s corporate repository as a PTP-inhibiting pTyr mimetic[23]. Structure-based drug design led to AKB-9778, which displays remarkable potency on VE-PTP (IC50=17 pM) and selectivity over most other phosphatases, with notable exceptions being receptor PTPs (RPTPs) density enhanced phosphatase 1 (DEP-1) and RPTPγ[21]. AKB-9778 administration in mice promoted Tie2 tyrosine phosphorylation in retinal endothelial cells and reduced ocular neovascularization and VEGF-induced vascular permeability[21,22]. Evidence supporting specific VE-PTP targeting in vivo includes: 1) AKB-9778 treatment phenocopied the effect of inducible VE-PTP deletion in adult mice -causing increased Tie2 phosphorylation and reduced vascularization- yet had no effect in mice lacking VE-PTP; 2) AKB-9778 inhibited vascular permeability equally in WT and DEP-1 knockout (KO) mice; 3) administration of anti-VE-PTP blocking antibody to mice led to similar phenotypic results as AKB-9778, reducing VEGF-induced vascular permeability and reduced ocular neovascularization[21,22].
Box 2. Clinical Trials with vascular endothelial protein tyrosine phosphatase inhibitor AKB-9778.
- In a Phase IB Trial in diabetic macular edema (DME) patients, vascular endothelial protein tyrosine phosphatase (VE-PTP) inhibitor AKB-9778 was safely self-administered subcutaneously twice daily for 4 weeks at doses up to 30 mg[19].
-
○At doses ≥22.5 mg, there was a modest transient decrease in blood pressure, presumably an on-target effect as activation of the VE-PTP substrate angiopoietin 1 receptor 2 (Tie2) is expected to stimulate vasodilation.
-
○Pharmacokinetics in DME patients showed a dose-dependent increase in AKB-9778 levels that peaked ~1 hr post-administration and decreased to low levels by 4 hr post-injection, and plasma half-life ~1 hr.
-
○
- In a Phase IIA Trial in DME patients, 15 mg AKB-9778 was self-administered subcutaneously twice daily alone or in combination with monthly intraocular injections of 0.3 mg ranibizumab, a VEGF neutralizing first-line DME treatment[20].
-
○AKB-9778 as a monotherapy did not cause improvement of macular edema after 12 weeks.
-
○AKB-9778 treatment combined with ranibizumab significantly reduced macular edema compared to ranibizumab treatment alone (29% of study eyes showed resolution in edema vs 17%, respectively).
-
○No significant improvement in visual acuity was observed with AKB-9778 treatment. The study authors commented that this should occur after edema resolution, likely requiring a longer and more-powered study.
-
○Although mild-to-moderate transient dizziness and fatigue occurred after AKB-9778 dosings, AKB-9778 demonstrated a favorable safety profile at this regimen. No patient discontinued treatment early due to complications of the study drug treatment.
-
○
Reversible bidentate inhibitors
Zhong-Yin Zhang’s laboratory pioneered the development of PTP bidentate inhibitors, which are small-molecules containing a core group for interaction with a PTP active-site and peripheral group for interaction with a proximal –ideally non-conserved- secondary site[24]. The SHP-2 inhibitor 11a-1 resulted from an attempt to generate bidentate SHP-2 inhibitors, and was recently employed to demonstrate that SHP-2 –a known target for cancer–is also a promising target for rheumatoid arthritis[25]. A precursor inhibitor, II-B08, was generated from screening a combinatorial library containing a pTyr mimetic salicylic acid core for binding the PTP active-site and a structurally diverse set of amines and hydrazines for additional interactions[26]. II-B08 inhibits non-competitively; co-crystallization revealed the salicylic acid core interacts with the phosphate-binding loop of the SHP-2 active-site, and the distal phenyl ring interacts within the SHP-2 active-site with the β5–β6 loop (aa 362–365), or E-loop. The E-loop is a component of the PTP active-site named after a conserved glutamate residue in classical PTPs that in many structures forms a β-hairpin[5]. Pairing an oxalic linker to a biaryl substituent led to 11a-1, which displays ≥5-fold selectivity for other PTPs[27]. Daily administration of 15 mg/kg i.p. significantly reduced growth of established melanoma tumors in a mouse xenograft model without affecting body weight[28]. We demonstrated that SHP-2 expression is increased in fibroblast-like synoviocytes (FLS) –joint-lining cells that become invasive and contribute to joint destruction during rheumatoid arthritis (RA)- from RA patients, and that SHP-2 promotes platelet-derived growth factor and tumor necrosis factor (TNF) signaling in these cells[29]. Heterozygous deletion of SHP-2 in radioresistant cells (which include FLS) or acute heterozygous deletion in myeloid cells significantly reduced arthritis development in the K/BxN serum transfer mouse model, which is dependent on actions of FLS and myeloid cells[25]. 11a-1 treatment reduced RA FLS migration and expression of mediators of invasiveness in response to TNF and interleukin (IL)-1, and daily administration substantially decreased K/BxN arthritis[25].
Reversible uncompetitive inhibitors
We recently discovered an orthosteric uncompetitive inhibitor of human LMPTP[30]. Uncompetitive inhibition occurs when an inhibitor binds to an enzyme-substrate complex[8]. Consistent with the above-mentioned role of LMPTP in dephosphorylating the IR, we found that global and liver-specific LMPTP KO protects mice from high-fat diet induced diabetes without affecting body weight[30]. To validate LMPTP as a new type 2 diabetes target, we developed an LMPTP inhibitor[30]. This series was identified by compound screening using a high concentration of OMFP substrate, such that the enzyme was at Vmax. Counterscreening and medicinal chemistry led to a quinoline core-based series of LMPTP inhibitors, exemplified by compound (Compd.) 23. Through isothermal titration calorimetry (ITC), nuclear magenetic resonance (NMR) spectroscopy, X-ray crystallography, hydroxyl radical footprinting, and mutagenesis, we determined that during catalysis, these inhibitors bind to the entrance of the active-site of the LMPTP phospho-Cys intermediate, excluding water from the active-site and preventing the release of phosphate required for the final step in catalysis. Other features include exquisite selectivity for LMPTP over other PTPs, oral bioavailability, and efficacy at increasing liver IR tyrosine phosphorylation and reversing high-fat diet-induced diabetes when administered to mice as a food admixture. Compd. 23 did not cause weight loss in mice and had no effect on diabetes or liver IR tyrosine phosphorylation levels in mice lacking liver-specific expression of LMPTP, confirming the specificity of the inhibitor in vivo and the action of LMPTP in the liver[30]. These findings suggest LMPTP as a key promoter of insulin resistance and that LMPTP inhibitors could have potential for ameliorating type 2 diabetes.
Irreversible inhibitors
PTPs can be irreversibly inhibited by covalent binding or oxidation of the active-site Cys, and this method has been used for inhibition of striatal-enriched PTP (STEP)[31] and cell division cycle 25 (CDC25)[32]. Deletion of central nervous system-expressed STEP improves cognition in Alzheimer’s disease (AD) mouse models, pointing to STEP as a promising target for AD[33]. Identification of STEP inhibitor TC-2153 resulted from serendipitous contamination of elemental sulfur in a library used to screen for STEP inhibitors[31]. The cyclicpolysulfide-containing TC-2153 -a reported anti-anxiolytic and anti-convulsant in mice- was subsequently sought after because of its similar chemistry. The inhibition mechanism of TC-2153 likely involves a covalent interaction between the STEP catalytic Cys472 and a TC-2153 sulfur atom. TC-2153 displays moderate selectivity for STEP against the closely-related hematopoietic PTP (HePTP) and STEP-like PTP (PTP-SL), and greater selectivity towards other PTPs. TC-2153 increased tyrosine phosphorylation of STEP substrates glutamate ionotropic receptor NMDA type subunit 2B, protein tyrosine kinase 2 beta (Pyk2) and extracellular signal-related kinase (ERK) 1/2 in cortical neuron cultures from WT but not STEP KO mice. In vivo TC-2153 administration caused increased ERK1/2 and Pyk2 phosphorylation only in brain tissues expressing STEP, and improved novel object recognition and reference memory in the 3xTg-AD model. TC-2153 treatment also improved cognitive and motor function in the phencyclidine-induced mouse model of schizophrenia[34].
CDC25 enzymes are overexpressed is different types of cancer, and though most available inhibitors are not selective among the 3 CDC25A/B/C proteins, collective inhibition is viewed as a cancer therapy strategy[35]. The most potent reported CDC25 inhibitor IRC-083864 (IC50~20–50 nM) was developed by improvement of quinone-based BN82685[32]. IRC-083864 is a heterocyclic bis-quinone with high selectivity over other phosphatases. Its inhibitory mechanism was not reported, but BN82865 irreversibly inhibits CDC25 in vitro, and these compounds were suggested to deactivate CDC25 enzymes by binding covalently to or oxidizing the active-site Cys. Oral administration of BN82865 and IRC-083864 inhibited growth of pancreatic and prostate carcinoma tumors, respectively, in nude mouse xenograft models, and intravenous (i.v.) administration of IRC-083864 inhibited pancreatic carcinoma tumor growth[35]. Recently, IRC-083864 was shown to inhibit clonogenic capacity of primary acute myeloid leukemia (AML) cells expressing the transforming Fms-like tyrosine kinase 3 internal tandem duplication[36]. IRC-083864 was licensed by Debiopharm Group as Debio 0931 for clinical development.
Allosteric small-molecule inhibitors
A trend surfacing in PTP inhibitor development is the appearance of allosteric small-molecules. In theory, allostery offers occasions for selective and cell-permeable inhibition by avoiding the charged PTP active-site. The first report occurred when Sunesis Pharmaceuticals published a crystal structure of PTP1B in complex with a benzofuran sulfonamide derivative of benzbromarone[37], revealing an allosteric site that can be exploited to inhibit PTP1B by blocking closure of the WPD loop[38]. However, while initial allosteric inhibitors targeting PTP1B[38] and PTPN22[39] achieved cell-permeability, these early compounds still lacked potency and selectivity. Persistent attempts resulted in several improved PTP inhibitors (Table 3). These compounds bind outside the active-site, and most exploit unique structural features to lock the enzyme in a catalytically unfavorable conformation. Since crystallization of PTP regions outside the catalytic domain can prove difficult, these studies illuminate the importance of biophysical methods for identifying compound binding sites when co-crystallization may not be feasible.
Table 3.
Features of recent small-molecule allosteric PTP inhibitors
| BCI: DUSP6 inhibitor | Compound 211: CD45 inhibitor | SHP099: SHP-2 inhibitor | MSI-1436: PTP1B inhibitor | |
|---|---|---|---|---|
| Chemical structure |
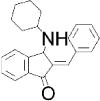
|
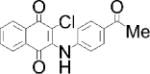
|
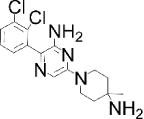
|
|
| Special features | Inhibits FGF signaling in zebrafish embryo reporter assay; inhibits ERK-mediated DUSP6 activation; induces death of human ALL cancer cells | Potent, selective; a single 3 mg/kg dose i.p. reduced inflammation in mouse delayed-type hypersensitivity model | Potent, highly selective, orally bioavailable; 75–100 mg/kg p.o. for 10 days decreased tumor cell growth in mouse xenograft models | Binds disordered PTP1B C-terminus, 5 mg/kg i.p. every 3 days inhibited breast tumor growth and metastasis in xenograft models; in Phase I Trial for metastatic breast cancer |
| Potency & selectivity | EC50=10.6 μM in zebrafish assay; inhibits DUSP6 and DUSP1, but not DUSP5, CCDC25B, PTP1B or VHR | CD45 IC50 =290 nM; no inhibition of PTPs LAR, PTP1B, RPTPσ, SHP-1 or DUSP22 | SHP-2 IC50=71 nM; IC50>100 μM on panel of 21 PTPs; IC50>10 μM on panel of 66 kinases; no reactivity against most targets in preclinical safety pharmacology panel up to 30 μM | PTP1B IC50 and Ki=600 nM; 10-fold selective for PTP1B vs TCPTP; 30-fold selective vs CD45, even greater for LAR & PTP-PEST; no activity on RPTPα or RPTPμ |
| Discovery | BCI identified in screen of 5,000 diverse compounds using live zebrafish embryo reporter for FGF activity; DUSP6 identified as molecular target; analog BCI-215 with similar potency and reduced toxicity identified in SAR analysis using zebrafish reporter assay | 120,000-compound in silico screen of NCI database for potential binding in groove at interface between CD45 D1 & D2 domains; hits tested for in vitro inhibition of intracellular region of CD45 using SRC peptide substrate; hit 37p analog 211 identified by SAR analysis | 100,000-compound screen of Novartis archive for inhibitors of full-length SHP-2 (in the presence of 0.5 μM bisphosphorylated IRS-1 peptide) but not SHP-2 PTP domain using DiFMUP substrate; medicinal chemistry improved initial hit SHP836 to SHP099 | Identified as appetite suppressant in mice; PTP1B later identified as molecular target |
| MOA | Inhibits substrate-induced stimulation of DUSP6 activity; predicted to bind crevice between general acid loop and helix α7 in low-activity DUSP6 form, preventing positioning of residue Asp262 for catalysis | Irreversible, non-competitive; causes conformational change of protein; predicted to bind in goove near interface between CD45 D1 & D2 domains | Binds allosteric pocket formed at interface of C-terminal SH2, N-terminal SH2 and PTP domains when enzyme is in closed, inactive conformation; stabilizes enzyme in inactive conformation | Reversible, non-competitive; likely binds to C-terminal helix α9′ and to another site close to catalytic region; induces conformational change in protein |
| Binding validation | Binding predicted by molecular docking | Circular dichroism suggested dramatic change in secondary structure of CD45 but not LAR; binding supported by mutation | Differential scanning fluorimetry: ΔTm=3.02°C; Xray co-crystallization with SHP-2 aa 1–525 (PDB 5EHP); mutation | Binding & conformational change shown by ITC, trypsin sensitivity, FRET, NMR spectroscopy and mutation |
| Cellular efficacy & specificity | Promoted FGF signaling in zebrafish embryos; restored ERK phosphorylation in phorbol ester-stimulated HeLa cells overexpressing DUSP6 (EC50=13.3 μM) or DUSP1 (EC50=8.0 μM), while inactive analogs did not; induced ALL cancer cell death (IC50=2.1 μM) | At 0.5 μM, increased phosphorylation of LCK-Y394 in Jurkat but not CD45-null J.45 T cells, and blocked TCR-induced phosphorylation of LCK-Y394, ZAP-70-Y319 and ERK1/2, IL-2 release and proliferation of primary mouse splenocytes | At [low-μM], slowed growth of hematopoietic cancer cells dependent on RTK or JAK1/2 signaling & colorectal cancer cells sensitive to Lapatinib; did not inhibit growth of cells carrying mutations in RAS or BRAF or KYSE520 cells carrying SHP-2-T253M/Q257L mutant | At [low-μM], blocked HER2 activation in MCF10A mammary epithelial cells; reduced migration of HER2 positive cell lines but not in MDF10A-NeuNT cells carrying PTP1B-L192A/S372A mutant; MSI-1436, but not inactive analog, pulled-down PTP1B from tumor lysates |
| Refs. | [40,42,43] | [45] | [46,47] | [48,50] |
BRAF, B-raf proto-oncogene; HAD, haloacid dehalogenase; HER2, human epidermal growth factor receptor 2; IRS-1, insulin receptor substrate 1; JAK, Janus kinase; NIH, National Institutes of Health; MLPCN, Molecular Libraries Probe Centers Network; NCI, National Cancer Institute; PPM1A, protein phosphatase magnesium-dependent 1A; Refs., references; RTK, receptor tyrosine kinase; SCP1, small C-terminal domain phosphatase 1; SRC, proto-oncogene tyrosine protein kinase; ZAP-70, zeta-chain-associated protein kinase of 70 kDa
An allosteric dual-specific phosphatase (DUSP) inhibitor was found by compound screening using transgenic zebrafish embryos expressing GFP as a reporter for fibroblast growth factor (FGF) signaling. (E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one (BCI) showed EC50=10.6 μM in this assay, and was subsequently identified as a DUSP6 inhibitor[40]. BCI also inhibits DUSP1, and is selective over vaccinia H1-related phosphatase (VHR) and DUSP5. DUSP6 is catalytically activated by binding to ERK substrate[41], and in vitro, BCI inhibited DUSP6 dephosphorylation of OMFP only in the presence of ERK[40]. Although biophysical data to explain the mechanism of action of BCI on DUSP6 is not yet available, BCI was predicted by molecular docking to allosterically inhibit ERK-induced DUSP6 activation by binding the crevice between the DUSP6 general acid loop and helix α7, preventing the positioning needed for Asp262 to act as an acid during catalysis[40,42]. BCI was used to implicate DUSP6 in acute lymphoblastic leukemia (ALL) cell transformation through negative regulation of ERK[43]. BCI treatment of patient-derived ALL cells caused ERK hyperactivation and induced cell death, suggesting potential for DUSP6 as an ALL therapeutic target[43].
Compound 211 is a selective inhibitor of CD45 –a PTP that promotes antigen receptor signaling in lymphocytes and is considered a drug target for autoimmunity[44]- identified by in silico screening for compounds predicted to bind the interface between CD45-D1 and -D2 domains[45]. Compound 211 is an irreversible, non-competitive inhibitor. Circular dichroism analysis indicated that Compound 211 produces dramatic changes in D1–D2 secondary structure suggestive of protein unfolding in CD45 but not leukocyte antigen-related PTP (LAR). This compound increased phosphorylation of CD45 substrate lymphocyte-specific protein tyrosine kinase (LCK) Y394 in Jurkat, but not CD45-null (J.45) cells, and blocked early and proximal TCR signaling. A 3 mg/kg i.p. dose substantially reduced inflammation in the delayed-type hypersensitivity mouse model[45].
Exploiting an auto-inhibitory mechanism of SHP-2, an allosteric approach led to a major progression in SHP-2 inhibitor development[46,47]. Compounds were screened for inhibition of full-length SHP-2 using the small-molecule substrate 6,8-difluoro-4-methylumbelliferyl phosphate (DiFMUP) in the presence of a bis-phosphorylated peptide that released SHP-2 from its autoinhibited form, while counterscreening assays eliminated hits acting only on the PTP domain. Multiple rounds of medicinal chemistry improved inhibitor potency, resulting in SHP099. Co-crystallization revealed SHP099 binds the pocket created by the active-site and SH2 domains in the closed SHP-2 conformation, stabilizing the auto-inhibited form. Other features of SHP099 include its excellent selectivity, efficacy in cell-based assays dependent upon SHP-2 expression, oral bioavailability, and tumor growth inhibition in mouse xenograft models without affecting body weight[46].
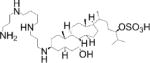
The discovery of PTP1B as the target of the aminosterol MSI-1436 (Trodusquemine)[48], which acts as an appetite suppressant in mice[49], raised interest in defining MSI-1436’s inhibitory mechanism. The Tonks and Peti groups found that MSI-1436 allosterically inhibited PTP1B by targeting its disordered C-terminal region[50]. MSI-1436 induced a PTP1B conformational change detected by trypsin sensitivity and fluorescence resonance energy transfer (FRET). ITC revealed MSI-1436 bound PTP1B within the C-terminal region (Kd=0.3 μM) and catalytic domain (Kd=2 μM). Biomolecular NMR spectroscopy demonstrated that PTP1B C-terminal residues (300–393) were flexible, predominantly disordered, and contained 2 α-helices. Residues in the C-terminal helix α9′ were most perturbed by MSI-1436 binding; helix-destabilizing mutations confirmed that this helix was crucial for inhibition. NMR spectroscopy followed by mutagenesis localized the second binding site, which was within the PTP1B catalytic domain close to the catalytic pocket and was partly overlapping with the previously described allosteric PTP1B site[38]. Administration of MSI-1436 markedly inhibited tumor growth and metastasis in mouse models of breast cancer[50]. MSI-1436 is currently in a Phase I Clinical Trial for metastatic breast cancer.
Allosteric oxidation-stabilizing inhibitors
An up-and-coming approach is PTP-targeting with oxidation-stabilizing inhibitors. While such small-molecules are not yet reported, Nicholas Tonks’ group provided proof-of-principle that intracellular oxidized PTP1B (PTP1B-OX) can be stabilized with antibodies[51,52]. PTPs are oxidized and inactivated by reactive oxygen species (ROS) produced in response to a variety of stimuli[53]. Mild oxidation is reversible and leads to conformational changes in PTP structure. In the presence of ROS, the PTP1B catalytic Cys undergoes oxidation-induced sulfenylamide formation, which inactivates the enzyme[54,55]. Single-chain variable antibody fragments against PTP1B-OX were generated by leveraging active-site double mutant PTP1B-C215A/S216A (CASA), which adopts a conformation similar to PTP1B-OX, as an epitope[51,52]. Clone scFv45 reacted with PTP1B-OX and PTP1B-CASA, but not with reduced PTP1B, and inhibited PTP1B-OX reactivation (IC50=19 nM) by reducing agent. When expressed in mammalian cells, scFv45 immunoprecipitated PTP1B-OX after H2O2 or insulin stimulation and colocalized with PTP1B-OX. scFv45 showed notable selectivity for PTP1B-OX over oxidized TCPTP[53]. Expression of scFv45, but not a non-targeting intrabody, enhanced IR phosphorylation and downstream signaling, suggesting PTP1B-OX can be stabilized intracellularly. Given the numerous PTPs whose activities are affected by reversible oxidization and nitrosylation[53], this study indicates the untapped potential that underlies various molecular PTP conformations.
Oligomerization small-molecule inhibitors
The Zhang group developed a small-molecule oligomerization inhibitor to inhibit the function of phosphatase of regenerating liver (PRL) PTPs, which are targets for tumor growth and metastasis [56,57], by disrupting their trimerization[58]. This compound series, exemplified by Compound 43, was identified from in silico library screening for PRL-1 trimerization disruptors, inhibited PRL trimerization without affecting its catalytic activity, and reduced viability and migration of cancer cell lines at low-μM concentrations[58]. Daily treatment of mice with 30 mg/kg Compound 43 i.p. blocked tumor growth in a melanoma xenograft model. This series disrupts trimerization of all three PRL proteins, however was well-tolerated in mice after 3 weeks of treatment[58], suggesting collective inhibition of PRL trimerization might be a viable option for cancer.
Trends in PTP-targeted biologics development
Here we will discuss recent progress in the development of biologic agents targeting RPTPs.
Radioimmunotherapy-delivering antibodies
As a transmembrane PTP highly expressed on hematopoietic cells, for decades CD45 has been the object of radioimmunotherapy strategies[59,60]. Studies are currently ongoing to employ anti-CD45 mAbs to improve outcome during hematopoietic cell transplantation (HCT). These include efforts to reduce the conditioning regimen for patients undergoing allogeneic transplantation[61], minimizing graft-versus-host disease without need of total-body irradiation[62], and delivering α-emitting radionuclides to hematopoietic tissues[63,64].
RPTP decoy biologics
The RPTP subfamily is unique among PTPs in their potential to be targeted through their intracellular wedge motifs or extracellular regions. Recent studies show that administration of an RPTPσ cell-penetrating wedge peptide mimetic (ISP) promoted innervation and functional restoration in mice following spinal cord injury[65,66]. RPTPσ is a receptor for chondroitin sulphate proteoglycans (CSPGs), an abundant extracellular matrix (ECM) component in the scar tissue that is generated after CNS injury. Activation of RPTPσ by CSPG transduces an inhibitory signal that inhibits axon regeneration. ISP treatment of cultured adult sensory neurons reduced CSPG-mediated inhibition of these cells -but not neurons lacking RPTPσ-, allowing their axonic extension through a CSPG gradient. ISP s.c. administration to rats following contusive spinal cord injury promoted axon regrowth within the CSPG-rich scar, restoring innervation to the spinal cord and recovery of locomotor and urinary systems[65]. ISP administration also improved motoneuron axon regeneration and motor function in rats subjected to spinal root avulsion injury, in which spinal nerves are disconnected from the spinal cord[66]. ISP administration also promoted cardiac innervation in a mouse model of myocardial infarction[67]. Daily i.p. injection in mice subjected to ischaemia-reperfusion restored sympathetic innervation to the CSPG-rich cardiac scar and the cardiac infarct and reduced cardiac arrhythmias[67]. The mode of action of ISP is not yet clear. ISP pulled-down RPTPσ from rodent brain and spinal cord lysates, and ISP likely inhibited RPTPσ function given that ISP treatment phenocopied RPTPσ KO in allowing axons to penetrate CSPG-rich glial scars[67,68]. It remains to be determined whether ISP acts by inhibiting RPTPσ catalytic activity.
RPTPσ also binds transmembrane heparan-sulfate proteoglycans (HSPGs), which inhibit RPTPσ by inducing oligomerization. The opposing effects of CSPGs and HSPGs on RPTPσ function is termed the “proteoglycan switch”[69]. RPTPσ binds proteoglycans through its N-terminal extracellular immunoglobulin-like domains Ig1&Ig2. RPTPσ is expressed in joint FLS, and the joint ECM comprises abundant proteoglycans. We found that RPTPσ was constitutively bound to HSPG syndecan-4 on the surface of FLS. Disruption of the RPTPσ/syndecan-4 interaction by treatment with 20 nM of RPTPσ-Ig1&Ig2 decoy protein impaired FLS invasiveness and cartilage attachment in a manner requiring RPTPσ catalytic activity. Treatment with RPTPσ-Ig1&Ig2 i.v. also blocked human RA FLS invasion into cartilage in a mouse xenograft model and reversed established arthritis in the K/BxN serum transfer model. Importantly, RPTPσ-Ig1&Ig2 did not affect the invasiveness of RPTPσ-null FLS or arthritis severity in RPTPσ-null mice[70]. These findings indicate the RPTPσ/syndecan-4 interaction as a potential novel therapeutic target for RA.
Concluding Remarks
The past few years have witnessed substantial progress in the development of PTP inhibitors. These advances signify that enzymes deemed undruggable may actually provide unique solutions for treating human disease. The continued generation of high-quality, selective probes to modulate PTP activity is paramount for successful growth of the PTP field. Persistent efforts to generate chemical inhibitors are providing opportunities to re-examine historical targets such as PTP1B and SHP-2, substantiate suspected targets in cases like STEP and PTPN22, and foster the emergence of new targets such as VE-PTP and LMPTP. Novel studies of RPTPσ are revealing potential opportunities for manipulating the function of PTPs within the receptor subfamily through the use of biologics. New approaches to inhibit PTPs, for example through small-molecule oxidation stabilizers, may provide additional options for attacking current targets. Furthermore, for cases where enhancing PTP enzymatic activity would be of therapeutic benefit, development of small-molecule PTP activators would expand our repertoire of PTP drug targets.
How can PTP-targeted probes be more rapidly and effectively developed (see Outstanding Questions)? These recent studies impart that there is no single formula for attacking PTPs. The successful tactic will likely be unique for each enzyme and involve exploiting distinctive active-site features, surrounding determinants, or auto-inhibition mechanisms. Thus molecular knowledge of each target will be of critical importance. We anticipate that the renewed interest in drugging PTPs will soon be followed by a resolve to more deeply understand the fundamental biochemistry of PTPs and result in a rise in studies on the structures and post-translational modifications of these enzymes, as well as their intramolecular and intermolecular interactions. This insight will be key to the intentional design of strategies to drug PTPs that take advantage of the unique biochemical traits of each member of the family.
Outstanding Questions Box.
Can small-molecules be developed as oxidation-stabilizing PTP inhibitors?
How can we develop small-molecule PTP activators to expand our repertoire of PTP drug targets?
How can we develop methods to more rapidly discover selective, bioavailable probes?
Can high-throughput methods be developed to discover inhibitors for multiple protein tyrosine phosphatases (PTPs) at a time?
How can we more efficiently isolate full-length recombinant PTP proteins for allosteric inhibitor development?
PTPs remain an unconquered territory, but their exploration has expanded dramatically in the last few years. Persistent efforts have reinvigorated the field, providing fresh expectation that while harnessing PTPs for therapy will be difficult, it is still within our reach.
Figure 2. Targeting PTPs with orthosteric small-molecule inhibitors.
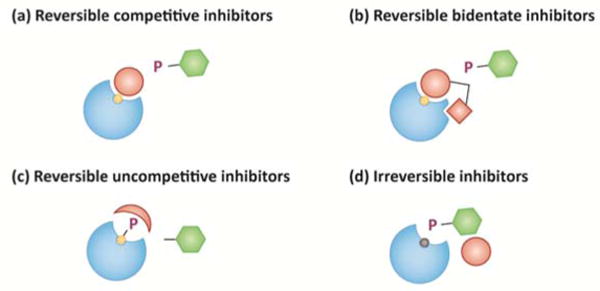
(a) Reversible competitive inhibitors bind to enzymes with rapid association and dissociation rates at the site of substrate binding, and thus compete with substrate for binding to the enzyme. (b) Bidentate inhibitors consist of two chemical moieties, and bind to the enzyme active-site and a proximal secondary site (in some cases this can occur within the active-site). (c) Uncompetitive inhibitors bind to an enzyme after formation of an enzyme-substrate complex, preventing completion of catalysis. (d) Irreversible inhibitors modify the enzyme active-site, rendering the enzyme non-functional.
Figure 3. Targeting RPTPσ with biologics.
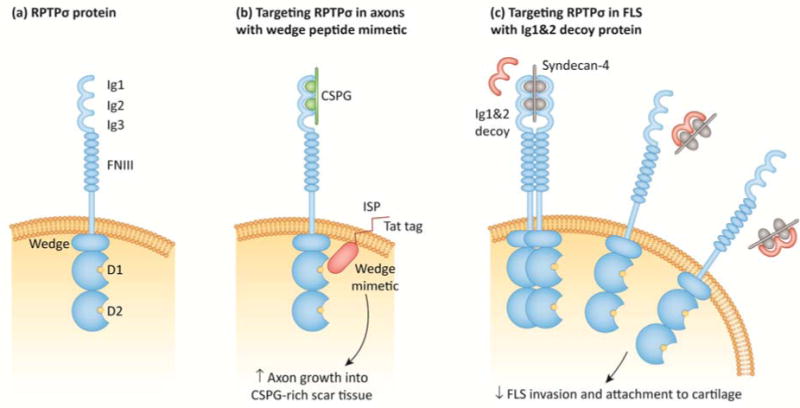
(a) Schematic of RPTPσ protein domains. RPTPσ consists of extracellular, transmembrane, and intracellular regions. The extracellular region consists of amino-terminal immunoglobulin-like domains (Ig1–Ig3) and multiple fibronectin type III (FNIII) repeats. As described in (b–c), the Ig1&2 domains mediate RPTPσ interactions with proteoglycans in the extracellular matrix (ECM). The intracellular region consists of a juxtamembrane helix-loop-helix “wedge” motif and two PTP domains (D1 and D2). (b) Targeting RPTPσ with a cell-penetrating wedge peptide mimetic (ISP). In neuronal cells, RPTPσ Ig1&2 domains act as a receptor for chondroitin sulfate proteoglycans (CSPG), an ECM component that is abundant in scar tissue and inhibits axon regeneration. Treatment with ISP, a wedge peptide mimetic conjugated to a cell-penetrating transactivator of transcription (TAT) peptide, alleviates CSPG-mediated inhibition of axon extension into CSPG-rich scars. The mechanism of action of ISP is currently unknown, although it likely inhibits RPTPσ function. (c) Targeting RPTPσ with an Ig1&2 decoy protein. On the surface of fibroblast-like synoviocytes (FLS), RPTPσ Ig1&2 domains bind to the heparan sulfate proteoglycan syndecan-4, which inhibits RPTPσ by inducing oligomerization. Treatment with recombinant Ig1&2 protein disrupts the interaction between RPTPσ and syndecan-4, inhibiting the invasiveness and cartilage attachment of FLS through RPTPσ catalytic activity.
Trends Box.
PTPs are critical for numerous cellular processes in health and disease, and several PTPs are validated drug targets.
Despite historical difficulties in drugging PTPs, efforts have persisted and led to development of new probes that are being used for biological examination of old and new PTP targets.
Allosteric PTP inhibitors are emerging, most of which exploit catalytically unfavorable conformations of the targeted enzyme.
New studies of receptor PTPs reveal the unique potential in targeting this PTP subfamily with decoy biologics.
Small-molecule inhibitors of VE-PTP and PTP1B are currently undergoing clinical trials for diabetic macular edema and metastatic breast cancer, respectively.
Acknowledgments
This work was supported by grants R01AR066053, R01AI070544 and R01DK106233 to N.B. from the National Institutes of Health, and Pathway to Stop Diabetes Grant 1–15-INI-13 to S.M.S. from the American Diabetes Association.
Glossary
- Allosteric inhibitor
inhibitor that binds outside of the enzyme active-site and induces or stabilizes a catalytically unfavorable enzyme conformation
- Bidentate inhibitor
inhibitor that contains a core group that interacts with the enzyme active-site and a peripheral group that interacts with a proximal –ideally non-conserved- secondary site
- Biologic agent
a substance derived from a living organism, used to modulate the function of a protein; examples include antibodies, proteins and peptides
- Cell-penetrating wedge peptide mimetic
a peptide consisting of 24 amino acids of the helix-loop-helix found in RPTP wedge motifs, conjugated to a cell-penetrating peptide derived from the sequence of the transactivator of transcription (TAT) protein of the human immunodeficiency virus
- Competitive inhibitor
inhibitor that binds to an enzyme at the site of substrate binding
- E-loop
a loop in the PTP active-site named after a conserved glutamate residue in classical PTPs
- Irreversible inhibitor
inhibitor that modifies the enzyme to render it non-functional
- Oligomerization inhibitor
inhibitor that disrupts a complex consisting of several protein monomers; examples include covalent interaction with or oxidation of the active-site
- Orthosteric inhibitor
inhibitor that binds at the enzyme active-site
- Oxidation-stabilizing inhibitor
inhibitor that binds to and stabilizes the reversible oxidation of a PTP on the catalytic Cys residue, maintaining the enzyme in an inactive state
- Proteoglycan switch
the reciprocal regulation of RPTPσ function that occurs when chondroitin-sulfate proteoglycans (CSPGs) or heparan sulfate proteoglycans (HSPGs) bind the RPTPσ extracellular region
- PTK
protein tyrosine kinase, enzyme that catalyzes phosphorylation of proteins on tyrosine residues
- PTP
protein tyrosine phosphatase, enzyme that catalyzes hydrolytic dephosphorylation of proteins on tyrosine residues
- Radioimmunotherapy
targeted therapy involving conjugation of an antibody to a radioactive agent in order to specifically deliver radiation to hematopoietic cells and tissues in patients with leukemias, lymphomas, or myelodysplasias
- Reversible inhibitor
inhibitor that binds to an enzyme with rapid association and dissociation rates
- RPTP
receptor protein tyrosine phosphatase, transmembrane enzyme that catalyzes hydrolytic dephosphorylation of proteins on tyrosine residues through an intracellular catalytic domain
- Uncompetitive inhibitor
inhibitor that binds to an enzyme-substrate complex
- WPD loop
a highly conserved loop in PTPs -named after the tryptophan-proline-aspartate residues present in most phosphotyrosine-specific PTPs- that closes around the PTP active-site upon substrate binding to place a catalytic aspartate residue in position for participation in catalysis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to declare.
References
- 1.Hunter T. The genesis of tyrosine phosphorylation. Cold Spring Harb Perspect Biol. 2014;6:a020644. doi: 10.1101/cshperspect.a020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonks NK. Protein tyrosine phosphatases–from housekeeping enzymes to master regulators of signal transduction. Febs J. 2013;280:346–378. doi: 10.1111/febs.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roskoski R., Jr A historical overview of protein kinases and their targeted small molecule inhibitors. Pharmacol Res. 2015;100:1–23. doi: 10.1016/j.phrs.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Alonso A, et al. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 5.Tautz L, et al. Protein tyrosine phosphatases: structure, function, and implication in human disease. Methods Mol Biol. 2013;1053:179–221. doi: 10.1007/978-1-62703-562-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He RJ, et al. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol Sin. 2014;35:1227–1246. doi: 10.1038/aps.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Copeland RA. Evaluation of enzyme inhibitors in drug discovery. A guide for medicinal chemists and pharmacologists. Methods Biochem Anal. 2005;46:1–265. [PubMed] [Google Scholar]
- 8.Ring B, et al. Reversible mechanisms of enzyme inhibition and resulting clinical significance. Methods Mol Biol. 2014;1113:37–56. doi: 10.1007/978-1-62703-758-7_4. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan N, et al. PTP1B inhibition suggests a therapeutic strategy for Rett syndrome. The Journal of clinical investigation. 2015;125:3163–3177. doi: 10.1172/JCI80323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lombardi LM, et al. MECP2 disorders: from the clinic to mice and back. The Journal of clinical investigation. 2015;125:2914–2923. doi: 10.1172/JCI78167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozek C, et al. Protein-tyrosine phosphatase 1B (PTP1B) is a novel regulator of central brain-derived neurotrophic factor and tropomyosin receptor kinase B (TrkB) signaling. The Journal of biological chemistry. 2014;289:31682–31692. doi: 10.1074/jbc.M114.603621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bottini N, Peterson EJ. Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease. Annu Rev Immunol. 2014;32:83–119. doi: 10.1146/annurev-immunol-032713-120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vang T, et al. LYP inhibits T-cell activation when dissociated from CSK. Nature chemical biology. 2012;8:437–446. doi: 10.1038/nchembio.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schickel JN, et al. TPN22 inhibition resets defective human central B cell tolerance. Sci Immunol. 2016;1 doi: 10.1126/sciimmunol.aaf7153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He R, et al. Inhibition of Low Molecular Weight Protein Tyrosine Phosphatase by an Induced-Fit Mechanism. J Med Chem. 2016 doi: 10.1021/acs.jmedchem.6b00993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caselli A, et al. Low molecular weight protein tyrosine phosphatase: Multifaceted functions of an evolutionarily conserved enzyme. Biochimica et biophysica acta. 2016;1864:1339–1355. doi: 10.1016/j.bbapap.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Dutta NK, et al. Mycobacterial Protein Tyrosine Phosphatases A and B Inhibitors Augment the Bactericidal Activity of the Standard Anti-tuberculosis Regimen. ACS Infect Dis. 2016;2:231–239. doi: 10.1021/acsinfecdis.5b00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, et al. Discovery and evaluation of novel inhibitors of mycobacterium protein tyrosine phosphatase B from the 6-Hydroxy-benzofuran-5-carboxylic acid scaffold. J Med Chem. 2013;56:832–842. doi: 10.1021/jm301781p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campochiaro PA, et al. Treatment of diabetic macular edema with an inhibitor of vascular endothelial-protein tyrosine phosphatase that activates Tie2. Ophthalmology. 2015;122:545–554. doi: 10.1016/j.ophtha.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Campochiaro PA, et al. Enhanced Benefit in Diabetic Macular Edema from AKB-9778 Tie2 Activation Combined with Vascular Endothelial Growth Factor Suppression. Ophthalmology. 2016;123:1722–1730. doi: 10.1016/j.ophtha.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Shen J, et al. Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. The Journal of clinical investigation. 2014;124:4564–4576. doi: 10.1172/JCI74527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frye M, et al. Interfering with VE-PTP stabilizes endothelial junctions in vivo via Tie-2 in the absence of VE-cadherin. J Exp Med. 2015;212:2267–2287. doi: 10.1084/jem.20150718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klopfenstein SR, et al. 1,2,3,4-Tetrahydroisoquinolinyl sulfamic acids as phosphatase PTP1B inhibitors. Bioorganic & medicinal chemistry letters. 2006;16:1574–1578. doi: 10.1016/j.bmcl.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 24.Low JL, et al. Bidentate inhibitors of protein tyrosine phosphatases. Antioxidants & redox signaling. 2014;20:2225–2250. doi: 10.1089/ars.2013.5710. [DOI] [PubMed] [Google Scholar]
- 25.Maeshima K, et al. Abnormal TPN11 enhancer methylation promotes rheumatoid arthritis fibroblast-like synoviocyte aggressiveness and joint inflammation. JCI Insight. 2016;1 doi: 10.1172/jci.insight.86580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, et al. Salicylic acid based small molecule inhibitor for the oncogenic Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2) J Med Chem. 2010;53:2482–2493. doi: 10.1021/jm901645u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng LF, et al. Therapeutic potential of targeting the oncogenic SHP2 phosphatase. J Med Chem. 2014;57:6594–6609. doi: 10.1021/jm5006176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang RY, et al. SHP2 phosphatase as a novel therapeutic target for melanoma treatment. Oncotarget. 2016 doi: 10.18632/oncotarget.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanford SM, et al. Protein tyrosine phosphatase expression profile of rheumatoid arthritis fibroblast-like synoviocytes: a novel role of SH2 domain-containing phosphatase 2 as a modulator of invasion and survival. Arthritis Rheum. 2013;65:1171–1180. doi: 10.1002/art.37872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stanford SM, et al. Diabetes reversal by inhibition of the low molecular weight tyrosine phosphatase. Nature chemical biology. 2017 doi: 10.1038/nchembio.2344. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu J, et al. Inhibitor of the tyrosine phosphatase STEP reverses cognitive deficits in a mouse model of Alzheimer’s disease. PLoS Biol. 2014;12:e1001923. doi: 10.1371/journal.pbio.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brezak MC, et al. IRC-083864, a novel bis quinone inhibitor of CDC25 phosphatases active against human cancer cells. Int J Cancer. 2009;124:1449–1456. doi: 10.1002/ijc.24080. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, et al. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer’s disease mouse model. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:19014–19019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, et al. Inhibition of STEP61 ameliorates deficits in mouse and hiPSC-based schizophrenia models. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brenner AK, et al. Therapeutic targeting the cell division cycle 25 (CDC25) phosphatases in human acute myeloid leukemia–the possibility to target several kinases through inhibition of the various CDC25 isoforms. Molecules. 2014;19:18414–18447. doi: 10.3390/molecules191118414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertoli S, et al. CDC25A governs proliferation and differentiation of FLT3-ITD acute myeloid leukemia. Oncotarget. 2015;6:38061–38078. doi: 10.18632/oncotarget.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wrobel J, et al. PTP1B inhibition and antihyperglycemic activity in the ob/ob mouse model of novel 11-arylbenzo[b]naphtho[2,3-d]furans and 11-arylbenzo[b]naphtho[2,3-d]thiophenes. J Med Chem. 1999;42:3199–3202. doi: 10.1021/jm990260v. [DOI] [PubMed] [Google Scholar]
- 38.Wiesmann C, et al. Allosteric inhibition of protein tyrosine phosphatase 1B. Nat Struct Mol Biol. 2004;11:730–737. doi: 10.1038/nsmb803. [DOI] [PubMed] [Google Scholar]
- 39.Stanford SM, et al. Discovery of a novel series of inhibitors of lymphoid tyrosine phosphatase with activity in human T cells. J Med Chem. 2011;54:1640–1654. doi: 10.1021/jm101202j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molina G, et al. Zebrafish chemical screening reveals an inhibitor of Dusp6 that expands cardiac cell lineages. Nature chemical biology. 2009;5:680–687. doi: 10.1038/nchembio.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 42.Korotchenko VN, et al. In vivo structure-activity relationship studies support allosteric targeting of a dual specificity phosphatase. Chembiochem. 2014;15:1436–1445. doi: 10.1002/cbic.201402000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shojaee S, et al. Erk Negative Feedback Control Enables Pre-B Cell Transformation and Represents a Therapeutic Target in Acute Lymphoblastic Leukemia. Cancer Cell. 2015;28:114–128. doi: 10.1016/j.ccell.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hermiston ML, et al. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 45.Perron MD, et al. Allosteric noncompetitive small molecule selective inhibitors of CD45 tyrosine phosphatase suppress T-cell receptor signals and inflammation in vivo. Mol Pharmacol. 2014;85:553–563. doi: 10.1124/mol.113.089847. [DOI] [PubMed] [Google Scholar]
- 46.Chen YN, et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature. 2016;535:148–152. doi: 10.1038/nature18621. [DOI] [PubMed] [Google Scholar]
- 47.Garcia Fortanet J, et al. Allosteric Inhibition of SHP2: Identification of a Potent, Selective, and Orally Efficacious Phosphatase Inhibitor. J Med Chem. 2016 doi: 10.1021/acs.jmedchem.6b00680. [DOI] [PubMed] [Google Scholar]
- 48.Lantz KA, et al. Inhibition of PTP1B by trodusquemine (MSI-1436) causes fat-specific weight loss in diet-induced obese mice. Obesity (Silver Spring) 2010;18:1516–1523. doi: 10.1038/oby.2009.444. [DOI] [PubMed] [Google Scholar]
- 49.Zasloff M, et al. A spermine-coupled cholesterol metabolite from the shark with potent appetite suppressant and antidiabetic properties. Int J Obes Relat Metab Disord. 2001;25:689–697. doi: 10.1038/sj.ijo.0801599. [DOI] [PubMed] [Google Scholar]
- 50.Krishnan N, et al. Targeting the disordered C terminus of PTP1B with an allosteric inhibitor. Nature chemical biology. 2014;10:558–566. doi: 10.1038/nchembio.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haque A, et al. Conformation-sensing antibodies stabilize the oxidized form of PTP1B and inhibit its phosphatase activity. Cell. 2011;147:185–198. doi: 10.1016/j.cell.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haque A, Tonks NK. The use of phage display to generate conformation-sensor recombinant antibodies. Nature protocols. 2012;7:2127–2143. doi: 10.1038/nprot.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karisch R, et al. Global proteomic assessment of the classical protein-tyrosine phosphatome and “Redoxome”. Cell. 2011;146:826–840. doi: 10.1016/j.cell.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salmeen A, et al. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 55.van Montfort RL, et al. Oxidation state of the active-site cysteine in protein tyrosine phosphatase 1B. Nature. 2003;423:773–777. doi: 10.1038/nature01681. [DOI] [PubMed] [Google Scholar]
- 56.Campbell AM, Zhang ZY. Phosphatase of regenerating liver: a novel target for cancer therapy. Expert Opin Ther Targets. 2014;18:555–569. doi: 10.1517/14728222.2014.892926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobayashi M, et al. Phosphatase of regenerating liver in hematopoietic stem cells and hematological malignancies. Cell cycle. 2014;13:2827–2835. doi: 10.4161/15384101.2014.954448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bai Y, et al. Novel Anticancer Agents Based on Targeting the Trimer Interface of the PRL Phosphatase. Cancer Res. 2016;76:4805–4815. doi: 10.1158/0008-5472.CAN-15-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jurcic JG, Rosenblat TL. Targeted alpha-particle immunotherapy for acute myeloid leukemia. Am Soc Clin Oncol Educ Book. 2014:e126–131. doi: 10.14694/EdBook_AM.2014.34.e126. [DOI] [PubMed] [Google Scholar]
- 60.Jurcic JG. Radioimmunotherapy for hematopoietic cell transplantation. Immunotherapy. 2013;5:383–394. doi: 10.2217/imt.13.11. [DOI] [PubMed] [Google Scholar]
- 61.Mawad R, et al. Radiolabeled anti-CD45 antibody with reduced-intensity conditioning and allogeneic transplantation for younger patients with advanced acute myeloid leukemia or myelodysplastic syndrome. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20:1363–1368. doi: 10.1016/j.bbmt.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orozco JJ, et al. Anti-CD45 radioimmunotherapy without TBI before transplantation facilitates persistent haploidentical donor engraftment. Blood. 2016;127:352–359. doi: 10.1182/blood-2014-12-617019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burtner CR, et al. (211)Astatine-Conjugated Monoclonal CD45 Antibody-Based Nonmyeloablative Conditioning for Stem Cell Gene Therapy. Hum Gene Ther. 2015;26:399–406. doi: 10.1089/hum.2015.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Frost SH, et al. alpha-Imaging Confirmed Efficient Targeting of CD45-Positive Cells After 211At-Radioimmunotherapy for Hematopoietic Cell Transplantation. J Nucl Med. 2015;56:1766–1773. doi: 10.2967/jnumed.115.162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lang BT, et al. Modulation of the proteoglycan receptor PTPsigma promotes recovery after spinal cord injury. Nature. 2015;518:404–408. doi: 10.1038/nature13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li H, et al. Enhanced regeneration and functional recovery after spinal root avulsion by manipulation of the proteoglycan receptor PTPsigma. Sci Rep. 2015;5:14923. doi: 10.1038/srep14923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gardner RT, et al. Targeting protein tyrosine phosphatase sigma after myocardial infarction restores cardiac sympathetic innervation and prevents arrhythmias. Nat Commun. 2015;6:6235. doi: 10.1038/ncomms7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen Y, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coles CH, et al. Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science. 2011;332:484–488. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Doody KM, et al. Targeting phosphatase-dependent proteoglycan switch for rheumatoid arthritis therapy. Science translational medicine. 2015;7:288ra276. doi: 10.1126/scitranslmed.aaa4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Campochiaro PA. Molecular pathogenesis of retinal and choroidal vascular diseases. Prog Retin Eye Res. 2015;49:67–81. doi: 10.1016/j.preteyeres.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panchal RG, et al. Reduced levels of protein tyrosine phosphatase CD45 protect mice from the lethal effects of Ebola virus infection. Cell Host Microbe. 2009;6:162–173. doi: 10.1016/j.chom.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 73.Panchal RG, et al. Reduced expression of CD45 protein-tyrosine phosphatase provides protection against anthrax pathogenesis. The Journal of biological chemistry. 2009;284:12874–12885. doi: 10.1074/jbc.M809633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qin Z, et al. Chronic stress induces anxiety via an amygdalar intracellular cascade that impairs endocannabinoid signaling. Neuron. 2015;85:1319–1331. doi: 10.1016/j.neuron.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 75.Xu J, et al. Down-regulation of BDNF in cell and animal models increases striatal-enriched protein tyrosine phosphatase 61 (STEP61) levels. J Neurochem. 2016;136:285–294. doi: 10.1111/jnc.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kamceva M, et al. Role of Striatal-Enriched Tyrosine Phosphatase in Neuronal Function. Neural Plast. 2016;2016:8136925. doi: 10.1155/2016/8136925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan G, et al. The tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis Rev. 2008;27:179–192. doi: 10.1007/s10555-008-9126-y. [DOI] [PubMed] [Google Scholar]
- 78.Wade F, et al. Deletion of low molecular weight protein tyrosine phosphatase (Acp1) protects against stress-induced cardiomyopathy. The Journal of pathology. 2015 doi: 10.1002/path.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fanzani L, et al. Mycobacterium tuberculosis Low Molecular Weight Phosphatases (MPtpA and MPtpB): From Biological Insight to Inhibitors. Curr Med Chem. 2015;22:3110–3132. doi: 10.2174/0929867322666150812150036. [DOI] [PubMed] [Google Scholar]
- 80.Xu J, et al. Inhibition of the tyrosine phosphatase STEP61 restores BDNF expression and reverses motor and cognitive deficits in phencyclidine-treated mice. Cell Mol Life Sci. 2016;73:1503–1514. doi: 10.1007/s00018-015-2057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


