Abstract
All arthropods periodically molt to replace their exoskeleton (cuticle). Immediately after shedding the old cuticle, the neurohormone bursicon causes the hardening and darkening of the new cuticle. Here we show that bursicon, to our knowledge the first heterodimeric cystine knot hormone found in insects, consists of two proteins encoded by the genes burs and pburs (partner of burs). The pburs/burs heterodimer from Drosophila melanogaster binds with high affinity and specificity to activate the G protein-coupled receptor DLGR2, leading to the stimulation of cAMP signaling in vitro and tanning in neck-ligated blowflies. Native bursicon from Periplaneta americana is also a heterodimer. In D. melanogaster the levels of pburs, burs, and DLGR2 transcripts are increased before ecdysis, consistent with their role in postecdysial cuticle changes. Immunohistochemical analyses in diverse insect species revealed the colocalization of pburs- and burs-immunoreactivity in some of the neurosecretory neurons that also express crustacean cardioactive peptide. Forty-three years after its initial description, the elucidation of the molecular identity of bursicon and the verification of its receptor allow for studies of bursicon actions in regulating cuticle tanning, wing expansion, and as yet unknown functions. Because bursicon subunit genes are homologous to the vertebrate bone morphogenetic protein antagonists, our findings also facilitate investigation on the function of these proteins during vertebrate development.
Keywords: BMP antagonist, heterodimeric polypeptide ligand, LGR
Insects comprise >90% of all animal species and are of great economical and ecological importance. The evolutionary success of insects is due partially to their exoskeleton, which provides protection, mechanical support, and an effective barrier to desiccation and infections. During immature stages, continued growth requires that insects replace their exoskeleton. During this molting process, a new cuticle is synthesized and secreted by underlying epidermal cells and then is hardened after the remains of the old cuticle are shed at ecdysis. Molting and ecdysis are regulated by at least six different hormones (1). The steroid 20-hydroxyecdysone directs the synthesis of the new cuticle (2), and then a cascade of peptide hormones orchestrates the events surrounding ecdysis.
According to current knowledge, the last uncharacterized hormone in this cascade, bursicon, is an ≈30-kDa neurohormone that is released after the completion of ecdysis (3, 4) and triggers the tanning (melanization and sclerotization) of the new cuticle as well as wing expansion (5). Bursicon of diverse insects, including blowf ly (Calliphora erythrocephala), cockroach (Periplaneta americana), cricket (Gryllus bimaculatus), locust (Locusta migratoria), and meal beetle (Tenebrio molitor) (4), initiates tanning in neck-ligated flies (6, 7), suggesting the conservation of this signaling system that is essential for insect survival.
Recently, a subfamily of G protein-coupled receptors with a large ectodomain containing leucine-rich repeats was identified and named as LGRs. The LGR genes are conserved in invertebrates and vertebrates (8) and can be divided into three groups as follows: group A, vertebrate glycoprotein hormone receptors; group B, Drosophila melanogaster LGR2 (DLGR2) and vertebrate orphan receptors LGR4/5/6 (9); and group C, mammalian relaxin/INSL3 receptors (10, 11). Based on genetic analyses in D. melanogaster, bursicon was proposed to act through the G protein-coupled receptor DLGR2, encoded by the rickets gene (12).
Based on the comparison with three P. americana partial peptide sequences (13), Dewey et al. (5) reported the identification of burs, an ≈15-kDa protein encoded by the D. melanogaster gene CG13419 as a bursicon candidate. Our preliminary data, however, indicated that recombinant burs was not bioactive. Because burs is a cystine knot protein with an uneven number of cysteine residues and because many ligands of this family form heterodimers (14), we hypothesized that the burs gene product could heterodimerize with another cystine knot protein encoded by the D. melanogaster gene CG15284, which we call pburs (partner of burs). Here, we show that the bursicon (pburs/burs) is, to our knowledge, the first heterodimeric cystine knot hormone discovered in insects. Recombinant pburs/burs is capable of high-affinity binding to the receptor DLGR2 to stimulate cAMP production in vitro and to induce tanning in the neck-ligated fly bioassay in vivo.
Materials and Methods
Isolation of D. melanogaster burs and pburs cDNAs and Identification of Their Orthologs in Other Insect Species. The protein sequence of pburs was identified by using the burs sequence against the D. melanogaster database with blast (15). For the amplification of the pburs cDNA, primers 5′-TGGGAGTGGGTCAGCATGCA-3′ and 5′-TAGTTTATGTTGAGGCATTA-3′ were used. The resulting PCR products were cloned into the pcDNA3.1/Zeo(+) expression vector (Invitrogen) and confirmed by sequencing. Similarly, primers 5′-AAGGACACTCGCAGTCGGGCCGA-3′ and 5′-CTATTGCAGAGCAATGCGCCGGA-3′ were used to amplify the burs cDNA from D. melanogaster. To identify its orthologs, the pburs protein sequence was used in tblastn searches against different insect genomes. The sequences of D. melanogaster burs and mammalian bone morphogenetic protein (BMP) antagonists were identified from the GenBank database [accession nos. AY672905 (burs), AAC39725 (gremlin), BAA92265 (DAN; differential screening-selected gene aberrative in neuroblastoma), AAH46632 (PRDC; protein related to DAN and Cerberus); BAC82440 (Coco), AAK92484 (cerberus), AAN45848 (USAG1), and AAQ88990 (SOST)]. The sequence alignment and phylogenetic tree were constructed by the neighbor-joining method using the megalign program in the dnastar software package (DNA-STAR, Madison, WI).
Expression and Purification of D. melanogaster pburs and burs. The expression vector pcDNA3.1 containing D. melanogaster pburs or burs was transfected into mammalian 293T cells by using Lipofectamine 2000 (Invitrogen), and transfected cells were selected by the Zeocin-containing medium. Selected cells were allowed to reach confluence and then cultured for 72 h in a serum-free medium (DMEM/Ham's F12 medium containing 100 μg/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamate). After filtration, the conditioned media were concentrated. To facilitate purification, D. melanogaster burs and pburs constructs were tagged with His-6- and FLAG-tagged epitopes, respectively, at the N terminus by replacing the endogenous signal peptide with a prolactin signal peptide and the epitope tags. To generate recombinant bursicon, cells coexpressing pburs and burs were clonally selected and confirmed based on tagged epitopes. Conditioned media were further purified by using metal chelating Sepharose (Amersham Pharmacia) against His-6-burs followed by anti-FLAG M1 affinity gel (Sigma) against FLAG-pburs. Protein purity and biochemical characteristics were analyzed after electrophoresis by using a 12% SDS-polyacrylamide gel.
In Vitro and in Vivo Bioassays of Bursicon. For assessing the cAMP production mediated through DLGR2 (8, 16), full-length DLGR2 cDNA was cloned into the pcDNA3.1/Zeo(+) expression vector (Invitrogen) and confirmed by sequencing. The DLGR2-containing plasmid then was transfected into human 293T cells by using Lipofectamine 2000 (Invitrogen). After 18–24 h of transfection, cells were harvested in the cAMP assay buffer (DMEM/F12 supplemented with 0.1% BSA and 0.25 mM 3-isobutyl-1-methylxanthine) and plated on 24-well culture plates (2 × 105 cells per ml). After 30 min of culture, cells were treated with ligands for 16 h before measurement of total cAMP in triplicate by a specific RIA (17).
In vivo bursicon activity was assessed by using the neck-ligated fly bioassay (3). The concentration of recombinant pburs, burs, or bursicon (pburs/burs heterodimer) was adjusted to 10 ng/μl. A 5-μl test solution of recombinant proteins or ganglia extracts from P. americana was injected into ligated flies (Sarcophaga bullata) at adult eclosion. The extent of tanning bioactivity was quantitated by assigning a score between 0 (no tanning) and 6 (dark tanning) as described in ref. 4. In each experimental series, 6–10 flies were injected and scored, and the mean was calculated. Phosphate buffer (pH 7.3) was injected as a control. The average of the means of three experiments ± SD is shown to indicate the variance of the data.
Antibody generation, receptor binding assay, quantitative real-time PCR, immunohistochemistry, and in situ hybridization procedures are described in Supporting Text, which is published as supporting information on the PNAS web site.
Results
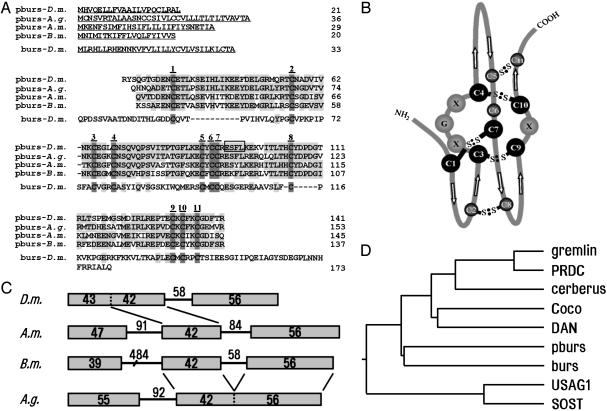
Identification of pburs and Its Relationship with Other Cystine Knot Proteins. Based on GenBank searches for novel cystine knot proteins as potential dimerization partners for burs, we identified the CG15284 gene in the fruit fly (D. melanogaster), which we have named pburs (partner of burs). As shown in Fig. 1A, the predicted pburs protein is highly conserved among D. melanogaster, mosquito (Anopheles gambiae), honey bee (Apis mellifera), and silkworm (Bombyx mori), and all contained the 11 cysteine residues that are also present in the D. melanogaster burs. A stretch of four residues (boxed) in D. melanogaster pburs is identical to a partial peptide of purified bursicon (GT28) found in P. americana (13). Based on the known cystine knot structures of TGF-β and CG-β (14), the structure of pburs could be predicted (Fig. 1B). Both pburs and burs are likely to form a cystine knot and contain two intramolecular disulfide bonds between C2–C8 and C5–C11. In addition, a free cysteine at position 6 may be involved in the formation of an intermolecular bridge. Analyses of the pburs gene structures from the four insects indicated that they consist of two or three exons (Fig. 1C). Of interest, the first exon in D. melanogaster is split into two exons in A. mellifera and B. mori, whereas exons 2 and 3 in A. mellifera and B. mori are combined into one exon in A. gambiae. Phylogenetic analyses based on the cystine knot region of several related proteins further indicated that pburs, together with burs, showed close sequence relationships with cystine knot proteins in the human BMP antagonist family (Fig. 1D). Some of these proteins have been found to antagonize the actions of BMP ligands during embryonic development and organogenesis (18, 19).
Fig. 1.
Predicted sequences, gene structure, and phylogenetic analyses of pburs from different insects. (A) Sequence alignment of pburs from different insects and burs from D. melanogaster (D.m.). Conserved residues are shaded, and cysteine residues are numbered. A.g., Anopheles gambiae; A.m., Apis mellifera; B.m., Bombyx mori. A stretch of four residues (boxed) in D.m. pburs is identical to that of a fragment purified from protease-treated bursicon from P. americana.(B) Predicted cystine knot structure of pburs. (C) Intron–exon arrangement of pburs genes in different insects. Exons are represented by boxes. The numbers of amino acid residues for each exon and nucleotides for each intron are shown. (D) Phylogenetic tree of D.m. pburs and burs together with seven human BMP antagonists.
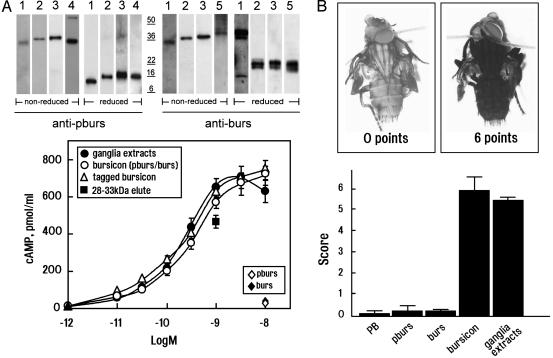
Bursicon Is a Heterodimer of pburs and burs Capable of Activating DLGR2. We generated specific antibodies against pburs and burs. As shown in Fig. 2A (Upper), in blots of SDS-polyacrylamide gels, a single high-molecular mass band of ≈30 kDa could be recognized by both anti-pburs and anti-burs antibodies in ganglia extracts of P. americana (lane 1) run under nonreducing conditions. In contrast, two smaller bands corresponding to pburs (≈14 kDa) and burs (≈16 kDa) monomers were found under reducing conditions (the nature of the two bands at ≈44 kDa, detected by using anti-burs antibodies under reducing conditions, is presently unknown). In addition, 293T cells were transfected with plasmids containing pburs and/or burs with or without epitope tags to generate recombinant proteins. Immunoblotting showed that the conditioned medium from transfected cells contained heterodimers or homodimers (lanes 2–5). When cells were cotransfected with pburs and burs (lane 3), only the heterodimers were detected. The molecular masses of the recombinant proteins were slightly larger than those found in the ganglia extracts from P. americana. Differential posttranslational modifications of native and recombinant proteins may account for these differences.
Fig. 2.
Bursicon, a heterodimer of two cystine-knot-containing polypeptides, is the cognate ligand for DLGR2. (A)(Upper) Immunoblot analyses of bursicon from P. americana ganglia extracts and conditioned media from 293T cells transfected with pburs and/or burs. Samples were run under nonreducing and reducing conditions. Lane 1, ganglia extracts from P. americana; lane 2, recombinant pburs/burs without epitope tags; lane 3, recombinant epitope-tagged pburs/burs; lane 4, recombinant pburs without a tag; and lane 5, recombinant burs without a tag. (Lower) Stimulation of DLGR2 by P. americana ganglia extracts or conditioned media from cells cotransfected with pburs/burs expression constructs (bursicon heterodimer), but not by the individual plasmid (pburs or burs). Both wild-type and epitope-tagged bursicon heterodimers were tested. In addition, proteins eluted from the 28- to 33-kDa region of a SDS gel loaded with P. americana ganglia extracts also were tested (▪). Ligand levels were determined from immunoblots by using purified tagged bursicon heterodimers as a standard. (B) Neck-ligated fly bioassay. Recombinant bursicon or P. americana ganglia extracts stimulated complete tanning of flies with a maximum score (5–6 points), whereas flies injected with phosphate buffer (PB), pburs, and burs alone did not tan and received an average score of 0–0.3 points. In three separate experiments, 6–10 flies were injected, and their tanning score was averaged.
To test the bioactivity of these proteins, 293T cells overexpressing DLGR2 were treated with medium containing heterodimeric pburs/burs with or without epitope tags. Both heterodimeric preparations stimulated dose-dependent increases in cAMP production, comparable to levels induced by P. americana ganglia extracts (Fig. 2 A Lower). In contrast, medium from cells transfected with either pburs or burs alone were ineffective. By using the neck-ligated fly bioassay, injections with recombinant heterodimer preparations or with P. americana ganglia extracts stimulated maximal tanning in a 0- to 6-point scoring system (Fig. 2B). In contrast, treatment with recombinant pburs or burs alone induced minimal tanning, similar to that obtained by using phosphate buffer. Protein elutions of P. americana ganglia extracts from SDS gels run under nonreducing conditions showed that only the 28- to 33-kDa fraction (bursicon) was functional in the neck-ligated fly bioassay (4) and activated DLGR2 (Fig. 2 A Lower). The recombinant heterodimer of pburs and burs therefore corresponds to bursicon.
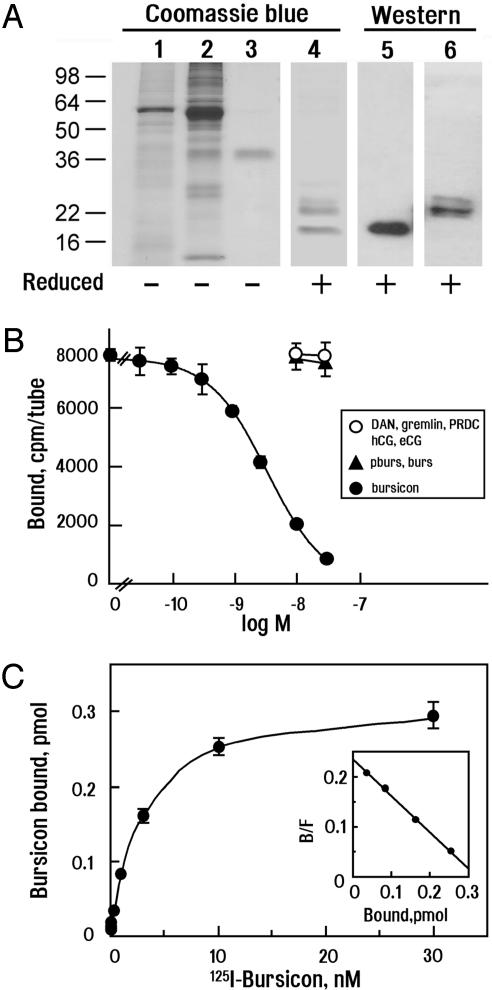
Specific High-Affinity Binding of Labeled Bursicon to DLGR2. To assess receptor-binding ability, we purified recombinant epitope-tagged bursicon by using two sequential affinity columns against polyHis and the FLAG epitope appended to burs and pburs, respectively. The purity of the bursicon heterodimer was confirmed by using SDS/PAGE followed by Coomassie blue staining (Fig. 3A, lanes 1–3). Under nonreducing conditions, purified bursicon migrated ≈38 kDa (Fig. 3A, lane 3), whereas two lower bands were evident under reducing conditions (Fig. 3A, lane 4) and confirmed to be pburs and burs monomers by using specific antibodies (Fig. 3A, lanes 5 and 6). Purified bursicon was iodinated and found to bind to cells expressing DLGR2 (Fig. 3B). 125I-bursicon binding was displaced in a dose-dependent manner by nonlabeled bursicon but was not affected by pburs or burs alone. Likewise, several BMP antagonists, gremlin, DAN, or PRDC, as well as gonadotropins (human chorionic gonadotropin or equine chorionic gonadotropin), did not compete for bursicon binding. Conversion of the displacement curve to the saturation and Scatchard plots (Fig. 3C) indicated that bursicon binds to DLGR2 with high affinity (Kd = 2.5 × 10–9 M).
Fig. 3.
Purification of bursicon and high-affinity binding of labeled bursicon to recombinant DLGR2 receptors. (A) Purification of tagged recombinant bursicon heterodimers. SDS/PAGE analyses of conditioned media containing tagged bursicon (lane 1) followed by sequential affinity purification using metal chelating (lane 2) and anti-FLAG M1 affinity chromatography (lane 3, nonreducing conditions; lane 4, reducing conditions). Samples were detected by using Coomassie blue staining. Purified bursicon proteins were further confirmed as a heterodimer by using antibodies against pburs (lane 5) or burs (lane 6) under reducing conditions. (B) Binding of 125I-labeled recombinant bursicon to DLGR2 was competed by nonlabeled bursicon, whereas coincubation with conditioned media containing only pburs, burs, or other proteins such as BMP antagonists (DAN, gremlin, or PRDC) or glycoprotein hormones (human chorionic gonadotropin, hCG, or equine chorionic gonadotropin, eCG) were not effective. (C) Saturation and Scatchard plot (Inset) analyses of bursicon binding to DLGR2.
Developmental Changes in the Expression of pburs, burs, and DLGR2 in D. melanogaster. To examine the expression of pburs, burs, and DLGR2, we performed real-time PCR analyses by using RNA preparations from different developmental stages of D. melanogaster. As shown in Fig. 5, which is published as supporting information on the PNAS web site, the transcript levels for pburs and burs showed a similar pattern of regulation. They were both low in larva (stages 1 and 2) and gradually increased in pupal stages (3 and 4) before reaching the highest levels in pharate adults (stage 7), in preparation for tanning after adult eclosion. For DLGR2 transcripts, an increase was found in early pupae (stage 4), followed by a decrease in the late pupae (stage 6), before increasing dramatically in pharate adults. In the adult animals (stage 8), all three transcripts were low.
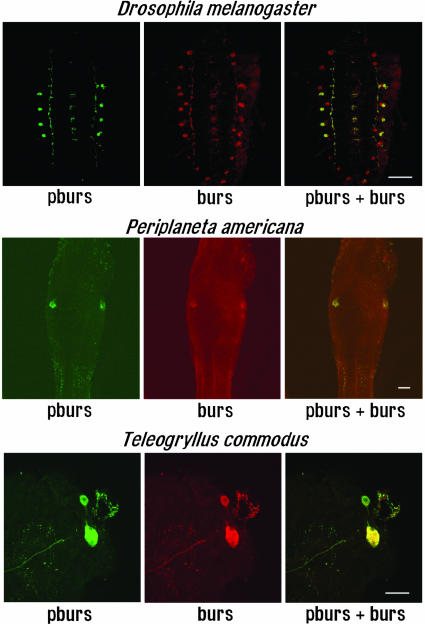
Colocalization of pburs, burs, and Crustacean Cardioactive Peptide (CCAP) in the Nervous System of Different Insects. Because the sequences of both pburs and burs are highly conserved in insects, we used antibodies against D. melanogaster pburs or burs to detect neurons expressing these proteins in the central nervous systems of three different insect species. Immunohistochemical staining for pburs and burs in the third-instar larva of D. melanogaster (Fig. 4 Top) revealed that pburs was coexpressed with burs in four bilateral neurons in thoracic and abdominal neuromeres of the ventral nervous system. However, the somata in the subesophageal and posterior abdominal neuromeres expressed only burs. Likewise, colocalization of pburs and burs occurred in the large bilateral lateral neurosecretory neurons of the first three unfused abdominal ganglia and in all anterior bilateral cell pairs in the thoracic ganglia of P. americana and in homologous neurons in the thoracic and abdominal ganglia of the cricket Teleogryllus commodus (Fig. 4 Middle and Bottom). In these somata, the immunostaining was found in large granules that characterize these endocrine cells.
Fig. 4.
Colocalization of pburs and burs in different insect nervous systems. Immunohistochemical detection of pburs and burs immunoreactivities in the ventral nervous system of D. melanogaster, P. americana, and T. commodus. (Top) Double labeling experiments in the third-instar larva of D. melanogaster. (Middle) pburs and burs colocalized in the large bilateral lateral somata of the second unfused abdominal ganglion of P. americana.(Bottom) pburs and burs colocalized in the large anterior bilateral neuron pair in the second thoracic ganglion of T. commodus. The structure at right is a large trachea. (Confocal imaging; scale bars, 100 μm.)
Further, by using antibodies against pburs and CCAP in P. americana and in situ hybridization of pburs in wild-type and CCAP neuron-ablated fly mutants (Fig. 6, which is published as supporting information on the PNAS web site), our data suggest that pburs, like burs (5), is colocalized with CCAP in some CCAP-immunoreactive neurons. Further information can be obtained in Supporting Text.
Discussion
The present study demonstrates the heterodimeric nature of bursicon, a neuroendocrine hormone essential for insect tanning and wing expansion. Bursicon consists of two cystine knot polypeptides, pburs and burs, capable of activating a G protein-coupled receptor, DLGR2. Under reducing conditions, purified recombinant bursicon heterodimers could be separated into pburs and burs monomers. Although the size of native cockroach bursicon recognized by both anti-pburs and -burs antibodies is consistent with previous findings (4, 20), the recombinant D. melanogaster bursicon expressed in mammalian cells showed a higher molecular mass, suggesting posttranslational modifications such as phosphorylation. Both recombinant and native bursicon induced cAMP production in DLGR2-expressing cells and stimulated tanning in the in vivo neck-ligated fly bioassay. Specific, high-affinity interactions between bursicon and DLGR2 were confirmed by using a radioligand receptor assay.
In D. melanogaster, mutations in the rickets gene show crossed postscutellar bristles and kinked femurs (21). These mutant flies carry lesions in the DLGR2 gene and fail to initiate tanning and wing expansion after adult emergence. Although rickets mutants do not melanize when injected with ganglia extracts containing bursicon, they do tan in response to injection of an analog of cAMP (12), consistent with our findings of the stimulation of cAMP production by bursicon in cells transfected with DLGR2. Because flies with burs mutations showed the same phenotypes as rickets mutants, burs was proposed to encode a ligand for DLGR2 (5, 12). The present findings demonstrate, however, that burs needs pburs as a partner for DLGR2 activation.
In addition to the induction of cuticle tanning, bursicon also has been implicated in a stereotyped behavioral program for wing expansion (12) and in the postecdysial cell death of wing epidermal cells (22, 23). Our real-time PCR results showing increases of pburs, burs, and DLGR2 transcripts at pupariation and eclosion stages of D. melanogaster are consistent with the importance of these genes during insect metamorphosis.
Immunohistochemical analyses of pburs and burs demonstrated that both polypeptides are colocalized in four bilateral neurons of the D. melanogaster ventral nervous system and in select neurons of P. americana and T. commodus. However, in D. melanogaster and T. commodus, some neurons express only burs, but not pburs. The neurons containing solely burs may serve different, yet-unknown functions. Sequence conservation of both pburs (Fig. 1) and burs in diverse insects (5) along with the immunohistochemical results indicate the evolutionary conservation of both genes. In addition to insects, bursicon activity has also been found in the abdominal nerve cord of the crustacean Homarus americanus (4), suggesting that both pburs and burs are likely conserved throughout arthropods.
Our real-time PCR analyses of burs, pburs, and DLGR2 showed that these transcripts were present before pupal and adult ecdysis. Maximal levels were observed just before adult emergence, consistent with the known role of bursicon in posteclosion tanning and sclerotization of the cuticle, wing inflation, and epidermal cell death (22). Earlier studies in moth (Manduca sexta) (24), locust (Schistocerca gregaria) (25), and Drosophila (26) demonstrated that most CCAP-expressing neurons undergo apoptosis after adult ecdysis, consistent with our findings of low transcript levels for pburs and burs in mature adult D. melanogaster. This low level of pburs and burs expression was accompanied by a reduction in the levels of DLGR2 message. Although the exact identity of bursicon targets is unclear, this change could reflect the down-regulation of bursicon receptor expression and/or the death of bursicon target cells. In other insects, the CCAP- and bursicon-expressing neurons survive into adulthood (13, 27). This finding may indicate that bursicon has additional unknown functions that can now be investigated.
D. melanogaster bearing targeted ablations of CCAP neurons showed defects in wing expansion and tanning (28), as well as lower bursicon bioactivity, indicating that bursicon expression is also disrupted. We have found that both pburs and burs are expressed in CCAP neurons, consistent with previous work showing that CCAP neurons express bursicon bioactivity in crickets (27) and cockroaches (13), and some neurons containing bursicon bioactivity in Manduca (29) also express CCAP (30). Further discussions of the potential relationship between bursicon and CCAP can be obtained in Supporting Text.
In D. melanogaster, three LGR genes have been identified (8, 16). They encode seven transmembrane proteins with a large ectodomain containing leucine-rich repeats. These three fly LGRs showed sequence homology with the three subgroups of mammalian LGRs, including the group A glycoprotein hormone receptors, group B LGR4/5/6, and group C LGR7 and -8 (31). Fly DLGR2, like mammalian LGR4/5/6, has 17–18 leucine-rich repeats, likely involved in ligand binding. A recent study identified a group B LGR ortholog in the gastropod Crassostrea gigas (oyster) (32), suggesting that these receptors have ancient origins. Although the ligands for LGR7 and LGR8 recently have been identified (10, 11), the ligands for the group B vertebrate LGRs are unknown. The insect pburs and burs genes are homologous to the vertebrate BMP antagonist family of genes found to be important during embryonic development and organogenesis (18, 19, 33). Only some members of the BMP antagonist family are competitive antagonists capable of direct binding to BMPs (18, 33), and the exact mechanisms of actions of most proteins in this family are still unknown. Future studies will reveal whether these cystine knot proteins are ligands for vertebrate orphans LGR4/5/6. Of interest, recent studies demonstrated that mutations in the LGR4 and LGR5 genes in mice are associated with perinatal lethality, suggesting important roles during embryonic development (34, 35).
The present identification of two cystine knot polypeptides as subunits for the heterodimeric bursicon in insects, together with the demonstration of specific binding and activation of a G protein-coupled receptor with leucine-rich repeats, finally allow for an investigation of the diverse aspects of the physiological functions of this important neuroendocrine hormone.
Supplementary Material
Acknowledgments
We thank C. Spencer for editorial assistance. The idea that bursicon might be a heterodimer of two cystine knot proteins was originally mentioned to one of us (H.-W.H.) by Dr. J. W. Truman. This work was supported by National Institutes of Health Grant HD23273 (to A.J.W.H.), National Science Foundation Grants 0217637 (to H.-W.H.) and 0343699 (to J.E.), and by the Bioinformatic Core of U54-HD-31398 as part of the Specialized Cooperative Centers Program in Reproduction Research. Confocal images were performed through the use of the Vanderbilt University Medical Center Cell Imaging Core Resource (supported by National Institutes of Health Grants CA68485, DK20593, and DK58404).
Abbreviations: BMP, bone morphogenetic protein; CCAP, crustacean cardioactive peptide.
Data deposition: The sequences for pburs reported in this paper have been deposited in the GenBank database [accession nos. AY823257 (D. melanogaster), AY823258 (A. mellifera), AY823259 (A. gambiae), and AY823260 (B. mori)].
References
- 1.Ewer, J. & Reynolds, S. (2002) in Hormones, Brain and Behavior, eds. Pfaff, D., Arnold, A., Etgen, A. M., Fahrbach, S. & Rubin, R. T. (Academic, San Diego) Vol. 3, pp. 1–92. [Google Scholar]
- 2.Riddiford, L. M. (1989) in Ecdysone: From Chemistry to Mode of Action, ed. Koolman, J. (Thieme, Stuttgart), pp. 407–413.
- 3.Fraenkel, G. & Hsiao, C. (1965) J. Insect Physiol. 11, 513–556. [Google Scholar]
- 4.Kostron, B., Marquardt, K., Kaltenhauser, U. & Honegger, H. W. (1995) J. Insect Physiol. 41, 1045–1053. [Google Scholar]
- 5.Dewey, E. M., McNabb, S. L., Ewer, J., Kuo, G. R., Takanishi, C. L., Truman, J. W. & Honegger, H. W. (2004) Curr. Biol. 14, 1208–1213. [DOI] [PubMed] [Google Scholar]
- 6.Cottrell, C. B. (1962) J. Exp. Biol. 39, 413–430. [Google Scholar]
- 7.Fraenkel, G. & Hsiao, C. (1962) Science 138, 27–29. [DOI] [PubMed] [Google Scholar]
- 8.Nishi, S., Hsu, S. Y., Zell, K. & Hsueh, A. J. (2000) Endocrinology 141, 4081–4090. [DOI] [PubMed] [Google Scholar]
- 9.Hsu, S. Y. (2003) Trends Endocrinol. Metab. 14, 303–309. [DOI] [PubMed] [Google Scholar]
- 10.Hsu, S. Y., Nakabayashi, K., Nishi, S., Kumagai, J., Kudo, M., Sherwood, O. D. & Hsueh, A. J. (2002) Science 295, 671–674. [DOI] [PubMed] [Google Scholar]
- 11.Kumagai, J., Hsu, S. Y., Matsumi, H., Roh, J. S., Fu, P., Wade, J. D., Bathgate, R. A. & Hsueh, A. J. (2002) J. Biol. Chem. 277, 31283–31286. [DOI] [PubMed] [Google Scholar]
- 12.Baker, J. D. & Truman, J. W. (2002) J. Exp. Biol. 205, 2555–2565. [DOI] [PubMed] [Google Scholar]
- 13.Honegger, H. W., Market, D., Pierce, L. A., Dewey, E. M., Kostron, B., Wilson, M., Choi, D., Klukas, K. A. & Mesce, K. A. (2002) J. Comp. Neurol. 452, 163–177. [DOI] [PubMed] [Google Scholar]
- 14.Vitt, U. A., Hsu, S. Y. & Hsueh, A. J. (2001) Mol. Endocrinol. 15, 681–694. [DOI] [PubMed] [Google Scholar]
- 15.Altschul, S. F., Madden, T. L., Schaffer, A. A., Zhang, J., Zhang, Z., Miller, W. & Lipman, D. J. (1997) Nucleic Acids Res. 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eriksen, K. K., Hauser, F., Schiott, M., Pedersen, K. M., Sondergaard, L. & Grimmelikhuijzen, C. J. (2000) Genome Res. 10, 924–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo, C. W., Kawamura, K., Klein, C. & Hsueh, A. J. (2004) Mol. Endocrinol. 18, 2085–2096. [DOI] [PubMed] [Google Scholar]
- 18.Sudo, S., Avsian-Kretchmer, O., Wang, L. S. & Hsueh, A. J. (2004) J. Biol. Chem. 279, 23134–23141. [DOI] [PubMed] [Google Scholar]
- 19.Canalis, E., Economides, A. N. & Gazzerro, E. (2003) Endocr. Rev. 24, 218–235. [DOI] [PubMed] [Google Scholar]
- 20.Taghert, P. H. & Truman, J. W. (1982) J. Exp. Biol. 98, 373–383. [Google Scholar]
- 21.Edmondson, M. E. (1948) Drosophila Inf. Serv. 22, 53. [Google Scholar]
- 22.Seligman, I. M., Filshie, B. K., Doy, F. A. & Crossley, A. C. (1975) Tissue Cell 7, 281–296. [DOI] [PubMed] [Google Scholar]
- 23.Kimura, K., Kodama, A., Hayasaka, Y. & Ohta, T. (2004) Development (Cambridge, U.K.) 131, 1597–1606. [DOI] [PubMed] [Google Scholar]
- 24.Ewer, J., Wang, C. M., Klukas, K. A., Mesce, K. A., Truman, J. W. & Fahrbach, S. E. (1998) J. Neurobiol. 37, 265–280. [DOI] [PubMed] [Google Scholar]
- 25.Braunig, P. (1990) Cell Tissue Res. 260, 95–108. [Google Scholar]
- 26.Draizen, T. A., Ewer, J. & Robinow, S. (1999) J. Neurobiol. 38, 455–465. [DOI] [PubMed] [Google Scholar]
- 27.Kostron, B., Kaltenhauser, U., Seibel, B., Braunig, P. & Honegger, H. (1996) J. Exp. Biol. 199, 367–377. [DOI] [PubMed] [Google Scholar]
- 28.Park, J. H., Schroeder, A. J., Helfrich-Forster, C., Jackson, F. R. & Ewer, J. (2003) Development (Cambridge, U.K.) 130, 2645–2656. [DOI] [PubMed] [Google Scholar]
- 29.Taghert, P. H. & Truman, J. W. (1982) J. Exp. Biol. 98, 385–401. [Google Scholar]
- 30.Ewer, J. & Truman, J. W. (1996) J. Comp. Neurol. 370, 330–341. [DOI] [PubMed] [Google Scholar]
- 31.Hsu, S. Y., Kudo, M., Chen, T., Nakabayashi, K., Bhalla, A., van der Spek, P. J., van Duin, M. & Hsueh, A. J. (2000) Mol. Endocrinol. 14, 1257–1271. [DOI] [PubMed] [Google Scholar]
- 32.Herpin, A., Badariotti, F., Rodet, F. & Favrel, P. (2004) Biochim. Biophys. Acta 1680, 137–144. [DOI] [PubMed] [Google Scholar]
- 33.Avsian-Kretchmer, O. & Hsueh, A. J. (2004) Mol. Endocrinol. 18, 1–12. [DOI] [PubMed] [Google Scholar]
- 34.Mazerbourg, S., Bouley, D. M., Sudo, S., Klein, C. A., Zhang, J. V., Kawamura, K., Goodrich, L. V., Rayburn, H., Tessier-Lavigne, M. & Hsueh, A. J. (2004) Mol. Endocrinol. 18, 2241–2254. [DOI] [PubMed] [Google Scholar]
- 35.Morita, H., Mazerbourg, S., Bouley, D. M., Luo, C. W., Kawamura, K., Kuwabara, Y., Baribault, H., Tian, H. & Hsueh, A. J. (2004) Mol. Cell. Biol. 24, 9736–9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.