Summary
Sickle cell disease (SCD) is a group of recessively inherited disorders of erythrocyte function that presents an ongoing threat to reducing childhood mortality around the world. While decades of research have led to improved survival for SCD patients in wealthy countries, survival remains dismal in low- and middle-income countries. Much of the early mortality associated with SCD is attributed to increased risk of infections due to early loss of splenic function. In the West, bacterial infections with encapsulated organisms are a primary concern. In sub-Saharan Africa, where the majority of infants with SCD are born, the same is true; however malaria presents an additional threat to survival. The search for factors that define variability in sickle cell phenotypes should include environmental modifiers, such as malaria. Further exploration of this relationship could lead to novel strategies to reduce morbidity and mortality attributable to infections. In this review, we explore the interactions between SCD, malaria and the spleen to better understand how splenomegaly and splenic (dys)function may co-exist in patients with SCD living in malaria-endemic areas.
Keywords: sickle cell disease, spleen, malaria, marginal zone B cell, immune function
Sickle Cell Disease is a Global Health Concern
Approximately 400,000 infants annually are born with sickle cell disease (SCD) worldwide, including over 300,000 in sub-Saharan Africa (SSA; Piel et al, 2013). In all SCD patients, haemoglobin S (HbS) is the predominant haemoglobin component. In carrier states, HbS or other haemoglobin variants (e.g. HbC and thalassemia) coexist with HbA. These heterozygous traits confer a survival advantage against severe clinical malaria due to Plasmodium falciparum (Gong et al, 2013; Fleming et al, 1979). Some West African countries report HbS carrier rates as high as 25% (Grosse et al, 2011). The mechanisms of sickle trait protection against malaria are not completely understood but include plasmodium-induced sickling, impaired parasite growth, and decreased adherence of parasitized erythrocytes to microvascular endothelium (Bunn, 2013).
An estimate of survival in successive SCD cohorts in the U.S., Europe and Caribbean in 2010 demonstrated that survival through childhood has increased markedly over the last 40 years and is partially attributable to improvements in newborn screening and preventive care (Quinn et al, 2010). In a similar period, survival for children with SCD in SSA has not improved. An earlier survey in Northern Nigeria identified 98% mortality in the first two years of life for children with SCD (Fleming et al, 1979). In a recent Tanzanian SCD cohort, the observed under-5 mortality rate, of 7.3 per 100 person-years, was 5-fold higher than those that died at later ages, consistent with limited childhood survival (Makani et al, 2011). A Nigerian preventive care programme for children and adults with SCD reported an annual mortality rate of 20% during the first year of operation (Akinyanju et al, 2005). Overall, an estimated 50–90% of children born with SCD in SSA die before 5 years of age (Grosse et al, 2011). Whereas newborn screening and clinical care programmes are operating in select locations across the continent (Ohene-Frempong et al, 2008; McGann et al, 2013; Tubman et al, 2016; Kafando et al, 2009; Rahimy et al, 2009), an estimated 50% survival may represent the best-case scenario in parts of SSA where care programmes have not been established. Unfortunately, no country in SSA has introduced a national newborn screening programme, making preventive care an important intervention for improving early childhood survival.
Loss of splenic function increases the risk of early death due to infections among SCD patients. Neither encapsulated bacteria nor parasitized erythrocytes can be cleared effectively by the spleen in SCD. In patients living outside of SSA, the spleen becomes fibrotic and atrophied early in life. In contrast, several malaria-endemic countries have reported splenomegaly in children with SCD, including among children with HbSS. Splenomegaly is attributed to recurrent infections with Plasmodium species (Adekile et al, 1988; McAuley et al, 2010; Sadarangani et al, 2009). Hyperreactive malarial splenomegaly among patients without SCD is associated with expansion and dysfunction of both the red pulp and the lymphoid tissues in the spleen. More detailed studies of splenic function in SCD in SSA would elucidate a role for malaria in SCD mortality and highlight immune mechanisms critical to sickle pathogenesis.
The spleen
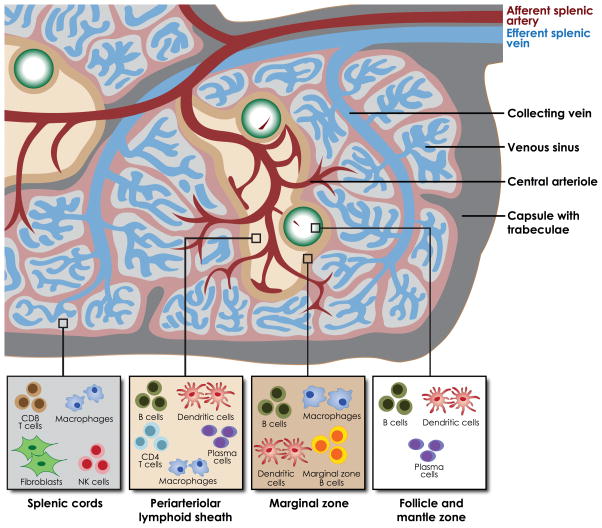
The spleen is organized to facilitate interactions between lymphocytes and antigen-presenting cells, allowing for the development of humoral immunity, though B lymphocyte proliferation, and for innate immunity, through cytokine signalling and phagocytosis (Fig. 1, 2A). Approximately 5% of the cardiac output is directed to the spleen each minute (William and Corazza, 2007). Through the splenic artery, blood enters the spleen through the perifollicular zone. Ninety per cent of blood travels to the red pulp, where blood flow slows as cells squeeze through splenic cords to re-enter circulation through the splenic vein. Cells with impaired deformability, including sickled cells and parasitized cells, cannot pass through the cords. Red pulp macrophages engulf and digest these damaged or senescent cells. The remaining 10% of blood is directed to the white pulp, crossing the periarteriolar lymphoid sheath and follicle. In the follicle, blood interacts with follicular T and B lymphocytes and dendritic cells en route to the marginal zone.
Figure 1. Architecture of the spleen.
Blood enters the spleen through the afferent splenic artery and is directed either to the periarteriolar lymphoid sheath and follicles, or to the red pulp and sinuses. Antigen-presenting cells interact with phagocytic cells and lymphocytes to mount humoral and innate responses to malaria. Figure adapted by permission from Macmillan Publishers Ltd: Nat Rev Immunol, Mebius, R. E., Kraal, G. Structure and function of the spleen. 5, 606–616, ©2005
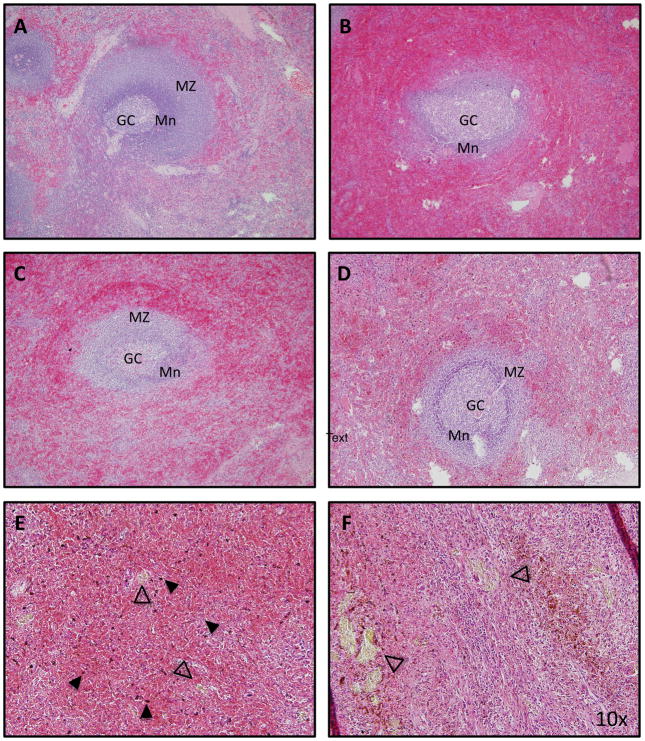
Figure 2. Histopathology of the spleen from patients with sickle cell disease.
(A) In normal spleen tissue, the follicle is organized into germinal centre (GC), mantle zone (Mn) and marginal zone (MZ).Senescent red cells are trapped and destroyed in the surrounding red pulp. (B) In a US patient with SCD who was splenectomised following recovery from splenic sequestration, the red pulp is congested with sickled cells. The structure of the follicle is less well defined. (C) An African patient with SCD who was splenectomised for splenic sequestration demonstrates congested of red pulp and preserved follicular architecture. (D) An African SCD patient who was splenectomised for hypersplenism similarly demonstrates engulfment of red pulp and preserved follicular architecture. In this patient, haemozoin deposits (E, solid arrowhead) and gamna gandy bodies are prominent (E and F, open arrowhead). All images are stained with haematoxylin and eosin stain. Magnification: 10x.
In the marginal zone, pathogens encounter lymphocytes, dendritic cells, and macrophages. The marginal zone is home to a specialized subset of non-switched memory B cells, or marginal zone B (MZB) lymphocytes (MZBs). MZBs facilitate T-cell independent humoral immune responses, detecting blood-borne pathogens and differentiating into plasma cells that produce immunoglobulin M (IgM). This action is critical for response to polysaccharide antigens, such as the pneumococcal polysaccharide vaccine. Absence of splenic MZBs is associated with increased risk of invasive pneumococcal disease and impaired response to pneumococcal vaccines (Wardemann et al, 2002). MZBs also function as antigen-presenting cells, recognizing pathogens and migrating into the follicle and periarteriolar lymphoid sheath for a T-cell dependent response.
MZB lymphocytes are critical for the development of the spleen and for normal immune function in murine models and humans. Absence of signalling from Notch-2, a regulator of cellular differentiation, leads to almost complete absence of MZBs in a conditional knockout mouse model (Saito et al, 2003). When MZBs are absent from a transgenic murine model, marginal zone macrophages and the structure of the marginal zone fail to develop (Nolte et al, 2004; Mebius and Kraal, 2005). Mice lacking tumour necrosis factor (TNF) or lymphotoxin-α demonstrate absent or disorganized marginal zone architecture (Korner et al, 2001; Martin and Kearney, 2002). Human studies suggest that the spleen is necessary to maintain the MZB subset. After splenectomy, adults do not maintain normal levels of circulating MZBs in the peripheral blood (pbMZB; Lammers et al, 2012). Similarly, in patients with congenital asplenia, the pbMZB compartment is appropriate for age below 4 years, but does not persist at normal levels as patient age increases (Weill et al, 2009). Without MZB, and thus without a functional spleen, protection against S. pneumoniae is diminished or wanes (Weller et al, 2005).
Measuring splenic function
Splenic function is measured by microscopic, biochemical, flow cytometric and radiographic techniques. Howell Jolly bodies (HJB) are intraerythrocytic nucleic acid remnants whose presence in erythrocytes marks splenic dysfunction. Cells containing HJBs are usually trapped by the spleen as their impaired deformability prevents passage through splenic cords. Patients with normal splenic function are expected to have no more than 300 HJB per 106 erythrocytes (Rogers et al, 2011). HJBs are typically identified by manual counting on a peripheral blood smear, which is labour-intense and poorly reproducible (Casper et al, 1976). A method for determining HJBs by flow cytometry has been described, but is not widely available (Harrod et al, 2007). Furthermore, as the flow cytometry assay described identifies DNA in erythrocytes, it is probably confounded by the presence of intraerythrocytic parasites in the setting of malaria (Dertinger et al, 2000).
Counting pitted red blood cells (RBC) visualized by direct interference contrast microscopy measures splenic dysfunction and correlates well with HJB count by flow cytometry (Rogers et al, 2011). Senescent erythrocytes, which accumulate microvesicles (i.e. ‘pits’) under the cell membrane, are normally cleared by the spleen. Patients with functional spleens should have less than 3.5% pitted RBCs when counted manually (Rogers et al, 1982; Rogers et al, 2011). Assessment of pitted RBCs is labour intensive and requires specialized instruments. In the malaria-exposed patient, counting is also potentially confounded by erythrocytes that have similar microvesicles due to prior removal of intraerythrocytic parasites after crossing the spleen (Anyona et al, 2006).
Flow cytometry and radiographic techniques are least commonly used. Quantifying pbMZB by flow cytometry has been used to define splenic function in disorders, such as coeliac disease, autoimmune lymphoproliferative syndrome, immune thrombocytopenia, and in adults with SCD (Di Sabatino et al, 2013; Wasserstrom et al, 2008; Lammers et al, 2012; Neven et al, 2014). MZBs develop in the spleen throughout infancy and circulate in the peripheral blood. MZBs, characterized by surface markers IgM+IgDloCD27+ are present in the spleen, lymph nodes, tonsils and gut-associated lymphoid tissues (Weill et al, 2009). PbMZB comprise 1% of the peripheral B lymphocyte population in cord blood, but increase to adult levels (15–20%) by 2 years of age (Weill et al, 2009; Timens et al, 1987). Widespread use of pbMZB quantification in paediatric patients must be adjusted for the variability of normal values with age. 99m Technectium (99mTc) scan correlates with the percentage of non-switched memory B cells (Lammers et al, 2012). Measuring macrophage uptake of 99mTc by liver/spleen (L/S) scan has been used to assess spleen function in multiple clinical investigations (Pearson et al, 1969; Rogers et al, 2011). Exposure to radiation, though limited, inhibits widespread use of this technique for monitoring.
The spleen in SCD
The spleen is one of the first organs affected in children with SCD. Children with SCD demonstrate abnormal uptake on L/S scan during infancy (Pearson et al, 1969). On histopathologic review, the spleen tissue from patients with SCD reveals red pulp engorged with sickled erythrocytes and fibrosis in the microvasculature (Fig. 2B). In some SCD patients exposed to malaria, the architecture of the follicle appears to be maintained and engorging of the red pulp is apparent (Figs. 2C, 2D). Malaria pigment, or haemozoin, is prominently observed throughout the spleen in a patient exposed to malaria (Fig. 2E). Also striking are the presence of gamna gandy bodies, deposits of haemosiderin, fibrous tissue and cellular debris, which are seen in both SCD and in malaria (Fig. 2F). Once the red pulp becomes fully engorged, erythrocytes redirect toward the white pulp, where they are engulfed by macrophages (William and Corazza, 2007; Pearson et al, 1969). In both human studies and murine models, disorganization of the white pulp has also been observed (Brousse et al, 2014; Szczepanek et al, 2012). Disorganization of both compartments probably contributes to splenic dysfunction.
Splenic dysfunction occurs commonly, but may not be permanent. In a US-based study, 75% of paediatric patients with HbSS demonstrated decreased or absent splenic function by 9 months of age, rising to 90% by age 15 months (Rogers et al, 2011). Patients in the US and Europe exhibit transient splenomegaly followed by splenic atrophy and fibrosis with corresponding increase in markers of splenic dysfunction by age 6 years (Rogers et al, 2011; Pearson et al, 1969). Splenic dysfunction has been reversed with chronic transfusion (Wang et al, 2001; Pearson et al, 1970) and with hydroxycarbamide (also termed hydroxyurea) (Hankins et al, 2008).
Clinical markers of splenic dysfunction correlate well with patient outcomes in non-African cohorts. In a retrospective investigation, patients with SCD and recurrent infection had higher pitted RBC counts than those without infection (De Ceulaer et al, 1985). US adults with HbSS and splenectomized patients were found to have significantly lower portions of non-switched memory B cells than controls or patients with HbSC. These findings correlated with response to pneumococcal polysaccharide vaccine (Lammers et al, 2012). In a meta-analysis of studies on infections, a 36-fold increased risk of infection with S. pneumoniae was observed amongst patients with SCD in SSA compared to patients without SCD, mirroring the risk of infection seen in the USA (Ramakrishnan et al, 2010; Wong et al, 1992).
A unique course of spleen growth among SCD patients in SSA suggests a physiology different than what is observed outside of SSA. Contrary to the US experience of splenic atrophy within the first 6 years, multiple descriptive and cross-sectional studies of SCD cohorts in SSA have described splenomegaly into the second decade of life. In a study of Nigerian adults with SCD, 4% had splenomegaly at a mean age of 23 years (Babadoko et al, 2012). In Kenya, splenomegaly was present in 10% of children with SCD under 2 years of age, which parallels the transient growth described in the US. However, in this cohort, splenomegaly peaked in prevalence at 44% in children aged 6–8 years (Sadarangani et al, 2009). In the Muhimbili Sickle Cohort (MSC), splenomegaly was noted in 10% of outpatient visits for patients without clinical malaria, where the average age was 13 years (Makani et al, 2010). Splenomegaly has similarly been described in SCD cohorts in malarious regions of India and the Middle East (Al-Salem, 2011; Chopra et al, 2005).
Functional studies paired with clinical observations are needed to clarify the impact of splenomegaly on infections and on the immune function of the spleen. Reports describing immune function in enlarged spleens in SSA have yielded conflicting results. Elevated HJB-containing RBCs were observed in one cohort of patients with splenomegaly, suggesting splenomegaly is associated with impaired splenic function (Awotua-Efebo et al, 2004). In another cohort, a statistically significant inverse relationship between spleen size and pitted RBC count was observed, suggesting that splenic function is retained as spleen size increases (Adekile et al, 1993).
Splenomegaly has been associated with numerous complications among patients with SCD (Al-Salem, 2011). Acute splenic sequestration occurs in early childhood and is known to recur frequently with high mortality (Rogers et al, 1978; Gill et al, 1995). Splenomegaly may co-exist with hypersplenism or trapping of whole blood in the spleen, thereby exacerbating anaemia. In patients with SCD, hypersplenism is associated with more frequent hospitalization and increased transfusion requirement (Brousse et al, 2014). Splenic infarctions occur in patients with sickle cell trait and in patients with ‘milder’, compound heterozygous forms of SCD, who are less likely to undergo early splenic atrophy.
The finding of persistent splenomegaly in patients with HbSS has been attributed to malaria exposure, though data is limited. Among Nigerian patients with HbSS, those with splenomegaly had a higher mean parasite density in the peripheral blood than patients with HbSS without splenomegaly (Awotua-Efebo et al, 2004). An African patient found to have splenomegaly after emigrating to the US was hypothesized to have splenomegaly to allow for some clearance/sequestering of malaria, suggesting that the parasite exacts a change on the host that counteracts the natural progression of SCD (De Franceschi et al, 2005). Anti-malarial IgG titres increased with spleen size in a Nigerian SCD cohort, suggesting a relationship between size and malaria infections (Adekile et al, 1993). However, during two years of observation in a Kenyan SCD cohort, spleen size did not correlate with clinical episodes of malaria (Sadarangani et al, 2009).
P. falciparum malaria
P. falciparum is an intraerythrocytic protozoan parasite endemic to SSA. The clinical syndrome involves cyclic fevers and anaemia, but can include severe neurological, renal and other end organ toxicities. The asexual stage of the parasite infects humans and matures in erythrocytes. The parasite feeds on haem, causing cell lysis as it matures and releases merozoites and pro-inflammatory parasite proteins into the serum. These proteins coat non-infected erythrocytes, stimulating a systemic inflammatory response. P. falciparum erythrocyte membrane protein-1 (PfEMP1), a parasite-derived protein, is expressed on the ERYTHROCYTE membrane during later stages of parasite development. PfEMP1 is highly immunogenic. It facilitates adhesion to vascular endothelium, preventing presentation of later-stage infected erythrocytes to the spleen. Early-stage parasitized erythrocytes and coated erythrocytes travel to the spleen and sequester in the spleen through macrophage activation and by mechanical inability to traverse splenic slits (Haldar and Mohandas, 2009).
Patients with SCD do not share the protective effect of HbS against malaria seen in patients with sickle cell trait. Patients with SCD develop clinical malaria at lower frequency than non-SCD patients, but experience higher mortality (Williams and Obaro 2011; Makani et al, 2011; Komba et al, 2009). In a 10-year retrospective review of patients hospitalized with SCD in the Democratic Republic of Congo, 63% of patients with SCD were diagnosed with clinical malaria by blood smear review and clinical criteria (Aloni et al, 2013). Splenomegaly was noted in 38% of children with SCD and clinical malaria. In a case-control study in Kenya, mortality among children with HbSS hospitalized for malaria was 8-fold higher than patients with HbAA, and 25-fold higher than patients with HbAS (McAuley et al, 2010). Despite higher mortality, patients with SCD have lower prevalence of parasitaemia and lower parasite density relative to patients without SCD (Komba et al, 2009; Awotua-Efebo et al, 2004).
Excess morbidity and mortality attributable to malaria in patients with SCD is multi-factorial. Fever, jaundice and haemoglobin < 50 g/l were more frequently identified in patients with SCD and malaria than those without malaria. (Aloni et al, 2013; Scott et al, 2011). Severe anaemia, which may result from malaria or SCD, is associated with increased risk of mortality. Haemoglobin <50 g/l is an independent predictor of death (Makani et al, 2011). Bacteraemia is widely understood to complicate acute malaria, and compounds the morbidity of malaria infections in patients with SCD (Scott et al, 2011; Berkley et al, 1999; Williams et al, 2009). Sickling and inflammation is increased by infected erythrocytes adhering to the vascular endothelium, thus reducing distal tissue perfusion. P. falciparum preferentially invades younger erythrocytes, including abundant reticulocytes in patients with SCD (Pasvol et al, 1980). Impaired splenic function probably retards uptake of parasites by the spleen as evidenced by the correlation between parasite load and pitted red cell count in patients with SCD (Awotua-Efebo et al, 2004).
The spleen in malaria
The immune response to malaria requires both antibody-mediated immunity and cell-mediated immunity (Riley and Stewart, 2013). Acute malaria stimulates a massive immune response in lymphoid tissues, especially the spleen. During a malaria infection in a non-SCD patient, brisk immune responses can lead to expansion of the white and red pulp and hypersplenism. After inoculation via mosquito bite, malaria sporozoites are directed to lymph tissues. In the spleen, parasites and parasitized cells activate dendritic cells to release inflammatory cytokines, which induce TH1 helper cell differentiation (Fig. 1). TH1 helper cells activate B cell differentiation and secretion of interferon-γ. Interferon gamma activates macrophages, causing TNF-α production and subsequently nitric oxide synthesis. Higher levels of TNF-α in the spleen are associated with splenomegaly in acute murine malaria (Jacobs et al, 1996). Over time, phagocytic cells loose efficacy as undigested haemosiderin deposits limit their function (Figs. 2E, 2F). The spleen may act as a reservoir for parasites (Linares et al, 2011; De Franceschi et al, 2005), which may place an additional burden on the innate system to control the infection. Developing antibodies to malaria takes decades, due to the wide variety of immunogenic proteins produced by the parasite (Wipasa et al, 2002).
Historically, the prevalence of splenomegaly in a population, termed the ‘spleen rate’, had been used as a marker of malaria prevalence (Edington, 1967; Zingman and Viner 1993; Shukla et al, 2011). At the extreme end of the spectrum, patients can develop hyperreactive malarial splenomegaly, a syndrome of excessive immune response to malaria marked by extreme organ enlargement, high total IgM and elevated malaria antibody titres. Hyperreactive malarial splenomegaly has been associated with human leucocyte antigen (HLA)–DR2 and the immunoglobulin haplotype, IgHG3 (Kelly, 1996; Tano et al, 2014).
Acute malaria infection is associated with shifts in peripheral blood B lymphocyte subclasses, including the pbMZB subset. Memory B lymphocytes rise acutely, and then return to normal levels during the recovery phase of malaria infection in children without SCD (Asito et al, 2008). Absolute pbMZB counts are lower in infants from malaria-endemic areas than in controls from a non-endemic area (Scholzen and Sauerwein 2013). In a murine model with P. chaubaudi, splenic MZBs decreased during acute infection and returned after clearance of infection with disordered morphology (Achtman et al, 2003; Stephens et al, 2009). Though peripheral blood lymphocyte subclasses have not been characterized in patients with SCD exposed to malaria, high immunoglobulin levels have been reported in patients with splenomegaly (Adekile et al, 1988).
The spleen in SCD in Africa: What is the impact of malaria?
The finding of unexpected splenomegaly in patients with SCD living in malaria-endemic areas suggests a ‘’turf war”: malaria is presumed to cause expansion and inefficacy of macrophages and lymphocytes in the spleen that are typically destroyed or damaged by sickling. Histopathology of the spleen shows both sickled cells and malaria pigment in some patients. Where splenic function may be restored with agents such as hydroxycarbamide, the impact of malaria on immunity may be an important consideration as hydroxycarbamide use expands. Malaria causes expansion of the MZB compartment, while sickling causes it to contract. Could recurrent malaria infections alter lymphocyte populations in patients with SCD? Given the clinical observation of increased mortality from malaria amongst patients with SCD, it is likely that the combined effect of malaria and sickling is detrimental. However, a prospective investigation into the combined effect of SCD and malaria on the immune systen would verify this relationship and elucidate mechanisms central to loss or preservation of splenic function.
In this review, we have shown how these processes overlap and may result in altered splenic function. Additional laboratory tools may need to be developed to assess splenic function in the presence of malaria. Known predictors of splenomegaly in SCD (e.g. HbF and HbA2) and genetic modifiers of malarial splenomegaly (e.g. HLA-DR2 and IgHG3) should be investigated in SCD patients with splenomegaly (Bhatia & Crane, 1989; Kelly, 1996; Steinberg & Sebastiani, 2012). For example, the HLA-DR2 phenotype is associated with hyperreactive splenomegaly (Bhatia and Crane, 1989) and protects against stroke in patients with SCD (Styles et al, 2000) but has not been defined in patients with both splenomegaly and SCD. Other infections that cause splenomegaly, such as schistosomiasis, human immunodeficiency vrius, cytomegalovirus and Epstein–Barr virus may also have a role in splenomegaly in SCD. Investigations into shared mechanisms of sickling and malaria pathogensis have produced useful tools for therapeutic drug design (Tubman et al, 2015). If malaria is associated with splenomegaly in SCD, there is potential to exploit this interaction to enhance understanding of SCD in Africa and to reduce the impact of infections on patients with SCD around the world.
Acknowledgments
VNT received support from the National Institutes of Health (K12HL087164). JM received funding from the Wellcome Trust, UK (WT093727MA). The authors would like to thank Dr. Marian Harris and Amos Mwakigonja for the pathology slides. The authors would like to thank Dr. Emmanuel Balandya and Dr. H. Franklin Bunn for reviewing this work.
Footnotes
Authorship
VNT wrote the paper. VNT and JM designed the search strategy. VNT and JM revised and approved of the final manuscript.
Disclosures
The authors have no relevant conflicts of interest to disclose.
References
- Achtman AH, Khan M, MacLennan IC, Langhorne J. Plasmodium chabaudi chabaudi infection in mice induces strong B cell responses and striking but temporary changes in splenic cell distribution. J Immunol. 2003;171(1):317–324. doi: 10.4049/jimmunol.171.1.317. [DOI] [PubMed] [Google Scholar]
- Adekile A, Adedodu O, Jeje A, Odesanmi W. Persistent gross splenomegaly in Nigeiran patients with sickle cell anaemia: relationship to malaria. Ann Trop Paediatr. 1988;8:103–107. doi: 10.1080/02724936.1988.11748549. [DOI] [PubMed] [Google Scholar]
- Adekile AD, McKie KM, Adeodu OO, Sulzer AJ, Liu JS, McKie VC, Kutlar F, Ramachandran M, Kaine W, Akenzua GI, Okolo AA, Obinyan EA, Ogala WN, Ibrahim M, Huisman THJl. Spleen in sickle cell anemia: comparative studies of Nigerian and U.S. patients. Am J Hematol. 1993;42(3):316–321. doi: 10.1002/ajh.2830420313. [DOI] [PubMed] [Google Scholar]
- Akinyanju OO, Otaigbe AI, Ibidapo MO. Outcome of holistic care in Nigerian patients with sickle cell anaemia. Clin Lab Haematol. 2005;27(3):195–199. doi: 10.1111/j.1365-2257.2005.00683.x. [DOI] [PubMed] [Google Scholar]
- Al-Salem AH. Splenic complications of sickle cell anemia and the role of splenectomy. ISRN Hematol. 2011;2011 doi: 10.5402/2011/864257. Article 864257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloni MN, Tshimanga BK, Ekulu PM, Ehungu JL, Ngiyulu RM. Malaria, clinical features and acute crisis in children suffering from sickle cell disease in resource-limited settings: a retrospective description of 90 cases. Pathog Glob Health. 2013;107(4):198–201. doi: 10.1179/2047773213Y.0000000089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anyona SB, Schrier SL, Gichuki CW, Waitumbi JN. Pitting of malaria parasites and spherocyte formation. Malar J. 2006;5:64. doi: 10.1186/1475-2875-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asito AS, Moormann AM, Kiprotich C, Ng’ang’a ZW, Ploutz-Snyder R, Rochford R. Alterations on peripheral B cell subsets following an acute uncomplicated clinical malaria infection in children. Malar J. 2008;7:238. doi: 10.1186/1475-2875-7-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awotua-Efebo O, Alikor EA, Nkanginieme KE. Malaria parasite density and splenic status by ultrasonography in stable sickle-cell anaemia (HbSS) children. Niger J Med. 2004;13(1):40–43. [PubMed] [Google Scholar]
- Babadoko AA, Ibinaye PO, Hassan A, Yusuf R, Ijei IP, Aiyekomogbon J, Aminu SM, Hamidu AU. Autosplenectomy of sickle cell disease in zaria, Nigeria: an ultrasonographic assessment. Oman Med J. 2012;27(2):121–123. doi: 10.5001/omj.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley J, Mwarumba S, Bramham K, Lowe B, Marsh K. Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg. 1999;93(3):283–286. doi: 10.1016/s0035-9203(99)90024-x. [DOI] [PubMed] [Google Scholar]
- Bhatia KK, Crane GG. HLA heterozygosity and hyperreactive malarious splenomegaly in the Upper Watut Valley of Papua New Guinea. P N G Med J. 1989;32(4):277–286. [PubMed] [Google Scholar]
- Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick(led) spleen. Br J Haematol. 2014;166(2):165–176. doi: 10.1111/bjh.12950. [DOI] [PubMed] [Google Scholar]
- Bunn HF. The triumph of good over evil: protection by the sickle gene against malaria. Blood. 2013;121(1):20–25. doi: 10.1182/blood-2012-08-449397. [DOI] [PubMed] [Google Scholar]
- Casper JT, Koethe S, Rodey GE, Thatcher LG. A new method for studying splenic reticuloendothelial dysfunction in sickle cell disease patients and its clinical application: a brief report. Blood. 1976;47(2):183–188. [PubMed] [Google Scholar]
- Chopra R, Al-Mulhim AR, Al-Baharani AT. Fibrocongestive splenomegaly in sickle cell disease: a distinct clinicopathological entity in the Eastern province of Saudi Arabia. Am J Hematol. 2005;79(3):180–186. doi: 10.1002/ajh.20380. [DOI] [PubMed] [Google Scholar]
- De Ceulaer K, Pagliuca A, Forbes M, Maude GH, Serjeant BE, Serjeant GR. Recurrent infections in sickle cell disease: haematological and immune studies. Clin Chim Acta. 1985;148(3):161–165. doi: 10.1016/0009-8981(85)90142-1. [DOI] [PubMed] [Google Scholar]
- De Franceschi L, Sada S, Andreoli A, Angheben A, Marocco S, Bisoffi Z. Sickle cell disease and hyperreactive malarial splenomegaly (HMS) in young immigrants from Africa. Blood. 2005;106(13):4415–4416. doi: 10.1182/blood-2005-08-3109. [DOI] [PubMed] [Google Scholar]
- Dertinger SD, Torous DK, Hall NE, Tometsko CR, Gasiewicz TA. Malaria-infected erythrocytes serve as biological standards to ensure reliable and consistent scoring of micronucleated erythrocytes by flow cytometry. Mutation Research. 2000;464:195–200. doi: 10.1016/s1383-5718(99)00183-7. [DOI] [PubMed] [Google Scholar]
- Di Sabatino A, Brunetti L, Carnevale Maffe G, Giuffrida P, Corazza GR. Is it worth investigating splenic function in patients with celiac disease? World J Gastroenterol. 2013;19(15):2313–2318. doi: 10.3748/wjg.v19.i15.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edington GM. Pathology of malaria in West Africa. Br Med J. 1967;1(5542):715–718. doi: 10.1136/bmj.1.5542.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AF, Storey J, Molineaux L, Iroko EA, Attai ED. Abnormal haemoglobins in the Sudan savanna of Nigeria. I. Prevalence of haemoglobins and relationships between sickle cell trait, malaria and survival. Ann Trop Med Parasitol. 1979;73(2):161–172. doi: 10.1080/00034983.1979.11687243. [DOI] [PubMed] [Google Scholar]
- Gill FM, Sleeper LA, Weiner SJ, Brown AK, Bellevue R, Grover R, Pegelow CH, Vichinsky E. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood. 1995;86(2):776–783. [PubMed] [Google Scholar]
- Gong L, Parikh S, Rosenthal PJ, Greenhouse B. Biochemical and immunological mechanisms by which sickle cell trait protects against malaria. Malar J. 2013;12:317. doi: 10.1186/1475-2875-12-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in Africa: a neglected cause of early childhood mortality. Am J Prev Med. 2011;41(6 Suppl 4):S398–405. doi: 10.1016/j.amepre.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar K, Mohandas N. Malaria, erythrocytic infection, and anemia. Hematology Am Soc Hematol Educ Program. 2009;2009:87–93. doi: 10.1182/asheducation-2009.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankins JS, Helton KJ, McCarville MB, Li CS, Wang WC, Ware RE. Preservation of spleen and brain function in children with sickle cell anemia treated with hydroxyurea. Pediatr Blood Cancer. 2008;50(2):293–297. doi: 10.1002/pbc.21271. [DOI] [PubMed] [Google Scholar]
- Harrod VL, Howard TA, Zimmerman SA, Dertinger SD, Ware RE. Quantitative analysis of Howell-Jolly bodies in children with sickle cell disease. Exp Hematol. 2007;35(2):179–183. doi: 10.1016/j.exphem.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Jacobs P, Radzioch D, Stevenson MM. In vivo regulation of nitric oxide production by tumor necrosis factor alpha and gamma interferon, but not by interleukin-4, during blood stage malaria in mice. Infect Immun. 1996;64(1):44–49. doi: 10.1128/iai.64.1.44-49.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafando E, Nacoulma E, Ouattara Y, Ayeroue J, Cotton F, Sawadogo M, Gulbis B. Neonatal haemoglobinopathy screening in Burkina Faso. J Clin Pathol. 2009;62(1):39–41. doi: 10.1136/jcp.2008.058966. [DOI] [PubMed] [Google Scholar]
- Kelly KM. IGHG3 G and the pathogenesis of hyperreactive malarious splenomegaly. Med Hypotheses. 1996;46(2):135–139. doi: 10.1016/s0306-9877(96)90013-4. [DOI] [PubMed] [Google Scholar]
- Komba AN, Makani J, Sadarangani M, Ajala-Agbo T, Berkley JA, Newton CR, Marsh K, Williams TN. Malaria as a cause of morbidity and mortality in children with homozygous sickle cell disease on the coast of Kenya. Clin Infect Dis. 2009;49(2):216–222. doi: 10.1086/599834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner H, Winkler TH, Sedgwick JD, Rollinghoff M, Basten A, Cook MC. Recirculating and marginal zone B cell populations can be established and maintained independently of primary and secondary follicles. Immunol Cell Biol. 2001;79(1):54–61. doi: 10.1046/j.1440-1711.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- Lammers AJ, de Porto AP, Bennink RJ, van Leeuwen EM, Biemond BJ, Goslings JC, van Marle J, ten Berge IJ, Speelman P, Hoekstra JB. Hyposplenism: comparison of different methods for determining splenic function. Am J Hematol. 2012;87(5):484–489. doi: 10.1002/ajh.23154. [DOI] [PubMed] [Google Scholar]
- Linares M, Albizua E, Mendez D, Rubio JM, Martinez-Serna A, Martinez MA, … Bautista JM. Malaria hidden in a patient with diffuse large-B-cell lymphoma and sickle-cell trait. J Clin Microbiol. 2011;49(12):4401–4404. doi: 10.1128/JCM.00911-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani J, Komba AN, Cox SE, Oruo J, Mwamtemi K, Kitundu J, Magesa P, Rwezaula S, Meda E, Mgaya J, Pallangyo K, Okiro E, Muturi D, Newton CR, Fegan G, Marsh K, Williams TN. Malaria in patients with sickle cell anemia: burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood. 2010;115(2):215–220. doi: 10.1182/blood-2009-07-233528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani J, Cox SE, Soka D, Komba AN, Oruo J, Mwamtemi H, Magesa P, Rwezaula S, Meda E, Mgaya J, Lowe B, Muturi D, Roberts DJ, Williams TN, Pallangyo K, Kitundu J, Fegan G, Kirkham FJ, Marsh K, Newton CR. Mortality in sickle cell anemia in Africa: a prospective cohort study in Tanzania. PLoS One. 2011;6(2):e14699. doi: 10.1371/journal.pone.0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F, Kearney JF. Marginal-zone B cells. Nat Rev Immunol. 2002;2(5):323–335. doi: 10.1038/nri799. [DOI] [PubMed] [Google Scholar]
- McAuley CF, Webb C, Makani J, Macharia A, Uyoga S, Opi DH, Ndila C, Ngatia A, Scott JA, Marsh K, Williams TN. High mortality from Plasmodium falciparum malaria in children living with sickle cell anemia on the coast of Kenya. Blood. 2010;116(10):1663–1668. doi: 10.1182/blood-2010-01-265249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann PT, Ferris MG, Ramamurthy U, Santos B, de Oliveira V, Bernardino L, Ware RE. A prospective newborn screening and treatment program for sickle cell anemia in Luanda, Angola. Am J Hematol. 2013;88(12):984–989. doi: 10.1002/ajh.23578. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5(8):606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- Neven B, Bruneau J, Stolzenberg MC, Meyts I, Magerus-Chatinet A, Moens L, Lanzarotti N, Weller S, Amiranoff D, Florkin B, Bader-Meunier B, Leverger G, Ferster A, Chantrain C, Blanche S, Picard C, Molina TJ, Brousse N, Durandy A, Rizzi M, Bossuyt X, Fischer A, Rieux-Laucat F. Defective anti-polysaccharide response and splenic marginal zone disorganization in ALPS patients. Blood. 2014;124(10):1597–1609. doi: 10.1182/blood-2014-02-553834. [DOI] [PubMed] [Google Scholar]
- Nolte MA, Arens R, Kraus M, van Oers MH, Kraal G, van Lier RA, Mebius RE. B cells are crucial for both development and maintenance of the splenic marginal zone. J Immunol. 2004;172(6):3620–3627. doi: 10.4049/jimmunol.172.6.3620. [DOI] [PubMed] [Google Scholar]
- Ohene-Frempong K, Oduro J, Tetteh H, Nkrumah F. Screening Newborns for Sickle Cell Disease in Ghana. Pediatrics. 2008;121(Supplement):S120–S121. [Google Scholar]
- Pasvol G, Weatherall DJ, Wilson RJ. The increased susceptibility of young red cells to invasion by the malarial parasite Plasmodium falciparum. Br J Haematol. 1980;45(2):285–295. doi: 10.1111/j.1365-2141.1980.tb07148.x. [DOI] [PubMed] [Google Scholar]
- Pearson HA, Spencer RP, Cornelius EA. Functional asplenia in sickle-cell anemia. N Engl J Med. 1969;281(17):923–926. doi: 10.1056/NEJM196910232811703. [DOI] [PubMed] [Google Scholar]
- Pearson HA, Cornelius EA, Schwartz AD, Zelson JH, Wolfson SL, Spencer RP. Transfusion-reversible functional asplenia in young children with sickle-cell anemia. N Engl J Med. 1970;283(7):334–337. doi: 10.1056/NEJM197008132830703. [DOI] [PubMed] [Google Scholar]
- Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, Temperley WH, Williams TN, Weatherall DJ, Hay SI. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood. 2010;115(17):3447–3452. doi: 10.1182/blood-2009-07-233700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimy MC, Gangbo A, Ahouignan G, Alihonou E. Newborn screening for sickle cell disease in the Republic of Benin. J Clin Pathol. 2009;62(1):46–48. doi: 10.1136/jcp.2008.059113. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan M, Moisi JC, Klugman KP, Iglesias JM, Grant LR, Mpoudi-Etame M, Levine OS. Increased risk of invasive bacterial infections in African people with sickle-cell disease: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(5):329–337. doi: 10.1016/S1473-3099(10)70055-4. [DOI] [PubMed] [Google Scholar]
- Riley EM, Stewart VA. Immune mechanisms in malaria: new insights in vaccine development. Nat Med. 2013;19(2):168–178. doi: 10.1038/nm.3083. [DOI] [PubMed] [Google Scholar]
- Rogers DW, Clarke JM, Cupidore L, Ramlal AM, Sparke BR, Serjeant GR. Early deaths in Jamaican children with sickle cell disease. Br Med J. 1978;1(6126):1515–1516. doi: 10.1136/bmj.1.6126.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DW, Serjeant BE, Serjeant GR. Early rise in the “pitted” red cell count as a guide to susceptibility to infection in childhood sickle cell anaemia. Arch Dis Child. 1982;57(5):338–342. doi: 10.1136/adc.57.5.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers ZR, Wang WC, Luo Z, Iyer RV, Shalaby-Rana E, Dertinger SD, Shulkin BL, Miller JH, Files B, Lane PA, Thompson BW, Miller ST, Ware RE for the BABY HUG. Biomarkers of splenic function in infants with sickle cell anemia: baseline data from the BABY HUG Trial. Blood. 2011;117(9):2614–2617. doi: 10.1182/blood-2010-04-278747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadarangani M, Makani J, Komba AN, Ajala-Agbo T, Newton CR, Marsh K, Williams TN. An observational study of children with sickle cell disease in Kilifi, Kenya. Br J Haematol. 2009;146(6):675–682. doi: 10.1111/j.1365-2141.2009.07771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Chiba S, Ichikawa M, Kunisato A, Asai T, Shimizu K, Yamaguchi T, Yamamoto G, Seo S, Kumano K, Nakagami-Yamaguchi E, Hamada Y, Aizawa S, Hirai H. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18(5):675–685. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- Scholzen A, Sauerwein RW. How malaria modulates memory: activation and dysregulation of B cells in Plasmodium infection. Trends Parasitol. 2013;29(5):252–262. doi: 10.1016/j.pt.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Scott JAG, Berkley JA, Mwangi I, Ochola L, Uyoga S, Macharia A, Ndila C, Lowe BS, Mwarumba S, Bauni E, Marsh K, Williams TN. Relation between falciparum malaria and bacteraemia in Kenyan children: a population-based, case-control study and a longitudinal study. Lancet. 2011;378(9799):1316–1323. doi: 10.1016/S0140-6736(11)60888-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla M, Singh N, Singh MP. Spleen rates and infant parasite rates as surveillance tool for malaria control in remote hard to reach areas of central India. Malar J. 2011;10:381. doi: 10.1186/1475-2875-10-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MH, Sebastiani P. Genetic modifiers of sickle cell disease. Am J Hematol. 2012;87(8):795–803. doi: 10.1002/ajh.23232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R, Ndungu FM, Langhorne J. Germinal centre and marginal zone B cells expand quickly in a second Plasmodium chabaudi malaria infection producing mature plasma cells. Parasite Immunol. 2009;31(1):20–31. doi: 10.1111/j.1365-3024.2008.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styles LA, Hoppe C, Klitz W, Vichinsky E, Lubin B, Trachtenberg E. Evidence for HLA-related susceptibility for stroke in children with sickle cell disease. Blood. 2000;95(11):3562–3567. [PubMed] [Google Scholar]
- Szczepanek SM, McNamara JT, Secor ER, Jr, Natarajan P, Guernsey LA, Miller LA, Ballesteros E, Jellison E, Thrall RS, Andemariam B. Splenic morphological changes are accompanied by altered baseline immunity in a mouse model of sickle-cell disease. Am J Pathol. 2012;181(5):1725–1734. doi: 10.1016/j.ajpath.2012.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tano ZN, Filho CE, Bregano RM, Pavanelli WR, Ruzon UG. Hyperreactive Malarious Splenomegaly and AIDS: a case report. Braz J Infect Dis. 2014;18(5):565–567. doi: 10.1016/j.bjid.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timens W, Rozeboom T, Poppema S. Fetal and neonatal development of human spleen: an immunohistological study. Immunology. 1987;60(4):603–609. [PMC free article] [PubMed] [Google Scholar]
- Tubman VN, Mejia P, Shmukler BE, Bei AK, Alper SL, Mitchell JR, Brugnara C, Duraisingh MT. The Clinically Tested Gardos Channel Inhibitor Senicapoc Exhibits Antimalarial Activity. Antimicrob Agents Chemother. 2015;60(1):613–616. doi: 10.1128/AAC.01668-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubman VN, Marshall R, Jallah W, Guo D, Ma C, Ohene-Frempong K, London WB, Heeney MM. Newborn Screening for Sickle Cell Disease in Liberia: A Pilot Study. Pediatr Blood Cancer. 2016;63(4):671–676. doi: 10.1002/pbc.25875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WC, Wynn LW, Rogers ZR, Scott JP, Lane PA, Ware RE. A two-year pilot trial of hydroxyurea in very young children with sickle-cell anemia. J Pediatr. 2001;139(6):790–796. doi: 10.1067/mpd.2001.119590. [DOI] [PubMed] [Google Scholar]
- Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195(6):771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserstrom H, Bussel J, Lim LC, Cunningham-Rundles C. Memory B cells and pneumococcal antibody after splenectomy. J Immunol. 2008;181(5):3684–3689. doi: 10.4049/jimmunol.181.5.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- Weller S, Reynaud CA, Weill JC. Vaccination against encapsulated bacteria in humans: paradoxes. Trends Immunol. 2005;26(2):85–89. doi: 10.1016/j.it.2004.11.004. [DOI] [PubMed] [Google Scholar]
- William BM, Corazza GR. Hyposplenism: a comprehensive review. Part I: basic concepts and causes. Hematology. 2007;12(1):1–13. doi: 10.1080/10245330600938422. [DOI] [PubMed] [Google Scholar]
- Williams TN, Obaro SK. Sickle cell disease and malaria morbidity: a tale with two tails. Trends Parasitol. 2011;27(7):315–320. doi: 10.1016/j.pt.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Williams TN, Uyoga S, Macharia A, Ndila C, McAuley CF, Opi DH, Mwarumba S, Makani J, Komba A, Ndiritu MN, Sharif SK, Marsh K, Berkley JA, Scott JAG. Bacteraemia in Kenyan children with sickle-cell anaemia: a retrospective cohort and case-control study. The Lancet. 2009;374(9698):1364–1370. doi: 10.1016/S0140-6736(09)61374-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipasa J, Elliott S, Xu H, Good MF. Immunity to asexual blood stage malaria and vaccine approaches. Immunol Cell Biol. 2002;80(5):401–414. doi: 10.1046/j.1440-1711.2002.01107.x. [DOI] [PubMed] [Google Scholar]
- Wong WY, Overturf GD, Powars DR. Infection caused by Streptococcus pneumoniae in children with sickle cell disease: epidemiology, immunologic mechanisms, prophylaxis, and vaccination. Clin Infect Dis. 1992;14(5):1124–1136. doi: 10.1093/clinids/14.5.1124. [DOI] [PubMed] [Google Scholar]
- Zingman BS, Viner BL. Splenic complications in malaria: case report and review. Clin Infect Dis. 1993;16(2):223–232. doi: 10.1093/clind/16.2.223. [DOI] [PubMed] [Google Scholar]