Abstract
In patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) treated with chemotherapy plus a tyrosine kinase inhibitor (TKI), the prognostic impact of additional chromosomal abnormalities (ACAs) is not well-established. We evaluated the prognostic impact of individual ACAs in 152 patients with Ph+ ALL receiving first-line intensive chemotherapy plus either imatinib (n=36), dasatinib (n=74) or ponatinib (n=42). ACAs were identified in 118 patients (78%). Compared to outcomes of patients without ACAs, ACAs were not associated with differences in either relapse-free survival (RFS; P=0.42) or overall survival (OS; P=0.51). When individual ACAs were evaluated, +der(22)t(9;22) and/or -9/9p in the absence of high hyperdiploidy (HeH) was present in 16% of patients and constituted a poor-risk ACA group. Patients with 1 or more poor-risk ACA in the absence of HeH had significantly shorter RFS (5-year RFS rate 33% versus 59%, P=0.01) and OS (5-year OS rate 24% versus 63%, P=0.003). These ACAs were prognostic in patients who received imatinib and dasatinib but not in those who received ponatinib. By multivariate analysis, this poor-risk ACA group was independently associated with worse RFS (HR 2.03 [95% CI 1.08-3.30], P=0.03) and OS (HR 2.02 [95% CI 1.10-3.71], P=0.02). Patients with Ph+ ALL who have +der(22)t(9;22) and/or -9/9p in the absence of HeH have relatively poor outcomes when treated with chemotherapy plus a TKI.
Keywords: Acute lymphoblastic, leukemia, Philadelphia chromosome, karyotype, cytogenetics, prognosis
Introduction
The presence of t(9;22) is the hallmark of Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL). Additional chromosomal abnormalities (ACAs) are also present at diagnosis in 40-70% of patients with Ph+ ALL.[1-8] Prior to the routine use of tyrosine kinase inhibitors (TKIs), the presence of ACAs in Ph+ ALL was associated with worse outcomes in some [1, 3, 4, 6] but not all [2, 5, 9] studies. In small reports of patients treated with chemotherapy plus imatinib, ACAs have been associated with shorter relapse-free survival (RFS) and overall survival (OS) [6-8]. However, few studies have evaluated the relative impact of individual ACAs in Ph+ ALL. The relevance of ACAs for patients treated with newer-generation TKIs is also unknown. To clarify the prognostic role of ACAs in Ph+ ALL, we investigated the impact of specific ACAs in adults with Ph+ ALL receiving frontline chemotherapy plus a TKI with imatinib, dasatinib or ponatinib.
Methods
Patients
Between June 2001 and January 2016, 182 adults with previously untreated Ph+ ALL received induction with hyperfractionated cyclophosphamide, vincristine (or liposomal vincristine), doxorubicin and dexamethasone alternating with methotrexate and high-dose cytarabine (i.e. the hyper-CVAD regimen) plus a TKI at our institution [10-12] The TKI was continued indefinitely as maintenance therapy. Allogeneic stem cell transplantation (ASCT) was performed at the discretion of the treating physician. Thirty patients positive for BCR-ABL1 by fluorescent in situ hybridization and/or polymerase chain reaction (PCR) but who had diploid cytogenetics or insufficient metaphases were excluded from the analysis. Of the 152 evaluable patients, the TKI received was imatinib in 36 (24%), dasatinib in 74 (49%) and ponatinib in 42 (28%). Median duration of follow-up was 43 months (range, 2-173 months).
Response Definitions
A complete molecular response (CMR) was defined as the absence of a quantifiable BCR-ABL1 transcript by PCR with a sensitivity of 0.01%. Relapse was defined by recurrence of >5% blasts in a bone marrow aspirate or by the presence of extramedullary disease. RFS was calculated from the time of complete remission until relapse or death. OS was calculated from the time of treatment initiation until death.
Statistical Methods
RFS and OS were calculated using Kaplan-Meier estimates, and survival estimates were compared using the log-rank test. Univariate Cox proportional hazards regression models were used to assess the association between patient characteristics and RFS or OS. Patient characteristics with P<0.10 in the univariate analyses were included in the multivariate model; backward elimination was used until all predictors had a P<0.05.
Results
ACA Frequencies
Baseline characteristics are shown in Table 1. Of the 152 evaluable patients in whom the Ph was detected, 34 (22%) had t(9;22) alone and 118 (78%) also had one or more ACAs. Median age was similar between patients with and without ACAs (55 years and 54 years, respectively, P=0.98). Among the 118 patients with ACAs, 25 (17%) were high hyperdiploid (HeH; defined as 51-65 chromosomes); no patients with low hypodiploidy (defined as 30-39 chromosomes) were identified. Excluding ACAs associated with chromosomal gain in the 25 patients with HeH, the recurrent ACAs identified in ≥5% of the remaining ACA population (n=118) were: -7/7q in 24 (20%), +der(22)t(9;22)in 15 (16%), -9/9p in 15 (16%), +21 in 8 (7%), +8 in 7 (6%) and translocations of 1q21 in 7 (6%). Among patients with translocations of 1q21, none had identical translocations.
Table 1. Baseline characteristics.
| Characteristics | Ph alone (N=34) | Ph plus ACAs (N=118) | Overall (N=152) |
|---|---|---|---|
|
| |||
| Median age, years (range) | 54 (26-80) | 55 (19-85) | 55 (19-85) |
|
| |||
| Median WBC, × 109/L (range) | 14.7 (0.9-243.6) | 8.3 (0.3-232.3) | 9.5 (0.3-243.6) |
|
| |||
| Performance status, n (%) | |||
| 0-1 | 28 (82) | 102 (86) | 130 (86) |
| 2 | 4 (12) | 9 (8) | 15 (10) |
| Unknown | 2 (6) | 5 (4) | 7 (5) |
|
| |||
| CD20 expression ≥20%, n/N (%) | 10/30 (33) | 55/110 (50) | 65/140 (46) |
|
| |||
| CNS leukemia, n/N (%) | 3/34 (9) | 18/117 (15) | 21/151 (14) |
|
| |||
| BCR-ABL transcript, n (%) | |||
| e1a2 or e1a3 (p190) | 17 (52) | 92 (78) | 109 (72) |
| e13a2 or e14a2 (p210) | 16 (48) | 26 (22) | 42 (28) |
|
| |||
| TKI | |||
| Imatinib | 7 (21) | 29 (25) | 36 (24) |
| Dasatinib | 18 (53) | 56 (47) | 74 (49) |
| Ponatinib | 9 (26) | 33 (28) | 42 (28) |
Ph, Philadelphia chromosome; ACAs, additional chromosomal abnormalities; WBC, white blood cell; CNS, central nervous system; TKI, tyrosine kinase inhibitor
Outcomes by Individual ACAs
The 5-year RFS and OS rates were similar between the t(9;22) alone and ACA groups (RFS: 59% and 53%, respectively, P=0.42; OS: 57% and 56%, respectively, P=0.51). When individual ACA groups were compared, distinct prognostic groups were identified (Table 2). In the absence of HeH, patients with +der(22)t(9;22)or -9/9p had a particularly poor prognosis and constituted a poor-risk ACA group with a median RFS of 11 months and 17 months and a median OS of 22 months and 28 months, respectively. Five patients had both +der(22) and -9/9p. The presence of +der(22) and/or -9/9p constituted a poor-risk ACA group (n=25, 21% of the ACA cohort and 16% of the evaluable population).
Table 2. Frequency and outcomes of additional chromosomal abnormalities.
| ACA Group1 | N (%)2 | RFS | OS | ||
|---|---|---|---|---|---|
| Median RFS (months) | 5-year RFS rate (%) | Median OS (months) | 5-year OS rate (%) | ||
| HeH | 25 (21) | 124 | 68 | 125 | 67 |
| -7/7q | 24 (20) | 58 | 49 | 76 | 58 |
| +der(22)t(9;22) | 15 (16) | 11 | 31 | 22 | 22 |
| -9/9p | 15 (16) | 17 | 34 | 28 | 26 |
| +21 | 8 (7) | not reached | 75 | not reached | 75 |
| +8 | 7 (6) | 70 | 56 | 53 | 48 |
| Translocation of 1q21 | 7 (6) | 38 | 50 | not reached | 63 |
Patients with more than one ACA are included in each individual ACA category. However, patients with HeH were excluded from analysis of individual ACAs.
Percentages represent proportion of patients with given abnormality out of evaluable population of 118 patients with ACAs
ACA, additional chromosomal abnormalities; RFS, relapse-free survival; OS, overall survival; HeH, high hyperdiploidy
Outcomes of Patients with +der(22)(9;22) and/or -9/9p
The median RFS and OS for this poor-risk group were 16 and 22 months, and 5-year RFS and OS rates were 33% and 24%, respectively. In contrast, patients with ACAs other than +der(22)t(9;22) or -9/9p (n=93, 79% of the ACA cohort and 61% of the evaluable population) had a median RFS and OS of 97 and 125 months, and 5-year RFS and OS rates of 59% and 65%, respectively. Among the 25 patients with HeH, 21 (84%) had at least 1 poor-risk ACA; these patients had a favorable survival despite the presence of concomitant poor risk ACA, with 5-year RFS and OS rates of 64% and 66%, respectively (P=0.08 and P=0.06 compared to patients with poor-risk ACAs without HeH).
Patients with ACAs other than +der(22)t(9;22) or -9/9p had similar RFS and OS to those with t(9;22) alone (P=0.64 and P=0.99, respectively); these groups were therefore pooled for additional analyses (n=127). The 3-month CMR rates for the pooled group of patients with t(9;22) alone or non-poor-risk ACAs and those with poor-risk ACAs were similar (61% versus 60%; P=0.93). Patients with poor-risk ACAs had significantly shorter RFS (median 16 versus 124 months; 5-year RFS rate 33% versus 59%, respectively; P=0.01; Fig. 1A) and OS (median 22 versus 125 months; 5-year OS rate 24% versus 63%, respectively; P=0.003; Fig. 1B). The 5-year cumulative relapse rate for the poor-risk ACA group versus others was 52% and 25%, respectively (P=0.003). The rate of ASCT was similar between t(9;22) alone or non-poor-risk ACA group and the poor-risk ACA group (23% versus 16%, respectively; P=0.45). Due to the small number of patients in the poor-risk ACA group who underwent ASCT (n=4), an analysis of the impact of ASCT by ACA risk group was not possible.
Figure 1. Outcomes for patients based on additional chromosomal abnormalities.
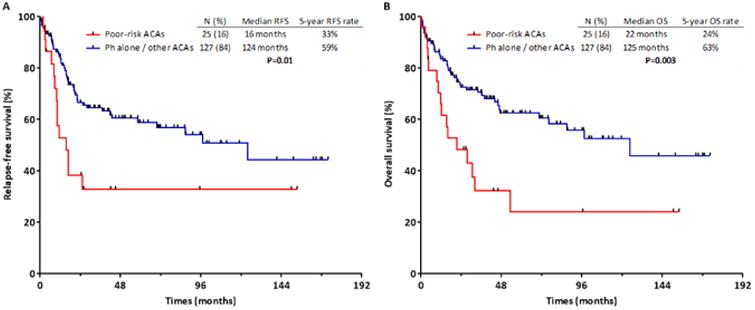
Relapse-free survival (A) and overall survival (B) for patients with and without poor-risk additional chromosomal abnormalities at diagnosis.
A differential prognostic impact of these poor-risk ACAs was observed according to the TKI received. The presence of +der(22)t(9;22) and/or -9/9p in the absence of HeH was associated with worse RFS and OS for patients treated with imatinib or dasatinib, but not for those who received ponatinib. Among those with and without poor-risk ACAs, the median RFS by TKI received was 11 versus 97 months for imatinib (P=0.06), 17 months versus not reached for dasatinib (P=0.06), and not reached versus not reached for ponatinib (P=0.81), although notably there were only 5 patients with poor-risk ACAs who received ponatinib (Fig. 2 A-C). The median OS was 14 versus 98 months for imatinib (P=0.02), 31 versus 76 months for dasatinib (P=0.10) and not reached versus not reached for ponatinib (P=0.38).
Figure 2. Relapse-free survival for patients based on additional chromosomal abnormalities and tyrosine kinase inhibitor.
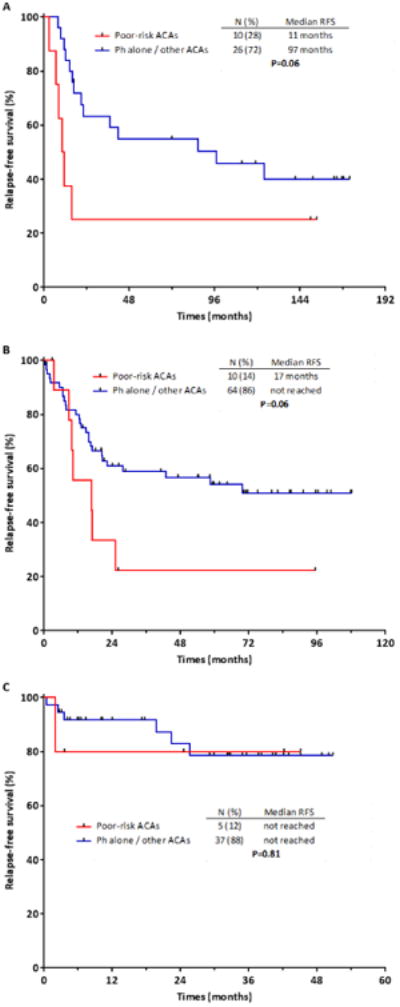
Relapse-free survival for patients with and without poor-risk additional chromosomal abnormalities treated with imatinib (A), dasatinib (B) or ponatinib (C).
Univariate and Multivariate Analysis for RFS and OS
Univariate analysis of variables associated with RFS and OS is shown in Table 3. By multivariate analysis, the factors associated with worse RFS were performance status ≥2 (HR 2.15 [95% CI 1.01-4.57], P=0.05) and poor-risk ACAs (HR 2.03 [95% CI 1.08-3.30], P=0.03), and the factors associated with worse OS were age (HR 1.02 [95% CI 1.00-1.04], P=0.02), treatment an earlier-generation TKI (HR 1.75 [1.15-2.66], P=0.01) and poor-risk ACAs (HR 2.02 [95% CI 1.10-3.71], P=0.02).
Table 3. Univariate analysis for relapse-free and overall survival.
| Characteristics | Risk of relapse or death | Risk of death | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age, years | 1.01 | 1.00-1.03 | 0.10 | 1.02 | 1.01-1.04 | 0.01 |
| Log WBC | 1.06 | 0.70-1.60 | 0.77 | 1.07 | 0.62-1.40 | 0.72 |
| Platelets | 1.00 | 0.99-1.00 | 0.24 | 1.00 | 1.00-1.00 | 0.37 |
| BM blast (%) | 1.02 | 1.00-1.03 | 0.13 | 1.02 | 1.00-1.04 | 0.09 |
| Performance status ≥2 | 2.15 | 1.02-4.56 | 0.05 | 2.20 | 1.07-4.50 | 0.03 |
| CD20 expression ≥20% | 1.29 | 0.77-2.18 | 0.36 | 1.54 | 0.91-2.63 | 0.11 |
| CNS leukemia | 1.21 | 0.60-2.47 | 0.59 | 1.60 | 0.83-3.09 | 0.16 |
| p190 BCR-ABL1 transcript | 0.67 | 0.37-1.22 | 0.19 | 0.57 | 0.30-1.07 | 0.08 |
| TKI: ponatinib vs. dasatinib vs. imatinib | 0.68 | 0.47-0.99 | 0.04 | 0.61 | 0.42-0.89 | 0.01 |
| Poor-risk ACAs | 2.16 | 1.19-3.94 | 0.01 | 2.35 | 1.32-4.17 | 0.003 |
HR, hazard ratio; CI, confidence interval; WBC, white blood cell; BM, bone marrow; CNS, central nervous system; TKI, tyrosine kinase inhibitor; ACAs, additional chromosomal abnormalities
Discussion
We have shown that +der(22)t(9;22) and -9/9p in the absence of HeH constitute a group of poor-risk ACAs that are independently associated with a 2-fold higher risk of relapse or death in patients with Ph+ ALL who are treated with chemotherapy plus a TKI. Notably, the prognostic impact of these poor-risk ACAs did not appear to be mediated by differences in rates of CMR or ASCT. These findings suggest that the presence of these poor-risk ACAs, which are present in 16% of patients with Ph+ ALL, should be considered when planning post-remission therapies.
The presence of +der(22)t(9;22)has previously been shown to confer an inferior prognosis in patients with chronic myeloid leukemia receiving TKI treatment [13] although its significance in Ph+ ALL in the pre-TKI era has been mixed [3, 4, 9]. Similarly, reports of the clinical significance of abnormalities of chromosome 9 in Ph+ ALL have yielded conflicting results [2-4, 6, 9]. Many of these studies were performed in the pre-TKI era and were limited by relatively small sample sizes. Our study, which includes a large number of patients, defines of the impact of ACAs in Ph+ ALL in the TKI era and shows that these 2 ACAs are independently associated with poor outcomes.
In our study, the prognostic impact of +der(22)t(9;22) and -9/9p was restricted to patients without HeH. We found that HeH was associated with favorable outcomes, even when +der(22)t(9;22) or -9/9p were also present. HeH has been well-established to be associated with superior survival in adults with ALL, although most studies have primarily evaluated patients with Philadelphia chromosome-negative disease [14-16]. Our finding that the negative prognostic influence of +der(22)t(9;22) and -9/9p is restricted to patients without HeH highlights that HeH ALL is a unique clinical entity, including among patients with Ph+ ALL. Furthermore, our exclusion of patients with HeH from analysis of the impact of individual ACAs likely explains the discrepancy between our findings and some previous studies that did not find a negative impact of +der(22)t(9;22) or -9/9p. For example, one prior study of ACAs in Ph+ ALL suggested that the presence of +der(22)t(9;22) was associated with a lower risk for relapse [9]. However, there was significant overlap of cases with HeH and +der(22)t(9;22), which may have confounded the results.
To our knowledge, only one prior report has described the negative prognostic impact of +der(22)t(9;22) and -9/9p in patients with Ph+ ALL treated with chemotherapy plus a TKI [7]. Our study corroborates these findings using a cohort of patients who received 3 different TKIs and which had a notably lower rate of ASCT in first remission (75% versus 22%). While we found that poor-risk ACAs were independently prognostic for RFS and OS when baseline characteristics and TKI were considered, the negative outcomes of these poor-risk ACAs was driven primarily by the patients treated with imatinib and dasatinib. Although the number of patients treated with ponatinib was relatively small, it is notable that poor-risk ACAs appeared not to have an adverse prognostic impact in the ponatinib cohort. These results therefore suggest that ponatinib may be able to negate the unfavorable prognostic influence of these poor-risk ACAs seen when earlier-generation TKIs are used.
Although achievement of CMR at 3 months has been established as a strong prognostic factor for patients with Ph+ ALL [17], the CMR rates were similar between patients with and without poor-risk ACAs, suggesting that the differential outcomes of these 2 groups is not mediated by initial depth of response. This raises the question of whether the higher rates of relapse seen in the poor-risk ACA group may be due in part to an increased likelihood of TKI resistance in patients harboring these poor-risk ACAs. To address this question, future studies should integrate genomic profiling and ABL1 kinase mutation analysis to elucidate the mechanisms by which these poor-risk ACAs lead to inferior outcomes in patients with Ph+ ALL.
In conclusion, in patients with Ph+ ALL receiving chemotherapy plus a TKI, the presence of +der(22)t(9;22)or -9/9p in the absence of HeH is associated with inferior RFS and OS. These poor-risk ACAs should be considered when planning post-remission strategies in patients with Ph+ ALL.
Acknowledgments
Funding source: Supported by the MD Anderson Cancer Center Support Grant CA016672 and the MD Anderson Cancer Center Leukemia SPORE CA10063
Disclosure of Conflicts of Interest: H.K., F.R. and J.C. have received research funding from Bristol Myers Squibb; H.K, J.C. and E.J. have received research funding from Ariad Pharmaceuticals; H.K., F.R., J.C. and E.J. have received research funding from Novartis Pharmaceuticals
Footnotes
Authorship Contributions: N.J.S. designed the study, collected and analyzed the data, and wrote the manuscript; E.J. designed the study, collected and analyzed the data, treated patients, and wrote the manuscript; H.M.K. designed the study, treated patients, and wrote the manuscript; K.S. performed the statistical analysis; H.K., G.C.I and R.G. collected and analyzed the data; F.R. designed the study and treated patients; C.C.Y and K.P. performed the cytogenetic and molecular analyses; J.E.C., S.M.O, G.G-M, D.T., N.J., T.M.K, N.D., C.B.B. and M.K. treated patients. All authors reviewed and approved the manuscript.
References
- 1.Rieder H, Ludwig WD, Gassmann W, et al. Prognostic significance of additional chromosome abnormalities in adult patients with Philadelphia chromosome positive acute lymphoblastic leukaemia. British journal of haematology. 1996;95:678–691. doi: 10.1046/j.1365-2141.1996.d01-1968.x. [DOI] [PubMed] [Google Scholar]
- 2.Ko BS, Tang JL, Lee FY, et al. Additional chromosomal abnormalities and variability of BCR breakpoints in Philadelphia chromosome/BCR-ABL-positive acute lymphoblastic leukemia in Taiwan. American journal of hematology. 2002;71:291–299. doi: 10.1002/ajh.10227. [DOI] [PubMed] [Google Scholar]
- 3.Heerema NA, Harbott J, Galimberti S, et al. Secondary cytogenetic aberrations in childhood Philadelphia chromosome positive acute lymphoblastic leukemia are nonrandom and may be associated with outcome. Leukemia. 2004;18:693–702. doi: 10.1038/sj.leu.2403324. [DOI] [PubMed] [Google Scholar]
- 4.Wetzler M, Dodge RK, Mrozek K, et al. Additional cytogenetic abnormalities in adults with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a study of the Cancer and Leukaemia Group B. British journal of haematology. 2004;124:275–288. doi: 10.1046/j.1365-2141.2003.04736.x. [DOI] [PubMed] [Google Scholar]
- 5.Wrzesien-Kus A, Robak T, Pluta A, et al. Outcome of treatment in adults with Philadelphia chromosome-positive and/or BCR-ABL--positive acute lymphoblastic leukemia-retrospective analysis of Polish Adult Leukemia Group (PALG) Annals of hematology. 2006;85:366–373. doi: 10.1007/s00277-006-0099-z. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Qiu L, Zou D, et al. Additional chromosomal abnormalities and their prognostic significance in adult Philadelphia-positive acute lymphoblastic leukemia: with or without imatinib in chemotherapy. Annals of hematology. 2009;88:1069–1077. doi: 10.1007/s00277-009-0720-z. [DOI] [PubMed] [Google Scholar]
- 7.Yanada M, Takeuchi J, Sugiura I, et al. Karyotype at diagnosis is the major prognostic factor predicting relapse-free survival for patients with Philadelphia chromosome-positive acute lymphoblastic leukemia treated with imatinib-combined chemotherapy. Haematologica. 2008;93:287–290. doi: 10.3324/haematol.11891. [DOI] [PubMed] [Google Scholar]
- 8.Schultz KR, Carroll A, Heerema NA, et al. Long-term follow-up of imatinib in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia: Children's Oncology Group study AALL0031. Leukemia. 2014;28:1467–1471. doi: 10.1038/leu.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fielding AK, Rowe JM, Richards SM, et al. Prospective outcome data on 267 unselected adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia confirms superiority of allogeneic transplantation over chemotherapy in the pre-imatinib era: results from the International ALL Trial MRC UKALLXII/ECOG2993. Blood. 2009;113:4489–4496. doi: 10.1182/blood-2009-01-199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravandi F, O'Brien SM, Cortes JE, et al. Long-term follow-up of a phase 2 study of chemotherapy plus dasatinib for the initial treatment of patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Cancer. 2015;121:4158–4164. doi: 10.1002/cncr.29646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jabbour E, Kantarjian H, Ravandi F, et al. Combination of hyper-CVAD with ponatinib as first-line therapy for patients with Philadelphia chromosome-positive acute lymphoblastic leukaemia: a single-centre, phase 2 study. The Lancet Oncology. 2015;16:1547–1555. doi: 10.1016/S1470-2045(15)00207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daver N, Thomas D, Ravandi F, et al. Final report of a phase II study of imatinib mesylate with hyper-CVAD for the front-line treatment of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Haematologica. 2015;100:653–661. doi: 10.3324/haematol.2014.118588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fabarius A, Leitner A, Hochhaus A, et al. Impact of additional cytogenetic aberrations at diagnosis on prognosis of CML: long-term observation of 1151 patients from the randomized CML Study IV. Blood. 2011;118:6760–6768. doi: 10.1182/blood-2011-08-373902. [DOI] [PubMed] [Google Scholar]
- 14.Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 15.Issa GC, Kantarjian HM, Yin CC, et al. Prognostic impact of pretreatment cytogenetics in adult Philadelphia chromosome-negative acute lymphoblastic leukemia in the era of minimal residual disease. Cancer. 2016 doi: 10.1002/cncr.30376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chilton L, Buck G, Harrison CJ, et al. High hyperdiploidy among adolescents and adults with acute lymphoblastic leukaemia (ALL): cytogenetic features, clinical characteristics and outcome. Leukemia. 2014;28:1511–1518. doi: 10.1038/leu.2013.379. [DOI] [PubMed] [Google Scholar]
- 17.Short NJ, Jabbour E, Sasaki K, et al. Impact of complete molecular response on survival in patients with Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood. 2016;128:504–507. doi: 10.1182/blood-2016-03-707562. [DOI] [PMC free article] [PubMed] [Google Scholar]


