Abstract
The ciliopathies Bardet–Biedl syndrome and Alström syndrome cause obesity. How ciliary dysfunction leads to obesity has remained mysterious, partly because of a lack of understanding of the physiological roles of primary cilia in the organs and pathways involved in the regulation of metabolism and energy homeostasis. Historically, the study of rare monogenetic disorders that present with obesity has informed our molecular understanding of the mechanisms involved in nonsyndromic forms of obesity. Here, we present a framework, based on genetic studies in mice and humans, of the molecular and cellular pathways underlying long-term regulation of energy homeostasis. We focus on recent progress linking these pathways to the function of the primary cilia with a particular emphasis on the roles of neuronal primary cilia in the regulation of satiety.
Cell signaling machinery involved in feeding behavior and energy homeostasis localizes to certain neuronal cilia. This may help explain why ciliary dysfunction can lead to obesity (e.g., in Bardet–Biedl Syndrome).
The primary cilium has emerged as a clinically important organelle with ciliary dysfunction underlying several human syndromes collectively called the ciliopathies. Ciliopathies are associated with diverse phenotypes affecting nearly every tissue and organ system (see Braun and Hildebrandt 2016). Ciliopathies such as Bardet–Biedl syndrome (BBS) and Alström syndrome (ALMS) present with pediatric obesity as a clinical feature. Although an understanding of how ciliary defects cause certain ciliopathy-associated phenotypes, such as limb and neural tube defects, is emerging (see Bangs and Anderson 2016), how disrupted ciliary function leads to obesity remains poorly understood.
Here, we describe some neuroendocrine signaling pathways involved in the control of energy homeostasis in mammals, defects in which cause obesity in humans. We then describe how the use of mouse genetic models of ciliopathies has yielded interesting and sometimes contradictory results about the mechanisms through which primary cilia regulate body weight.
In particular, we examine a potential role for cilia in the leptin–melanocortin signaling axis. We also discuss the FTO locus and a neighboring gene involved in ciliary function, RPGRIP1L, as influencing obesity through effects on leptin signaling. We close with a discussion on the emerging literature on the potential of adipocyte or preadipocyte cilia to impact metabolism and obesity. It is clear that the primary cilium, a long-neglected organelle, plays important roles in controlling mammalian energy homeostasis. Future studies on the roles of cilia in energy homeostasis may reveal therapeutic targets for this common disease.
HUMAN OBESITY AND CILIOPATHIES
Pathophysiology of Obesity
Obesity is an increase in energy stored as fat in sufficient magnitude to result in adverse health consequences, such as diabetes and cardiovascular disease. Mechanistically, obesity is caused by long-term caloric intake in excess of energy expenditure. The control of food intake and energy expenditure is accomplished through afferent signals that sense the energy status of the individual, integration in the brain including the hypothalamus, and efferent signals including those determining the intensity of hunger. One common misconception is that this physiological system is dedicated to the prevention of obesity. Instead, this system’s essential role is the prevention of starvation and ensuring adequate energy intake to meet the energy requirements of basal metabolism, physical activity, growth, and reproduction.
Genetic Predisposition to Human Obesity
Environmental influences, such as diet and exercise, interact with genetic factors to influence the onset and progression of weight gain. Genetic studies, including twin studies, have revealed that genetic variation accounts for 40%–70% of weight variation, and that the heritability of obesity increases with its severity (Allison et al. 1996; O’Rahilly and Farooqi 2000). Both common genetic variants with small effects and rare genetic variants of larger effect contribute to the predisposition to obesity. The common genetic variants identified by genome-wide association studies (GWAS) have small, clinically insignificant effects individually. Most of these variants are located outside gene-coding regions, making it difficult to identify how they act. A case in point is the variant most strongly associated with obesity in multiple populations, a polymorphism present within an intron of the FTO gene (Frayling et al. 2007). One FTO-associated variant predisposes to obesity with an odds ratio of 1.3 to 1.7, which translates into a 2–3 kg weight gain. Even after extensive research efforts, the mechanism behind FTO-associated increases in weight remains unclear. We discuss below how the FTO-associated SNP may impact a neighboring gene, RPGRIP1L, encoding a critical component of the ciliary transition zone.
On the other end of the genetic effect spectrum from GWAS-identified single-nucleotide polymorphisms (SNPs), are single-gene defects that cause severe obesity, both in humans and mice. Analysis of these single genes has provided valuable insights into how energy stores are regulated in response to variable access to nutrition and demands for energy expenditure. Examples of single genes in which mutations cause human obesity include LEPTIN (LEP), its receptor (LEPR), PROOPIOMELANOCORTIN (POMC), MELANOCORTIN RECEPTOR 4 (MC4R), and SIM1, all of which are components of the leptin–melanocortin system.
The Leptin–Melanocortin Pathway Regulates Energy Homeostasis
One major afferent signal allowing the brain to sense the level of energy stores is the hormone leptin (Zhang et al. 1994). This cytokine-like 167-amino-acid protein is released by adipocytes in proportion to fat mass. Decreasing leptin levels inform the brain of diminishing fat storage resulting from a negative energy balance, and compensatory effects on appetite and energy expenditure that can replenish the stores and reestablish energy balance. Leptin’s action is mediated by the leptin receptor (LEPR), a single-transmembrane domain member of the class I cytokine receptor family. On binding to leptin, LEPR homodimerizes, leading to phosphorylation of JAK and STAT3. On phosphorylation, STAT3 dimerizes, translocates to the nucleus, and activates transcription of LEPTIN target genes (for a review on leptin signaling, see Myers et al. 2010).
The LEPTIN-responsive isoform of LEPR is expressed mainly in the hypothalamus, a brain region that interprets and integrates the peripheral signals that communicate energy balance. Within the hypothalamus, LEPTIN differentially affects the activity of two adjacent groups of neurons in the arcuate nucleus (ARC). LEPTIN inhibits the orexigenic agouti-related peptide (AgRP) and neuropeptide Y (NPY)–producing neurons, and activates the anorexigenic proopiomelanocortin (POMC)-producing neurons. In these latter neurons, POMC is cleaved by the proteases proconvertase-1 and proconvertase-2 to generate the anorexigenic neuropeptide α-melanocyte-stimulating hormone (α-MSH).
Interactions between these neuronal populations within the ARC allow for cross talk and modulation of the neuronal output. The development of sophisticated inducible conditional alleles and reporters in mouse models has begun to reveal the complex regulation of subpopulations of neurons within the ARC (for recent reviews on ARC-mediated control of food intake, see Begg and Woods 2013; Mountjoy 2015).
AgRP/NPY- and POMC-producing neurons in the ARC appear to respond directly to circulating signaling factors such as LEPTIN. These ARC neurons send axonal projections to second-order neurons in other regions of the hypothalamus, such as the paraventricular nucleus (PVN) and the lateral hypothalamic area (LHA), as well as to the hindbrain. Both α-MSH and AgRP act on a common G-protein-coupled receptor, melanocortin 4 receptor (MC4R), expressed by a subset of PVN neurons. α-MSH binds to and activates MC4R, whereas AgRP inhibits MC4R activity. To date, mutations in MC4R are the most common monogenic cause of severe human obesity, accounting for ∼2.5% of cases. Similar to the POMC and AgRP neurons of the ARC, new tools have begun to reveal diversity among MC4R-expressing neurons and signaling mechanisms (Garfield et al. 2015; Ghamari-Langroudi et al. 2015). MC4R signaling and downstream neuronal circuitry remains an attractive target for therapeutics to treat obesity.
Human patients and mouse models mutant for components of the leptin–melanocortin pathway have helped reveal the physiological functions of this signaling system. For example, rare humans with complete LEPTIN deficiency show behaviors and physiological signs of starvation despite being extremely obese. LEPTIN replacement abolishes the hyperphagia, leading to normalization of weight, showing the necessary role for LEPTIN in regulating human energy homeostasis (Farooqi et al. 1999).
Although LEPTIN was initially thought to be a potential treatment for common obesity, all obese humans and animal models develop a resistance to LEPTIN’s anorectic actions, despite having elevated adipose and thus increased circulating serum leptin levels. The molecular mechanisms associated with LEPTIN resistance are an active area of research and include altered transport of leptin across the blood–brain barrier, hypothalamic inflammation and ER stress, and diminished hypothalamic LEPTIN signaling (for a recent review of leptin resistance, see Aragones et al. 2016).
Ciliopathies Associated with Obesity
Ciliopathies for which obesity is an integral component of the clinical presentation include BBS (OMIM #209900) and ALMS (OMIM #203800). In addition to obesity, BBS is characterized by retinal degeneration, postaxial polydactyly, and kidney cysts. Other associated findings include anosmia, mental retardation, hepatic fibrosis, and type 2 diabetes mellitus (Forsythe and Beales 2013). Obesity in patients with BBS ranges from mild to severe, and is reversible with caloric restriction and exercise (Beales et al. 1999). BBS is rare and genetically heterogeneous. To date, mutations in 21 genes, BBS1–21, have been identified that contribute to the development of the phenotype (see Braun and Hildebrandt 2016). Many BBS gene products form a large protein complex termed the BBSome (Nachury et al. 2007). The BBSome functions in the localization of select transmembrane proteins to, and removal from, the cilium (Berbari et al. 2008; Jin et al. 2010; Domire et al. 2011; Koemeter-Cox et al. 2014; Liew et al. 2014; Mourao et al. 2014).
Unlike the genetic heterogeneity underlying BBS, ALMS is associated with mutations in a single gene, ALMS1. Together with retinal degeneration and hearing loss, early-onset obesity is also one of the hallmarks. In addition, ALMS is associated with cardiomyopathy, liver and kidney dysfunction, and delayed puberty (for a review on ALMS, see Girard and Petrovsky 2011). Like BBS, the pathogenesis of ALMS has been linked to dysfunction of the primary cilium. The ALMS1 protein localizes to the centrosome and ciliary basal body and likely has a role in the formation or maintenance of primary cilia (Hearn et al. 2002; Li et al. 2007; Knorz et al. 2010). The human mutations that cause ALMS truncate ALMS1. These truncated proteins are able to support ciliogenesis, but may alter ciliary function or long-term maintenance, leading to the development of ALMS.
LESSONS FROM MOUSE MODELS OF CILIA-ASSOCIATED OBESITY
Mouse models of monogenic forms of obesity, which recapitulate the human phenotypes, have been invaluable for elucidating molecular mechanisms underlying the pathogenesis of obesity. Similarly, a better understanding of the role of the primary cilia in obesity and the relationship between cilia function and the leptin–melanocortin pathway has been driven by the study of mice carrying mutations affecting Alms1 or BBS-associated genes, as well as by mice carrying conditional mutations affecting the formation and maintenance of primary cilia.
BBS and ALMS Ciliopathy Mouse Models Are Obese
As in humans, mice mutant for many of the BBS-associated genes show obesity (Table 1) (Mykytyn et al. 2004; Nishimura et al. 2004; Davis et al. 2007). Mouse mutations that model ALMS include a gene-trap (Alms1Gt(XH152)Byg) and a spontaneous mutation (fat aussie, foz) (Collin et al. 2005; Arsov et al. 2006). Unlike Bbs mutant mice, Alms1 mutant mice are born at a normal weight, similar to the clinical observations of ALMS in humans. Hyperphagia and obesity accompanied by hyperinsulinemia and type 2 diabetes occur postnatally in Alms1 mutant mice.
Table 1.
Cilia-associated mouse models of obesity
| Gene | Allele (MGI) | Type of allele | Mouse phenotypes | References |
|---|---|---|---|---|
| Adcy3 | Adcy3tm1Drs | Knockout | Obesity, anosmia | Wang et al. 2009 |
| Adcy3 | Adcy3Jll | ENU gain-of- function | Resistant to diet-induced obesity, less adipose | Pitman et al. 2014 |
| Alms1 | Alms1Gt(XH152)Byg | Genetrap | Obesity, retinopathy, male infertility, late-onset hearing loss | Collin et al. 2005 |
| Alms1 | Alms1foz | Spontaneous | Obesity, male infertility, late-onset hearing loss | Arsov et al. 2006 |
| Bbs1 | Bbs1tm1Vcs | Knockin | Obesity, retinopathy, male infertility, ventriculomegaly | Davis et al. 2007 |
| Bbs2 | Bbs2tm1Vcs | Knockout | Obesity, retinopathy, renal cysts, male infertility, anosmia, social submissiveness, ventriculomegaly | Nishimura et al. 2004; Davis et al. 2007 |
| Bbs3/Arl6a | Arl6tm2Vcs | Knockout | Increased fat mass, retinopathy, male infertility, hydrocephalus, elevated blood pressure | Zhang et al. 2011 |
| Bbs4b | Bbs4tm1Vcs | Knockout | Obesity, retinopathy, male infertility, social submissiveness, ventriculomegaly | Mykytyn et al. 2004; Nishimura et al. 2004; Davis et al. 2007 |
| Bbs4b | Bbs4Gt1Nk | Genetrap | Obesity (sex-dependent penetrance and severity), retinopathy, social submissiveness, increased anxiety | Eichers et al. 2006 |
| Bbs6/Mkks | Mkkstm1Vcs | Knockout | Obesity, retinopathy, male infertility, anosmia, elevated blood pressure, social submissiveness, ventriculomegaly | Fath et al. 2005; Davis et al. 2007 |
| Bbs7 | Bbs7tm1Vcs | Knockout | Obesity, male infertility, ventriculomegaly | Zhang et al. 2013 |
| Bbs8/Ttc8 | Ttc8tm1Reed | Knockout | Obesity, anosmia, retinal degeneration, renal tubule anomalies | Tadenev et al. 2011 |
| Bbs11/Trim32 | Trim32Gt(BGA355)Byg | Genetrap | Increased body weight, muscular myopathy | Kudryashova et al. 2009 |
| Bbs12 | Bbs12tm1.1Vmar | Knockout | Obesity, retinal degeneration | Marion et al. 2012 |
| Ift88 | Ift88tm1.1Bky | Conditional knockout | Obesity, renal cysts, hepatic cysts | Davenport et al. 2007 |
| Kif3a | Kif3atm1Gsn | Conditional knockout | Obesity, renal cysts, hepatic cysts | Davenport et al. 2007 |
| Rpgrip1l | Rpgrip1ltm1a(EUCOMM)Wtsi | Knockout | Obesity | Stratigopoulos et al. 2014 |
aBbs3 mutant mice do not become obese, but do display increased fat mass. Likewise, Bbs11 mutant mice have not been reported to be obese, but do display a significant and persistent increase in body weight starting at 2 months of age.
bTwo different Bbs4−/− knockout mouse lines have been independently generated and different penetrance and severity of obesity have been reported for each.
Although Bbs and Alms1 gene mutations cause obesity and Bbs and Alms1 proteins are implicated in the function of the primary cilia, these observations do not show that the associated obesity is caused by alteration in cilia function, or that cilia are essential for the regulation of energy homeostasis.
Conditional Disruption of Primary Cilia Causes Obesity in Mice
Intraflagellar transport (IFT) is the process of protein transport within cilia critical for both their formation and maintenance. Conditional deletion of genes essential for IFT, such as Ift88 or Kif3a, is useful for delineating when and where cilia are required for a specific phenotype. Inducing organism-wide loss of cilia in adult mice causes hyperphagia and subsequent obesity (Davenport et al. 2007). Restricting mice lacking cilia to the diet of control animals prevented obesity, indicating that cilia restrict weight gain by inhibiting the consumption of food rather than by affecting metabolism or locomotor activity. To test where cilia function to restrict food consumption, Ift88 or Kif3a were removed exclusively in neurons using synapsin1-Cre (Davenport et al. 2007). As with organism-wide loss of cilia, removing cilia specifically in neurons causes obesity, strongly implicating neuronal cilia in the regulation of appetite and satiety.
The Neurons Involved in Obesity-Associated Ciliopathy
As discussed above, the hypothalamus regulates appetite through neurons that make POMC or AgRP. Importantly, hypothalamic neurons each possess a single primary cilium, although the precise functions of these cilia are largely unknown. To address the role of cilia in these hypothalamic neurons, Ift88 or Kif3a were conditionally removed in POMC- or AgRP-expressing neurons (Xu et al. 2005). By 6 weeks of age, mice lacking cilia on POMC-expressing neurons weighed significantly more than control mice, and continued to become obese during adulthood (Davenport et al. 2007). Mice lacking cilia on AgRP-expressing neurons did not show increased weight (NF Berbari and BK Yoder, unpubl.). In addition to obesity, mice lacking cilia on POMC-expressing neurons displayed increased levels of leptin, fasting serum glucose, and insulin. These increases were only present in obese mutants, not those kept at the control weight by pair feeding, indicating that these changes were secondary to obesity.
Potential Lepr Involvement in Ciliopathy-Associated Obesity
Although this work indicates that hypothalamic cilia restrain feeding, it does not reveal how they do so (Davenport et al. 2007). A suggestion of a molecular mechanism, Bbs1, a component of the BBSome, directly binds to Lepr and may participate in Lepr trafficking (Seo et al. 2009). Like mice lacking cilia on POMC-expressing neurons, ad libitum–fed Bbs2, Bbs4, and Bbs6 mutant mice show elevated leptin levels (Rahmouni et al. 2008; Seo et al. 2009). Importantly, these Bbs mutants fail to reduce food intake in response to injection of leptin, raising the possibility that a diminished response to leptin contributes to obesity in BBS (Rahmouni et al. 2008).
Nearly all obese mice and humans show elevated levels of circulating leptin, but this leptin is insufficient to suppress appetite, a phenomenon known as leptin resistance (Maffei et al. 1995; Considine et al. 1996). Thus, leptin resistance can either be a cause or a consequence of obesity. Interestingly, when caloric restriction was used to normalize leptin levels in Bbs mutant mice they still failed to respond to leptin with diminished food intake (Seo et al. 2009). The investigators concluded that leptin resistance was the primary deficit initiating hyperphagia and obesity in Bbs mice, but did not take into account the food anticipatory behavior that is observed on calorie restriction in mice. A growing literature reports that maintaining calorie restriction in rodents can have prolonged effects on meal structure and circadian rhythm (for a review on anticipatory feeding behavior, see Mistlberger 2009).
If both body composition and anticipatory feeding behavior are controlled for, adult mice lacking Ift88, or Bbs mutant mice, before the onset of obesity, display unaltered responses to leptin injected intraperitoneally (Berbari et al. 2013). Lepr activity was similar between these ciliopathy models and control mice, and other phenotypes associated with leptin and LEPR mutations, such as changes in thermoregulation and locomotor activity, are not present. These data suggest that cilia are not directly involved in leptin signaling (Berbari et al. 2013).
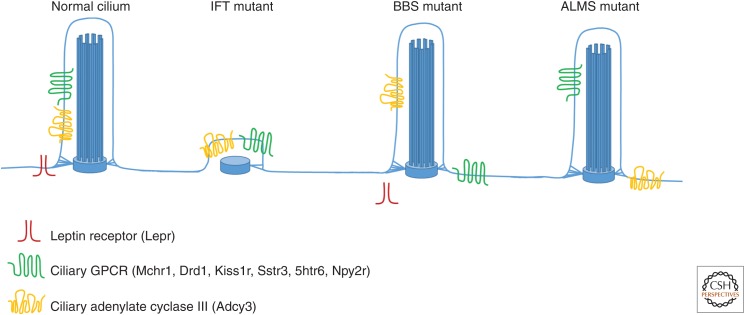
Very recent work by Guo et al. (2016) report a cilium-independent function of the BBSome. It appears to be required for trafficking of Lepr to the plasma membrane, and in Bbs mutants the obesity appears to be primarily a result of deficits in leptin sensitivity. This is in contrast to their findings with conditional loss of IFT88 in which they report leptin resistance on increases in adiposity. This work begins to show that cilia mutant mouse models may display obesity through different and independent mechanisms (Fig. 1). This raises the exciting potential for cilia-mediated signaling and obesity to be broadly relevant beyond the rare ciliopathies.
Figure 1.
Model of how ciliary signaling may contribute to energy homeostasis. Certain G-protein-coupled receptors (GPCRs) and signaling machinery, such as Sstr3, Mchr1, Drd1, Kiss1r, Htr6, and Adcy3, localize to the cilia membrane of neurons in specific brain regions. Intraflagellar transport (IFT) mutation results in loss of the cilium. In mouse models of Bardet–Biedl syndrome (BBS), both ciliary GPCRs and membrane-associated Lepr localization are perturbed. In Alström syndrome (ALMS) mouse models, GPCRs remain at cilia, but Adcy3 no longer localizes appropriately to cilia. Taken together, these models convey the complexity of the cilium as a signaling center and indicate that there are differing requirements for membrane protein localization to cilia.
Leptin-Independent Mechanisms by Which Neuronal Cilia May Affect Energy Homeostasis
Mutations affecting the mouse orthologs of BBS-associated genes, including Bbs2, Bbs3, and Bbs4, disrupt the localization of at least some GPCRs to their cilia, including melanin-concentrating hormone receptor 1 (Mchr1) (Berbari et al. 2008; Zhang et al. 2011).
Defective Mchr1 signaling is an attractive candidate for mediating obesity in Bbs mutants, as Mchr1 regulates feeding behavior. Either pharmacological or genetic activation of the Mchr1 pathway is associated with hyperphagia, whereas repression is associated with anorectic behavior (Qu et al. 1996; Shimada et al. 1998; Ludwig et al. 2001; Borowsky et al. 2002; Chen et al. 2002). Thus, for Mchr1 signaling to underlie obesity in Bbs mutants, the failure of Mchr1 to reach the cilium would have to be associated with ectopic activation of Mchr1 signaling. This is possible, although inactivation through sequestration in the cilium has not been previously described for GPCRs.
Apart from Mchr1, there is a growing list of other GPCRs that can preferentially localize to neuronal cilia. Some of these ciliary GPCRs include somatostatin receptor 3 (Sstr3), serotonin receptor 6 (5HT6), dopamine receptor 1 (Drd1), neuropeptide Y receptor 2 (Npy2r), and kisspeptin receptor 1 (Kiss1r) (Hamon et al. 1999; Handel et al. 1999; Brailov et al. 2000; Schulz et al. 2000; Marley and von Zastrow 2010; Domire et al. 2011; Loktev and Jackson 2013; Koemeter-Cox et al. 2014). While the functional significance of the localization of these receptors to cilia remains unclear, it is possible that they affect appetite, satiety, or metabolism, especially when given that somatostatin, serotonin, dopamine, neuropeptide Y, and kisspeptin, have all been implicated in either reward, feeding behaviors, metabolism, or glucose handling (Vijayan and McCann 1977; Pollock and Rowland 1981; Aponte et al. 1984; Salamone et al. 1990; Tolson et al. 2014).
Interestingly, Alms1foz/foz mice display a progressive loss of ciliary adenylyl cyclase III (Adcy3) in their hypothalamus, but no changes in Sstr3 or Mchr1 localization to cilia (Bishop et al. 2007; Heydet et al. 2013). Loss of Adcy3 function can itself cause obesity in mice through alterations in activity, hyperphagia, and leptin resistance (Wang et al. 2009). Conversely, a gain-of-function mutation in Adcy3 confers protection from diet-induced obesity (Pitman et al. 2014). Future studies aimed at dissecting the roles of cilia GPCRs and their associated signaling proteins will shed light on cilia-associated changes in feeding behaviors, and other behaviors that neuronal cilia may modulate.
RELATIONSHIP OF THE TRANSITION ZONE PROTEIN RPGRIP1L TO OBESITY
Several common SNPs associated with obesity occur within the first intron of the Fat Mass and Obesity-Associated (FTO) gene (Dina et al. 2007; Frayling et al. 2007; Scuteri et al. 2007; Meyre et al. 2010). While studies have suggested mechanisms by which Fto can affect body weight, SNPs can alter regulatory elements that control the expression of distant genes, raising the possibility that FTO-associated SNPs may alter the expression of other genes. Chromatin conformation capture (3C) and circular chromosome conformation capture followed by high-throughput sequencing (4C-seq) revealed that, in addition to the Fto promoter, the region of the obesity-associated SNPs also interacts with the promoter of Irx3, a distant gene encoding a homeobox transcription factor (Smemo et al. 2014). At least in the human cerebellum, the presence of weight-associated SNPs and Irx3 expression, but not Fto expression, were correlated (Smemo et al. 2014). Thus, SNPs within the FTO gene may affect the brain expression of IRX3 to affect human body weight.
In addition to IRX3, FTO is nearby to retinitis pigmentosa GTPase regulator-interacting protein-1 like (RPGRIP1L). RPGRIP1L encodes a key transition zone component involved in the localization of many other transition zone proteins (Liu et al. 2011; Williams et al. 2011). Like Fto, fasting lowers expression of Rpgrip1l in the mouse hypothalamus (Stratigopoulos et al. 2011), suggesting that transcriptional regulation of Rpgrip1l may control energy homeostasis. Homozygous mutations of mouse Rpgrip1l cause embryonic phenotypes consistent with a severe disruption in ciliogenesis, complicating the analysis of how Rpgrip1l may impact energy metabolism (Delous et al. 2007). However, heterozygous mice express half the normal levels of Rpgrip1l protein (and wild-type levels of Fto and Irx3), and are hyperphagic and fatter than wild-type controls (Stratigopoulos et al. 2014). The number of Adcy3-positive cilia in the ARC of Rpgrip1l heterozygotes is modestly reduced, suggesting that reduced expression of Rpgrip1l may alter neuronal cilia, increasing feeding (Stratigopoulos et al. 2014). One of the FTO-associated SNPs overlaps with a binding site for the p110 isoform of CUX1, a long-range transcriptional regulator that can affect RPGRIP1L expression in vitro (Stratigopoulos et al. 2011; Vadnais et al. 2013). Thus, diminished expression of this key transition zone component may alter ciliogenesis to account for how the FTO-associated SNPs affect human weight. Future studies looking at conditional changes in Rpgrip1l expression in development and the adult animal will help to elucidate the potential role for Rgprip1l in energy homeostasis.
POTENTIAL ROLES FOR PERIPHERAL CILIA IN METABOLIC REGULATION AND OBESITY
Functions for cilia in the central control of energy metabolism do not preclude additional roles for cilia relevant to obesity in peripheral tissues, including adipocytes. Like many mesenchymal cell types, preadipocytes can be ciliated (Marion et al. 2009; Zhu et al. 2009; Dalbay et al. 2015). Interestingly, preadipocytes are transiently ciliated during the transition from proliferation to terminal differentiation, leading to mature adipocytes that lack cilia (Marion et al. 2009). Interfering with ciliary function by knocking down BBS10 or BBS12 in human preadipocytes increased PPARγ nuclear localization, a marker of adipogenesis (Marion et al. 2009). In contrast, knocking down Ift88 in preadipocytes decreased PPARγ levels and nuclear localization, and inhibited fat droplet formation, a marker of mature adipocytes (Zhu et al. 2009; Dalbay et al. 2015). Although it is not clear how these different manipulations of BBS-associated and ciliogenic genes relate to each other, one possibility is that cilia promote adipogenesis in a way that is restrained by the activity of BBS proteins.
One candidate for mediating the effects of cilia on adipogenesis is Hedgehog (Hh) signaling (see Bangs and Anderson 2016). Sustained activation of Hh signaling can suppress adipogenesis, and down-regulation of Hh signaling is concomitant with terminal differentiation of adipocytes (Cousin et al. 2006; Suh et al. 2006; Fontaine et al. 2008; James et al. 2010). The Hh pathway mediator Smoothened (Smo) localizes to preadipocyte cilia, suggesting that active ciliary Hh signaling may restrain premature differentiation into mature adipocytes (Marion et al. 2009), although there are differing data on whether Smo inhibition is sufficient to promote adipocyte differentiation (Suh et al. 2006; Fontaine et al. 2008; James et al. 2010). In most cell types, BBS proteins have minor roles in Hh signaling, but it will be interesting to determine whether down-regulation of Hh signaling in preadipocytes may account for the increased adipogenesis caused by BBS protein loss-of-function.
The decision of preadipocytes to terminally differentiate may depend on ciliary signaling beyond Hh signaling. One pathway that may oppose antiadipogenic Hh signaling may be insulin-like growth factor 1 receptor (IGF1R) signaling. IGF1R, a receptor tyrosine kinase, is an important activator of both the expansion and differentiation of 3T3-L1 preadipocytes (Qiu et al. 2001; Xu and Liao 2004). IGF1R phosphorylates its adapter protein, insulin receptor substrate 1 (IRS1), to indirectly activate AKT1, a serine/threonine protein kinase involved in adipocyte metabolism (Fischer-Posovszky et al. 2012). Activated IGF1R localizes to 3T3-L1 cilia, and activated IRS1 and AKT1 to the basal body (Zhu et al. 2009), suggesting an important role for cilia in sensitizing preadipocytes to the prodifferentiative effects of insulin. Consistent with this hypothesis, inhibiting ciliogenesis by knockdown of either Ift88 or Kif3a inhibits the activation of IGF1R or AKT1 in 3T3-L1 preadipocytes the same (Zhu et al. 2009).
In addition to effects on the differentiation of preadipocyte into mature adipocytes, ciliary signaling may impact adipogenesis through regulation of cellular metabolism. Stimulating 3T3-L1 adipocytes with Sonic Hedgehog ligand promoted aerobic glycolysis on a time scale inconsistent with a transcriptional effect, suggesting that Hh signals can impact metabolism through Gli-independent “noncanonical” mechanisms (Teperino et al. 2012).
Could disruption of a nontranscriptional, noncanonical ciliary Hh signaling pathway decrease aerobic glycolysis and thereby at least partially account for how ciliary defects cause obesity? SAG and cyclopamine, two small molecules that promote ciliary localization of Smo but have opposite effects on the Gli-dependent Hh transcriptional program, both promote glucose uptake in 3T3-L1 cells (Teperino et al. 2012). Moreover, cyclopamine increases glucose uptake by mouse brown adipose tissue and muscle in vivo and increases core body temperature by ∼1°C, consistent with increased thermogenesis. It will be interesting to determine whether genetic removal of Smo or cilia from brown adipose tissue and muscle blocks these effects, confirming that cyclopamine acts through a ciliary Hh pathway to activate thermogenesis.
One way that ciliary Smo may affect metabolism is through AMP-activated protein kinase (AMPK), a critical regulator of cellular energy homeostasis. Pharmacological stimulation of Smo activated AMPK in a way that was dependent on Ift88 and Kif3a, two genes required for ciliogenesis (Teperino et al. 2012). AMPK and its upstream regulator, LKB1, have been identified as proteins that can, at least partially, localize to cilia (Boehlke et al. 2010; Mick et al. 2015), further suggesting that Smo may regulate AMPK at the cilium. However, small molecule antagonists of Smo that block its localization to cilia also induced AMPK activation, indicating that the cilium may not be crucial for Hh-mediated modulation of AMPK (Teperino et al. 2012). In contrast to Hh pathway stimulation, AMPK reduces glucose uptake and restricts aerobic glycolysis, at least in some cell types (Faubert et al. 2013), suggesting that the effects of Hh signaling on metabolism may be at least partly mediated through an AMPK-independent mechanism. Although neuronal cilia clearly have important roles in satiety control, alterations in ciliary signaling in preadipocytes, adipocytes, or muscle may contribute to ciliopathy-associated obesity, a possibility that remains to be examined in ciliopathy models.
CONCLUSIONS
Important questions about the pathogenesis of ciliopathy-associated obesity concern some of the following: which tissues are involved, which signaling pathways are involved, and whether the obesity arises from developmental or physiological changes. Conditional genetic ablation of the primary cilia in mice has established that neuronal primary cilia suppress feeding behavior, suggesting that some form of ciliary signaling promotes satiety. A clear hypothesis is that human ciliopathy mutations could alter the localization and/or function of receptor(s) involved in energy homeostasis at the primary cilia. While LEPR is an excellent candidate for such a role, it may not be one of the direct culprits. Moreover, it is not yet clear whether the obesity caused by these mutations results from a developmental or postdevelopmental alteration of cilia function, with the possibility that it may be both. Of interest will be the systematic conditional removal of both ciliogenic and BBS-associated genes in adult hypothalamic neuronal populations implicated in feeding behavior to elucidate which neurons possess the cilia that function in appetite and satiety.
Footnotes
Editors: Wallace Marshall and Renata Basto
Additional Perspectives on Cilia available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Allison DB, Kaprio J, Korkeila M, Koskenvuo M, Neale MC, Hayakawa K. 1996. The heritability of body mass index among an international sample of monozygotic twins reared apart. Int J Obes Relat Metab Disord 20: 501–506. [PubMed] [Google Scholar]
- Aponte G, Leung P, Gross D, Yamada T. 1984. Effects of somatostatin on food intake in rats. Life Sci 35: 741–746. [DOI] [PubMed] [Google Scholar]
- Aragones G, Ardid-Ruiz A, Ibars M, Suarez M, Blade C. 2016. Modulation of leptin resistance by food compounds. Mol Nutr Food Res 60: 1789–1803. [DOI] [PubMed] [Google Scholar]
- Arsov T, Silva DG, O'Bryan MK, Sainsbury A, Lee NJ, Kennedy C, Manji SS, Nelms K, Liu C, Vinuesa CG, et al. 2006. Fat aussie—A new Alström syndrome mouse showing a critical role for ALMS1 in obesity, diabetes, and spermatogenesis. Mol Endocrinol 20: 1610–1622. [DOI] [PubMed] [Google Scholar]
- *.Bangs F, Anderson KV. 2016. Primary cilia and mammalian Hedgehog signaling. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales PL, Elcioglu N, Woolf AS, Parker D, Flinter FA. 1999. New criteria for improved diagnosis of Bardet–Biedl syndrome: Results of a population survey. J Med Genet 36: 437–446. [PMC free article] [PubMed] [Google Scholar]
- Begg DP, Woods SC. 2013. The endocrinology of food intake. Nat Rev Endocrinol 9: 584–597. [DOI] [PubMed] [Google Scholar]
- Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. 2008. Bardet–Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci 105: 4242–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berbari NF, Pasek RC, Malarkey EB, Yazdi SM, McNair AD, Lewis WR, Nagy TR, Kesterson RA, Yoder BK. 2013. Leptin resistance is a secondary consequence of the obesity in ciliopathy mutant mice. Proc Natl Acad Sci 110: 7796–7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GA, Berbari NF, Lewis J, Mykytyn K. 2007. Type III adenylyl cyclase localizes to primary cilia throughout the adult mouse brain. J Comp Neurol 505: 562–571. [DOI] [PubMed] [Google Scholar]
- Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, Bredt S, Beyer T, Janusch H, Hamann C, Godel M, et al. 2010. Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol 12: 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, et al. 2002. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med 8: 825–830. [DOI] [PubMed] [Google Scholar]
- Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Verge D. 2000. Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res 872: 271–275. [DOI] [PubMed] [Google Scholar]
- *.Braun DA, Hildebrandt F. 2016. Ciliopathies. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a028191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Hu C, Hsu CK, Zhang Q, Bi C, Asnicar M, Hsiung HM, Fox N, Slieker LJ, Yang DD, et al. 2002. Targeted disruption of the melanin-concentrating hormone receptor-1 results in hyperphagia and resistance to diet-induced obesity. Endocrinology 143: 2469–2477. [DOI] [PubMed] [Google Scholar]
- Collin GB, Cyr E, Bronson R, Marshall JD, Gifford EJ, Hicks W, Murray SA, Zheng QY, Smith RS, Nishina PM, et al. 2005. Alms1-disrupted mice recapitulate human Alström syndrome. Hum Mol Genet 14: 2323–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. 1996. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 334: 292–295. [DOI] [PubMed] [Google Scholar]
- Cousin W, Dani C, Peraldi P. 2006. Inhibition of the anti-adipogenic Hedgehog signaling pathway by cyclopamine does not trigger adipocyte differentiation. Biochem Biophys Res Commun 349: 799–803. [DOI] [PubMed] [Google Scholar]
- Dalbay MT, Thorpe SD, Connelly JT, Chapple JP, Knight MM. 2015. Adipogenic differentiation of hMSCs is mediated by recruitment of IGF-1r onto the primary cilium associated with cilia elongation. Stem Cells 33: 1952–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport JR, Watts AJ, Roper VC, Croyle MJ, van Groen T, Wyss JM, Nagy TR, Kesterson RA, Yoder BK. 2007. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol 17: 1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, Agassandian K, Philp AR, Searby CC, Andrews MP, Thompson S, et al. 2007. A knockin mouse model of the Bardet–Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proc Natl Acad Sci 104: 19422–19427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delous M, Baala L, Salomon R, Laclef C, Vierkotten J, Tory K, Golzio C, Lacoste T, Besse L, Ozilou C, et al. 2007. The ciliary gene RPGRIP1L is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet 39: 875–881. [DOI] [PubMed] [Google Scholar]
- Dina C, Meyre D, Gallina S, Durand E, Korner A, Jacobson P, Carlsson LM, Kiess W, Vatin V, Lecoeur C, et al. 2007. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 39: 724–726. [DOI] [PubMed] [Google Scholar]
- Domire JS, Green JA, Lee KG, Johnson AD, Askwith CC, Mykytyn K. 2011. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet–Biedl syndrome proteins. Cell Mol Life Sci 68: 2951–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichers ER, Abd-El-Barr MM, Paylor R, Lewis RA, Bi W, Lin X, Meehan TP, Stockton DW, Wu SM, Lindsay E, et al. 2006. Phenotypic characterization of Bbs4 null mice reveals age-dependent penetrance and variable expressivity. Hum Genet 120: 211–226. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. 1999. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341: 879–884. [DOI] [PubMed] [Google Scholar]
- Fath MA, Mullins RF, Searby C, Nishimura DY, Wei J, Rahmouni K, Davis RE, Tayeh MK, Andrews M, Yang B, et al. 2005. Mkks-null mice have a phenotype resembling Bardet–Biedl syndrome. Hum Mol Genet 14: 1109–1118. [DOI] [PubMed] [Google Scholar]
- Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B, et al. 2013. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab 17: 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Posovszky P, Tews D, Horenburg S, Debatin KM, Wabitsch M. 2012. Differential function of Akt1 and Akt2 in human adipocytes. Mol Cell Endocrinol 358: 135–143. [DOI] [PubMed] [Google Scholar]
- Fontaine C, Cousin W, Plaisant M, Dani C, Peraldi P. 2008. Hedgehog signaling alters adipocyte maturation of human mesenchymal stem cells. Stem Cells 26: 1037–1046. [DOI] [PubMed] [Google Scholar]
- Forsythe E, Beales PL. 2013. Bardet–Biedl syndrome. Eur J Hum Genet 21: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, et al. 2007. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316: 889–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Li C, Madara JC, Shah BP, Webber E, Steger JS, Campbell JN, Gavrilova O, Lee CE, Olson DP, et al. 2015. A neural basis for melanocortin-4 receptor-regulated appetite. Nat Neurosci 18: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghamari-Langroudi M, Digby GJ, Sebag JA, Millhauser GL, Palomino R, Matthews R, Gillyard T, Panaro BL, Tough IR, Cox HM, et al. 2015. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature 520: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard D, Petrovsky N. 2011. Alström syndrome: Insights into the pathogenesis of metabolic disorders. Nat Rev Endocrinol 7: 77–88. [DOI] [PubMed] [Google Scholar]
- Guo DF, Cui H, Zhang Q, Morgan DA, Thedens DR, Nishimura D, Grobe JL, Sheffield VC, Rahmouni K. 2016. The BBSome controls energy homeostasis by mediating the transport of the leptin receptor to the plasma membrane. PLoS Genet 12: e1005890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon M, Doucet E, Lefevre K, Miquel MC, Lanfumey L, Insausti R, Frechilla D, Del Rio J, Verge D. 1999. Antibodies and antisense oligonucleotide for probing the distribution and putative functions of central 5-HT6 receptors. Neuropsychopharmacology 21: 68S–76S. [DOI] [PubMed] [Google Scholar]
- Handel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Hollt V. 1999. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience 89: 909–926. [DOI] [PubMed] [Google Scholar]
- Hearn T, Renforth GL, Spalluto C, Hanley NA, Piper K, Brickwood S, White C, Connolly V, Taylor JF, Russell-Eggitt I, et al. 2002. Mutation of ALMS1, a large gene with a tandem repeat encoding 47 amino acids, causes Alström syndrome. Nat Genet 31: 79–83. [DOI] [PubMed] [Google Scholar]
- Heydet D, Chen LX, Larter CZ, Inglis C, Silverman MA, Farrell GC, Leroux MR. 2013. A truncating mutation of Alms1 reduces the number of hypothalamic neuronal cilia in obese mice. Dev Neurobiol 73: 1–13. [DOI] [PubMed] [Google Scholar]
- James AW, Leucht P, Levi B, Carre AL, Xu Y, Helms JA, Longaker MT. 2010. Sonic Hedgehog influences the balance of osteogenesis and adipogenesis in mouse adipose-derived stromal cells. Tissue Eng Part A 16: 2605–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. 2010. The conserved Bardet–Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 141: 1208–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorz VJ, Spalluto C, Lessard M, Purvis TL, Adigun FF, Collin GB, Hanley NA, Wilson DI, Hearn T. 2010. Centriolar association of ALMS1 and likely centrosomal functions of the ALMS motif-containing proteins C10orf90 and KIAA1731. Mol Biol Cell 21: 3617–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koemeter-Cox AI, Sherwood TW, Green JA, Steiner RA, Berbari NF, Yoder BK, Kauffman AS, Monsma PC, Brown A, Askwith CC, et al. 2014. Primary cilia enhance kisspeptin receptor signaling on gonadotropin-releasing hormone neurons. Proc Natl Acad Sci 111: 10335–10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryashova E, Wu J, Havton LA, Spencer MJ. 2009. Deficiency of the E3 ubiquitin ligase TRIM32 in mice leads to a myopathy with a neurogenic component. Hum Mol Genet 18: 1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Vega R, Nelms K, Gekakis N, Goodnow C, McNamara P, Wu H, Hong NA, Glynne R. 2007. A role for Alström syndrome protein, alms1, in kidney ciliogenesis and cellular quiescence. PLoS Genet 3: e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew GM, Ye F, Nager AR, Murphy JP, Lee JS, Aguiar M, Breslow DK, Gygi SP, Nachury MV. 2014. The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev Cell 31: 265–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhang M, Xia Z, Xu P, Chen L, Xu T. 2011. Caenorhabditis elegans ciliary protein NPHP-8, the homologue of human RPGRIP1L, is required for ciliogenesis and chemosensation. Biochem Biophys Res Commun 410: 626–631. [DOI] [PubMed] [Google Scholar]
- Loktev AV, Jackson PK. 2013. Neuropeptide Y family receptors traffic via the Bardet–Biedl syndrome pathway to signal in neuronal primary cilia. Cell Rep 5: 1316–1329. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Tritos NA, Mastaitis JW, Kulkarni R, Kokkotou E, Elmquist J, Lowell B, Flier JS, Maratos-Flier E. 2001. Melanin-concentrating hormone overexpression in transgenic mice leads to obesity and insulin resistance. J Clin Invest 107: 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. 1995. Leptin levels in human and rodent: Measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1: 1155–1161. [DOI] [PubMed] [Google Scholar]
- Marion V, Stoetzel C, Schlicht D, Messaddeq N, Koch M, Flori E, Danse JM, Mandel JL, Dollfus H. 2009. Transient ciliogenesis involving Bardet–Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc Natl Acad Sci 106: 1820–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion V, Mockel A, De Melo C, Obringer C, Claussmann A, Simon A, Messaddeq N, Durand M, Dupuis L, Loeffler JP, et al. 2012. BBS-induced ciliary defect enhances adipogenesis, causing paradoxical higher-insulin sensitivity, glucose usage, and decreased inflammatory response. Cell Metab 16: 363–377. [DOI] [PubMed] [Google Scholar]
- Marley A, von Zastrow M. 2010. DISC1 regulates primary cilia that display specific dopamine receptors. PLoS ONE 5: e10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyre D, Proulx K, Kawagoe-Takaki H, Vatin V, Gutierrez-Aguilar R, Lyon D, Ma M, Choquet H, Horber F, Van Hul W, et al. 2010. Prevalence of loss-of-function FTO mutations in lean and obese individuals. Diabetes 59: 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, Nachury MV. 2015. Proteomics of primary cilia by proximity labeling. Dev Cell 35: 497–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE. 2009. Food-anticipatory circadian rhythms: Concepts and methods. Eur J Neurosci 30: 1718–1729. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG. 2015. Pro-Opiomelanocortin (POMC) neurones, POMC-derived peptides, melanocortin receptors and obesity: How understanding of this system has changed over the last decade. J Neuroendocrinol 27: 406–418. [DOI] [PubMed] [Google Scholar]
- Mourao A, Nager AR, Nachury MV, Lorentzen E. 2014. Structural basis for membrane targeting of the BBSome by ARL6. Nat Struct Mol Biol 21: 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG Jr, Leibel RL, Seeley RJ, Schwartz MW. 2010. Obesity and leptin resistance: Distinguishing cause from effect. Trends Endocrinol Metab 21: 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, Yang B, Braun T, Casavant T, Stone EM, Sheffield VC. 2004. Bardet–Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci 101: 8664–8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, et al. 2007. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129: 1201–1213. [DOI] [PubMed] [Google Scholar]
- Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang B, et al. 2004. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci 101: 16588–16593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Rahilly S, Farooqi IS. 2000. The genetics of obesity in humans. In Endotext (ed. De Groot LJ, et al. ). MDText, South Dartmouth, MA. [Google Scholar]
- Pitman JL, Wheeler MC, Lloyd DJ, Walker JR, Glynne RJ, Gekakis N. 2014. A gain-of-function mutation in adenylate cyclase 3 protects mice from diet-induced obesity. PLoS ONE 9: e110226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock JD, Rowland N. 1981. Peripherally administered serotonin decreases food intake in rats. Pharmacol Biochem Behav 15: 179–183. [DOI] [PubMed] [Google Scholar]
- Qiu Z, Wei Y, Chen N, Jiang M, Wu J, Liao K. 2001. DNA synthesis and mitotic clonal expansion is not a required step for 3T3-L1 preadipocyte differentiation into adipocytes. J Biol Chem 276: 11988–11995. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. 1996. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature 380: 243–247. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Fath MA, Seo S, Thedens DR, Berry CJ, Weiss R, Nishimura DY, Sheffield VC. 2008. Leptin resistance contributes to obesity and hypertension in mouse models of Bardet–Biedl syndrome. J Clin Invest 118: 1458–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Zigmond MJ, Stricker EM. 1990. Characterization of the impaired feeding behavior in rats given haloperidol or dopamine-depleting brain lesions. Neuroscience 39: 17–24. [DOI] [PubMed] [Google Scholar]
- Schulz S, Handel M, Schreff M, Schmidt H, Hollt V. 2000. Localization of five somatostatin receptors in the rat central nervous system using subtype-specific antibodies. J Physiol Paris 94: 259–264. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, Najjar S, Nagaraja R, Orru M, Usala G, et al. 2007. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 3: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. 2009. Requirement of Bardet–Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet 18: 1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada M, Tritos NA, Lowell BB, Flier JS, Maratos-Flier E. 1998. Mice lacking melanin-concentrating hormone are hypophagic and lean. Nature 396: 670–674. [DOI] [PubMed] [Google Scholar]
- Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gomez-Marin C, Aneas I, Credidio FL, Sobreira DR, Wasserman NF, et al. 2014. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature 507: 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratigopoulos G, LeDuc CA, Cremona ML, Chung WK, Leibel RL. 2011. Cut-like homeobox 1 (CUX1) regulates expression of the fat mass and obesity-associated and retinitis pigmentosa GTPase regulator-interacting protein-1-like (RPGRIP1L) genes and coordinates leptin receptor signaling. J Biol Chem 286: 2155–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratigopoulos G, Martin Carli JF, O'Day DR, Wang L, Leduc CA, Lanzano P, Chung WK, Rosenbaum M, Egli D, Doherty DA, et al. 2014. Hypomorphism for RPGRIP1L, a ciliary gene vicinal to the FTO locus, causes increased adiposity in mice. Cell Metab 19: 767–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JM, Gao X, McKay J, McKay R, Salo Z, Graff JM. 2006. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab 3: 25–34. [DOI] [PubMed] [Google Scholar]
- Tadenev AL, Kulaga HM, May-Simera HL, Kelley MW, Katsanis N, Reed RR. 2011. Loss of Bardet–Biedl syndrome protein-8 (BBS8) perturbs olfactory function, protein localization, and axon targeting. Proc Natl Acad Sci 108: 10320–10325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teperino R, Amann S, Bayer M, McGee SL, Loipetzberger A, Connor T, Jaeger C, Kammerer B, Winter L, Wiche G, et al. 2012. Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell 151: 414–426. [DOI] [PubMed] [Google Scholar]
- Tolson KP, Garcia C, Yen S, Simonds S, Stefanidis A, Lawrence A, Smith JT, Kauffman AS. 2014. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J Clin Invest 124: 3075–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadnais C, Awan AA, Harada R, Clermont PL, Leduy L, Berube G, Nepveu A. 2013. Long-range transcriptional regulation by the p110 CUX1 homeodomain protein on the ENCODE array. BMC Genomics 14: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan E, McCann SM. 1977. Suppression of feeding and drinking activity in rats following intraventricular injection of thyrotropin releasing hormone (TRH). Endocrinology 100: 1727–1730. [DOI] [PubMed] [Google Scholar]
- Wang Z, Li V, Chan GC, Phan T, Nudelman AS, Xia Z, Storm DR. 2009. Adult type 3 adenylyl cyclase-deficient mice are obese. PLoS ONE 4: e6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, et al. 2011. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol 192: 1023–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liao K. 2004. Protein kinase B/AKT 1 plays a pivotal role in insulin-like growth factor-1 receptor signaling induced 3T3-L1 adipocyte differentiation. J Biol Chem 279: 35914–35922. [DOI] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. 2005. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest 115: 951–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Nishimura D, Seo S, Vogel T, Morgan DA, Searby C, Bugge K, Stone EM, Rahmouni K, Sheffield VC. 2011. Bardet–Biedl syndrome 3 (Bbs3) knockout mouse model reveals common BBS-associated phenotypes and Bbs3 unique phenotypes. Proc Natl Acad Sci 108: 20678–20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Nishimura D, Vogel T, Shao J, Swiderski R, Yin T, Searby C, Carter CS, Kim G, Bugge K, et al. 2013. BBS7 is required for BBSome formation and its absence in mice results in Bardet–Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking. J Cell Sci 126: 2372–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Shi S, Wang H, Liao K. 2009. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J Cell Sci 122: 2760–2768. [DOI] [PubMed] [Google Scholar]