Abstract
Chronic hepatitis B (CHB) affects over 350 million individuals worldwide and is the most common cause of liver cancer. In the United States, CHB affects at least 2 to 3 million individuals, and current therapies can control the disease but not cure it. There are over 30 new molecules being studied in CHB in preclinical to phase 2 studies, targeting specific parts of the hepatitis B virus (HBV) life cycle and the host immune response. When discussing new therapies for CHB, it is critical to understand both the various phases of CHB and the life cycle of HBV. This article will discuss both of these issues, as well as mechanisms of action of potential therapies and possible ways to combine such therapies in the various phases of CHB.
Keywords: HBV life cycle, capsid inhibitors, RNA silencing, HBsAg release inhibitors, TLR agonists, checkpoint inhibitors, therapeutic vaccines
Natural History of Hepatitis B Virus
After natural infection in adults, the majority of individuals lose serum hepatitis B surface antigen (HBsAg) and gain antibody to hepatitis B surface antigen (anti-HBs), a so-called functional cure (Table 1). In contrast, exposure to hepatitis B virus (HBV) as neonates and children usually leads to chronic hepatitis B (CHB) that initially is characterized by very high HBV DNA levels, serum hepatitis B e antigen (HBeAg) positivity, normal alanine aminotransferase (ALT) levels, and normal liver biopsy findings, the so-called immune tolerant phase, which lasts several decades.1 This phase is followed by an immune active phase, in which HBV DNA levels are above 2000 IU/mL, ALT levels are elevated, and active inflammation and fibrosis are present on liver biopsy. The majority of patients evolve into an inactive phase of disease, characterized by normal ALT levels, loss of HBeAg, acquisition of antibody to hepatitis B e antigen (anti-HBe), and HBV DNA levels less than 2000 IU/mL. Some patients will reactivate with HBeAg-positive or -negative disease, and these patients are at higher risk for progression to cirrhosis and hepatocellular carcinoma. Few patients (<1% per year) lose HBsAg; those who do usually have inactive CHB.
Table 1.
Types of Chronic HBV Control and Cure
| Inactive State |
Sustained, off drug:
|
| Functional Cure (Clinical Resolution) |
Sustained, off drug:
|
| Complete Cure (Virologic Cure) |
|
ALT, alanine aminotransferase; anti-HBs, antibody to hepatitis B surface antigen; cccDNA, covalently closed circular DNA; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus.
Current treatment of CHB targets the immune active phase. Control can be achieved in a number of ways: inflammatory control with normalization of serum ALT levels and decreased activity on liver biopsy, virologic control with suppression of serum HBV DNA levels, or immunologic control with seroconversion from HBeAg to anti-HBe and from HBsAg to anti-HBs. As of 2017, CHB cannot be cured, but it can be controlled with improvement in morbidity and mortality from cirrhosis and hepatocellular carcinoma.2 Current nucleos(t)ide (nucs) therapies are very successful in suppressing HBV DNA levels. Both nucs and interferon (IFN) decrease inflammation and fibrosis. However, the achievement of a functional cure in patients with CHB (off therapy, loss of HBsAg, and normal liver function with or without anti-HBs) occurs in a minority of patients.3 This means that many or most patients face long-term, even lifelong, therapy to control CHB.
Life Cycle of Hepatitis B Virus
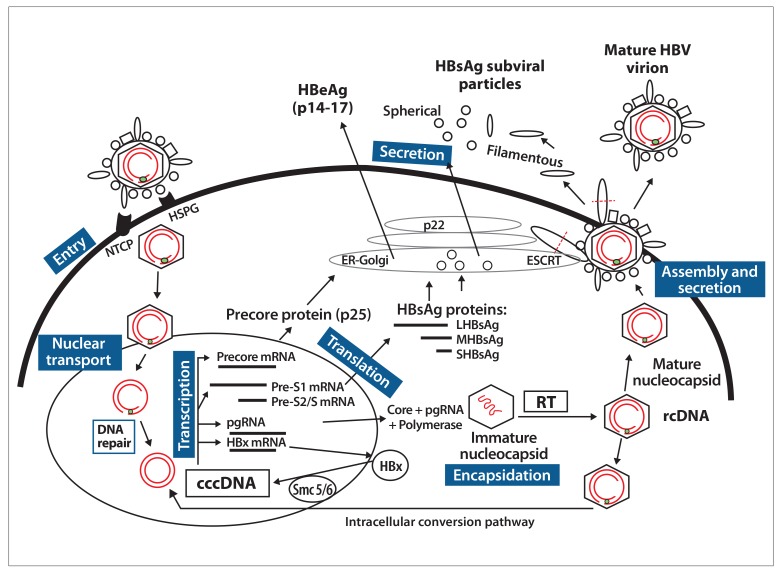
Understanding new therapies requires an understanding of the HBV life cycle, as different therapies will target different parts of the HBV life cycle, as shown in the Figure and Table 2. Current therapeutic targets are shown in Table 3. HBV is a partially double-stranded, enveloped DNA virus that replicates its genome via reverse transcription (RT).4 It enters the hepatocyte via a 2-step process involving binding to the sodium taurocholate cotransporting polypeptide (NTCP),5 a high affinity receptor, after the virus has bound to heparan sulfate proteoglycans (HSPG), a low affinity receptor. The current model of entry is that, in the space of Disse, HBV is first tethered in a reversible manner by the “a” determinant on the HBsAg, the main envelope protein of the virus, to HSPG, and this facilitates interaction of the NTCP binding site on the pre-S1 protein of the HBV to NTCP. Following binding, HBV is endocytosed into the cell and released into the cytoplasm of the hepatocyte.
Figure.
Life cycle of hepatitis B virus.
Entry: HBV is tethered to HSPG, and this facilitates entry via its receptor NTCP. HBV is endocytosed into the cell and released into the cytoplasm of the hepatocyte.
Nuclear transport: After uncoating of the HBV virion in the cytoplasm, the partially double-stranded DNA is transported to the nucleus, where its genomic rcDNA is repaired by host enzymes to form cccDNA, the template for HBV replication.
Transcription: cccDNA generates pgRNA that produces the core protein and viral polymerase, precore mRNA that produces HBeAg, and the subgenomic RNAs that produce HBsAg and HBx proteins.
Encapsidation: The HBV core protein (core) is critical for formation of replication complexes containing the pgRNA and polymerase, forming an immature nucleocapsid. After RT of the pgRNA, a mature nucleocapsid containing DNA is formed. Core is also required for intracellular trafficking, and encapsidated HBV genomes are imported back into the nucleus via the intracellular conversion pathway, partly replenishing the pool of cccDNA minichromosomes.
Assembly and secretion: Mature nucleocapsids are enveloped with HBsAg (pre-S1, pre-S2, and S) proteins through the ESCRT machinery in the Golgi, resulting in the secretion of mature virions and releasing excess HBsAg as filamentous subviral particles. The HBsAg subviral 22-nm particles are produced in great excess and secreted via the ER-Golgi apparatus. HBeAg is a soluble and secreted product of precore mRNA.
cccDNA, covalently closed circular DNA; ER, endoplasmic reticulum; ESCRT, endosomal sorting complex required for transport; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HBx, hepatitis B x; HSPG, heparan sulfate proteoglycans; LHBsAg, large hepatitis B surface antigen; MHBsAg, medium hepatitis B surface antigen; mRNA, messenger RNA; NTCP, sodium taurocholate cotransporting polypeptide; pgRNA, pregenomic RNA; rcDNA, relaxed circular DNA; RT, reverse transcription; SHBsAg, small hepatitis B surface antigen; smc, structural maintenance of chromosomes.
Table 2.
Strategies to Eradicate Chronic HBV Infection
| Virologic Approaches | Host Immune Approaches |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
APOBEC3A/3B, apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like 3A or 3B; cccDNA, covalently closed circular DNA; HBV, hepatitis B virus; IL, interleukin; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; TLR, toll-like receptor.
Table 3.
Potential HBV Therapies in the Pipeline With Known Site of Action
| Site of Action | Drug | Company | Status |
|---|---|---|---|
| Antiviral | |||
| Entry inhibitor | Myrcludex B | Hepatera/MYR GmbH | Phase 2 |
| cccDNA inhibitor | Preclinical | ||
| RNA silencers | ARC-520/-521 | Arrowhead | Terminated |
| ARB-1467 | Arbutus | Phase 2 | |
| ARB-1740 | Arbutus | Preclinical | |
| ALN-HBV | Alnylam | Phase 1/2 | |
| GSK 3228836 | GlaxoSmithKline/Ionis | Phase 1 | |
| Core protein inhibitors | AL-3778 | Alios/Johnson & Johnson | Phase 1/2 |
| ABI-H0731 | Assembly Biosciences | Phase 1 | |
| BAY 41-4109 | AiCuris (Germany) | Phase 1 | |
| GLS4 | HEC | Phase 1 | |
| JNJ-56136379 | Johnson & Johnson | Phase 1 | |
| AB-423 | Arbutus | Preclinical | |
| HBsAg release inhibitors | REP 2139 | Replicor | Phase 2 |
| REP 2165 | Replicor | Phase 2 | |
| Immunomodulators | |||
| TLR-7 agonist | GS-9620 | Gilead Sciences | Phase 2 |
| Therapeutic vaccines | GS-4774 | Gilead Sciences | Complete phase 2 |
| ABX 203 | ABIVAX (France) | Phase 2/3 | |
| AIC649 | AiCuris (Germany) | Phase 1 | |
| FB-02.2 | Altimmune | Phase 1 | |
| INO-1800 | Inovio | Phase 1 | |
| TG1050 | Transgene (France) | Phase 1 | |
| Small molecules | |||
|
SB 9200 | Spring Bank Pharmaceuticals | Phase 2 |
|
Birinapant | TetraLogic | Terminated |
|
PD-1/PDL-2 mAb | Merck Sharp and Dohme; Bristol-Myers Squibb | |
| CTLA-4 mAb | |||
cccDNA, covalently closed circular DNA; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; mAb, monoclonal antibody; PD-1, programmed cell death protein 1; PDL-2, programmed death-ligand 2; RIG-1 NOD2, retinoic acid-inducible gene I nucleotide-binding oligomerization domain-containing protein 2; SMAC, second mitochondrial activator of caspase; TLR, toll-like receptor.
After uncoating of the HBV virion in the cytoplasm, the partially double-stranded DNA is transported to the nucleus, where its genomic relaxed circular DNA (rcDNA) is repaired by host enzymes to form covalently closed circular DNA (cccDNA), the template for HBV replication, which exists as a minichromosome. The HBV DNA does not contain an origin of replication, so the cccDNA cannot replicate in the conventional manner of double-stranded DNA, but is instead replenished from cytoplasmic rcDNA originating from RT of the pregenomic RNA. In the nucleus, the cccDNA minichromosome associates with the hepatitis B core antigen (HBcAg), histones, and other DNA-chromatin complexes, including topoisomerases, to form a functional minichromosome.6 These minichromosomes are not static but exist as multiple topoisomers (up to 21), half of which are transcriptionally active and half inactive.7 The cccDNA has a long half-life, especially the transcriptionally silent cccDNA molecules, is stable in quiescent cells, and survives cell turnover, but cellular proliferation does dilute cccDNA copy number. The cccDNA minichromosome is the template for transcription, generating 2 classes of RNA: (1) pregenomic RNA (greater than genomic length) that produces the core protein and viral polymerase and (2) subgenomic RNAs that produce envelope proteins and hepatitis B x (HBx) protein. The HBx protein is a crucial regulator of HBV transcriptional activity and is absolutely required for maintenance of active and persistent replication. Recently, it has been shown that HBx protein inactivates the cellular chromatin complex of structural maintenance of chromosome 5/6, which inhibits HBV transcription.8 For a functional cure of CHB, HBx protein potentially represents an excellent target.
The HBV core protein (HBcAg) is critical for formation of replication complexes containing the pregenomic RNA and polymerase, forming an immature nucleocapsid that, after RT of the pregenomic RNA, forms the mature nucleocapsid containing rcDNA. The core protein is not only required for RT, but also for intracellular trafficking and maintenance of chronic infection, as encapsidated HBV genomes are imported back into the nucleus via the intracellular conversion pathway, partly replenishing the pool of cccDNA minichromosomes. The majority of mature nucleocapsids are enveloped with HBsAg proteins (pre-S1, pre-S2, and S) through the endosomal sorting complex required for transport machinery in the Golgi, resulting in the secretion of mature virions. It is possible that some of these released virions can re-infect hepatocytes, further amplifying the pool of cccDNA molecules in each cell. The subviral 22-nm particles are produced in great excess and released via the endoplasmic reticulum–Golgi apparatus.9
HBeAg is a soluble and secreted product of precore messenger RNA (mRNA) and is the other greater-than-genomic-length RNA. HBeAg has been shown to promote immunologic tolerance10 and directly modify toll-like receptor (TLR) signaling and antigen presentation in the liver.11 HBV DNA can integrate into the host through a process of illegitimate recombination assisted by host enzymes acting on double-stranded linear DNA.12 Integrated HBV sequences do not provide an adequate template for productive replication of whole virions, but the open reading frame of the S gene, with its regulatory elements, is usually still intact, and so HBsAg can be produced. This is of great relevance to new direct-acting antiviral agents and treatment goals, as 2 sources of HBsAg can be identified: those from cccDNA minichromosomes (episomal) and those from integrated HBV DNA.
Direct-Acting Antiviral Agents
Entry Inhibitors
As outlined above, following binding of HBV to NTCP on the hepatocyte surface, HBV is endocytosed into the cytoplasm of the hepatocyte. Small molecule compounds that block this binding to the NTCP receptor have been engineered and studied in patients with CHB, with and without hepatitis D virus (HDV), a defective virus that utilizes the HBV surface proteins to enter and exit the hepatocyte. Myrcludex B (Hepatera/MYR GmbH) is a HBV pre-S1–derived lipoprotein polypeptide that competes with HBV and HDV for binding of the pre-S1 protein of HBsAg to the NTCP, preventing HBV and/or HDV entry. This agent blocks entry of HBV and HDV at picomolar concentrations and, not surprisingly, can increase serum bile acids. A phase 1b/2a study has been reported in 24 CHB patients with elevated levels of ALT and HBV DNA.13 Twenty-four patients with HBeAg-negative CHB who were coinfected with HDV were randomized to the following: 2 mg of subcutaneous Myrcludex B daily for 24 weeks followed by pegylated interferon α-2a (pegIFN α-2a) weekly for 48 weeks, Myrcludex B daily in combination with pegIFN α-2a weekly for 24 weeks followed by 24 weeks of pegIFN α-2a alone, or pegIFN α-2a for 48 weeks alone. Myrcludex B as monotherapy had no effect on serum HBsAg levels. However, Myrcludex B therapy led to significant decreases in serum HDV RNA in all cohorts: HDV RNA became negative in 5 of 7 patients treated with the agent followed by pegIFN α-2a, and serum ALT levels normalized in 6 of 8 patients in this group. Myrcludex B pre-S antibodies were detected in 9 of 14 patients. Myrcludex B had no effect on unconjugated bile acids but did impair uptake of taurine and glycine conjugated bile acids. The agent was well tolerated and offers a new paradigm for controlling HBV burden in the liver.
Nuclear Transport and Covalently Closed Circular DNA Inhibitors
There are no therapeutic drugs currently available in humans that target cccDNA directly, but there is activity and interest in this area in preclinical studies. Potential mechanisms to target cccDNA include the prevention of cccDNA formation, inhibition of entry of the rcDNA precursor into the nucleus, inhibition of conversion of rcDNA to cccDNA and formation of a minichromosome, elimination of cccDNA, silencing of its transcription, or inhibition of viral or cellular factors that contribute to cccDNA stability and/or formation (such as HBcAg and HBx protein inhibition). Disubstituted sulfonamide compounds have been reported to inhibit cccDNA in cell-based assays in vitro by apparently interfering with rcDNA conversion to cccDNA.14 DNA cleavage enzymes can specifically target the cccDNA, such as homing endonucleases,15 zinc finger nucleases (which introduce double-stranded breaks in DNA),16,17 and transcription activator-like effector nucleases (TALENs).18 TALENs have been shown in vitro to inhibit transcription and formation of HBeAg and HBsAg.18 Most recently, the clustered regularly interspaced short palindromic repeats/Cas9 system is being studied as a platform to mutate or inactivate viral genomes,19 including HBV cccDNA.20,21
RNA Silencers
Silencing of HBV gene expression using RNA interference–based therapy has entered phase 1 and 2 clinical trials. ARC-520 (Arrowhead) was a first-in-clinic combination of small interfering RNAs (siRNAs) directed against conserved HBV RNA sequences, which efficiently knocked down HBV RNA, HBV proteins, and DNA levels.22 This agent was comprised of 2 siRNAs that covered approximately 99.6% of known HBV sequences. The siRNAs were then conjugated to cholesterol and hepatocyte-targeted ligands. This complex was then taken up by endosomes in hepatocytes and subsequently released into cytoplasm after lysis of the endosomal membrane. However, the drug was withdrawn due to carrier toxicity. A mouse model of HBV recently found that the combination of the capsid inhibitor AB-423 (Arbutus), the second-generation siRNA agent ARB-1740 (Arbutus), and entecavir resulted in a 3 log10 reduction in serum HBV DNA levels and up to a 2 log10 reduction in serum HBsAg and HBeAg in vitro.23
Hepatitis B Virus Capsid Inhibitors
Core inhibitors have been described by multiple names, including capsid assembly modulators (CAMs), core protein allosteric modifiers (Assembly Biosciences), and nucleocapsid inhibitors such as heteroaryldihydropyrimidines (HAPs). As discussed above, the core protein has multiple functions in the HBV life cycle that are critical for genome packaging, RT, intracellular trafficking, and maintenance of chronic infection because encapsidated HBV genomes are imported into the nucleus and core protein forms part of cccDNA in the minichromosome. AL-3778 (Alios/Johnson & Johnson) is an orally administered, first-in-class HBV CAM whose efficacy in 73 HBeAg-positive CHB patients was reported at the 2017 meeting of the Asian Pacific Association for the Study of the Liver.24 The investigators reported dose-related reductions in the levels of serum HBV DNA and serum HBV RNA, with additive reductions when combined with pegIFN, but without reduction in HBsAg levels. AL-3778 is a small molecule and direct-acting antiviral agent acting through aberrant core protein processing, thereby resulting in capsid misassembly and subsequent inhibition of HBV DNA replication.
HAPs are nucleocapsid inhibitors that bind to core particles to reduce both HBV DNA and HBcAg levels, the latter via degradation by the proteasome pathway.25,26 HAPs also enhance viral assembly but favor assembly of aberrant particles, indicating that HAPs interfere with capsid formation/stability in a complex manner. Similar to phenylpropenamide derivatives, HAPs are able to efficiently inhibit nuc-resistant viral variants in vitro.27 Morphothiadine mesilate (GLS4, HEC) is another HAP nucleocapsid compound and triggers aberrant core particle assembly in vitro in Hep AD38 cells; phase 1/2 trials have been initiated in China.25
Hepatitis B Surface Antigen Release Inhibitors
The nucleic acid polymer (NAP) REP 2139 (Replicor) is taken up by hepatocytes, targets apolipoprotein, and blocks entry and formation of subviral particles but not virion production. The REP 401 protocol (NCT02565719) is a randomized, controlled trial assessing the safety and efficacy of the first-in-class NAP HBsAg release inhibitors REP 2139 and REP 2165 (Replicor) in combination with tenofovir disoproxil fumarate (TDF) and pegIFN α-2a in treatment-naive HBeAg-negative CHB patients. Greater than 3 to 4 log10 reductions were noted in 7 of 9 patients who received REP 2139 and in 4 of 9 patients who received REP 2165 plus TDF and pegIFN.28 Anti-HBs levels were noted in patients who lost HBsAg. As seen in earlier studies, viral rebound after stopping the drug was noted.29
Other small molecules that can inhibit the secretory pathway of HBsAg include the benzimidazole compound BM60130 and triazol-o-pyrimidine derivatives.31 BM601 selectively inhibits intracellular relocalization of the HBV surface protein to the Golgi apparatus,30 which decreases HBsAg and HBV release without affecting HBeAg secretion. It is unknown whether suppression of HBsAg levels in serum leads to restoration of T-cell responsiveness, which may allow for host clearance of HBV.
Host Immune Responses
IFNs have been in clinical use for decades and will not be discussed here. However, potential targets have been studied via engagement and activation of interferon α receptor (IFNAR) or the lymphotoxin β receptor (LTBR), which has been shown to upregulate apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (APOBEC) 3A or APOBEC3B for IFNAR or LTBR, respectively.32 These cytidine deaminases can selectively bind to core protein, thus bringing them directly into contact with nuclear cccDNA, resulting in cytidine deamination, apurinic/apyrimidinic site formation, and finally cccDNA degradation that prevents HBV replication. APOBECs do not bind to inactive cccDNA. These may explain some of the beneficial effects of IFN therapy and could be new therapeutic targets.
Toll-Like Receptors
TLRs recognize structurally conserved molecules derived from microbes and play a key role in the innate immune system. They typically signal through myeloid differentiation primary response gene 88 and have both antiviral (activating IFN response factor 7 responsive genes) and proinflammatory effects (nuclear factor κ B activation and induction of proinflammatory cytokines). One such molecule, GS-9620 (Gilead Sciences), is an orally available TLR-7 agonist that was selected because its antiviral response dominated its proinflammatory response.33 In preclinical studies of woodchucks and chimpanzees, this agent reduced surface antigen and viral DNA, and some animals lost surface antigen and seroconverted to surface antibody.34 This agent also decreased HBeAg and induced IFN-α and IFN-stimulated genes (ISGs) as well as natural killer cells. However, the agent did not decrease HBsAg in humans in short-term studies, although it did induce ISG-15 production.35 When studied in the clinic in a group of 26 HBeAg-negative CHB patients who were suppressed on TDF for at least 3 years, the addition of 12 weeks of GS-9620 at 1, 2, or 4 mg orally each week did not alter HBsAg levels in serum.36 There were improvements in natural killer cell and specific T-cell responses observed with GS-9620 (eg, IFN-γ and interleukin-2 production).37
Host Cellular Targets
Second mitochondria-derived activator of caspases mimetics are targeted agents that activate apoptotic cell death and block prosurvival signaling. One such molecule, birinapant (TetraLogic), in combination with an antiviral, cleared HBV in a mouse model by promoting tumor necrosis factor–mediated apoptosis of infected hepatocytes.38 Phase 1 studies were terminated when cases of cranial nerve palsies were noted in humans.
Checkpoint Inhibitors
Activation of naive T cells through engagement of the T-cell receptor with major histocompatibility complex on the antigen-presenting cell leads to proliferation, cytokine production, and/or cytotoxicity. With continued antigen stimulus, programmed cell death protein 1 (PD-1) is expressed on the T cell and programmed death-ligand 1 (PD-L1) on the antigen-presenting cell, which downregulates T-cell activation, and the T cell becomes exhausted. Monoclonal antibodies (mAb) to PD-1 or PD-L1 will inhibit PD-1/PD-L1 activity and reinvigorate the T cell, allowing for cell proliferation, cytokine production, and, in the case of CD8 T cells, cytotoxicity. In vitro PD-1 mAb and PD-L1 mAb have been shown in CHB patients to induce IFN-γ–secreting CD8 T cells.39 PD-1 mAb and PD-L1 mAb have been used in oncology for the treatment of cancers, including hepatocellular carcinoma. In one such study of PD-1 mAb and CTLA-4 mAb in 23 CHB Chinese patients with melanoma, no patient stopped therapy, but immune-related adverse events were noted: endocrine disorders (39%, 9/23), liver function abnormalities (22%, 5/23), and dermatologic events (17%, 4/23).40 Although in the United States, PD-1 and PD-L1 inhibitors have been approved for specific cancers, there are no data published to date on CHB patients without hepatocellular carcinoma. CHB patients with hepatocellular carcinoma have been treated with nivolumab (Opdivo, Bristol-Myers Squibb), a human immunoglobulin G4 mAb PD-1 inhibitor, with some patients stopping therapy due to liver flares.41 This study was done in patients with end-stage liver disease and hepatocellular carcinoma, and it is not clear whether more adverse events would occur in CHB patients in other phases of disease. However, these data provide a potential approach to treatment of patients with CHB.
Therapeutic Vaccines
Standard HBV vaccines have been studied for many years without success in CHB. Newer vaccine approaches include immune complex vaccines, nasal vaccines, DNA vaccines, T-cell vaccines, adenovirus-based vaccines, and yeast-based vaccines. Tarmogens are yeast-based vaccines (including surface, X, and core proteins of HBV), which can induce HBV-specific T-cell responses in vitro in peripheral blood mononuclear cells from CHB patients.42 A phase 2 study of tarmogen GS-4774 (Gilead Sciences), with or without TDF, was evaluated in 195 CHB patients who were not on antivirals previously and had immune active disease with HBV DNA levels of at least 2000 IU/mL. Change in quantitative HBsAg was the endpoint. There was no change in quantitative HBsAg between those receiving TDF or TDF plus tarmogen through 48 weeks of therapy, and no patient lost HBsAg.43
How to Combine New Therapies
As seen in the management of HIV, new combination therapies will likely be needed in order to cure CHB, but the therapy(ies) may differ by age of patient, phase of CHB, fibrosis stage, or HBV genotype. Additionally, the choice of therapy may vary by level of HBV DNA or quantitative HBsAg, by the activity of immune response, or even by new HBV markers. CHB is a dynamic, heterogeneous disease, and the phases as noted above are not always clear or distinct, either virologically or immunologically.44 For example, there are varying levels of HBsAg even in inactive disease, and the immunologic status between stages has been shown to be fluid. In a recent study, a high level of HBV DNA integration in the context of clonal hepatocyte expansion was noted in young patients, even those thought to be immune tolerant, indicating that the scenario for hepatocarcinogenesis is initiated even in patients with early-stage CHB.44 Patients in similar phases have shown mixed responses, and recognition of core, polymerase, and envelope peptides did not distinguish between phases.44 Another study of HBsAg epitope changes in 25 HBeAg-positive, immune active CHB patients during treatment with TDF found 2 distinct populations: 14 patients who demonstrated a HBsAg clearance profile (reduced recognition/availability at both loops 1 and 2 regions of the “a” determinant), and 11 patients with a nonclearance profile (without change in epitope recognition or reduced binding at only 1 epitope).45 These HBsAg epitope changes positively correlated with HBsAg loss and anti-HBs seroconversion (P<.02; positive predictive value, 83%), and the clearance profile on antiviral therapy by week 24 or 48 was strongly associated with a significant decline (>1.0 log10 IU/mL) or loss of HBsAg. In another study, the immune inactive stage has been shown to correlate with expression of PD-1 on CD8 T cells in woodchucks.46 Anti–PD-1, in combination with entecavir and vaccine, led to virologic and immune control, with some woodchucks acquiring antibody to surface antigen and clearing the virus. Given this heterogeneity within and between phases, clinical trials will have to address which patients should and can be treated with which new drugs and whether patients need to be already suppressed on nucs. In addition, the risk/benefit ratio may differ depending on the type of therapy, the age of the patient, and/or the phase of disease. It may be that different phases need different therapies, and different surrogate markers of efficacy will be needed to monitor success. Much work needs to be done to determine ideal endpoints for these new therapies.47 Children and young adults are most likely to be in the immune tolerant phase, and cure will provide the most benefit with the most quality-adjusted life-years gained. However, the ideal time to treat these patients is not clear. They would benefit most from short-term finite therapy. Studies have shown that these patients are not tolerant but have altered and measurable immune responses, with activated monocytes and natural killer cells, as well as proinflammatory cytokines.44,47,48 Other phases of CHB have shown expression of inhibitory molecules on T cells, such as PD-1.49 If the immunologic profile varies in different CHB patients, then multiple HBV drugs may be needed to enhance clearance (eg, checkpoint inhibitors and different direct-acting antivirals that target RNA, HBcAg, HBx protein, or HBsAg).
Summary
Many of the new drugs discussed above have not shown positive outcomes when used alone in terms of antiviral efficacy, and some have been discarded for clinical toxicity, but they have provided proof-of-concept that targeting both the HBV life cycle and the host immune response may have profound effects on controlling HBV replication. A lack of response to an individual drug as monotherapy is not surprising, as combination therapy will likely be required for cure, using different antivirals targeted to different parts of the HBV life cycle or using a combination of both antivirals and immune modulation. It is also likely that the endpoints used will vary for different therapies, and new data are awaited to assist in optimizing how to study the efficacy of new drugs. Surrogate markers of efficacy will include immunologic, virologic, and pathologic endpoints and may vary with the drug studies and the phase of infection.47 In addition, selection of patients in clinical trials will be critical to determine which therapies are most active in which phases of CHB. The risk/benefit ratio may well depend on the toxicity of therapy, the age of the patient, and the phase of disease. Exciting new data are emerging for the treatment of this disease, which is the most prevalent chronic viral hepatitis worldwide.
References
- 1.McMahon BJ. Natural history of chronic hepatitis B. Clin Liver Dis. 2010;14(3):381–396. doi: 10.1016/j.cld.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Gordon SC, Lamerato LE, Rupp LB, et al. CHeCS Investigators. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol. 2014;12(5):885–893. doi: 10.1016/j.cgh.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcellin P, Ahn SH, Ma X, et al. Study 149 Investigators. Combination of tenofovir disoproxil fumarate and peginterferon α-2a increases loss of hepatitis B surface antigen in patients with chronic hepatitis B. Gastroenterology. 2016;150(1):134–144.e10. doi: 10.1053/j.gastro.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 4.Locarnini S. Molecular virology of hepatitis B virus. Semin Liver Dis. 2004;24(1):3–10. doi: 10.1055/s-2004-828672. [DOI] [PubMed] [Google Scholar]
- 5.Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife. 2012;1:e00049. doi: 10.7554/eLife.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bock CT, Schranz P, Schröder CH, Zentgraf H. Hepatitis B virus genome is organized into nucleosomes in the nucleus of the infected cell. Virus Genes. 1994;8(3):215–229. doi: 10.1007/BF01703079. [DOI] [PubMed] [Google Scholar]
- 7.Newbold JE, Xin H, Tencza M, et al. The covalently closed duplex form of the hepadnavirus genome exists in situ as a heterogeneous population of viral minichromosomes. J Virol. 1995;69(6):3350–3357. doi: 10.1128/jvi.69.6.3350-3357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decorsière A, Mueller H, van Breugel PC, et al. Hepatitis B virus X protein identifies the Smc5/6 complex as a host restriction factor. Nature. 2016;531(7594):386–389. doi: 10.1038/nature17170. [DOI] [PubMed] [Google Scholar]
- 9.Blondot ML, Bruss V, Kann M. Intracellular transport and egress of hepatitis B virus. J Hepatol. 2016;64(1 suppl):S49–S59. doi: 10.1016/j.jhep.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Milich DR, Jones JE, Hughes JL, Price J, Raney AK, McLachlan A. Is a function of the secreted hepatitis B e antigen to induce immunologic tolerance in utero? Proc Natl Acad Sci U S A. 1990;87(17):6599–6603. doi: 10.1073/pnas.87.17.6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Visvanathan K, Skinner NA, Thompson AJ, et al. Regulation of toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology. 2007;45(1):102–110. doi: 10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 12.Bill CA, Summers J. Genomic DNA double-strand breaks are targets for hepadnaviral DNA integration. Proc Natl Acad Sci U S A. 2004;101(30):11135–11140. doi: 10.1073/pnas.0403925101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogomolov P, Alexandrov A, Voronkova N, et al. Treatment of chronic hepatitis D with the entry inhibitor Myrcludex B: first results of a phase Ib/IIa study. J Hepatol. 2016;65(3):490–498. doi: 10.1016/j.jhep.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Cai D, Mills C, Yu W, et al. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob Agents Chemother. 2012;56(8):4277–4288. doi: 10.1128/AAC.00473-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Zhang W, Lin J, et al. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Mol Ther. 2014;22(2):303–311. doi: 10.1038/mt.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber ND, Stone D, Sedlak RH, et al. AAV-mediated delivery of zinc finger nucleases targeting hepatitis B virus inhibits active replication. PLoS One. 2014;9(5):e97579. doi: 10.1371/journal.pone.0097579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmerman KA, Fischer KP, Joyce MA, Tyrrell DL. Zinc finger proteins designed to specifically target duck hepatitis B virus covalently closed circular DNA inhibit viral transcription in tissue culture. J Virol. 2008;82(16):8013–8021. doi: 10.1128/JVI.00366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreyer T, Nicholson S, Ely A, Arbuthnot P, Bloom K. Improved antiviral efficacy using TALEN-mediated homology directed recombination to introduce artificial primary miRNAs into DNA of hepatitis B virus. Biochem Biophys Res Commun. 2016;478(4):1563–1568. doi: 10.1016/j.bbrc.2016.08.152. [DOI] [PubMed] [Google Scholar]
- 19.Moyo B, Bloom K, Scott T, Ely A, Arbuthnot P. Advances with using CRISPR/Cas-mediated gene editing to treat infections with hepatitis B virus and hepatitis C virus [published online January 10, 2017] Virus Res. doi:10.1016/j.virusres.2017.01.003. [DOI] [PubMed]
- 20.Kennedy EM, Bassit LC, Mueller H, et al. Suppression of hepatitis B virus DNA accumulation in chronically infected cells using a bacterial CRISPR/Cas RNA-guided DNA endonuclease. Virology. 2015;476:196–205. doi: 10.1016/j.virol.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015:672–686. doi: 10.1016/j.virol.2015.02.031. 479-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schluep T, Lickliter J, Hamilton J, et al. Safety, tolerability, and pharmacokinetics of ARC-520 injection, an RNA interference-based therapeutic for the treatment of chronic hepatitis B virus infection, in healthy volunteers [published online October 14, 2016] Clin Pharmacol Drug Dev. doi:10.1002/cpdd.318. [DOI] [PMC free article] [PubMed]
- 23.Lee AC, Reid SP, Thi EP, et al. Exploring combination therapy for curing HBV: preclinical studies with capsid inhibitor AB-423 and a siRNA agent, ARB-1740. Hepatology. 2016;64:122A. [Google Scholar]
- 24.Kennedy WP, Yuen M-F, Kim DJ, et al. Safety, tolerability, pharmacokinetics and antiviral activity of AL-3778, a first-in-class, HBV core assembly modulator alone and in combination with peginterferon-alpha 2a, in treatment naive HBeAg-positive chronic hepatitis B patients. Hepatol Int. 2017;11:S7. [Google Scholar]
- 25.Ren Q, Liu X, Luo Z, et al. Discovery of hepatitis B virus capsid assembly inhibitors leading to a heteroaryldihydropyrimidine based clinical candidate (GLS4) Bioorg Med Chem. 2017;25(3):1042–1056. doi: 10.1016/j.bmc.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Stray SJ, Zlotnick A. BAY 41-4109 has multiple effects on hepatitis B virus capsid assembly. J Mol Recognit. 2006;19(6):542–548. doi: 10.1002/jmr.801. [DOI] [PubMed] [Google Scholar]
- 27.Wu G, Liu B, Zhang Y, et al. Preclinical characterization of GLS4, an inhibitor of hepatitis B virus core particle assembly. Antimicrob Agents Chemother. 2013;57(11):5344–5354. doi: 10.1128/AAC.01091-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bazinet M, Placinta G, Moscalu I, et al. Update on safety and efficacy in the REP 401 protocol: REP 2139-Mg or REP 2165-Mg used in combination with tenofovir disoproxil fumarate and pegylated interferon alpha 2A in treatment naive Caucasian patients with chronic HBeAg negative HBV infection. Hepatol Int. 2017;11:S85. [Google Scholar]
- 29.Al-Mahtab M, Bazinet M, Vaillant A. Safety and efficacy of nucleic acid polymers in monotherapy and combined with immunotherapy in treatment-naive Bangladeshi patients with HBeAg+ chronic hepatitis B infection. PLoS One. 2016;11(6):e0156667. doi: 10.1371/journal.pone.0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu YB, Yang L, Wang GF, et al. Benzimidazole derivative, BM601, a novel inhibitor of hepatitis B virus and HBsAg secretion. Antiviral Res. 2014;107:6–15. doi: 10.1016/j.antiviral.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 31.Dougherty AM, Guo H, Westby G, et al. A substituted tetrahydro-tetrazolopyrimidine is a specific and novel inhibitor of hepatitis B virus surface antigen secretion. Antimicrob Agents Chemother. 2007;51(12):4427–4437. doi: 10.1128/AAC.00541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucifora J, Xia Y, Reisinger F, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;343(6176):1221–1228. doi: 10.1126/science.1243462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopatin U, Wolfgang G, Tumas D, et al. Safety, pharmacokinetics and pharmacodynamics of GS-9620, an oral toll-like receptor 7 agonist. Antivir Ther. 2013;18(3):409–418. doi: 10.3851/IMP2548. [DOI] [PubMed] [Google Scholar]
- 34.Lanford RE, Guerra B, Chavez D, et al. GS-9620, an oral agonist of toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144(7):1508–1517. 1517.e1–10. doi: 10.1053/j.gastro.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gane EJ, Lim YS, Gordon SC, et al. The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. J Hepatol. 2015;63(2):320–328. doi: 10.1016/j.jhep.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 36.Janssen HL, Kim YJ, Ferrari C, et al. Safety and efficacy of GS-9620 in virally-suppressed patients with chronic hepatitis B. Hepatology. 2016;64:913A. doi: 10.1016/j.jhep.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Boni C, Vecchi A, Rossi M, et al. TLR-7 agonist GS-9620 can improve HBV-specific T cell and NK cell responses in nucleos(t)ide suppressed patients with chronic hepatitis B. Hepatology. 2016;64:7A. [Google Scholar]
- 38.Ebert G, Allison C, Preston S, et al. Eliminating hepatitis B by antagonizing cellular inhibitors of apoptosis. Proc Natl Acad Sci U S A. 2015;112(18):5803–5808. doi: 10.1073/pnas.1502400112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sherman AC, Trehanpati N, Daucher M, et al. Augmentation of hepatitis B virus-specific cellular immunity with programmed death receptor-1/programmed death receptor-L1 blockade in hepatitis B virus and HIV/hepatitis B virus coinfected patients treated with adefovir. AIDS Res Hum Retroviruses. 2013;29(4):665–672. doi: 10.1089/aid.2012.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen X, Wang Y, Ding Y, et al. Safety of immune checkpoint inhibitors in Chinese patients with melanoma. Melanoma Res. 2016;26(3):284–289. doi: 10.1097/CMR.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 41.El-Khoueiry AB, Melero I, Crocenzi TS, et al. Phase I/II safety and antitumor activity of nivolumab in patients with advanced hepatocellular carcinoma (HCC): CA209-040. J Clin Oncol. 2015;33 LBA101. [Google Scholar]
- 42.King TH, Kemmler CB, Guo Z, et al. A whole recombinant yeast-based therapeutic vaccine elicits HBV X, S and Core specific T cells in mice and activates human T cells recognizing epitopes linked to viral clearance. PLoS One. 2014;9(7):e101904. doi: 10.1371/journal.pone.0101904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janssen HL, Yoon SK, Yoshida EM, et al. Safety and efficacy of GS-4774 in combination with TDF in patients with chronic hepatitis B not on antiviral medication. Hepatology. 2016;64:122A. [Google Scholar]
- 44.Mason WS, Gill US, Litwin S, et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology. 2016;151(5):986–998.e4. doi: 10.1053/j.gastro.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walsh R, Hammond R, Yuen L, et al. Mapping HBsAg epitope profiles to predict HBsAg loss/seroconversion in a treatment naive cohort of genotype A chronic hepatitis B (CHB) patients receiving tenofovir disoproxil fumarate (TDF) therapy. Hepatology. 2015;62:966A. [Google Scholar]
- 46.Liu J, Zhang E, Ma Z, et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014;10(1):e1003856. doi: 10.1371/journal.ppat.1003856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Block TM, Locarnini S, McMahon BJ, Rehermann B, Peters MG. Use of current and new endpoints in the evaluation of experimental hepatitis B therapeutics. Clin Infect Dis. 2017;64(9):1283–1288. doi: 10.1093/cid/cix129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hong M, Sandalova E, Low D, et al. Trained immunity in newborn infants of HBV-infected mothers. Nat Commun. 2015;6:6588. doi: 10.1038/ncomms7588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park S-H, Rehermann B. Immune responses to HCV and other hepatitis viruses. Immunity. 2014;40(1):13–24. doi: 10.1016/j.immuni.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]