Abstract
Herpes simplex virus-1 has been identified as the trigger factor in certain cases of NMDA-receptor autoimmune encephalitis. We report on a 67-year-old female patient, who was severely affected by post-herpetic NMDA-receptor autoimmune encephalitis. Her symptoms did not improve under methylprednisolone pulse therapy and plasma exchange under acyclovir prophylaxis. She received protein A immunoadsorption and a long-term immunosuppression with rituximab. Under treatment, activated T-cells as well as B- and plasma cells decreased in peripheral blood and cerebrospinal fluid, and anti-NMDA-R IgG titers in serum and cerebrospinal fluid declined with near complete cessation of intrathecal autoantibody synthesis. The patient regained near complete independence and profoundly improved on formal neuropsychological assessment. Despite reduction of antiviral defense through of lowered activated T cells and concomitantly decreasing HSV-specific IgG antibodies, no evidence of viral reactivation was detected.
INTRODUCTION
In certain cases, relapsing symptoms following Herpes simplex virus-1 (HSV1) encephalitis are caused by the formation of autoantibodies directed against the GluN1 subunit of the human NMDA-receptor (NMDA-R), linking autoimmune and infective pathologies [1, 2]. Previously reported treatment options include methylprednisolone pulse therapy (MP), plasma exchange (PLEX) and most recently rituximab or cyclophosphamide under acyclovir prophylaxis [3]. Whilst patients with this dual pathology often suffer from residual deficits due to the HSV1 infection, immunosuppressive treatment can improve new or worsening symptoms caused by the autoimmune pathology [4]. Here, we present a patient with severe relapsing symptoms refractory to MP and PLEX, but with a remarkable improvement through protein A immunoadsorption (IA) [5] and rituximab.
CASE REPORT
A 67-year-old Caucasian woman, who was diagnosed with HSV1 encephalitis with positive HSV1-PCR in CSF as the cause of aphasia and reduced consciousness 3 months prior. Her preceding medical history was otherwise unremarkable. Following a steady improvement under antiviral and rehabilitation therapy, her caregivers reported a subsequent acute decline in cognitive and motor abilities. Clinical examination on admission to our hospital (T = 0 M, Fig. 1) showed the patient hallucinating and disorientated. She was aphasic and unable to verbally or non-verbally communicate. Muscle tone was increased with generalized rigidity, rendering the patient non-ambulatory. Under treatment with MP and PLEX, symptoms remained unchanged for 14 days but improved continuously under IA. Following acute treatment, the patient was discharged to a rehabilitation facility. She returned three months later (T = 3 M, Fig. 1) with a recurrence of the same clinical presentation. Treatment consisted of 14 days of MP and PLEX, which again did not change the symptoms. Once more, improvement could only be achieved through IA.
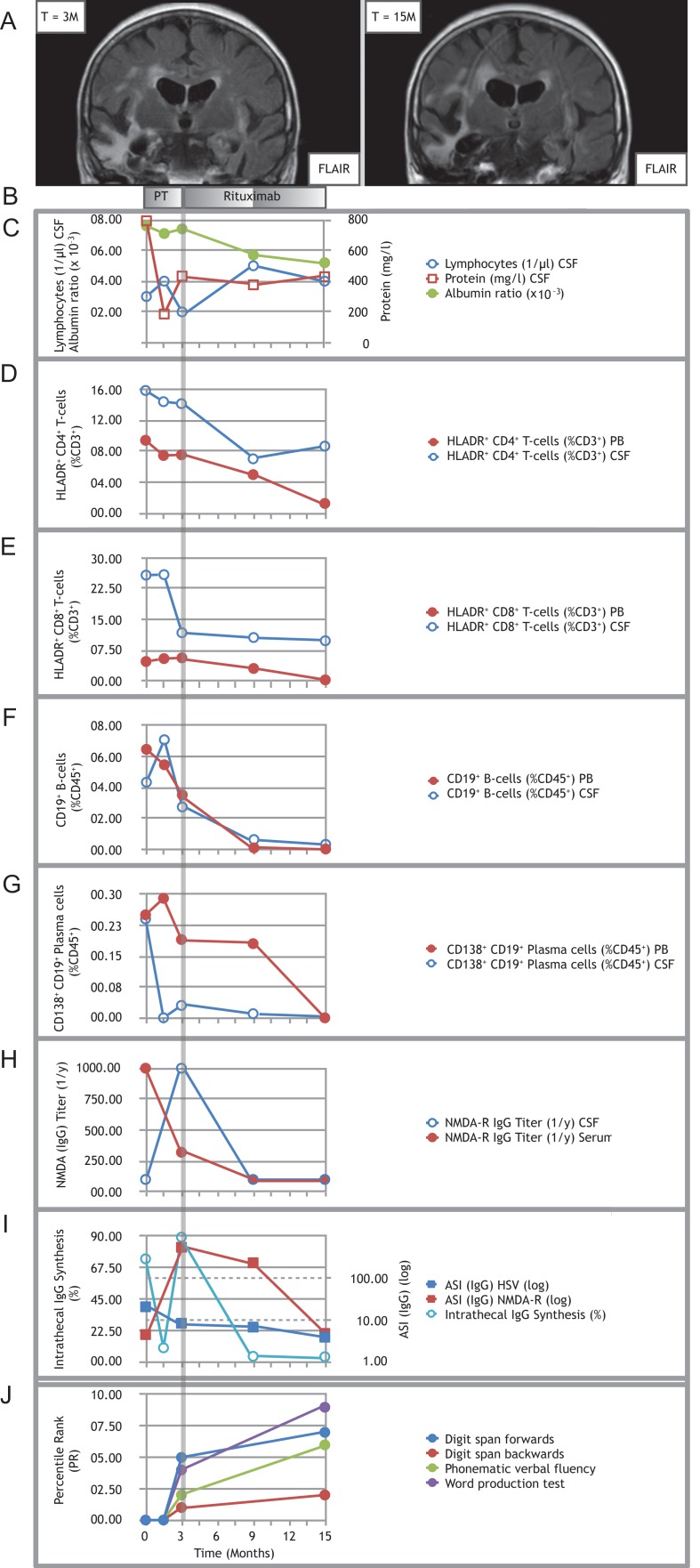
Figure 1:
Treating refractory post-herpetic anti-NMDA-receptor encephalitis with rituximab. (A) Representative MRI image (coronal FLAIR) showing right more than left temporal scarring before initiation of rituximab (left panel); follow-up MRI (coronal FLAIR) showing progressive atrophy involving both temporal lobes (right panel). (B) Pre-treatment (PT) consisted of multiple cycles of MP combined with PLEX and IA. Induction treatment with 2 × 200 mg rituximab was applied at month 3, maintenance doses with 1 × 200 mg were each applied at month 9 and 15. (C) Time course of CSF lymphocytes, CSF protein and albumin ratio. (D–G) Time courses of proportions of activated HLADR+ CD4+ T-cells (D), activated HLADR+ CD8+ T-cells (E), CD19+ B-cells (F) and CD138+ CD19+ plasma cells (G) in PB and CSF. Note: both CD8+ and CD4+ T-cells stained positive for CD3. Double labeling was avoided in the description and text for improved clarity. (H) Titers of anti-NMDA-R IgG autoantibodies over the course of the disease. (I) Time course of percentages of intrathecal IgG synthesis and the antigen-specific IgG antibody indices (ASI) for NMDA-R and HSV. All samples for antibody analysis at certain given time points were obtained before initiation of immunotherapy, especially before PLEX and IA. (J) Results of the neuropsychological assessment. Because of the severe impairment in the early stages of the disease, testing became first possible upon initiation of rituximab treatment (month 3) in a limited fashion with selected tests. It was repeated after 12 months of rituximab treatment (month 15). Percentile ranks for the word production test were calculated from internal control group data (N = 26). Note: In (C) and (I), circled data points are referenced by the lefthand y-axis (y1), whereas squared data points by the righthand y-axis (y2). Data T = −3 M is not shown, since the patient was not treated at our hospital at that stage.
On first presentation (T = 0 M, Fig. 1) and relapse (T = 3 M, Fig. 1), MRI showed temporal scarring predominantly on the right side caused by previous HSV1 infection, but no signs of viral reactivation or other acute pathologies (Fig. 1A).
Initial routine CSF analysis (T = 0M, Fig. 1) yielded a normal lymphocyte count (3/μl), elevated protein (793 mg/l), normal albumin ratio (7.6 × 10−3), increased intrathecal IgG-synthesis (74%) and type 3 oligoclonal bands (OCB) (Fig. 1C and I). During relapse (T = 3 M, Fig. 1), CSF analysis showed normal values for lymphocyte count (2/μl), CSF protein (433 mg/l) and albumin ratio (5.7 × 10−3), increased intrathecal IgG synthesis (89%) and type 3 OCB (Fig. 1C and I).
Flow cytometry [6] at first presentation (T = 0M, Fig. 1) and relapse (T = 3M, Fig. 1) showed increased percentages of activated HLADR+ CD8+ and HLADR+ CD4+ T-cells in peripheral blood (PB) and CSF (Fig. 1D and E). CD19+ B-cells and CD138+ CD19+ plasma cells were elevated in CSF (Fig. 1F and G). In absence of active HSV1 replication in CSF with a negative HSV-PCR throughout the course of her relapses (T0–15, Fig. 1), antigen-specific IgG indices (ASI) for HSV1 were 20.8 (T = 0M, Fig. 1) and 8.4 (T = 3M, Fig.1), respectively. Anti-NMDA-R IgG titers were elevated initially (serum 1:1000, CSF 1:100) corresponding to a NMDA-R IgG ASI of 4.5 (T = 0 M, Fig. 1). The relapse (T = 3 M, Fig.1) was marked with an increase in anti-NMDA-R IgG titers to 1:1000 in CSF and a decrease to 1:320 in serum resulting in an ASI of 552.9 (Fig. 1I).
We established long-term immunotherapy with rituximab (2 × 200 mg for induction) under anti-infective prophylaxis (acyclovir 3 × 400 mg/d; co-trimoxazole 1 × 800/160 mg/d), because of the severity of the relapsing symptoms (T = 3 M, Fig.1). The patient returned for follow-up and intravenous administration of 1 × 200 mg rituximab for maintenance therapy at 9 and 15 months after the initial presentation (T = 9 and 15, Fig. 1). Routine CSF parameters remained normal (T = 9 M: lymphocytes 5/μl, CSF protein 376 mg/l, albumin ratio 5.7 × 10−3; T = 15 M: lymphocytes 4/μl, CSF protein 429 mg/l, albumin ratio 5.2 × 10−3; Fig. 1C). Intrathecal IgG synthesis dropped to 4% (with type 3 OCB) at first (T = 9 M, Fig.1) and 3.7% (with type 2 OCB) at second follow-up (T = 15 M, Fig.1) (Fig. 1I). Flow cytometry revealed decreasing T-cell activation in the HLADR+ CD4+ and HLADR+ CD8+ population in PB and CSF at first (T = 9 M, Fig.1) and second (T = 15 M, Fig. 1) follow-up (Fig. 1D and E). Under rituximab treatment, CD19+ B-cells and CD138+ CD19+ plasma cells decreased in PB and CSF at first (T = 9 M, Fig.1) and second (T = 15 M, Fig. 1) follow-up (Fig. 1F and G). Anti-NMDA-R IgG titers and ASI declined (T = 9 M: serum 1:100, CSF 1:100, ASI 235; T = 15: serum 1:100, CSF 1:100, ASI 4.8) (Fig. 1H and I), illustrating fading of intrathecal anti-NMDA-R IgG synthesis [7]. Despite immunotherapy, HSV-ASI declined to 6.8 (T = 9 M, Fig. 1) and 3.9 (T = 15 M, Fig.1), respectively, and no evidence of viral reactivation was found throughout follow-up with a consistently negative HSV1-PCR in CSF. Formal neuropsychological assessment (NPA) [8] was first possible at initiation of rituximab treatment (T = 3 M, Fig. 1) in a limited fashion (digit span forwards and backwards, phonematic verbal fluency, word production test) and showed severe deficits in attention span, executive functions and language abilities. During treatment, the patient's cognitive and motor abilities improved consistently. She was able to walk without support and to complete the activities of daily living nearly independently. At the 15 months follow-up (T = 15 M, Fig. 1), whilst NPA performance had improved in all tests, certain residual deficits remained (Fig. 1J). MRI at the 15 months follow-up (T = 15 M, Fig. 1) demonstrated bilateral temporal atrophy, more pronounced on the right side (Fig. 1A). There were no adverse events under rituximab therapy.
DISCUSSION
Several studies have established that HSV1 encephalitis can trigger encephalitis with anti-NMDA-R autoantibodies [1, 2]. The infective form commonly antedates the autoimmune form, yet it remains uncertain which facet of HSV infection drives the autoimmune response. Testing for anti-NMDA-R antibodies in patients with relapses following HSV-encephalitis is recommended [9]. Overall the occurrence of such cases is rare, hence standardized treatment recommendations are not available yet. Treatment attempts regularly include antiviral and immunosuppressive agents, including combinations of acyclovir, steroids with and without PLEX [10] and secondary immunotherapy including rituximab or cyclophosphamide under acyclovir prophylaxis [3]. Patients with post-herpetic NMDA-autoimmune encephalitis often suffer from residual deficits due to the HSV1 infection and experience new or worsening symptoms through NMDA-R–autoantibody formation. Immunosuppressive treatment can improve NMD-R autoantibody mediated symptoms hence it is recommended once the diagnosis is established [4]. Patients with classic NMDA-R autoimmune encephalitis have been shown to recover to full health or minor residual deficits in up to 75% of cases [11, 12], it remains uncertain whether the prognosis of post-herpetic NMDA-R autoimmunencephalitis is as favorable. We describe an adult female patient suffering from severe relapsing post-herpetic anti-NMDA-R encephalitis, which failed to respond to MP and PLEX but improved remarkably under IA followed by induction of rituximab treatment at relapse. Both methods of therapeutic apheresis (PLEX and IA) have emerged as possible treatment options for autoimmune encephalitis [13]. In our case, IA seemed to be more effective as improvement occurred with short latency following this therapy. This could be explained by the very effective removal of intrathecal autoantibodies by IA as reported recently [5], however, a cumulative effect of PLEX and IA cannot be fully ruled out as both methods were applied subsequently at both times in this patient. During maintenance treatment, activated T-cells as well as B- and plasma cells decreased in PB and CSF. Anti-NMDA-R IgG titers in PB and CSF declined with near complete cessation of intrathecal autoantibody synthesis. Despite reduction of activated T cells in PB and CSF and concomitant decreasing HSV IgG ASI, no evidence of viral reactivation was detected under treatment with rituximab. Cognitive and motor abilities increased, as demonstrated by NPA and improvement on modified Rankin scale, which declined from 5 to 2 within 12 months. Hence, rituximab in combination with IA, presents a possible treatment option for refractory post-herpetic NMDA-encephalitis as previously reported [3]. However, further research in an increased number of patients is needed regarding its efficacy and safety.
FUNDING
This study was supported by the German Research Foundation (DFG)-funded Collaborative Research Centre TR128 ‘Initiating/Effector vs Regulatory Mechanisms in Multiple Sclerosis: Progress towards Tackling the Disease’ (Project Z2 to H.W.).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Armangue T, Leypoldt F, Málaga I, Raspall-Chaure M, Marti I, Nichter C, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol 2014;75:317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Linnoila JJ, Binnicker MJ, Majed M, Klein CJ, McKeon A. CSF herpes virus and autoantibody profiles in the evaluation of encephalitis. Neurol Neuroimmunol Neuroinflamm 2016;3:e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Armangue T, Titulaer MJ, Málaga I, Bataller L, Gabilondo I, Graus F, et al. Pediatric anti-N-methyl-d-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr 2013;162:850–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armangue T, Moris G, Cantarín-Extremera V, Conde CE, Rostasy K, Erro ME, et al. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology 2015;85:1736–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dogan Onugoren M, Golombeck KS, Bien C, Abu-Tair M, Brand M, Bulla-Hellwig M, et al. Immunoadsorption therapy in autoimmune encephalitides. Neurol Neuroimmunol Neuroinflamm 2016;3:e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Golombeck KS, Bönte K, Mönig C, van Loo KM, Hartwig M, Schwindt W, et al. Evidence of a pathogenic role for CD8(+) T cells in anti-GABAB receptor limbic encephalitis. Neurol Neuroimmunol Neuroinflamm 2016;3:e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reiber H, Peter JB. Cerebrospinal fluid analysis: disease-related data patterns and evaluation programs 2001;184:101–22. [DOI] [PubMed] [Google Scholar]
- 8. Lueg G, Gross CC, Lohmann H, Johnen A, Kemmling A, Deppe M, et al. Clinical relevance of specific T-cell activation in the blood and cerebrospinal fluid of patients with mild Alzheimer's disease. Neurobiol Aging 2015;36:81–9. [DOI] [PubMed] [Google Scholar]
- 9. DeSena A, Graves D, Warnack W, Greenberg BM. Herpes simplex encephalitis as a potential cause of anti-N-Methyl-d-aspartate receptor antibody encephalitis. JAMA Neurol 2014;71:344–3. [DOI] [PubMed] [Google Scholar]
- 10. Leypoldt F, Titulaer MJ, Aguilar E, Walther J, Bönstrup M, Havemeister S, et al. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology 2013;81:1637–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dalmau J, Tüzün E, Wu H, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heine J, Ly LT, Lieker I, Slowinski T, Finke C, Prüss H, et al. Immunoadsorption or plasma exchange in the treatment of autoimmune encephalitis: a pilot study. J Neurol 2016;263:2395. [DOI] [PubMed] [Google Scholar]