Abstract
Cell adhesions link cells to the extracellular matrix (ECM) and to each other and depend on interactions with the actin cytoskeleton. Both cell–ECM and cell–cell adhesion sites contain discrete, yet overlapping, functional modules. These modules establish physical associations with the actin cytoskeleton, locally modulate actin organization and dynamics, and trigger intracellular signaling pathways. Interplay between these modules generates distinct actin architectures that underlie different stages, types, and functions of cell–ECM and cell–cell adhesions. Actomyosin contractility is required to generate mature, stable adhesions, as well as to sense and translate the mechanical properties of the cellular environment into changes in cell organization and behavior. Here, we review the organization and function of different adhesion modules and how they interact with the actin cytoskeleton. We highlight the molecular mechanisms of mechanotransduction in adhesions and how adhesion molecules mediate cross talk between cell–ECM and cell–cell adhesion sites.
Discrete multiprotein complexes called cell adhesions link cells to the extracellular matrix and to each other. They depend on interactions with the actin cytoskeleton and serve as hubs for signaling and mechanotransduction.
1. INTRODUCTION
Cell adhesion to the extracellular matrix (ECM) and to neighboring cells is the hallmark of multicellularity and underlies the organization and distinct physiological functions of mammalian tissues (Gumbiner 1996). Aberrant cell adhesion contributes to diverse pathologies, including cancer metastasis, vascular disease, and inflammation (Hynes 2007; Ley et al. 2007; Friedl and Gilmour 2009).
Discrete macromolecular complexes mediate cell adhesions and form a link between the actin cytoskeleton and either the ECM or adjacent cells. The organization of the actin cytoskeleton at adhesion sites (e.g., filament nucleation, cross-linking, bundling, and actomyosin contractility) is tightly regulated and driven by adhesion proteins that are physically linked to the actin cytoskeleton (Schwarz and Gardel 2012; Wehrle-Haller 2012). Adhesions serve as signaling hubs; they trigger downstream pathways through a plethora of effectors, including kinases and the Rho family of GTPases, which regulate the organization and dynamics of the actin cytoskeleton (Hynes 2002; Burridge and Wennerberg 2004). In addition, these signaling pathways control cellular processes such as proliferation, survival, and gene expression, although these pathways will not be covered in this review (Schwartz and Assoian 2001).
Here, we discuss the interplay between the organization of the actin cytoskeleton and adhesions at cell–ECM and cell–cell contacts. We first present an overview of how cell adhesions were identified as sites of protein accumulation and physical linkage to the actin cytoskeleton, and then we discuss the distinct actin architectures that underlie these different adhesions. Furthermore, we highlight the important roles of actomyosin activity in force transmission through adhesions and in sensing and translating the properties of the ECM and forces from neighboring cells through specific cellular responses. Finally, we discuss the significance of cross talk between cell–ECM and cell–cell adhesions in cell behavior.
2. CELL ADHESIONS LINK ACTIN TO THE CELLULAR MICROENVIRONMENT: A HISTORICAL PERSPECTIVE
2.1. A Molecular Link between Actin Filaments and the ECM
The first imaging studies of fibroblasts on planar substrates in culture revealed discrete regions of close substratum contact and physical linkage between the ECM and actin filament bundles across the plasma membrane (Curtis 1964). Subsequent electron microscope (EM) images showed dense cytoplasmic fibrillar structures (actin filament bundles) that terminated in discrete areas of electron density and correlated with the “close contacts” that had been observed by light microscopy (Izzard and Lochner 1976; Heath and Dunn 1978). These sites were proposed to serve as traction points that supported the translocation of the cell body during migration (Izzard and Lochner 1980). Concurrent studies showed that fibronectin—an ECM protein secreted by cells and implicated in cell attachment to the substratum—localized adjacent to actin filament bundles and their termini (Hynes and Destree 1978; Singer 1979). This suggested the presence of a transmembrane linker molecule that connected the actin cytoskeleton and fibronectin and thereby served as an ECM adhesion molecule.
2.2. Identification of the Molecules That Mediate the Linkage between Actin and the ECM
In the late 1970s and early 1980s, a number of proteins were identified that localized in regions of close contact between cells and the ECM. These included α-actinin (Lazarides and Burridge 1975), which also decorated actin filaments, vinculin (Geiger 1979), talin (Burridge and Connell 1983), and integrin, a receptor for fibronectin (Chen et al. 1985; Damsky et al. 1985; Hynes 2002). These proteins interacted with each other and with actin, suggesting that they functioned as a protein complex mediating the fibronectin–actin linkage (Horwitz et al. 1986). Thus, these discrete regions of cell adhesion to the ECM, often termed focal contacts or focal adhesions (FAs), acquired a distinct molecular identity.
2.3. E-Cadherin Mediates Cell–Cell Attachment and Localizes with Actin
During the same period, electron microscopy studies of polarized epithelia revealed the presence of three types of intercellular junctions among adhering cells. They comprised the tight junction (TJ), adherens junction (AJ), and desmosomes (Farquhar and Palade 1963); the TJ and AJ localized at the juxta-lumenal region and are collectively called the apical junction complex. The TJ regulates the passage of ions and small solutes among epithelial cells, whereas desmosomes provide mechanical strength to epithelial sheets and connect with intermediate filaments. Here, we focus on the AJ as it mediates cell–cell adhesion through a linkage with actin and is the best characterized of the intracellular junctions (Takeichi 1995; Takeichi 2014).
E-cadherin was identified as a key transmembrane adhesion receptor involved in Ca2+-dependent cell–cell adhesion (Takeichi 1977; Yoshida-Noro et al. 1984). Subsequent immunocytochemical studies revealed that cadherin family proteins concentrate in the apical region of epithelial tissues where the AJ is primarily located. Today, the term “cadherin” refers to a large family of related transmembrane receptors involved in cell–cell adhesion in various types of cell junctions (reviewed in detail elsewhere [Takeichi 2014]). In addition to cadherins, members of the Ca2+-independent IgG superfamily termed nectins were also found at the AJ and are involved in the initial formation of cell–cell contacts (Rikitake et al. 2012). Cadherins colocalized with cortical actin bundles, which suggested that AJ linkage to the actin cytoskeleton is required for cell–cell adhesion (Hirano et al. 1987; Geiger and Ginsberg 1991). Thus, E-cadherin, and other classical cadherins (such as N-, P- and R-cadherin), emerged as the AJ transmembrane molecule that connected the actin cytoskeleton between neighboring cells, an idea that was further supported by the finding that deletion of the E-cadherin cytoplasmic domain resulted in loss of cell–cell adhesion (Nagafuchi and Takeichi 1988; Ozawa et al. 1989).
2.4. Catenins Mediate the Actin–Cadherin Linkage in Cell–Cell Adhesion
As with FAs, the AJ contains cytoplasmic proteins that are recruited to regions of cell–cell contacts. The amino acid sequence of the cytoplasmic domain of classical cadherins is highly conserved, indicating that different cadherins bind to similar cytoplasmic proteins. These proteins were identified as p120-catenin, which regulates cadherin turnover at the plasma membrane, and β-catenin (termed armadillo in Drosophila), which binds the actin-binding and bundling protein α-catenin (Ozawa et al. 1989; Peifer and Wieschaus 1990; McCrea and Gumbiner 1991; Nagafuchi et al. 1991; Davis et al. 2003). As α-catenin can directly bind actin (Rimm et al. 1995), it was originally thought to directly link the cadherin–catenin complex to the actin cytoskeleton. However, subsequent studies revealed that the interaction between actin and the AJ is more complex, and it involves many actin linkage and regulatory proteins that are dynamically recruited to the AJ (see below).
3. CELL ADHESIONS ARE MODULAR AND MULTIFUNCTIONAL STRUCTURES
The repertoire of molecules present at cell–ECM and cell–cell adhesions has greatly expanded since the early discoveries of the core complexes. It is now clear that both adhesions are highly complex, consisting of more than 150 proteins that can be categorized into common functional modules (Fig. 1A) (Kanchanawong et al. 2010; Patla et al. 2010; Murray and Zaidel-Bar 2014; Toret et al. 2014). These cytoplasmic modules perform three main functions: First, they establish the structural linkage of adhesions to the actin cytoskeleton; second, they modulate actin organization and dynamics; and, third, they trigger intracellular signaling pathways. Together they form an intricate network that facilitates the dynamic association of the actin cytoskeleton to either the ECM or adjacent cells.
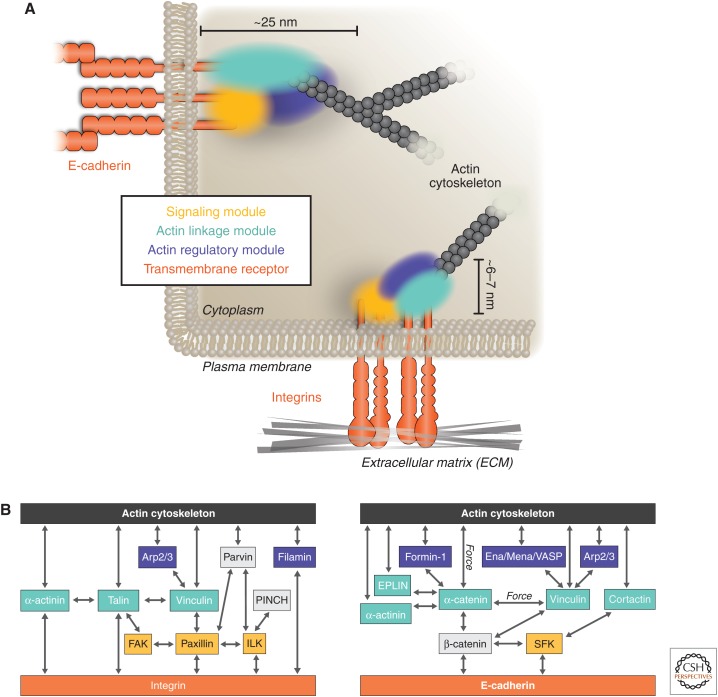
Figure 1.
Organization of functional modules at cell adhesions. (A) Cell–ECM and cell–cell adhesions are compartmentalized in modules with distinct functional properties. Physical linkage between the actin cytoskeleton (gray) and transmembrane adhesion receptors (orange) is mediated by the actin linkage module (cyan), which contains actin-binding molecules. Regulation of this linkage and local actin organization is mediated by components of the actin regulatory (blue) and signaling (yellow) modules. (B) Molecular interactions of proteins in the different adhesion modules between the actin cytoskeleton (gray) and adhesion receptors (orange). Functional modules at both adhesion sites are spatially segregated (with partial overlap) and, in cell–ECM adhesions (left), are organized within vertical organizational strata between the plasma membrane and the actin cytoskeleton. Arp, Actin-related protein; ECM, extracellular matrix; EPLIN, “epithelial protein lost in neoplasm” (LIMA1); FAK, focal adhesion kinase; ILK, integrin-linked kinase; SFK, Src-family kinases.
3.1. The Actin Linkage Module
At cell–ECM adhesions, linkages between the integrin adhesion receptors and actin are mediated by talin (Critchley 2009) and/or α-actinin (Otey et al. 1990), both of which can bind to vinculin (Ziegler et al. 2008), which itself also binds to actin (Humphries et al. 2007). The actin-binding and -cross-linking protein filamin can also link integrins to the actin cytoskeleton (Loo et al. 1998), either directly through binding to the β-integrin subunit or indirectly through interactions with migfilin that, in turn, binds the integrin-binding and -activating protein kindlin (Fig. 1B) (Tu et al. 2003).
Some actin-binding proteins at cell–ECM adhesions are also present in cell–cell adhesions, including vinculin and α-actinin (Knudsen et al. 1995; Watabe-Uchida et al. 1998; Weiss et al. 1998), as well as the membrane-associated actin-binding ERM proteins ezrin, radixin, and moesin (Clucas and Valderrama 2014). That these different adhesion complexes share some of the same proteins provides a potential mechanism of cross talk and regulation (see Sec. 7). The AJ also contains specific proteins not found at cell–ECM adhesions, including spectrin, ZO-1, Ajuba, afadin, and EPLIN (epithelial protein lost in neoplasm), most of which are recruited to the cadherin–catenin complex through interaction with α-catenin (Fig. 1B) (for detailed reviews, refer to Kobielak and Fuchs 2004; Maiden and Hardin 2011). The linkage between core adhesion molecules and the actin cytoskeleton at both adhesion sites can vary in composition and likely reflects different requirements for cadherin and integrin transmembrane receptors, different ECM molecules to which integrins bind (e.g., collagen, laminins, fibronectin), and different kinds or stages of adhesion.
3.2. The Actin Regulatory Module
Besides a physical link to the actin cytoskeleton, local modulation of actin organization is also essential for the integrity of adhesion. For example, inhibiting the activity of the Arp2/3 complex, which induces the assembly of branched actin arrays, impairs the formation of new cell–ECM adhesions (Wu et al. 2012; Chorev et al. 2014) and cell–cell contacts (Bailly et al. 2001; Beckham et al. 2014). Certain members of the formin family of actin nucleators, which promote the formation and elongation of linear actin bundles, also localize and regulate actin assembly at dorsal stress fibers (SFs) and FAs (Skau et al. 2015). Furthermore, formin-1 and diaphanous-related formin-1 (Dia1) localize to AJs and are required for the integrity of cell–cell adhesions (Sahai and Marshall 2002; Kobielak et al. 2004; Carramusa et al. 2007). The ENA/Mena/VASP (vasodilator-stimulated phosphoprotein) family of proteins is also important for regulating the actin network at adhesion sites. They have G- and F-actin-binding domains and bind to the barbed end of actin filaments to promote actin filament elongation by acting as processive barbed-end actin-filament nucleators (Krause et al. 2003). VASP is recruited to cell–ECM (Brindle et al. 1996) and cell–cell contacts (Leerberg et al. 2014) through binding to vinculin and likely contributes to actin stabilization at adhesion sites (Bear et al. 2002; Scott et al. 2006). In summary, actin-regulatory proteins organize the actin cytoskeleton at cell–ECM and cell–cell adhesions, thus influencing the physical linkage of actin to adhesions, and they are themselves regulated by components of the adhesion signaling modules.
3.3. The Signaling Module
Cell–ECM and cell–cell adhesions trigger diverse cytoplasmic signaling pathways. Numerous kinases, phosphatases, and small GTPases comprise these signaling modules, many of which are shared by both cell–ECM and cell–cell adhesion complexes. These modules influence actin polymerization and organization, as well as actomyosin contractility, which in turn regulate adhesion organization and strength. These pathways also affect a wide range of cellular processes such as proliferation, survival, and gene expression (Assoian and Schwartz 2001).
At cell–ECM adhesions, the FAK (focal adhesion kinase)–paxillin signaling module regulates actin nucleation, polymerization, and organization. This is a well-described pathway that includes the tyrosine kinases FAK, Src-family kinases, Abl, and phosphoinositide 3-kinase (PI3K). Paxillin serves as a scaffold for numerous proteins that regulate Rho-family GTPase activity; it also has binding activities for core adhesion components such as vinculin, p130Cas, Pix, and the Ser/Thr-protein kinase PAK (Turner 2000). FAK, in addition to its tyrosine kinase activity, acts as a Rho-GTPase regulatory scaffold by binding Rho GTPase-activating proteins (GAPs) such as p190RhoGAP. FAK also binds vinculin and talin (Fig. 1B) (Turner et al. 1990; Lawson et al. 2012), which highlights the spatial integration and interplay among different modules in adhesions to promote protein recruitment, signaling, and actin regulatory function.
The signaling modules in cell–cell adhesions are less well defined, although Src-family kinases, PI-3 kinase, EGF receptor kinase, and the Rho family of small GTPases (Rac1, RhoA, Cdc42) play an important role in regulating different components of the adhesion modules. For example, Src-mediated tyrosine phosphorylation of β-catenin dissociates it from E-cadherin and facilitates its entry into the nucleus where β-catenin activates genes involved in cell proliferation (Brembeck et al. 2004; Coluccia et al. 2006). In addition expression of “dominant-negative” or constitutively active Rho-family GTPases results in dramatic changes in cell–cell contacts through effects on actin organization and dynamics (Braga et al. 1997; Takaishi et al. 1997; Jou and Nelson 1998; Braga et al. 1999). The activities of Rac and Rho are spatially restricted at cell–cell contacts, as cadherin ligation results in transient Rac accumulation and activation (Nakagawa et al. 2001; Noren et al. 2001; Perez et al. 2008; Terry et al. 2011), whereas activated Rho appears to be excluded from sites of cadherin engagement and is instead localized to the edges of the expanding cell–cell contact (Yamada and Nelson 2007). Rho activity is required for myosin-II-dependent actin contractility, and inhibition of myosin light-chain kinase (MLCK), a downstream target of Rho, stalls the extension of cell–cell contact formation (Yamada and Nelson 2007).
Here, we have presented some examples of key integrin- and cadherin-associated modules that are present in adhesions and highlight the complexity of their interactions. However, many more components and interactions exist (for detailed reviews, see Harburger and Calderwood 2009; McCormack et al. 2013).
3.4. Functional Modules Are Spatially Compartmentalized in Cell Adhesions
Recent observations using cryo-electron microscopy and superresolution fluorescence microscopy have provided initial three-dimensional nanoscale snapshots of the architecture of adhesions (Kanchanawong et al. 2010; Patla et al. 2010). At cell–ECM adhesion sites, this ongoing work reveals a compartmentalized spatial organization of proteins in which proteins are organized vertically into horizontal layers of functional modules between the plasma membrane and actin filaments (Fig. 1A,B). The FAK–paxillin signaling module is at the integrin transmembrane receptor layer, whereas actin-associated components such as α-actinin, VASP, and zyxin are organized in a layer proximal to the actin filaments. The central linkage layer comprises vinculin and talin and has domains near both integrin and actin filaments, thereby connecting them. Vinculin activation coincides with an upward repositioning during FA maturation, whereas inactive vinculin localizes to the lower integrin transmembrane receptor layer (Case et al. 2015). Talin spans all the modules—the talin head and integrin-binding domain localize with the signaling and integrin-proximal region, whereas the talin rod and actin-binding domain span both the central and actin filament linkage layers (Kanchanawong et al. 2010). This spatial overlap is supportive of a role for talin in mediating the spatial organization of functional modules in adhesions.
The nanoscale architecture of cell–cell adhesion sites is less well defined. Recent superresolution imaging has revealed that E-cadherin organizes into distinct nanoscale precursor clusters that mature into adhesive contacts (Truong Quang et al. 2013; Wu et al. 2015). The cortical F-actin network is thought to surround clusters of E-cadherin. This organization might play a role in maintaining independent adhesion subunits, as E-cadherin clusters pack to form larger adhesion surfaces. However, additional work is required to elucidate the spatiotemporal organization of cytoplasmic components of cell–cell adhesions.
4. ADHESION ARCHITECTURE IS DICTATED BY ACTIN FILAMENT ORGANIZATION
Although both cell–ECM and cell–cell adhesions comprise similar protein modules, different assembly stages and types of adhesions exist, varying in morphology, molecular composition, and function. Here, we describe different types of cell–ECM and cell–cell adhesions, the actin filament organization associated with them, and the role of force and myosin II in their organization.
4.1. Cell Adhesions Have Diverse Morphologies and Molecular Compositions
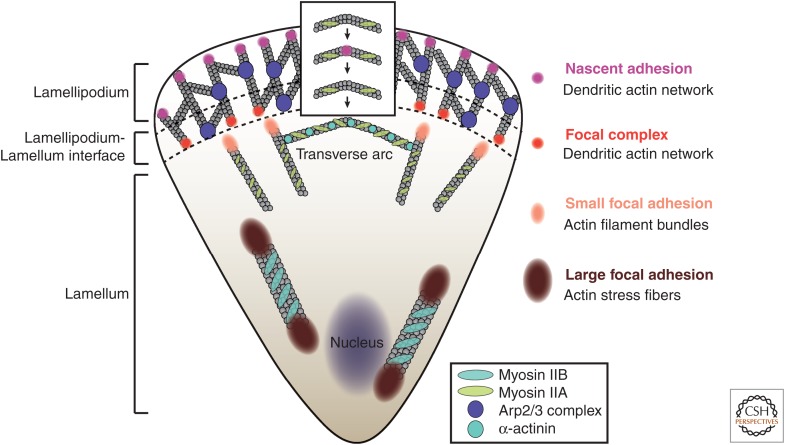
Cell–ECM adhesions have diverse morphologies, compositional variations, and functions (Geiger and Yamada 2011). Nascent adhesions are small diffraction-limited structures (<0.3 µm) present within the dendritic actin network in the lamellipodium of spreading or migrating cells (Nayal et al. 2006; Alexandrova et al. 2008; Choi et al. 2008). Focal complexes are slightly larger adhesions (0.5–1 µm) located posterior to nascent adhesions at the lamellipodium–lamellum interface. Both nascent adhesions and focal complexes contain integrin, talin, kindlin, ILK, α-actinin, and vinculin and are enriched in FAK, paxillin, and Src (Huveneers and Danen 2009; Elad et al. 2013). Focal adhesions are a class of large, elongated adhesions that reside outside the lamellipodium (Zaidel-Bar et al. 2003). Their sizes and morphology range from 1 to 10 µm, with variable aspect ratios, and they associate with linear actin filament bundles. Finally, fibrillar adhesions are very large (>5 µm), elongated adhesions located in the central region of the cell. They associate with large actomyosin bundles and are involved in organization of the ECM (Pankov et al. 2000). Fibrillar adhesions have relatively low tyrosine phosphorylation levels, high levels of vinculin, and α-actinin and contain unique molecules such as zyxin and tensin (Fig. 2) (Zaidel-Bar et al. 2003).
Figure 2.
Actin cytoskeleton architecture dictates the organization of cell–ECM adhesion. Diffraction-limited nascent adhesions emerge within the dendritic actin network, which is promoted by the action of the Arp2/3 complex in the lamellipodium of motile cells. Nascent adhesions are precursors for larger focal complexes (∼1 µm) located along the lamellipodium–lamellum interface and elongated focal adhesions (FAs) (1–5 µm) that are associated with actin filament bundles in the lamellum. The size and stability of actin filament bundles correlates with the size of FAs—small actin filament bundles located at the leading edge of the lamellum (associated with α-actinin and myosin IIA) correlate with small FAs, whereas large, stable (myosin IIB decorated) actin fibers along the center and rear of the cell promote large FAs. Transverse actin arcs that connect actin filaments and fibers presumably form in the front of the lamellipodium when myosin IIA emerges and bundles actin fibers in an arc that then recedes backward and couples with nascent adhesions as the edge protrudes. This arc eventually joins other transverse arcs in the lamellum.
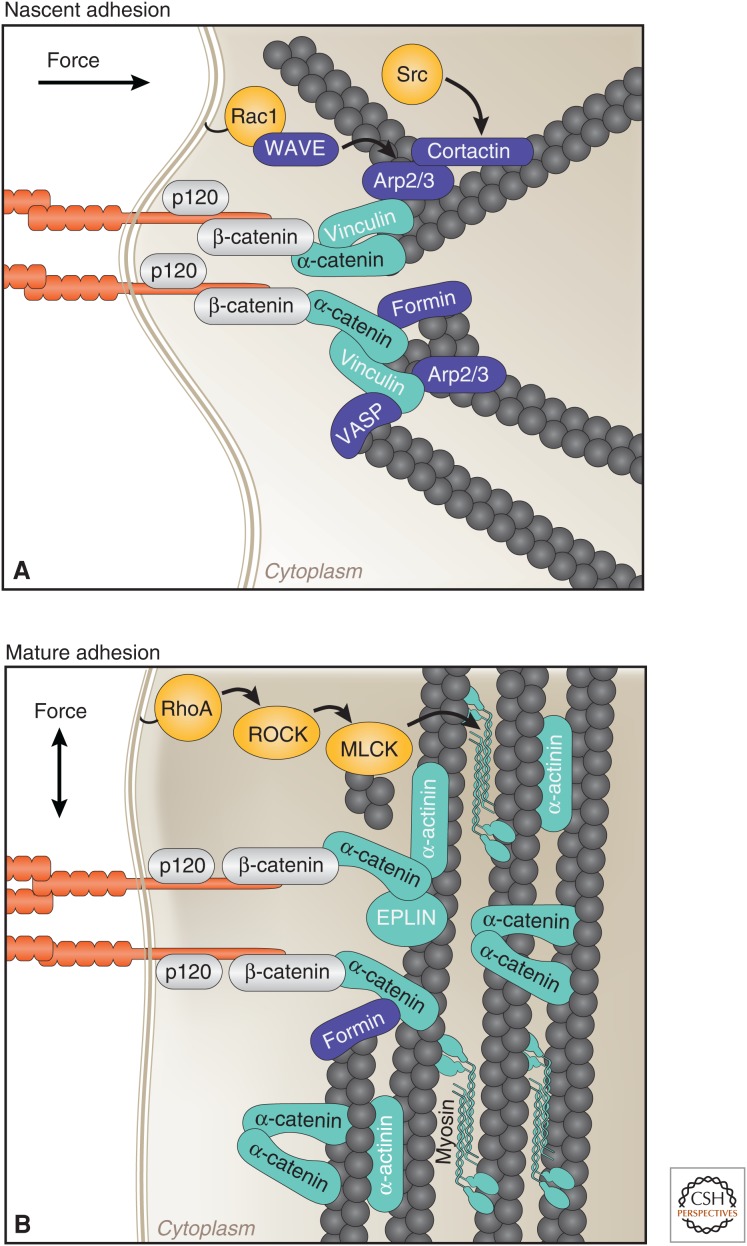
Similar to cell–ECM adhesions, different stages of cell–cell contact formation coincide with a distinct organization of the actin cytoskeleton (Fig. 3). Forming cell–cell junctions adopt a punctate morphology and are connected to radial actin bundles perpendicular to sites of contact (Adams et al. 1996; Vasioukhin et al. 2000), whereas, upon expansion of the contact, the peri-junctional actin is remodeled into parallel actin bundles (Zhang et al. 2005). Unique molecules also populate the different stages of cell–cell adhesion as vinculin, for example, is observed at newly forming junctions but is absent from mature, stable contacts (Fig. 3) (Miyoshi and Takai 2008). Although more work is required to fully resolve how different stages of cell–cell adhesion are formed, it is clear that the actin structure and protein composition contributes to the observed difference in the morphologies of cell–cell adhesion complexes.
Figure 3.
Stages of cell–cell contact formation coincide with distinct organizations of the actin cytoskeleton, molecular compositions, and directions of forces. (A) Nascent cell–cell adhesions are connected to radial actin bundles lying perpendicular to sites of contact. These sites contain high levels of active Rac1 and Src, and actin-regulatory proteins (Arp2/3, cortactin, VASP, formin-1) that cooperate to generate a branched actin network required for contact expansion. α-catenin is under tension, which results in association with actin, directly through a catch–bond interaction, and vinculin. (B) At mature adhesions, the peri-junctional actin cytoskeleton is rearranged into bundles parallel to the plasma membrane. RhoA activity increases, which activates myosin II, thereby generating tensile forces required for adhesion maturation. Locally high concentrations of α-catenin presumably recruit actin binding and bundling proteins (EPLIN and α-actinin), and α-catenin itself forms homodimers that bind and bundle actin. EPLIN, epithelial protein lost in neoplasm (LIMA1); MLCK, myosin light-chain kinase; VASP, vasodilator-stimulated phosphoprotein.
4.2. Assembly of Nascent Cell Adhesions Is Driven by Actin Polymerization
The formation and stability of nascent cell–ECM adhesions are coupled to the formation and stability of the dendritic actin network cytoskeleton. In migrating or spreading cells, cell–ECM adhesions persist until they reach the base of the lamellipodium, where dendritic actin disassembles or reorganizes (Choi et al. 2008). Some studies indicate that actin polymerization drives preactivated integrin clusters probing for sites of adhesion nucleation near the leading edge (Galbraith et al. 2007). Actin nucleation around integrin sites might be mediated by the Arp2/3 complex through its interaction with vinculin (DeMali et al. 2002) or FAK (Serrels et al. 2007; Swaminathan et al. 2016). In addition, actin nucleation can be mediated by direct interaction between actin and vinculin (Thievessen et al. 2013) or transient interaction between α-actinin and integrin (Bachir et al. 2014). These observations suggest an important, but poorly understood, role of actin branching and elongation in the formation of nascent cell–ECM adhesions.
Cadherins play an active role in shaping actin organization that mediates nascent cell–cell contact formation through dynamic recruitment and interaction with actin binding and regulatory proteins (Ratheesh et al. 2012). Cell–cell adhesion depends on actin polymerization. Cadherin homophilic ligation in epithelial cells in vivo (e.g., Drosophila) and tissue-culture cells (e.g., MDCK cells) directly recruits and activates the Arp2/3 complex (Kovacs et al. 2002; Verma et al. 2004), the nucleation-promoting factor WAVE (Yamazaki et al. 2007; Verma et al. 2012), and cortactin (Helwani et al. 2004), which coincides with rapid actin filament assembly (Yonemura et al. 1995; Adams et al. 1996; Vasioukhin et al. 2001). Arp2/3-induced actin filament branches can be stabilized by cortactin, thus preventing actin filament disassembly (Weaver et al. 2001). As the cell–cell contact expands, there is a large reorganization of the actin cytoskeleton adjacent to the adhering plasma membrane and it requires the activation and localization of myosin II to the edges of the cell–cell contacts that drives compaction (Shewan et al. 2005; Yamada and Nelson 2007).
4.3. Adhesion Sites Are Reinforced and Stabilized by Actin Bundling
Elongated cell–ECM adhesions form along actin bundles at the lamellipodium–lamellum interface (Choi et al. 2008). These actin bundles contain α-actinin and grow by formin-mediated actin polymerization, which nucleates unbranched actin filaments (Riveline et al. 2001; Oakes et al. 2012). Knockdown of α-actinin perturbs actin bundle integrity and inhibits the formation of large, elongated adhesions (Choi et al. 2008). Similarly, disruption of mDia1 activity using the formin inhibitor SMIFH2 or mDia1 knockdown inhibits actin filament polymerization and reduces adhesion elongation (Beckham et al. 2014).
The stabilization of cell–cell adhesions is also accompanied by a large structural reorganization of the actin cytoskeleton adjacent to adhering plasma membranes (Fig. 3). Formins localize to cell–cell contacts through direct interaction with α-catenin and promote linear actin cable assembly (Kobielak et al. 2004). The ENA/Mena/VASP family of actin-regulatory proteins also contributes to the stabilization of cell–cell adhesions as VASP promotes actin bundle formation (Vasioukhin et al. 2000). This occurs with a switch from Rac1 to RhoA activity during cell–cell contact reinforcement and expansion (Yamada and Nelson 2007), which is also observed at cell–ECM adhesions (Machacek et al. 2009). Active RhoA contributes to junction contraction and activates formins and Rho kinase (ROCK). Together, they lead to maturation of the cell–cell contact by further promoting the formation of actin filament bundles parallel to the plasma membrane (Zhang et al. 2005).
Actin filament reorganization at the AJ might also involve the actin binding and bundling activity of α-catenin homodimers, which are thought to form in the peri-junctional cytoplasm upon local disassociation from the cadherin–catenin complex (Drees et al. 2005). α-catenin homodimers bind to F-actin and inhibit Arp2/3-mediated polymerization and cofilin-mediated severing of actin. This is hypothesized to dampen membrane protrusive activity and promote contractility of the cell–cell junction and adhesion strength (Drees et al. 2005; Benjamin et al. 2010; Hansen et al. 2013; Bianchini et al. 2015). Taken together, these studies show an intimate relationship between actin filament bundles and the formation of elongated cell–ECM and extended cell–cell adhesions.
4.4. Adhesion Size and Maturation Are Regulated by Myosin II Activity
Myosin II activity is crucial for cell–ECM and cell–cell adhesion formation, maturation, and function. There are three isoforms of myosin II (A, B, and C), of which the A and B isoforms are expressed in most cells. These isoforms have different ATPase and contractility activities, and differentially regulate the size and stability of adhesion complexes. In general, myosin II organizes actin filaments into antiparallel filament bundles and can either maintain that organization by cross-linking the filaments together or can induce contractility through its ATPase-driven motor activity (Vicente-Manzanares et al. 2009). Here, we discuss the role of myosin II in regulating the organization of adhesion complexes and the associated actin cytoskeleton. In Section 5, we discuss the role of myosin II in mechanotransduction through adhesion complexes.
At cell–ECM adhesions, inhibition of myosin II activity blocks actomyosin filament assembly and impairs the formation of large, elongated adhesions (Riveline et al. 2001; Vicente-Manzanares et al. 2007). Both the actin-cross-linking and contractile functions of myosin are implicated in adhesion size. Myosin II mutants that can cross-link or bundle actin but do not induce contractility, can still serve as a structural template for adhesion growth and elongation (Choi et al. 2008). However, larger, more elongated and stable adhesions appear to require myosin-II-mediated actin network contraction (Chrzanowska-Wodnicka and Burridge 1996; Riveline et al. 2001). Myosin II activity also regulates the molecular composition of adhesions (Kuo et al. 2011; Schiller et al. 2011) as it promotes the recruitment of mechanosensitive proteins such as zyxin and vinculin and negatively regulates Rac activators such as β-Pix, which leads to small, dynamic adhesions (Kuo et al. 2011).
Myosin II isoforms associate with different kinds of adhesions. Myosin IIA binds to short actin filament bundles. Myosin IIA is concentrated at sites of smaller and less stable adhesions such as the front of the lamellar region of spreading or migrating cells (Vicente-Manzanares et al. 2007) and within the lamellipodium during edge protrusion (Burnette et al. 2011). Myosin IIA localization to FAs is driven by Rac1 activation (Pasapera et al. 2015). Myosin IIB is mostly absent from small adhesions at the leading edge of motile cells and associates with large actin filament bundles away from the leading edge of migrating cells.
At cell–cell adhesions, myosin II is activated at new adhesion sites, generates contractile forces at contact edges, and colocalizes with cadherins at mature junctions (Krendel and Bonder 1999; Yamada and Nelson 2007; Cavey et al. 2008). Depletion of myosin IIB results in a reduction of steady-state junctional F-actin in epithelial monolayers (Smutny et al. 2010). Furthermore, the actin-cross-linking function of myosin II is required to generate a dynamic contractile network that provides tensile forces required for adhesion maturation (Shewan et al. 2005; Liu et al. 2010; Borghi et al. 2012; Luo et al. 2013). The activity of different myosin II isoforms also influences the localization and activity of adhesion molecules. Myosin IIA is required for cadherin clustering, whereas myosin IIB controls the continuous distribution of cadherin clusters along the length of cell–cell contacts (for a detailed review, see Budnar and Yap 2013). Taken together, myosin II plays an important role in forming both cell–ECM and cell–cell adhesions, and actomyosin contractility is essential for cells to sense and respond to mechanical forces.
5. CELL ADHESIONS SENSE AND TRANSDUCE MECHANICAL FORCES
Cell adhesions both sense and transduce intracellular forces between cells and the ECM (Bershadsky et al. 2003; Oakes and Gardel 2014) and neighboring cells (Ladoux et al. 2010; le Duc et al. 2010; Liu et al. 2010; Tabdili et al. 2012; Weber et al. 2012; Thomas et al. 2013; DeMali et al. 2014). In the following section, we describe force measurements at adhesion sites and discuss some of the key mechanoresponsive adhesion components and molecular mechanisms that underlie force sensing and transmission in cell adhesions.
5.1. Actomyosin Force Propagation through Cell–ECM and Cell–Cell Adhesions
Traction forces transmitted by adhesions to the surrounding ECM can be estimated by the displacement of either micropatterned elastomeric substrate “posts” (Tan et al. 2003) or fiduciary fluorescent beads embedded in pliable substrates that are deformed by cell traction (Style et al. 2014). These “traction force” measurements reveal distinct and spatially resolved forces exerted at adhesions (in the range of 1–10 nN per adhesion) that align parallel to the long axis of elongated adhesions and actin bundles (Balaban et al. 2001; Nicolas et al. 2004). These forces depend on myosin II activity as the force decreases when cells are treated with inhibitors of actomyosin contractility (Balaban et al. 2001; Beningo et al. 2001; Gardel et al. 2008). Although a linear relationship between force and adhesion size has been reported (Balaban et al. 2001; Tan et al. 2003; Gardel et al. 2008; Stricker et al. 2011), significant traction forces are also detected near nascent adhesions and focal complexes associated with the lamellipodium of protruding cells, whereas less force is observed in large, central adhesions (Beningo et al. 2001; Tan et al. 2003; Gardel et al. 2008). Interestingly, as nascent adhesions are independent of myosin II, the forces on these adhesions are likely generated by actin polymerization against the membrane of the leading edge.
Actomyosin-mediated forces are also observed at cell–cell adhesion sites, but direct measurement of these forces is difficult because of the orthogonal organization of cell junctions relative to the substratum. Thus, measurements of rupture forces between isolated adhesion molecules or cell junctions have been used to determine forces at cell–cell adhesions. For example, pulling on single cadherin–cadherin bonds by atomic force microscopy (AFM) showed that 10–157 pN is required to separate the bonds (Baumgartner et al. 2000; Perret et al. 2004; Panorchan et al. 2006; Shi et al. 2008). However, higher forces (1–200 nN) were required to rupture adhesions between pairs of cells (Chu et al. 2004; Stockinger et al. 2011) or cells migrating across surfaces coated with the extracellular domain of cadherin (Ganz et al. 2006; Liu et al. 2010; Maruthamuthu et al. 2011). These results indicate that these larger forces might reflect the organization of large numbers (quasicrystalline arrays) of cadherins as junctions mature and therefore an increase in adhesive strength coincident with the reorganization of the actomyosin network around those junctions.
Qualitative studies that disrupted the cadherin-junction–actin network revealed that the direction of forces also changes with junction maturation. During the formation of cell–cell adhesions, the addition of low concentrations of cytochalasin D, which predominantly caps actin filament barbed ends, revealed actin contractile forces at the extremities of the contacts in MDCK doublets where activated Rho GTPase localized (Yamada and Nelson 2007) and hence were perpendicular to contact sites (Liu et al. 2010). Analysis of tension at mature cell–cell contacts in epithelial monolayers by laser ablation indicated, conversely, that actomyosin-based tension is oriented parallel to the contacts (Farhadifar et al. 2007; Cavey et al. 2008).
Thus, both cell–ECM and cell–cell adhesions serve as sites that shunt intracellular forces to the ECM and neighboring cells (Bershadsky et al. 2003; Gardel et al. 2010; Leckband and de Rooij 2014). As discussed below, these adhesion complexes sense and respond to force in a number of ways, which can result in force-dependent incorporation or release of specific adhesion-associated molecules, conformational changes, and posttranslational modifications of adhesion proteins, remodeling of adhesions, and changes in mechanical properties such as adhesion strength.
5.2. Molecular Mechanisms of Force Sensing and Response in Adhesions
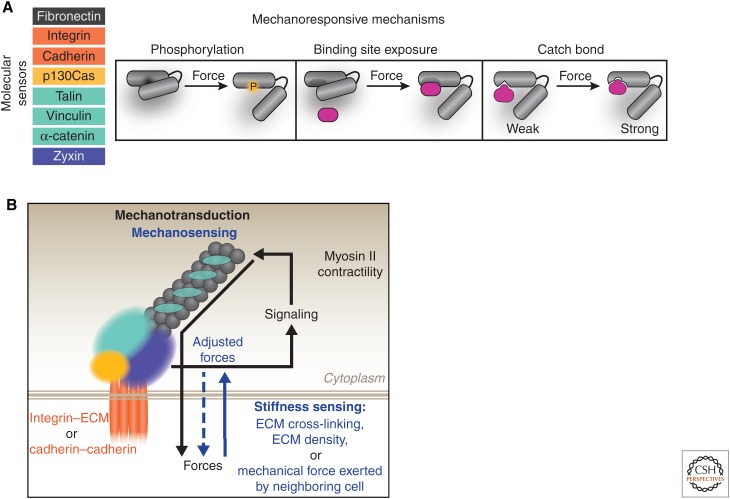
Forces on adhesion complexes appear to dictate adhesion size and strength, composition, and the signals that they generate (Balaban et al. 2001; Lele et al. 2006; Carisey et al. 2013; Hytonen and Wehrle-Haller 2015). Although understanding the mechanisms involved is an active area of investigation, conformational strain, or protein unfolding, is a likely explanation (Fig. 4A). Force-sensitive adhesion molecules include the adhesion receptors: integrin (Friedland et al. 2009; Morimatsu et al. 2013) and cadherin (Borghi et al. 2012; Cai et al. 2014; Manibog et al. 2014); actin-binding proteins: talin (del Rio et al. 2009), vinculin (Grashoff et al. 2010), zyxin (Lele et al. 2006), and α-catenin (Yonemura et al. 2010; Yao et al. 2014); and the Src kinase scaffold p130cas (Sawada et al. 2006).
Figure 4.
Mechanisms of mechanotransduction in adhesions. (A) Multiple key components within adhesions mediate force sensing and transmission. This is through force-induced conformational changes that can expose cryptic sites containing residues that can become phosphorylated or provide binding sites for other proteins. In addition, a number of proteins in adhesion complexes interact through a catch bond–binding mechanism in which force increases the bond lifetime, as reported for homotypic trans binding between cadherin extracellular domains and α-catenin–actin interactions. (B) Force sensing and transmission at cell–ECM and cell–cell adhesions converges on myosin II activity. Cell adhesions initiate signaling cascades that activate members of the Rho family of GTPases (RhoA, Cdc42), among other proteins, which activate myosin II. Actomyosin contractility results in traction forces that are transmitted through adhesion complexes to the ECM or neighboring cells—this is commonly referred to as “mechanotransduction.” Furthermore, adhesions sense mechanical forces in their microenvironment (“mechanosensing”), such as substrate stiffness and pulling forces from neighboring cells, and translate those forces into biochemical signals that, in turn, activate myosin II to regulate the forces transmitted through adhesions. Both mechanotransduction and mechanosensing occur in a dynamic, tightly regulated feedback loop mediated by myosin II activity and actin organization. ECM, extracellular matrix.
Force-dependent conformational unfolding of mechanosensitive molecules can result in the exposure of cryptic sites for tyrosine phosphorylation, such as in p130Cas (Sawada et al. 2006), or binding sites that promote protein localization and interaction, such as talin and vinculin at cell–ECM adhesions (del Rio et al. 2009; Carisey et al. 2013; Hirata et al. 2014), and vinculin, which is recruited to α-catenin at cell–cell contacts (Fig. 4A) (Choi et al. 2012). Conformational changes in α-catenin stretched with magnetic tweezers revealed a 1000-fold increase in the binding affinity to the vinculin head domain (Yao et al. 2014). Catch bonds, in which the bond lifetime increases under tension, have been detected in response to force-mediated conformational unfolding in fibronectin (Smith et al. 2007), resulting in enhanced interaction with integrin (Kong et al. 2009), as well as among homotypic interactions between the extracellular domains of cadherins (Rakshit et al. 2012). Similarly, a catch bond in α-catenin results in strong binding to actin filaments under force and facilitates the attachment of the cadherin–catenin complex to the actin cytoskeleton at cell–cell adhesions (Buckley et al. 2014). Thus, force-induced conformational changes and binding mechanisms of specific adhesion proteins alter their molecular associations and affinities, therefore influencing the composition of cell adhesions.
Although it is evident that adhesions transmit force to the substratum or other cells, the identity of the proteins that physiologically sense and transmit forces is less clear. To address this, fluorescence resonance energy transfer (FRET)-based biosensors have been developed for vinculin (Grashoff et al. 2010), cadherin (Borghi et al. 2012), and α-catenin (Kim et al. 2015). The application of these biosensors in cellular environments has led to interesting and unexpected observations. For example, the mechanical tension on vinculin does not consistently correlate with the forces transmitted by the adhesions—forces on vinculin are high in adhesions near the leading edge and in the center of the cell, which transmit less force. Furthermore, inhibition of myosin II activity, which reduces traction forces by adhesions, does not affect forces transmitted across vinculin (Grashoff et al. 2010). In addition, paxillin, not generally associated with mechanotransduction, has recently been shown to localize in regions of high force within FAs (Morimatsu et al. 2015), and this could suggest a potential role in mechanosensing to promote directed cell migration (Plotnikov et al. 2012).
E-cadherin tension on single cadherins at cell–cell junctions is in the low-picoNewton range (Borghi et al. 2012; Cai et al. 2014), similar to forces across vinculin in FAs (Grashoff et al. 2010). Interestingly, a variant E-cadherin engineered to contain a tension sensor module (“EcadTsMod”) was found to be under tension even at a plasma membrane that was not in contact with another cell (Borghi et al. 2012; Cai et al. 2014). A similar approach showed that vascular endothelial (VE)-cadherin is under tension at the junction between endothelial cells and this stress is modulated by fluid shear stress, although tension was not detected outside the junctional area (Conway et al. 2013). A FRET-based sensor in α-catenin revealed changes in α-catenin conformation at junctions during cell contact formation (Kim et al. 2015). Although α-catenin unfolding is likely central in F-actin (Buckley et al. 2014) and vinculin binding (Yao et al. 2014), this study found that vinculin recruitment to cell–cell contacts was delayed compared with the timing of α-catenin unfolding. The high-affinity binding between conformationally regulated proteins, such as α-catenin to vinculin in cell–cell adhesions, might be elicited by force for initial binding events. However, these interactions can be transient, and associated proteins such as vinculin might also be stably recruited to mature cell–cell adhesions (independent of its interaction with α-catenin) as an F-actin-bundling protein. Finally, the forces measured at single AJ adhesion proteins, using these FRET-based biosensors, are much lower than the forces (1–200 nN) required to rupture adhesions between cells and, perhaps, they point to a role for adhesion protein clustering in cell junction formation and stabilization.
5.3. Mechanosensing in Cell–ECM and Cell–Cell Adhesions
Cells sense substrate stiffness through cell–ECM adhesions and pulling forces from neighboring cells through cell–cell adhesions, and they respond through a signaling loop that includes actomyosin contractility (Fig. 4B). This mechanosensing mechanism is crucial for cells to adapt to forces from the ECM and to changes in tissue rigidity or compressive forces within their environment. For example, ECM pliability is implicated in many cell processes, including migration, proliferation, and differentiation (Discher et al. 2005). The differentiation of neurons from stem cells requires a soft substrate (<5 kPa), resembling the pliability of brain tissue, whereas osteoblasts differentiate on stiffer substrates (>20 kPa) that resemble bone (Engler et al. 2006). Substrate stiffness is also implicated in disease progression as migratory cell invasiveness during cancer metastasis correlates with stiff tissue environments (Huang and Ingber 2005).
Most cells adhering to stiff substrates display larger and more stable cell–ECM adhesions, as well as larger transmitted forces than cells on softer substrates (Pelham and Wang 1997). Actomyosin organization correlates with adhesion size and forces transmitted on substrates with variable stiffness, and a Rho–ROCK–myosin-II pathway mediates these changes (Schiller et al. 2013).
The cadherin adhesion complex, through linkage to the actin cytoskeleton, also responds to mechanical force through a number of mechanisms that remodel cell junctions and alter adhesion strength and junction stiffness. Evidence of junctional remodeling on tension came from studies in which cadherin junction stiffness was found to increase between cells and Fc–E-cadherin-coated beads when a twisting torque from the bead was exerted on the cell (le Duc et al. 2010). This stiffening response correlated with the accumulation of vinculin and actin in an actin- and α-catenin-dependent manner (Barry et al. 2014). Another line of evidence comes from dual micropipette measurements in which the adhesion between cell doublets increased after pulling on cell pairs, which also correlated with actomyosin remodeling at the junctions (Chu et al. 2004; Grashoff et al. 2010; Borghi et al. 2012; Kim et al. 2015). Cadherin-based adhesions can also sense the rigidity of the surrounding substrate. Direct measurement of forces applied by cells on cadherin-coated polydimethylsiloxane (PDMS) revealed that cells apply, through cadherin adhesions, tension in the range of 5 nN/µm2, similar to integrin-dependent stress applied to the ECM (10 nN/µm2) (Ganz et al. 2006). Force applied through N- or E-cadherin as a function of the compliance of the adhesive surface showed that stresses measured with the softer environments (1 kPa ∼ 10 nN/µm2) were an order of magnitude higher than that for cell–cell junctions (1 nN/µm2) (Liu et al. 2010; Tabdili et al. 2012; Maruthamuthu and Gardel 2014). Thus, cadherin-based cell–cell adhesions are mechanosensitive and can both transmit and adapt to mechanical load.
6. CELL–ECM ADHESIONS FUNCTION AS A MOLECULAR CLUTCH
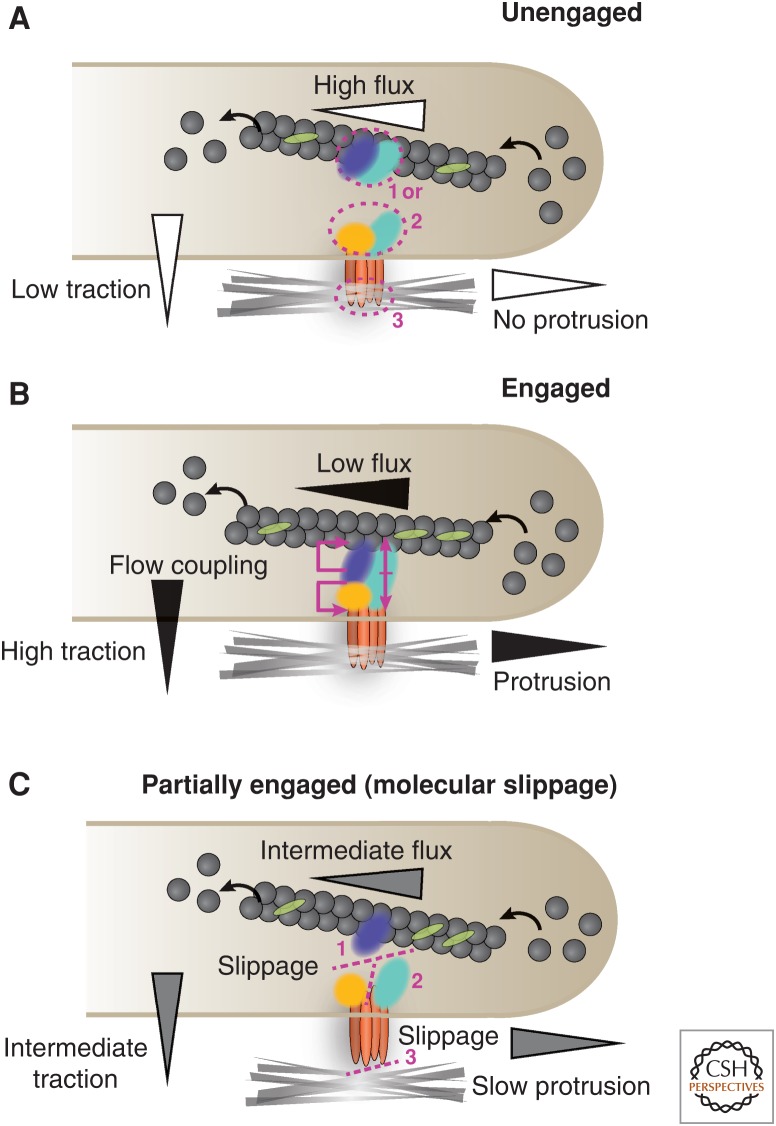
Although studies of forces and their functions at cell–cell adhesions are relatively recent, the role of forces and actomyosin activity in the organization and function of cell–ECM adhesions is more advanced. Traction forces at cell–ECM adhesions arise primarily from forces generated by the rearward (retrograde) translocation of polymerizing or contractile actin filaments (Wang 1985; Watanabe and Mitchison 2002; Ponti et al. 2004). This force is regulated through a “molecular clutch” mechanism, involving coupling between actin filaments and the ECM through adhesions, that inhibits actin flux (retrograde) movement and thereby shunts force to the substratum (Fig. 5A,B) (Mitchison and Kirschner 1988). “Clutch engagement” manifests as reduced retrograde or rearward actin flow (Watanabe and Mitchison 2002; Ponti et al. 2004) and increased traction forces in the vicinity of adhesions (Gardel et al. 2008).
Figure 5.
Schematic diagram of the states that comprise the “molecular clutch” model at cell–ECM adhesions. (A) In an unengaged clutch, the actin cytoskeleton is uncoupled from the cell membrane and integrin adhesion receptors and manifests as fast actin retrograde flux and low traction transmitted to the ECM. The dissociation between the actin cytoskeleton and the membrane is mediated by adhesion molecules within the actin linkage domain (cyan) that could either be associated with actin and the actin-regulatory module (blue) (1), integrin and the signaling module (yellow) (2), or integrin and the ECM (3). (B) In a fully engaged clutch, strong coupling occurs between the actin cytoskeleton, integrin, and various intracellular adhesion components. This presents as fast actin retrograde flows and high forces transmitted to the ECM. However, the differential coupling of the retrograde speeds of adhesion components with actin or integrin indicates that an intermediate and partial clutch engagement exists (C). This arises from molecular slippage that occurs at the actin linkage module level between the actin regulatory domain (1), the signaling domain (2), or integrin–ECM binding (3), and it presents as intermediate actin retrograde speeds and traction forces. The level of clutch engagement correlates with protrusion, as indicated in the diagram.
Recent observations reveal that this molecular clutch is complex and includes a variable “molecular slippage” in adhesions (Fig. 5C) (Brown et al. 2006; Guo and Wang 2007; Hu et al. 2007). Several ECM adhesion proteins show concurrent flux with the retrograde movement of actin and with speeds that correlate with their location relative to actin in the actin–ECM linkage. For example, actin-binding and regulatory proteins such as α-actinin move fast with the retrograde actin flow, whereas others such as paxillin, which resides in the membrane-proximal domain, have a slow retrograde flux similar to that of integrins (Brown et al. 2006; Guo and Wang 2007; Hu et al. 2007). Talin and vinculin have intermediate retrograde flux speeds between those of actin and integrins (Brown et al. 2006). The location of this molecular slippage in the adhesion–actin complex can also vary with the adhesion receptor–ligand pair. For example, large adhesions in cells adhering to laminin through α6β1 integrin have a rapid flux rate and low traction force; in these cells, the integrins are fluxing rapidly, suggesting a weak ligand–receptor interaction (Chen et al. 2012). Thus, modulating the actin–integrin linkage and the molecular clutch engagement in adhesions allows cells to sense and respond to forces from the ECM and supports a broad range of processes that accompany adhesions and actin cytoskeleton organization and function (for a detailed review, see Case and Waterman 2015).
7. CROSS TALK BETWEEN CELL–ECM AND CELL–CELL ADHESIONS
Integration of signals from cell–ECM and cell–cell adhesions is required for many multicellular processes such as cell migration. For example, the coordinated movements of individual and groups of cells within tissues during development (e.g., gastrulation) require controlled changes in intracellular junction strength, actomyosin dynamics, and cell–ECM adhesions as cells exchange neighbors and move past each other, for example, in the Drosophila blastoderm (Bertet et al. 2004; Blankenship et al. 2006; Cavey et al. 2008; Fernandez-Gonzalez et al. 2009; Lecuit et al. 2011).
Measurements of forces during collective cell migration using monolayer stress microscopy (MSM) revealed that collective migration in epithelia is driven by individual cells migrating along local axes of maximum principle stress that are correlated in an Nth nearest-neighbor fashion, which implies that neighboring cells must be transferring forces (“plithotaxis”) (Tambe et al. 2011). Recent modeling further suggests that, during collective migration, forces are propagated across neighboring cells, traversing intercellular junctions in a cooperative manner, and building up differentials of mechanical stress (Serra-Picamal et al. 2012). MSM studies have also implicated E-cadherin as a key component. For example, cells that had decreased expression of components of the cadherin complex—MDCK cells that were grown in the presence of low Ca2+ levels or E-cadherin antibodies to inhibit trans binding between cadherin extracellular domains—had reduced alignment between the orientations of local stresses and the orientations of local cell movements (Tambe et al. 2011). Thus, the assumption of force-based coupling is justified, and the transmission of mechanical stresses between cells during collective migration occurs through cadherin-based cell–cell adhesions.
As cell–cell junctions are orthogonal to cell–ECM adhesions, it has been difficult to analyze directly the effects of cell–cell adhesion on cell–ECM adhesion organization, and vice versa (Fig. 6). However, use of micropatterned, alternating stripes functionalized with ECM proteins and either E-cadherin (Borghi et al. 2010) or combinations of desmosomal cadherins (Dsg, Dsc) (Lowndes et al. 2014) has revealed strong effects of cadherin engagement on actin-dependent plasma membrane dynamics associated with both cadherin- and integrin-based adhesions, and on the rate and direction of cell migration. These experiments confirm that there is cross talk between cell–cell and cell–ECM adhesions.
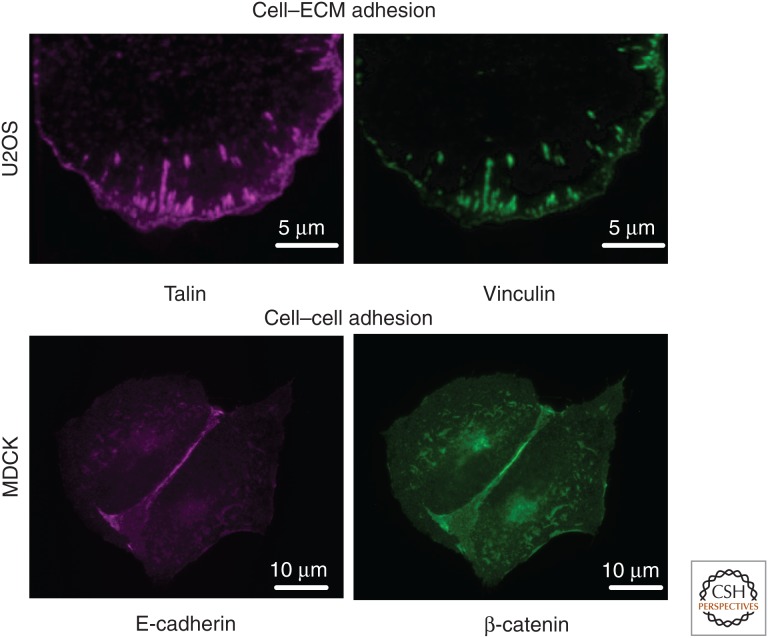
Figure 6.
Fluorescent images of an osteosarcoma U2OS cell expressing GFP–vinculin and mCh–talin showing colocalization at cell–ECM adhesions, and Madin–Darby canine kidney epithelial (MDCK) cells immunostained for E-cadherin and β-catenin showing their colocalization at cell–cell adhesions.
How the different adhesion types regulate each other is not understood. Integrin engagement can affect VE- (Wang et al. 2006) and E-cadherin (Martinez-Rico et al. 2010) expression and function and, reciprocally, activation of VE-cadherin (Tzima et al. 2005). As noted above, E-cadherin engagement can regulate integrin adhesion and cell migration; this likely occurs through regulation of actin dynamics by α-catenin (Borghi et al. 2010). Moreover, the cross talk between adhesions is regulated by the substrate stiffness; for example, cell adhesion to a rigid (5-mPa) two-dimensional surface of ECM spots filled in with E-cadherin extracellular domain results in the formation of predominantly integrin-based adhesions, but adhesion to a softer surface (60 kPa) allowed assembly of integrin and E-cadherin adhesions (Tsai and Kam 2009).
In summary, cell–ECM and cell–cell adhesions interact with and modulate the actin cytoskeleton at distinct adhesion sites. Yet how these adhesion sites cooperate to regulate signaling pathways and collective cell migration is only just beginning to emerge (Mui et al. 2016).
8. CONCLUSION
Cell–ECM and cell–cell adhesion sites contain overlapping functional modules comprising distinct and common proteins. These modules interact with, and regulate, the organization, dynamics, and function of the actin cytoskeleton and initiate intracellular signaling pathways. In turn, different actin architectures affect the organization of adhesion sites that are reflected in different stages, types, and functions of cell–ECM and cell–cell adhesions. Both types of adhesions sense and translate the mechanical properties of the cellular environment (the ECM or other cells) to changes in cell organization and behavior through activation of actomyosin contractility. Although the functions of cell–ECM and cell–cell adhesion complexes have been examined separately, it is clear that there is cross talk between these adhesion sites to coordinate cell migration with interactions between cells.
ACKNOWLEDGMENTS
J.M.B. was supported by a National Institutes of Health (NIH) Cell and Molecular Biology (CMB) Training Grant T32-GM007276 and a National Science Foundation Graduate Research Fellowship DGE-114747. Work in the Horwitz laboratory was supported by the NIH grants GM023244 and GM098412 and in the Nelson laboratory by the NIH grant GM35527.
Footnotes
Editors: Thomas D. Pollard and Robert D. Goldman
Additional Perspectives on The Cytoskeleton available at www.cshperspectives.org
REFERENCES
- Adams CL, Nelson WJ, Smith SJ. 1996. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell-cell adhesion. J Cell Biol 135: 1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrova AY, Arnold K, Schaub S, Vasiliev JM, Meister JJ, Bershadsky AD, Verkhovsky AB. 2008. Comparative dynamics of retrograde actin flow and focal adhesions: Formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE 3: e3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian RK, Schwartz MA. 2001. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev 11: 48–53. [DOI] [PubMed] [Google Scholar]
- Bachir AI, Zareno J, Moissoglu K, Plow EF, Gratton E, Horwitz AR. 2014. Integrin-associated complexes form hierarchically with variable stoichiometry in nascent adhesions. Curr Biol 24: 1845–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly M, Ichetovkin I, Grant W, Zebda N, Machesky LM, Segall JE, Condeelis J. 2001. The F-actin side binding activity of the Arp2/3 complex is essential for actin nucleation and lamellipod extension. Curr Biol 11: 620–625. [DOI] [PubMed] [Google Scholar]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. 2001. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat Cell Biol 3: 466–472. [DOI] [PubMed] [Google Scholar]
- Barry AK, Tabdili H, Muhamed I, Wu J, Shashikanth N, Gomez GA, Yap AS, Gottardi CJ, de Rooij J, Wang N, et al. 2014. α-catenin cytomechanics—Role in cadherin-dependent adhesion and mechanotransduction. J Cell Sci 127: 1779–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner W, Hinterdorfer P, Ness W, Raab A, Vestweber D, Schindler H, Drenckhahn D. 2000. Cadherin interaction probed by atomic force microscopy. Proc Natl Acad Sci 97: 4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, et al. 2002. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109: 509–521. [DOI] [PubMed] [Google Scholar]
- Beckham Y, Vasquez RJ, Stricker J, Sayegh K, Campillo C, Gardel ML. 2014. Arp2/3 inhibition induces amoeboid-like protrusions in MCF10A epithelial cells by reduced cytoskeletal-membrane coupling and focal adhesion assembly. PLoS ONE 9: e100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. 2001. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol 153: 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin JM, Kwiatkowski AV, Yang C, Korobova F, Pokutta S, Svitkina T, Weis WI, Nelson WJ. 2010. αE-catenin regulates actin dynamics independently of cadherin-mediated cell–cell adhesion. J Cell Biol 189: 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky AD, Balaban NQ, Geiger B. 2003. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol 19: 677–695. [DOI] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T. 2004. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429: 667–671. [DOI] [PubMed] [Google Scholar]
- Bianchini JM, Kitt KN, Gloerich M, Pokutta S, Weis WI, Nelson WJ. 2015. Reevaluating αE-catenin monomer and homodimer functions by characterizing E-cadherin/αE-catenin chimeras. J Cell Biol 210: 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. 2006. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell 11: 459–470. [DOI] [PubMed] [Google Scholar]
- Borghi N, Lowndes M, Maruthamuthu V, Gardel ML, Nelson WJ. 2010. Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions. Proc Natl Acad Sci 107: 13324–13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi N, Sorokina M, Shcherbakova OG, Weis WI, Pruitt BL, Nelson WJ, Dunn AR. 2012. E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc Natl Acad Sci 109: 12568–12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Machesky LM, Hall A, Hotchin NA. 1997. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol 137: 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga VM, Del Maschio A, Machesky L, Dejana E. 1999. Regulation of cadherin function by Rho and Rac: Modulation by junction maturation and cellular context. Mol Biol Cell 10: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembeck FH, Schwarz-Romond T, Bakkers J, Wilhelm S, Hammerschmidt M, Birchmeier W. 2004. Essential role of BCL9-2 in the switch between β-catenin's adhesive and transcriptional functions. Genes Dev 18: 2225–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle NP, Holt MR, Davies JE, Price CJ, Critchley DR. 1996. The focal-adhesion vasodilator-stimulated phosphoprotein (VASP) binds to the proline-rich domain in vinculin. Biochem J 318: 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CM, Hebert B, Kolin DL, Zareno J, Whitmore L, Horwitz AR, Wiseman PW. 2006. Probing the integrin-actin linkage using high-resolution protein velocity mapping. J Cell Sci 119: 5204–5214. [DOI] [PubMed] [Google Scholar]
- Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR. 2014. Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science 346: 1254211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnar S, Yap AS. 2013. A mechanobiological perspective on cadherins and the actin-myosin cytoskeleton. F1000Prime Rep 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette DT, Manley S, Sengupta P, Sougrat R, Davidson MW, Kachar B, Lippincott-Schwartz J. 2011. A role for actin arcs in the leading-edge advance of migrating cells. Nat Cell Biol 13: 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Connell L. 1983. Talin: A cytoskeletal component concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell Motil 3: 405–417. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. 2004. Rho and Rac take center stage. Cell 116: 167–179. [DOI] [PubMed] [Google Scholar]
- Cai D, Chen SC, Prasad M, He L, Wang X, Choesmel-Cadamuro V, Sawyer JK, Danuser G, Montell DJ. 2014. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 157: 1146–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carisey A, Tsang R, Greiner AM, Nijenhuis N, Heath N, Nazgiewicz A, Kemkemer R, Derby B, Spatz J, Ballestrem C. 2013. Vinculin regulates the recruitment and release of core focal adhesion proteins in a force-dependent manner. Curr Biol 23: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carramusa L, Ballestrem C, Zilberman Y, Bershadsky AD. 2007. Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J Cell Sci 120: 3870–3882. [DOI] [PubMed] [Google Scholar]
- Case LB, Waterman CM. 2015. Integration of actin dynamics and cell adhesion by a three-dimensional, mechanosensitive molecular clutch. Nat Cell Biol 17: 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LB, Baird MA, Shtengel G, Campbell SL, Hess HF, Davidson MW, Waterman CM. 2015. Molecular mechanism of vinculin activation and nanoscale spatial organization in focal adhesions. Nat Cell Biol 17: 880–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavey M, Rauzi M, Lenne PF, Lecuit T. 2008. A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453: 751–756. [DOI] [PubMed] [Google Scholar]
- Chen WT, Greve JM, Gottlieb DI, Singer SJ. 1985. Immunocytochemical localization of 140 kD cell adhesion molecules in cultured chicken fibroblasts, and in chicken smooth muscle and intestinal epithelial tissues. J Histochem Cytochem 33: 576–586. [DOI] [PubMed] [Google Scholar]
- Chen L, Vicente-Manzanares M, Potvin-Trottier L, Wiseman PW, Horwitz AR. 2012. The integrin-ligand interaction regulates adhesion and migration through a molecular clutch. PLoS ONE 7: e40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. 2008. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol 10: 1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Pokutta S, Cadwell GW, Bobkov AA, Bankston LA, Liddington RC, Weis WI. 2012. αE-catenin is an autoinhibited molecule that coactivates vinculin. Proc Natl Acad Sci 109: 8576–8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorev DS, Moscovitz O, Geiger B, Sharon M. 2014. Regulation of focal adhesion formation by a vinculin-Arp2/3 hybrid complex. Nat Commun 5: 3758. [DOI] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. 1996. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol 133: 1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dufour S. 2004. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol 167: 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clucas J, Valderrama F. 2014. ERM proteins in cancer progression. J Cell Sci 127: 267–275. [DOI] [PubMed] [Google Scholar]
- Coluccia AM, Benati D, Dekhil H, De Filippo A, Lan C, Gambacorti-Passerini C. 2006. SKI-606 decreases growth and motility of colorectal cancer cells by preventing pp60 (c-Src)-dependent tyrosine phosphorylation of β-catenin and its nuclear signaling. Cancer Res 66: 2279–2286. [DOI] [PubMed] [Google Scholar]
- Conway DE, Breckenridge MT, Hinde E, Gratton E, Chen CS, Schwartz MA. 2013. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Curr Biol 23: 1024–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley DR. 2009. Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu Rev Biophys 38: 235–254. [DOI] [PubMed] [Google Scholar]
- Curtis AS. 1964. The mechanism of adhesion of cells to glass. A study by interference reflection microscopy. J Cell Biol 20: 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky CH, Knudsen KA, Bradley D, Buck CA, Horwitz AF. 1985. Distribution of the cell substratum attachment (CSAT) antigen on myogenic and fibroblastic cells in culture. J Cell Biol 100: 1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB. 2003. A core function for p120-catenin in cadherin turnover. J Cell Biol 163: 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. 2009. Stretching single talin rod molecules activates vinculin binding. Science 323: 638–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali KA, Barlow CA, Burridge K. 2002. Recruitment of the Arp2/3 complex to vinculin: Coupling membrane protrusion to matrix adhesion. J Cell Biol 159: 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali KA, Sun X, Bui GA. 2014. Force transmission at cell-cell and cell-matrix adhesions. Biochemistry 53: 7706–7717. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. 2005. Tissue cells feel and respond to the stiffness of their substrate. Science 310: 1139–1143. [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. 2005. α-catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly. Cell 123: 903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elad N, Volberg T, Patla I, Hirschfeld-Warneken V, Grashoff C, Spatz JP, Fassler R, Geiger B, Medalia O. 2013. The role of integrin-linked kinase in the molecular architecture of focal adhesions. J Cell Sci 126: 4099–4107. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. 2006. Matrix elasticity directs stem cell lineage specification. Cell 126: 677–689. [DOI] [PubMed] [Google Scholar]
- Farhadifar R, Roper JC, Aigouy B, Eaton S, Julicher F. 2007. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr Biol 17: 2095–2104. [DOI] [PubMed] [Google Scholar]
- Farquhar MG, Palade GE. 1963. Junctional complexes in various epithelia. J Cell Biol 17: 375–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Simoes Sde M, Roper JC, Eaton S, Zallen JA. 2009. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell 17: 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P, Gilmour D. 2009. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10: 445–457. [DOI] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, Boettiger D. 2009. Mechanically activated integrin switch controls α5β1 function. Science 323: 642–644. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Galbraith JA. 2007. Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science 315: 992–995. [DOI] [PubMed] [Google Scholar]
- Ganz A, Lambert M, Saez A, Silberzan P, Buguin A, Mege RM, Ladoux B. 2006. Traction forces exerted through N-cadherin contacts. Biol Cell 98: 721–730. [DOI] [PubMed] [Google Scholar]
- Gardel ML, Sabass B, Ji L, Danuser G, Schwarz US, Waterman CM. 2008. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J Cell Biol 183: 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardel ML, Schneider IC, Aratyn-Schaus Y, Waterman CM. 2010. Mechanical integration of actin and adhesion dynamics in cell migration. Annu Rev Cell Dev Biol 26: 315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. 1979. A 130K protein from chicken gizzard: Its localization at the termini of microfilament bundles in cultured chicken cells. Cell 18: 193–205. [DOI] [PubMed] [Google Scholar]
- Geiger B, Ginsberg D. 1991. The cytoplasmic domain of adherens-type junctions. Cell Motil Cytoskeleton 20: 1–6. [DOI] [PubMed] [Google Scholar]
- Geiger B, Yamada KM. 2011. Molecular architecture and function of matrix adhesions. Cold Spring Harb Perspect Biol 3: pii: a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, et al. 2010. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 466: 263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. 1996. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 84: 345–357. [DOI] [PubMed] [Google Scholar]
- Guo WH, Wang YL. 2007. Retrograde fluxes of focal adhesion proteins in response to cell migration and mechanical signals. Mol Biol Cell 18: 4519–4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen SD, Kwiatkowski AV, Ouyang CY, Liu H, Pokutta S, Watkins SC, Volkmann N, Hanein D, Weis WI, Mullins RD, et al. 2013. αE-catenin actin-binding domain alters actin filament conformation and regulates binding of nucleation and disassembly factors. Mol Biol Cell 24: 3710–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harburger DS, Calderwood DA. 2009. Integrin signalling at a glance. J Cell Sci 122: 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath JP, Dunn GA. 1978. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high-voltage electron-microscope study. J Cell Sci 29: 197–212. [DOI] [PubMed] [Google Scholar]
- Helwani FM, Kovacs EM, Paterson AD, Verma S, Ali RG, Fanning AS, Weed SA, Yap AS. 2004. Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J Cell Biol 164: 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Nose A, Hatta K, Kawakami A, Takeichi M. 1987. Calcium-dependent cell-cell adhesion molecules (cadherins): Subclass specificities and possible involvement of actin bundles. J Cell Biol 105: 2501–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Tatsumi H, Lim CT, Sokabe M. 2014. Force-dependent vinculin binding to talin in live cells: A crucial step in anchoring the actin cytoskeleton to focal adhesions. Am J Physiol Cell Physiol 306: C607–C620. [DOI] [PubMed] [Google Scholar]
- Horwitz A, Duggan K, Buck C, Beckerle MC, Burridge K. 1986. Interaction of plasma membrane fibronectin receptor with talin—A transmembrane linkage. Nature 320: 531–533. [DOI] [PubMed] [Google Scholar]
- Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. 2007. Differential transmission of actin motion within focal adhesions. Science 315: 111–115. [DOI] [PubMed] [Google Scholar]
- Huang S, Ingber DE. 2005. Cell tension, matrix mechanics, and cancer development. Cancer Cell 8: 175–176. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C. 2007. Vinculin controls focal adhesion formation by direct interactions with talin and actin. J Cell Biol 179: 1043–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S, Danen EH. 2009. Adhesion signaling—Crosstalk between integrins, Src and Rho. J Cell Sci 122: 1059–1069. [DOI] [PubMed] [Google Scholar]
- Hynes RO. 2002. Integrins: Bidirectional, allosteric signaling machines. Cell 110: 673–687. [DOI] [PubMed] [Google Scholar]
- Hynes RO. 2007. Cell-matrix adhesion in vascular development. J Thromb Haemost 5: 32–40. [DOI] [PubMed] [Google Scholar]
- Hynes RO, Destree AT. 1978. Relationships between fibronectin (LETS protein) and actin. Cell 15: 875–886. [DOI] [PubMed] [Google Scholar]
- Hytonen VP, Wehrle-Haller B. 2015. Mechanosensing in cell-matrix adhesions—Converting tension into chemical signals. Exp Cell Res 343: 35–41. [DOI] [PubMed] [Google Scholar]
- Izzard CS, Lochner LR. 1976. Cell-to-substrate contacts in living fibroblasts: An interference reflexion study with an evaluation of the technique. J Cell Sci 21: 129–159. [DOI] [PubMed] [Google Scholar]
- Izzard CS, Lochner LR. 1980. Formation of cell-to-substrate contacts during fibroblast motility: An interference-reflexion study. J Cell Sci 42: 81–116. [DOI] [PubMed] [Google Scholar]
- Jou TS, Nelson WJ. 1998. Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J Cell Biol 142: 85–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. 2010. Nanoscale architecture of integrin-based cell adhesions. Nature 468: 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TJ, Zheng S, Sun J, Muhamed I, Wu J, Lei L, Kong X, Leckband DE, Wang Y. 2015. Dynamic visualization of α-catenin reveals rapid, reversible conformation switching between tension states. Curr Biol 25: 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. 1995. Interaction of α-actinin with the cadherin/catenin cell-cell adhesion complex via α-catenin. J Cell Biol 130: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A, Fuchs E. 2004. α-catenin: At the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol 5: 614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A, Pasolli HA, Fuchs E. 2004. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol 6: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F, Garcia AJ, Mould AP, Humphries MJ, Zhu C. 2009. Demonstration of catch bonds between an integrin and its ligand. J Cell Biol 185: 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS. 2002. Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr Biol 12: 379–382. [DOI] [PubMed] [Google Scholar]
- Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. 2003. Ena/VASP proteins: Regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol 19: 541–564. [DOI] [PubMed] [Google Scholar]
- Krendel MF, Bonder EM. 1999. Analysis of actin filament bundle dynamics during contact formation in live epithelial cells. Cell Motil Cytoskeleton 43: 296–309. [DOI] [PubMed] [Google Scholar]
- Kuo JC, Han X, Hsiao CT, Yates JR 3rd, Waterman CM. 2011. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for β-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol 13: 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladoux B, Anon E, Lambert M, Rabodzey A, Hersen P, Buguin A, Silberzan P, Mege RM. 2010. Strength dependence of cadherin-mediated adhesions. Biophys J 98: 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C, Lim ST, Uryu S, Chen XL, Calderwood DA, Schlaepfer DD. 2012. FAK promotes recruitment of talin to nascent adhesions to control cell motility. J Cell Biol 196: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E, Burridge K. 1975. α-actinin: Immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell 6: 289–298. [DOI] [PubMed] [Google Scholar]
- Leckband DE, de Rooij J. 2014. Cadherin adhesion and mechanotransduction. Annu Rev Cell Dev Biol 30: 291–315. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Lenne PF, Munro E. 2011. Force generation, transmission, and integration during cell and tissue morphogenesis. Annu Rev Cell Dev Biol 27: 157–184. [DOI] [PubMed] [Google Scholar]
- le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J. 2010. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol 189: 1107–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerberg JM, Gomez GA, Verma S, Moussa EJ, Wu SK, Priya R, Hoffman BD, Grashoff C, Schwartz MA, Yap AS. 2014. Tension-sensitive actin assembly supports contractility at the epithelial zonula adherens. Curr Biol 24: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lele TP, Pendse J, Kumar S, Salanga M, Karavitis J, Ingber DE. 2006. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J Cell Physiol 207: 187–194. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. 2007. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat Rev Immunol 7: 678–689. [DOI] [PubMed] [Google Scholar]
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS. 2010. Mechanical tugging force regulates the size of cell–cell junctions. Proc Natl Acad Sci 107: 9944–9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo DT, Kanner SB, Aruffo A. 1998. Filamin binds to the cytoplasmic domain of the β1-integrin. Identification of amino acids responsible for this interaction. J Biol Chem 273: 23304–23312. [DOI] [PubMed] [Google Scholar]
- Lowndes M, Rakshit S, Shafraz O, Borghi N, Harmon RM, Green KJ, Sivasankar S, Nelson WJ. 2014. Different roles of cadherins in the assembly and structural integrity of the desmosome complex. J Cell Sci 127: 2339–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Yu CH, Lieu ZZ, Allard J, Mogilner A, Sheetz MP, Bershadsky AD. 2013. Analysis of the local organization and dynamics of cellular actin networks. J Cell Biol 202: 1057–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, Abell A, Johnson GL, Hahn KM, Danuser G. 2009. Coordination of Rho GTPase activities during cell protrusion. Nature 461: 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiden SL, Hardin J. 2011. The secret life of α-catenin: Moonlighting in morphogenesis. J Cell Biol 195: 543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manibog K, Li H, Rakshit S, Sivasankar S. 2014. Resolving the molecular mechanism of cadherin catch bond formation. Nat Commun 5: 3941. [DOI] [PubMed] [Google Scholar]
- Martinez-Rico C, Pincet F, Thiery JP, Dufour S. 2010. Integrins stimulate E-cadherin-mediated intercellular adhesion by regulating Src-kinase activation and actomyosin contractility. J Cell Sci 123: 712–722. [DOI] [PubMed] [Google Scholar]
- Maruthamuthu V, Gardel ML. 2014. Protrusive activity guides changes in cell-cell tension during epithelial cell scattering. Biophys J 107: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruthamuthu V, Sabass B, Schwarz US, Gardel ML. 2011. Cell-ECM traction force modulates endogenous tension at cell-cell contacts. Proc Natl Acad Sci 108: 4708–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J, Welsh NJ, Braga VM. 2013. Cycling around cell–cell adhesion with Rho GTPase regulators. J Cell Sci 126: 379–391. [DOI] [PubMed] [Google Scholar]
- McCrea PD, Gumbiner BM. 1991. Purification of a 92-kDa cytoplasmic protein tightly associated with the cell-cell adhesion molecule E-cadherin (uvomorulin). Characterization and extractability of the protein complex from the cell cytostructure. J Biol Chem 266: 4514–4520. [PubMed] [Google Scholar]
- Mitchison T, Kirschner M. 1988. Cytoskeletal dynamics and nerve growth. Neuron 1: 761–772. [DOI] [PubMed] [Google Scholar]
- Miyoshi J, Takai Y. 2008. Structural and functional associations of apical junctions with cytoskeleton. Biochim Biophys Acta 1778: 670–691. [DOI] [PubMed] [Google Scholar]
- Morimatsu M, Mekhdjian AH, Adhikari AS, Dunn AR. 2013. Molecular tension sensors report forces generated by single integrin molecules in living cells. Nano Lett 13: 3985–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimatsu M, Mekhdjian AH, Chang AC, Tan SJ, Dunn AR. 2015. Visualizing the interior architecture of focal adhesions with high-resolution traction maps. Nano Lett 15: 2220–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui KL, Chen CS, Assoian RK. 2016. The mechanical regulation of integrin-cadherin crosstalk organizes cells, signaling and forces. J Cell Sci 129: 1093–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PS, Zaidel-Bar R. 2014. Pre-metazoan origins and evolution of the cadherin adhesome. Biol Open 3: 1183–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. 1988. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. Embo J 7: 3679–3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M, Tsukita S. 1991. The 102 kd cadherin-associated protein: Similarity to vinculin and posttranscriptional regulation of expression. Cell 65: 849–857. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Fukata M, Yamaga M, Itoh N, Kaibuchi K. 2001. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J Cell Sci 114: 1829–1838. [DOI] [PubMed] [Google Scholar]
- Nayal A, Webb DJ, Brown CM, Schaefer EM, Vicente-Manzanares M, Horwitz AR. 2006. Paxillin phosphorylation at Ser273 localizes a GIT1-PIX-PAK complex and regulates adhesion and protrusion dynamics. J Cell Biol 173: 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas A, Geiger B, Safran SA. 2004. Cell mechanosensitivity controls the anisotropy of focal adhesions. Proc Natl Acad Sci 101: 12520–12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren NK, Niessen CM, Gumbiner BM, Burridge K. 2001. Cadherin engagement regulates Rho family GTPases. J Biol Chem 276: 33305–33308. [DOI] [PubMed] [Google Scholar]
- Oakes PW, Gardel ML. 2014. Stressing the limits of focal adhesion mechanosensitivity. Curr Opin Cell Biol 30C: 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes PW, Beckham Y, Stricker J, Gardel ML. 2012. Tension is required but not sufficient for focal adhesion maturation without a stress fiber template. J Cell Biol 196: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey CA, Pavalko FM, Burridge K. 1990. An interaction between α-actinin and the β 1 integrin subunit in vitro. J Cell Biol 111: 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. 1989. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. Embo J 8: 1711–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankov R, Cukierman E, Katz BZ, Matsumoto K, Lin DC, Lin S, Hahn C, Yamada KM. 2000. Integrin dynamics and matrix assembly: Tensin-dependent translocation of α(5)β(1) integrins promotes early fibronectin fibrillogenesis. J Cell Biol 148: 1075–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panorchan P, Thompson MS, Davis KJ, Tseng Y, Konstantopoulos K, Wirtz D. 2006. Single-molecule analysis of cadherin-mediated cell-cell adhesion. J Cell Sci 119: 66–74. [DOI] [PubMed] [Google Scholar]
- Pasapera AM, Plotnikov SV, Fischer RS, Case LB, Egelhoff TT, Waterman CM. 2015. Rac1-dependent phosphorylation and focal adhesion recruitment of myosin IIA regulates migration and mechanosensing. Curr Biol 25: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patla I, Volberg T, Elad N, Hirschfeld-Warneken V, Grashoff C, Fassler R, Spatz JP, Geiger B, Medalia O. 2010. Dissecting the molecular architecture of integrin adhesion sites by cryo-electron tomography. Nat Cell Biol 12: 909–915. [DOI] [PubMed] [Google Scholar]
- Peifer M, Wieschaus E. 1990. The segment polarity gene armadillo encodes a functionally modular protein that is the Drosophila homolog of human plakoglobin. Cell 63: 1167–1176. [DOI] [PubMed] [Google Scholar]
- Pelham RJ Jr., Wang Y. 1997. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci 94: 13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez TD, Tamada M, Sheetz MP, Nelson WJ. 2008. Immediate-early signaling induced by E-cadherin engagement and adhesion. J Biol Chem 283: 5014–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret E, Leung A, Feracci H, Evans E. 2004. Trans-bonded pairs of E-cadherin exhibit a remarkable hierarchy of mechanical strengths. Proc Natl Acad Sci 101: 16472–16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov SV, Pasapera AM, Sabass B, Waterman CM. 2012. Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell 151: 1513–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. 2004. Two distinct actin networks drive the protrusion of migrating cells. Science 305: 1782–1786. [DOI] [PubMed] [Google Scholar]
- Rakshit S, Zhang Y, Manibog K, Shafraz O, Sivasankar S. 2012. Ideal, catch, and slip bonds in cadherin adhesion. Proc Natl Acad Sci 109: 18815–18820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratheesh A, Gomez GA, Priya R, Verma S, Kovacs EM, Jiang K, Brown NH, Akhmanova A, Stehbens SJ, Yap AS. 2012. Centralspindlin and α-catenin regulate Rho signalling at the epithelial zonula adherens. Nat Cell Biol 14: 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikitake Y, Mandai K, Takai Y. 2012. The role of nectins in different types of cell-cell adhesion. J Cell Sci 125: 3713–3722. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. 1995. α 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci 92: 8813–8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. 2001. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 153: 1175–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Marshall CJ. 2002. ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat Cell Biol 4: 408–415. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. 2006. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller HB, Friedel CC, Boulegue C, Fassler R. 2011. Quantitative proteomics of the integrin adhesome show a myosin II-dependent recruitment of LIM domain proteins. EMBO Rep 12: 259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller HB, Hermann MR, Polleux J, Vignaud T, Zanivan S, Friedel CC, Sun Z, Raducanu A, Gottschalk KE, Thery M, et al. 2013. β1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat Cell Biol 15: 625–636. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Assoian RK. 2001. Integrins and cell proliferation: Regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci 114: 2553–2560. [DOI] [PubMed] [Google Scholar]
- Schwarz US, Gardel ML. 2012. United we stand: Integrating the actin cytoskeleton and cell-matrix adhesions in cellular mechanotransduction. J Cell Sci 125: 3051–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA, Shewan AM, den Elzen NR, Loureiro JJ, Gertler FB, Yap AS. 2006. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol Biol Cell 17: 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Picamal X, Conte V, Vincent R, Anon E, Tambe DT, Bazellieres E, Butler JP, Fredberg JJ, Trepat X. 2012. Mechanical waves during tissue expansion. Nat Phys 8: 628–634. [Google Scholar]
- Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, Jones GE, Frame MC. 2007. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol 9: 1046–1056. [DOI] [PubMed] [Google Scholar]
- Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS. 2005. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell 16: 4531–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Chien YH, Leckband D. 2008. Biophysical properties of cadherin bonds do not predict cell sorting. J Biol Chem 283: 28454–28463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer II. 1979. The fibronexus: A transmembrane association of fibronectin-containing fibers and bundles of 5 nm microfilaments in hamster and human fibroblasts. Cell 16: 675–685. [DOI] [PubMed] [Google Scholar]
- Skau CT, Plotnikov SV, Doyle AD, Waterman CM. 2015. Inverted formin 2 in focal adhesions promotes dorsal stress fiber and fibrillar adhesion formation to drive extracellular matrix assembly. Proc Natl Acad Sci 112: E2447–E2456. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, Luna-Morris S, Vogel V. 2007. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol 5: e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS. 2010. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol 12: 696–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger P, Maitre JL, Heisenberg CP. 2011. Defective neuroepithelial cell cohesion affects tangential branchiomotor neuron migration in the zebrafish neural tube. Development 138: 4673–4683. [DOI] [PubMed] [Google Scholar]
- Stricker J, Aratyn-Schaus Y, Oakes PW, Gardel ML. 2011. Spatiotemporal constraints on the force-dependent growth of focal adhesions. Biophys J 100: 2883–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Style RW, Boltyanskiy R, German GK, Hyland C, MacMinn CW, Mertz AF, Wilen LA, Xu Y, Dufresne ER. 2014. Traction force microscopy in physics and biology. Soft Matter 10: 4047–4055. [DOI] [PubMed] [Google Scholar]
- Swaminathan V, Fischer RS, Waterman CM. 2016. The FAK-Arp2/3 interaction promotes leading edge advance and haptosensing by coupling nascent adhesions to lamellipodia actin. Mol Biol Cell 27: 1085–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabdili H, Langer M, Shi Q, Poh YC, Wang N, Leckband D. 2012. Cadherin-dependent mechanotransduction depends on ligand identity but not affinity. J Cell Sci 125: 4362–4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kotani H, Nishioka H, Takai Y. 1997. Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J Cell Biol 139: 1047–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. 1977. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol 75: 464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. 1995. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol 7: 619–627. [DOI] [PubMed] [Google Scholar]
- Takeichi M. 2014. Dynamic contacts: Rearranging adherens junctions to drive epithelial remodelling. Nat Rev Mol Cell Biol 15: 397–410. [DOI] [PubMed] [Google Scholar]
- Tambe DT, Hardin CC, Angelini TE, Rajendran K, Park CY, Serra-Picamal X, Zhou EH, Zaman MH, Butler JP, Weitz DA, et al. 2011. Collective cell guidance by cooperative intercellular forces. Nat Mater 10: 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. 2003. Cells lying on a bed of microneedles: An approach to isolate mechanical force. Proc Natl Acad Sci 100: 1484–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K. 2011. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol 13: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thievessen I, Thompson PM, Berlemont S, Plevock KM, Plotnikov SV, Zemljic-Harpf A, Ross RS, Davidson MW, Danuser G, Campbell SL, et al. 2013. Vinculin-actin interaction couples actin retrograde flow to focal adhesions, but is dispensable for focal adhesion growth. J Cell Biol 202: 163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas WA, Boscher C, Chu YS, Cuvelier D, Martinez-Rico C, Seddiki R, Heysch J, Ladoux B, Thiery JP, Mege RM, et al. 2013. α-Catenin and vinculin cooperate to promote high E-cadherin-based adhesion strength. J Biol Chem 288: 4957–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toret CP, D’Ambrosio MV, Vale RD, Simon MA, Nelson WJ. 2014. A genome-wide screen identifies conserved protein hubs required for cadherin-mediated cell-cell adhesion. J Cell Biol 204: 265–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong Quang BA, Mani M, Markova O, Lecuit T, Lenne PF. 2013. Principles of E-cadherin supramolecular organization in vivo. Curr Biol 23: 2197–2207. [DOI] [PubMed] [Google Scholar]
- Tsai J, Kam L. 2009. Rigidity-dependent cross talk between integrin and cadherin signaling. Biophys J 96: L39–L41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Wu S, Shi X, Chen K, Wu C. 2003. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell 113: 37–47. [DOI] [PubMed] [Google Scholar]
- Turner CE. 2000. Paxillin and focal adhesion signalling. Nat Cell Biol 2: E231–E236. [DOI] [PubMed] [Google Scholar]
- Turner CE, Glenney JR Jr., Burridge K. 1990. Paxillin: A new vinculin-binding protein present in focal adhesions. J Cell Biol 111: 1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. 2005. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437: 426–431. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. 2000. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100: 209–219. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Degenstein L, Wise B, Fuchs E. 2001. Hyperproliferation and defects in epithelial polarity upon conditional ablation of α-catenin in skin. Cell 104: 605–617. [DOI] [PubMed] [Google Scholar]
- Verma S, Shewan AM, Scott JA, Helwani FM, den Elzen NR, Miki H, Takenawa T, Yap AS. 2004. Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J Biol Chem 279: 34062–34070. [DOI] [PubMed] [Google Scholar]
- Verma S, Han SP, Michael M, Gomez GA, Yang Z, Teasdale RD, Ratheesh A, Kovacs EM, Ali RG, Yap AS. 2012. A WAVE2-Arp2/3 actin nucleator apparatus supports junctional tension at the epithelial zonula adherens. Mol Biol Cell 23: 4601–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. 2007. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol 176: 573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. 2009. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL. 1985. Exchange of actin subunits at the leading edge of living fibroblasts: Possible role of treadmilling. J Cell Biol 101: 597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jin G, Miao H, Li JY, Usami S, Chien S. 2006. Integrins regulate VE-cadherin and catenins: Dependence of this regulation on Src, but not on Ras. Proc Natl Acad Sci 103: 1774–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Uchida N, Imamura Y, Nagafuchi A, Fujimoto K, Uemura T, Vermeulen S, van Roy F, Adamson ED, Takeichi M. 1998. α-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J Cell Biol 142: 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe N, Mitchison TJ. 2002. Single-molecule speckle analysis of actin filament turnover in lamellipodia. Science 295: 1083–1086. [DOI] [PubMed] [Google Scholar]
- Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. 2001. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol 11: 370–374. [DOI] [PubMed] [Google Scholar]
- Weber GF, Bjerke MA, DeSimone DW. 2012. A mechanoresponsive cadherin-keratin complex directs polarized protrusive behavior and collective cell migration. Dev Cell 22: 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrle-Haller B. 2012. Structure and function of focal adhesions. Curr Opin Cell Biol 24: 116–124. [DOI] [PubMed] [Google Scholar]
- Weiss EE, Kroemker M, Rudiger AH, Jockusch BM, Rudiger M. 1998. Vinculin is part of the cadherin-catenin junctional complex: Complex formation between α-catenin and vinculin. J Cell Biol 141: 755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Asokan SB, Berginski ME, Haynes EM, Sharpless NE, Griffith JD, Gomez SM, Bear JE. 2012. Arp2/3 is critical for lamellipodia and response to extracellular matrix cues but is dispensable for chemotaxis. Cell 148: 973–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Kanchanawong P, Zaidel-Bar R. 2015. Actin-delimited adhesion-independent clustering of e-cadherin forms the nanoscale building blocks of adherens junctions. Dev Cell 32: 139–154. [DOI] [PubMed] [Google Scholar]
- Yamada S, Nelson WJ. 2007. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol 178: 517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D, Oikawa T, Takenawa T. 2007. Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell-cell adhesion. J Cell Sci 120: 86–100. [DOI] [PubMed] [Google Scholar]
- Yao M, Qiu W, Liu R, Efremov AK, Cong P, Seddiki R, Payre M, Lim CT, Ladoux B, Mege RM, et al. 2014. Force-dependent conformational switch of α-catenin controls vinculin binding. Nat Commun 5: 4525. [DOI] [PubMed] [Google Scholar]
- Yonemura S, Itoh M, Nagafuchi A, Tsukita S. 1995. Cell-to-cell adherens junction formation and actin filament organization: Similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci 108: 127–142. [DOI] [PubMed] [Google Scholar]
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. 2010. α-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 12: 533–542. [DOI] [PubMed] [Google Scholar]
- Yoshida-Noro C, Suzuki N, Takeichi M. 1984. Molecular nature of the calcium-dependent cell-cell adhesion system in mouse teratocarcinoma and embryonic cells studied with a monoclonal antibody. Dev Biol 101: 19–27. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. 2003. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci 116: 4605–4613. [DOI] [PubMed] [Google Scholar]
- Zhang J, Betson M, Erasmus J, Zeikos K, Bailly M, Cramer LP, Braga VM. 2005. Actin at cell-cell junctions is composed of two dynamic and functional populations. J Cell Sci 118: 5549–5562. [DOI] [PubMed] [Google Scholar]
- Ziegler WH, Gingras AR, Critchley DR, Emsley J. 2008. Integrin connections to the cytoskeleton through talin and vinculin. Biochem Soc Trans 36: 235–239. [DOI] [PubMed] [Google Scholar]