Abstract
The present study reports a young woman with acute ataxia, areflexia and ophthalmoplegia, accompanied by psychosis and involuntary movements (IVMs) from disease onset. Anti-GQ1b and anti-GT1a antibodies were detected allowing for a diagnosis of Miller Fisher syndrome (MFS). However, psychosis and IVMs are atypical MFS symptoms and often mimic symptoms of anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis. Interestingly, the autoantibodies against full-length glutamate receptor-ε2 (GluRε2) and glutamate NR2B- and NR2A-containing heteromers (NR1/NR2) of NMDAR were also detected in the patient serum and cerebrospinal fluid. It was concluded that psychosis and IVMs in this patient were associated with autoantibodies against various GluRs.
Background
Miller Fisher syndrome (MFS) is an autoimmune disorder accompanied by acute progressive ophthalmoplegia, ataxia and areflexia. Bickerstaff's brainstem encephalitis (BBE) is a related syndrome in which central nervous system (CNS) features, such as altered consciousness and/or long tract signs, accompany classic triad.1 2 Ganglioside GQ1b antibodies are often associated with both conditions, resulting in the proposal that MFS and BBE might be closely related and form a continuous disease.3 Among the clinical features of both MFS and BBE, psychosis and involuntary movements (IVMs) are atypical.4 The present case reports a young woman who presented with acute cerebellar ataxia, areflexia and ophthalmoplegia, which was accompanied by psychosis and IVMs from disease onset. Serum serological tests revealed high anti-GQ1b and anti-GT1a antibody titres. In addition, serum and cerebrospinal fluid (CSF) tests also detected high antibody titres against NR2B- and NR2A-containing heteromers of N-methyl-d-aspartate receptor (NMDAR) and antiglutamate receptor-ε2 (GluRε2).
Case presentation
A 23-year-old woman presented initially with double vision and a mild, unsteady gait and was admitted to our hospital in May 2008. One week earlier, the patient experienced a respiratory infection. On the first day of illness, the patient suffered from transient double vision and dizziness. The following day, the patient was restless, agitated and cried incomprehensively. Symptoms rapidly exacerbated following hospital admission (day 2); the patient was alert, but exhibited emotional incontinence and personality changes. Extraocular movement was inhibited in all directions and conjugate eye movements were impaired with coarse nystagmus. Facial muscles were weak and the patient presented with slurred speech and dysphagia. Marked cerebellar ataxia was present in the finger-nose and heel-knee test. Muscle tone decreased and deep tendon reflexes of the upper and lower extremities were absent. The Babinski response was bilaterally negative and limb muscle strength and sensory examination were normal.
On day 3, the patient became somnolent and screamed irritably like a child. Both pupils were dilated and light reflexes were diminished. The patient developed orolingual IVMs, such as lip-licking, chewing, dyskinesia and myoclonic movements. Psychosis and IVMs mimicked features of patients with ovarian teratoma-associated encephalitis5 (video 1). Hypersalivation was also exhibited, but hypoventilation or seizures did not develop.
Investigations
Routine blood analyses, including thyroid functions, were normal. Serum antibodies specific to HIV, syphilis, neurotrophic viruses, Mycoplasma pneumoniae, thyroid disease and autoimmune disease (antinuclear, anti-SS-A/SS-B antibodies, MPO-ANCA, PR3-ANCA and anticardiolipin antibodies) were insignificant. CSF analysis revealed a white blood cell count of 14/ml, 24 mg protein/dl and negative PCR results for herpes simplex virus genome. EEGs showed a slightly irregular basic pattern without epileptic discharge. Nerve conduction velocities and compound muscle action potentials were normal, with a significant decrease in F-wave amplitude of median and tibial nerves. Brainstem auditory-evoked potentials and cranial MRI, with or without gadolinium enhancement revealed no abnormalities. Chest, abdominal and pelvic CT were normal. 123I-iodoamphetamine single photon emission CT (123I-IMP SPECT), which was performed on day 13, revealed hypoperfusion in the brainstem, cerebellum, thalamus and frontotemporal cortices. Follow-up 123I-IMP SPECT on day 26 demonstrated perfusion recovery in these lesions (figure 1).
Figure 1.
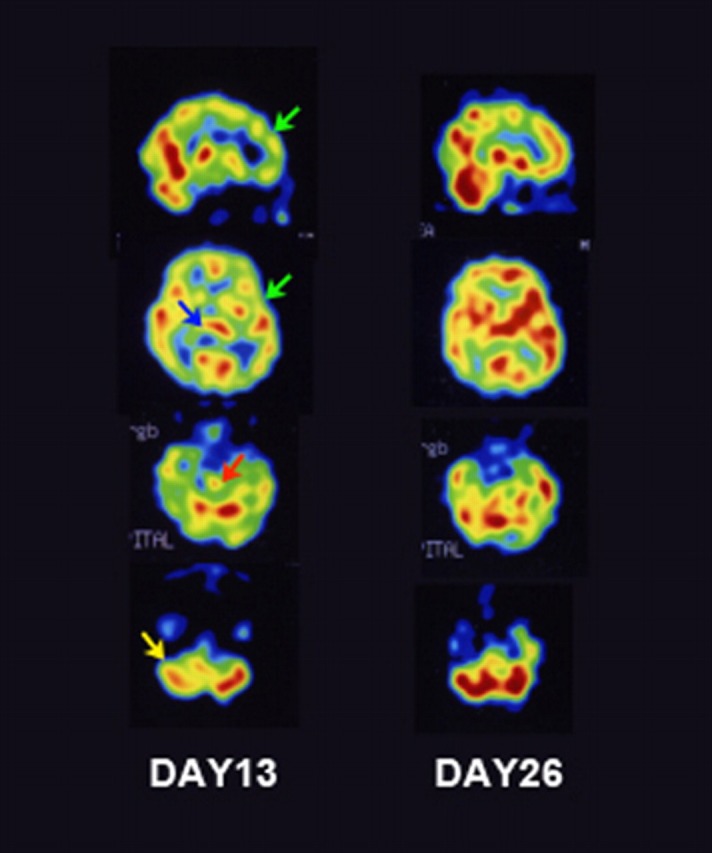
123I-iodoamphetamine single photon emission CT (123I-IMP SPECT) imaging of the patient. On day 13, 123I-IMP SPECT reveals decreased rCBF in the frontotemporal cortex (green arrows), thalamus (blue arrow), brainstem (red arrow) and cerebellum (yellow arrow) (A). Follow-up 123I-IMP SPECT on day 26 shows improvement in the damaged lesion (B).
Video 1.
The patient exhibits ophthalmoplegia, coarse nystagmus, sluggish papillary light reflex and involuntary movements around the eyebrows and mouth. The patient also screams irritably like a child.
Differential diagnosis and treatment
These findings suggested the diagnosis of atypical MFS-related disorder accompanied by psychosis and IVMs. Therefore, the patient was administered intravenous immunoglobulin (Ig) (20 g/day for 5 days), followed by intravenous methylprednisolone (1 g/day for 3 days). On day 10, the patient developed rough and coarse IVMs, which affected the left arm, despite improved psychiatric behaviour. Subsequently, the patient received intravenous methylprednisolone, accompanied by steroid tapering. ELISA revealed that serum samples from day 3 contained high IgG antibody titres to GQ1b and GT1a. Furthermore, antibodies to full-length GluRε2 (anti-GluRε2 antibodies) and glutamate NR2B- and NR2A-containing heteromers of NMDAR (anti-NR1/NR2 antibodies) were examined as described previously.6 Anti-NR1/NR2 antibodies, as well as anti-GluRε2 IgG and IgM antibodies, were expressed in serum and CSF on day 3. On day 16, serum anti-GluRε2 IgM antibody levels were reduced, but IgG antibodies remained present.
Outcome and follow-up
Although the symptoms lasted for 2 weeks, improvement was considerable with intact neurological symptoms within 6 weeks of disease onset. After 1 year, the patient exhibited no neurological complications and pelvic MRI was normal.
Discussion
Clinical findings fulfilled diagnostic criteria for MFS.2 However, the patient also presented with atypical clinical features, such as psychosis and IVMs. In addition, anti-NR1/NR2 antibodies and anti-GluRε2 antibodies were present. 123I-IMP SPECT examinations demonstrated that patient symptoms were due to impaired cortex, thalamus, cerebellum and brainstem. Interestingly, Wada et al, also described two BBE patients with delirium4; both patients fulfilled the diagnostic criteria for BBE, including positive serum titres for anti-GQ1b and -GT1a antibodies, as well as childish behaviours and emotional incontinence. The patients also exhibited rigidity and IVMs, such as tremors and hypertonia in the masseter muscles, which mimicked tetanus. SPECT analysis revealed hypoperfusion of the frontal lobe, brainstem and basal ganglia, which was similar to results from the present study. Although atypical psychosis and IVMs in these patients were possibly due to autoantibodies against various GluRs, including anti-GluRε2 antibodies and anti-NR1/NR2 antibodies, the association between these antibodies was not discussed in the paper.
Dalumau et al reported that limbic encephalitis is a result of anti-NR1/NR2 antibodies associated with ovarian teratoma, which has been termed anti-NMDAR encephalitis.5 Clinical characteristics of patients with anti-NMDAR encephalitis include psychosis, seizures, IVMs, autonomic instability and central hypoventilation. Anti-GluRε2 antibody is described as an association between non-herpetic limbic encephalitis6–8 and epilepsia partialis continua-related Rasmussen encephalitis.9 Several studies have shown that anti-GluRε2 and anti-NR1/NR2 antibodies are detectable in patients with non-herpetic limbic encephalitis.7 8 Kamei et al reported the clinical features of acute juvenile female non-herpetic encephalitis (AJFNHE) in Japan based on a nationwide questionnaire,8 with a detection rate of autoantibodies against several GluRs of 67%. Clinical AJFNHE phenotypes closely mimic anti-NMDAR encephalitis, including psychosis and IVMs, and anti-NR1/NR2 antibodies have been detected in several patients with AJFNHE.8 The GluRε2 subunit NR2A functions as an NMDAR component. Huerta et al reported that NR2 autoantibodies induce emotional abnormality in mice.10 These studies suggested that autoantibodies against some GluR subunits could result in psychosis and IVMs in our patient.
The mechanism by which ganglioside (anti-GQ1b and anti-GT1a) and GluR antibodies were co-expressed in the patient, thereby triggering clinical features, remains unknown. There is a high prevalence of prodromal viral-like symptoms in anti-NMDAR encephalitis and AJFNHE.5 8 Moreover, Gable et al reported that 4/10 patients with anti-NMDAR encephalitis exhibit serological evidence of acute Mycoplasma infection.11 Although the number of cases in this study was very small, with a high amount of false IgM positivity results, Mycoplasma infection should be considered, as Mycoplasma could contain epitopes mimicking both GQ1b ganglioside and GluRs. The patient did not express Mycoplasma-specific IgM antibodies; however, a prodromal respiratory infection was diagnosed. Therefore, it is possible that a microorganism could trigger antibody production against these antigens, as detected in the patient.
Learning points.
-
▶
Patients with MFS and related disorders rarely present with atypical psychosis and IVMs such as delirium, oral dyskinesia, tremor or myoclonus.
-
▶
CNS involvement, such as psychosis and IVMs in patients with atypical MFS-related disorders could be associated with various anti-GluR antibodies.
Acknowledgments
The authors thank Dr Susumu Kusunoki (Department of Neurology, Kinki University) and Dr Keiko Tanaka (Department of Neurology, Kanazawa Medical University) for measuring antiganglioside antibodies and anti-NMDA receptor antibody, respectively.
Footnotes
Competing interests: None.
Patient consent: Obtained.
References
- 1.Odaka M, Yuki N, Yamada M, et al. Bickerstaff's brainstem encephalitis: clinical features of 62 cases and a subgroup associated with Guillain-Barré syndrome. Brain 2003;126(Pt 10):2279–90. [DOI] [PubMed] [Google Scholar]
- 2.Overell JR, Willison HJ. Recent developments in Miller Fisher syndrome and related disorders. Curr Opin Neurol 2005;18:562–6. [DOI] [PubMed] [Google Scholar]
- 3.Odaka M, Yuki N, Hirata K. Anti-GQ1b IgG antibody syndrome: clinical and immunological range. J Neurol Neurosurg Psychiatr 2001;70:50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wada Y, Yanagihara C, Nishimura Y, et al. Delirium in two patients with Bickerstaff's brainstem encephalitis. J Neurol Sci 2008;269:184–6. [DOI] [PubMed] [Google Scholar]
- 5.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tachibana N, Shirakawa T, Ishii K, et al. Expression of various glutamate receptors including N-methyl-D-asparate receptor (NMDAR) in an ovarian teratoma removed from a young woman with anti-NMDAR encephalitis. Inter Med 2010;49:2167–73. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi Y. Epitope of autoantibodies to N-methyl-D-aspartate receptor heteromers in paraneoplastic limbic encephalitis. Ann Neurol 2008;64:110–11; author reply 111–12. [DOI] [PubMed] [Google Scholar]
- 8.Kamei S, Kuzuhara S, Ishihara M, et al. Nationwide survey of acute juvenile female non-herpetic encephalitis in Japan: relationship to anti-N-methyl-D-asparate receptor encephalitis. Inter Med 2009;48:673–9. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi Y, Mori H, Mishina M, et al. Autoantibodies and cell-mediated autoimmunity to NMDA-type GluRε2 in patients with Rasmussen's encephalitis and chronic progressive epilepsia partialis continua. Epilepsia 2005;46:152–8. [DOI] [PubMed] [Google Scholar]
- 10.Huerta PT, Kowal C, DeGiorgio LA, et al. Immunity and behavior: antibodies alter emotion. Proc Natl Acad Sci USA 2006;103:678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gable MS, Gable S, Radner A, et al. Anti-NMDA receptor encephalitis: report of ten cases and comparison with viral encephalitis. Eur J Clin Microbiol Infect Dis 2009;28:1421–9. [DOI] [PMC free article] [PubMed] [Google Scholar]


