Abstract
The final differentiation or maturation of dendritic cells (DCs) in response to environmental stimuli influences their ability to both initiate immunity and determine the quality of the response to antigens. Circulating immune complexes and cell-bound immunoglobulins present in normal human sera represent a potential stimulus for inadvertent DC activation in the steady state and during autoimmunity. Here, we show that selective blockade of the inhibitory Fcγ receptor (FcγR) FcγRIIb with recently developed monoclonal antibodies leads to maturation of human monocyte-derived DCs, which depends on the presence of IgG in normal human plasma. Plasma, in the presence of an FcγRIIb blockade, caused the DCs to up-regulate the expression of costimulatory molecules and to produce the inflammatory mediator IL-12p70. FcγRIIb blockade of DCs loaded with tumor cells led to increased tumor-specific T cell immunity without the need for exogenous stimuli other than human plasma. Therefore, the activation status of DCs in the presence of normal human serum depends on the balance between activating and inhibitory FcγRs and can be enhanced by new antibodies that react selectively with FcγRIIb. These data suggest an approach for modifying this balance to enhance immunity to immune complexes and antibody-coated tumor cells and to silence DC activation by immune complexes in autoimmune states.
Keywords: autoimmunity, monoclonal antibody, myeloma, vaccination, cross-presentation
Dendritic cells (DCs) are highly differentiated antigen-presenting cells that play a key role in the initiation and regulation of T cell immunity to pathogens and tumors while at the same time preventing immune responses against self-tissues or environmental antigens (1, 2). A critical property of DCs is that their ability to activate or inhibit immunity is linked to environmental stimuli, which determine their final differentiation or maturation status (3). Several stimuli, such as pathogens recognized by means of Toll-like receptors, CD40L, heat shock proteins, inflammatory cytokines, and innate lymphocytes, can lead to DC maturation and T cell immunity (2). However, under steady state, DCs must avoid inappropriate activation to prevent responses to self-antigens (“horror autotoxicus”) and harmless environmental antigens (4, 5). Specific pathways that prevent spontaneous DC activation are not as well understood as microbial and inflammatory stimuli.
Circulating immune complexes and cell-bound immunoglobulins present in normal human sera represent a potential stimulus for DC activation in the steady state (6). The physiologic consequences of cell-bound IgG and immune complexes are modulated by a balance between activating and inhibitory Fcγ receptors (FcγRs) and include immune regulatory and inflammatory responses (7–10). Engagement of activating FcγRs that contain an immune tyrosine-based activation motif on effector cells, including monocytes, neutrophils, natural killer cells, and mast cells, mediates phagocytosis, antibody-dependent cell-mediated cytotoxicity, and release of cytokines and other inflammatory mediators. In contrast, the inhibitory FcγR contains an immune tyrosine inhibitory motif. Signaling by means of this receptor leads to recruitment and phosphorylation of an SH2 domain containing inositol polyphosphate 5′ phosphatase that regulates signaling by activating receptors (11, 12). Recent studies have also shown an important maturation role for FcγR expression on mouse antigen-presenting cells, including DCs (13). In addition, targeting of antigens to FcγRs on human DCs, including immune complexes and antibody-coated tumor cells, leads to enhanced generation of both CD4+ and CD8+ T cell responses in culture (6, 14–18). Therefore, the targeting of antigens to DCs by means of immune complexes can mediate both maturation of DCs and antigen presentation, leading to immunity that includes cross-presentation of tumors and autoantigens to CD8+ T cells (17, 19–23).
The FcγR system represents a balance of activating and inhibitory receptors that determines the outcome of immune complex-mediated inflammation and immunity (24). Prior studies by Kalergis and Ravetch (25) have shown that targeting immune complexes to DCs from mice genetically lacking inhibitory FcγRIIb can lead to enhanced generation of antigen-specific CD8+ T cell immunity in vitro and in vivo. Likewise, genetic deletion of FcγRIIb leads to spontaneous autoimmunity in genetically prone mice (9). However, the FcγR system, i.e., both the number and type of activating and inhibitory receptors, differs significantly between mice and humans (24), and methods other than genetic deletion are required to manipulate the balance between activating and inhibitory FcγRs. This difference may complicate the translation of data generated in the mouse systems to humans. Here, we show that human monocyte-derived DCs express both activating and inhibitory FcγRs. Using new monoclonal antibodies that selectively block the inhibitory FcγR (M. C. Veri, S. Gorlatov, H. Li, S. Burke, S. Johnson, J. Stavenhagen, J.V.R., E.B., and S.K., unpublished work), we find that blockade of this FcγR leads to spontaneous and full DC maturation mediated through activating Ig ligands in normal human plasma. Through the aegis of an inhibitory FcγR blockade, DCs present antigen from antibody-coated tumor cells and generate tumor-specific T cells without the need for a further maturation stimulus. These data demonstrate that inhibitory FcγRs have important effects on the function of human DCs and suggest that DCs are modulated continuously by ligands in normal human serum.
Materials and Methods
Isolation of DCs. CD14+ cells were separated from peripheral blood mononuclear cells by using CD14 microbeads and columns (Miltenyi Biotec, Auburn, CA) following the manufacturer's protocol and were cultured in RPMI medium 1640 with l-glutamine (Mediatech, Herndon, VA) supplemented with 1% single donor plasma GMCSF (20 ng/ml, Immunex) and IL-4 (12.5 ng/ml, R & D Systems) to generate DCs. For some experiments, DC cultures were performed in serum-free media (AIM-V medium, GIBCO). Additional DC culture media as controls in some experiments included serum-free media supplemented with 1% plasma or RPMI medium 1640 supplemented with Ig-depleted 1% plasma. Igs were depleted from plasma by using affinity chromatography on a protein G-Sepharose column (Amersham Biosciences), and depletion was verified by using SDS/PAGE under reducing conditions. For some experiments, DCs were matured by using an inflammatory cytokine mixture (26) consisting of IL-1β (10 ng/ml), IL-6 (1,000 units/ml), and TNF-α (10 ng/ml) (all from R & D Systems) and prostaglandin E2 (1 μg/ml, Sigma).
To isolate blood-derived DCs, peripheral blood mononuclear cells were stained with a lineage antibody mixture (Lin-1 FITC, Becton Dickinson) containing anti-CD3, -CD14, -CD16, -CD19, -CD20, and -CD56. The lineage negative fraction was isolated by using anti-FITC magnetic microbeads (per the recommendations of the manufacturer, Miltenyi Biotec) followed by fluorescence-activated cell sorting. Myeloid DCs were identified as being Lin-1 negative, HLA-DRhigh, and CD11c+ cells. Plasmacytoid DCs were identified as being Lin-1 negative, HLA-DRhigh, and either CD123 or BDCA2+ cells.
FcγRIIb-Blocking Antibodies. Antibodies that selectively bind and block human FcγRIIb were obtained from MacroGenics. Initial experiments used a mouse monoclonal antibody (clone 2B6). In prior studies, these antibodies reacted specifically with CD32B and not CD32A, as revealed by ELISA, surface plasmon resonance, and FACS staining of cell lines and transfectants (M. C. Veri, S. Gorlatov, H. Li, S. Burke, S. Johnson, J. Stavenhagen, J.V.R., E.B., and S.K., unpublished work). Furthermore, the 2B6 mAb also competed for immune complex binding to the receptor in an ELISA format and on cells expressing the inhibitory receptor, and it mediated an inhibitory signal for CD32-mediated activation in model systems. Additional constructs that were tested included a human–mouse chimeric antibody (ch-2B6), as well as an aglycosylated version (agly-2B6) designed to minimize binding by means of the Fc region of the antibody. Antibodies were used at a concentration of 1–25 μg/ml, with 1 μg/ml saturating the capacity of 2B6 to induce DC maturation, which is consistent with prior studies in other test systems.
Blocking Inhibitory FcγRs on Human Monocyte-Derived DCs and Evaluation of Their Maturation. To block inhibitory FcγRs, immature DCs were harvested on day 5 of culture. Up to 2 × 106 DCs were suspended in 500 μl of DC culture medium and treated for 2 h at 37°C with anti-human FcγRIIb-blocking antibody (clone 2B6, 1 μg/ml), IgG1 isotype control antibody (1 μg/ml, Sigma), or anti-CD16 receptor-blocking antibody (clone 3G8, 1 μg/ml, Becton Dickinson) or left untreated. After this initial incubation, additional culture medium was added, and DCs were cultured overnight at 1 × 106 DCs per ml. DCs were harvested the next day to assess DC maturation. The following antibodies were used for evaluating the surface changes associated with DC maturation: CD11c-APC, CD80-PE, CD83-FITC, CD86-PE, and HLA-DR-FITC (all from Becton Dickinson). Immature DCs, as well as DCs matured by using the cytokine mixture, were tested for the presence of both the inhibitory receptor FcγRIIb (by using anti-FcγRIIb antibody 2B6-FITC, MacroGenics) and the activating FcγR FcγRIIa (clone IV.3, Medarex, Annandale, NJ).
Using ELISA to Measure the Production of IL-12p70 by DCs. DCs cultured from purified monocytes were treated with FcγRIIb-blocking antibody or isotype control, as described above. After overnight culture, supernatants were harvested and analyzed for the presence of IL-12p70 by ELISA (R & D Systems), following the manufacturer's recommendations.
Myeloma Cell Lines. Myeloma cell lines were obtained from the American Type Culture Collection (U266 cells) or provided by J. Epstein (Arkansas Cancer Research Center, Little Rock, AR) (cag cells). Both lines were maintained in RPMI medium 1640 supplemented with 10% FBS.
Loading of Antibody-Coated Dying Tumor Cells on DCs. Immature DCs were either cultured alone or fed on day 5 of culture with antibody-coated dying tumor cells, as described in ref. 14. Before culture, the DCs (4 × 106 DCs per ml) were either left untreated or treated with 1 μg/ml FcγRIIb-blocking antibody 2B6 or IgG1 isotype control antibody for 30 min at 37°C and then used to load tumor cells. Tumor cells were labeled with anti-syndecan-1 antibody (B-B4, 1 μg/ml, Serotec) for 30 min at 37°C and then washed and irradiated to 30 Gy. The irradiated tumor cells were immediately cocultured with the immature DCs alone (DC-totumor ratio, 1:1; DCs at 0.5 × 106 cells per ml in 200 μl of 5% pooled human serum) or DCs precoated with FcγR-blocking antibody or isotype control antibody, as above. After 8 h of coculture, maturation cytokines were added to some of the DC cultures. DCs were harvested and used for T cell stimulation after overnight culture with the tumor cells with or without the addition of maturation cytokines.
Evaluation of Tumor Cell Uptake. To evaluate phagocytosis of dying tumor cells by DCs, live tumor cells were labeled red with PKH26 (Sigma–Aldrich), and immature DCs were labeled green with PKH67 (Sigma–Aldrich), as described in ref. 14. The tumor cells were then coated with anti-syndecan-1 antibody, irradiated, and cocultured with the dye-labeled DCs (either untreated or treated with anti-FcγRIIb antibody or isotype control) at either 4°C or 37°C. After 4–8 h of coculture, tumor uptake was determined by evaluating the double positive cells seen by flow cytometry.
Stimulation of T Cells. CD14– blood mononuclear cells were used as the source of T cells. CD56 cells were depleted from the CD14– cells by using CD56 microbeads (Miltenyi Biotec). CD56-depleted T cells were stimulated in 24-well flat-bottom plates in RPMI medium 1640 with l-glutamine supplemented with 5% pooled human serum. Unpulsed or tumor-loaded DCs were added to the T cells at a ratio of 1:10–1:30 on days 0 and 7 of culture. IL-2 (25–50 units/ml, Chiron) was added on day 2 and 7 of culture. Cultures were tested for the presence of tumor-specific T cells 7–10 days after the last stimulation with DCs.
Evaluation of Tumor-Reactive, IFN-γ-Producing T Cells. The induction of tumor-reactive, IFN-γ-producing T cells by tumor-loaded DCs was assessed by using an enzyme-linked immunospot (ELISPOT) assay, as described in ref. 18. For ELISPOT assay, 105 T cells were cocultured overnight with tumor cells (T cell-to-tumor cell ratio, 30:1) in ELISPOT plates precoated with anti-IFN-γ antibody (MABTECH, Stockholm). To detect peptide-specific T cells, autologous DCs were pulsed with 10 μM HLA A2 restricted peptides derived from MAGE-A3 (271–279, FLWGPRALV), NY-ESO-1 (157–165, SLLMWITQC), or 2.5 μM of an overlapping peptide library (15-mer peptides overlapping by 11 aa) derived from survivin. The peptides were synthesized by the proteomics resource center at The Rockefeller University. The peptide-pulsed DCs were washed and used as antigen-presenting cells in the ELISPOT assay, as described in ref. 14.
Results
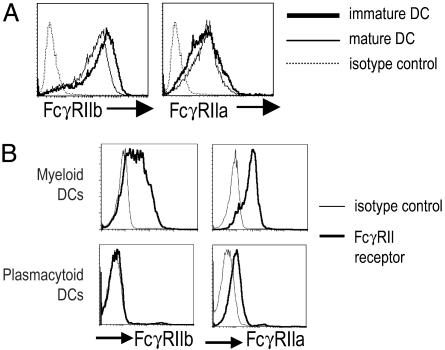
Expression of Inhibitory (FcγRIIb) and Activating (FcγRIIa) FcγRs on Immature and Mature Monocyte-Derived DCs and Blood-Derived DCs. Prior studies have shown that FcγRII is the most common FcγR expressed on human monocyte-derived DCs (17, 20, 22, 27). However, these studies did not specifically examine the pattern of activating versus inhibitory isoforms of this receptor. We therefore used new monoclonal antibodies to the inhibitory FcγRIIb (CD32B) to compare expression of this receptor to the activating FcγRIIa (CD32A) on pure populations of monocyte-derived (CD14+) DCs in the immature stage and after maturation with a cytokine mixture. In prior studies that used ELISA, surface plasmon resonance, and FACS staining of cell lines and transfectants to examine the isoform specificity of the recently developed antibodies, the new antibodies reacted specifically with CD32B and not CD32A (M. C. Veri, S. Gorlatov, H. Li, S. Burke, S. Johnson, J. Stavenhagen, J.V.R., E.B., and S.K., unpublished work). Immature and mature monocyte-derived DCs expressed both FcγRIIb and FcγRIIa by FACS (Fig. 1A). To confirm the ability of these antibodies to specifically bind the CD32B and not CD32A on human DCs, we analyzed the staining patterns by using these antibodies on myeloid (lineage negative, HLA-DR+, and CD11c+) and plasmacytoid (lineage negative, HLA-DR+, and CD123+/BDCA2+) subsets of blood DCs. Plasmacytoid DCs have been shown to express CD32A, but they lack CD32B at the mRNA level (28). Consistent with this, we found that plasmacytoid DCs expressed CD32A but did bind the 2B6 mAb to CD32B; in contrast, myeloid DCs stained well with specific antibodies against both the activating and inhibitory receptors (Fig. 1B). Thus, myeloid and plasmacytoid subsets of blood DCs differ in the expression of the activating and inhibitory isoforms of FcγRII.
Fig. 1.
Expression of FcγRIIa and FcγRIIb on human DCs. (A) Expression of FcγRIIa and FcγRIIb on human monocyte-derived DCs. Purified CD14+ monocytes were induced to differentiate into DCs in the presence of GMCSF and IL-4. On day 5 of culture, inflammatory cytokines were added to yield mature DCs. Expression of FcγRIIa and FcγRIIb on immature and mature DCs was determined by flow cytometry by using specific antibodies (IV.3 and 2B6, respectively). Data are representative of three experiments. (B) Expression of FcγRIIa and FcγRIIb on myeloid and plasmacytoid subsets of human blood-derived DCs. Myeloid (Lin-1–, DR+, and CD11c+) and plasmacytoid (Lin-1–, DR+, and CD123+/BDCA2+) DCs were isolated from peripheral blood mononuclear cells as described in Materials and Methods. Expression of FcγRIIa and FcγRIIb on immature and mature DCs was determined by flow cytometry by using specific antibodies (IV.3 and 2B6, respectively). Data are representative of three experiments.
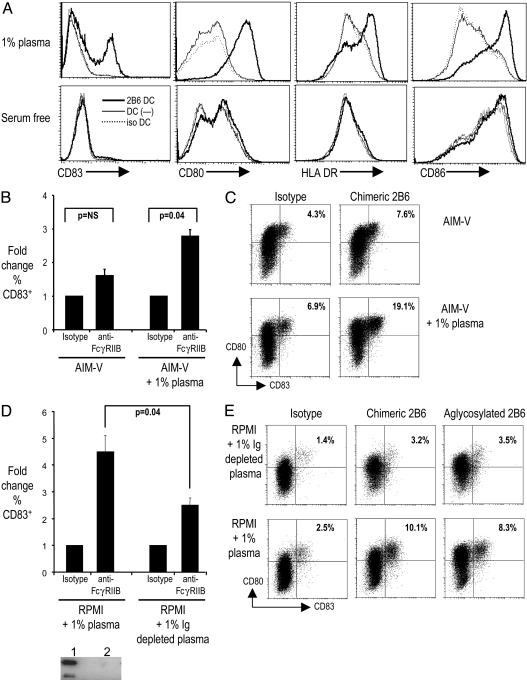
Blocking Inhibitory FcγRIIb on Immature DCs Leads to a Mature Cell Surface Phenotype. Serum from otherwise healthy adults can contain circulating immune complexes (up to 50–100 μg/ml) that, in principle, may engage FcγRs on DCs (29, 30). We hypothesized that the lack of spontaneous DC maturation during culture in human plasma was due to coengagement of activation and inhibitory receptors. To test this hypothesis more directly, we pretreated DCs cultured under standard conditions in the presence of 1% normal human plasma and with either a FcγRIIb-blocking antibody (2B6), an FcγRIII-blocking antibody (3G8), or an isotype control. We then monitored DC maturation by the up-regulation of surface markers HLA-DR, CD80, CD86, and CD83. FcγRIIb blockade was associated with up-regulation of CD83, as well as costimulatory molecules (CD80 and CD86) and HLA-DR. Neither 3G8 or isotype control resulted in significant up-regulation of these surface markers. The dose of antibody used in these experiments (1 μg/ml) was guided by prior studies testing inhibition of immune complex binding to CD32B. In pilot studies, we also tested higher doses of antibody, up to 25 μg/ml, which did not result in greater phenotypic DC maturation (data not shown). Importantly, DC maturation associated with FcγRIIb blockade was seen only when the DCs were cultured in the presence of human plasma, not in serum/plasma-free media (Fig. 2A). Addition of 1% plasma to serum-free media reconstituted the DC maturation seen after FcγRIIb blockade (Fig. 2 B and C). Depletion of Ig from the DC culture media by using a protein G-Sepharose column attenuated the up-regulation of DC maturation markers (Fig. 2 D and E). This effect was highly sensitive to the presence of residual Ig after depletion. The effect of Ig on DC maturation was still maintained after an initial depletion of >95% of the Ig in the DC culture medium (data not shown). However, further depletion of Ig to levels that were undetectable by Western blot in a 50× concentrated sample (see Western blot in Fig. 2D) led to an attenuation of DC maturation by human serum. To further characterize the effects on DC maturation, we used two additional constructs of the same antibody: a mouse–human chimeric antibody (2B6-ch) and an aglycosylated version (2B6-agly) designed to minimize binding by means of the Fc portion. Results similar to those obtained with 2B6 were obtained with these 2B6 modified antibodies (Fig. 2E). Thus, the blockade of FcγRIIb on human DCs leads to DC maturation in the presence of activating Ig ligands in normal human serum.
Fig. 2.
Blockade of FcγRIIb in the presence of human serum leads to maturation of human monocyte-derived DCs. (A) Monocyte-derived DCs cultured in RPMI medium 1640 with 1% plasma or serum-free media (AIM-V) were incubated overnight with anti-FcγRIIb antibody (2B6, 1 μg/ml) or isotype control antibody. Expression of HLA-DR, CD80, CD86, and CD83 on CD11c+ DCs was monitored by flow cytometry. Data are representative of three similar experiments. (B) Monocyte-derived DCs were cultured either in serum-free medium (AIM-V) or AIM-V supplemented with 1% plasma. DCs were cultured with chimeric (ch-2B6) anti-FcγRIIb antibody or isotype control. DC maturation was monitored by flow cytometry. Data are mean/SD of two similar experiments. (C) Representative FACS plot showing expression of maturation marker CD83/CD80 in DCs cultured under conditions described in B. Percentages of CD83+ cells are noted. (D) Monocyte-derived DCs were cultured in either RPMI medium 1640 with 1% plasma or RPMI medium 1640 with 1% Ig-depleted plasma. DCs were cultured with chimeric (ch-2B6) anti-FcγRIIb antibody or isotype control. DC maturation was monitored by flow cytometry. Data shown are mean/SD of two similar experiments. Western blot (Lower) shows depletion of Ig from plasma. Lane 1 is RPMI medium 1640 with 1% plasma, and lane 2 is 50× concentrate of RPMI medium 1640 with 1% Ig-depleted plasma. (E) Representative FACS plot showing expression of maturation marker CD83/CD80 in DCs cultured under conditions described in D, with isotype control or chimeric (ch-2B6) or aglycosylated anti-FcγRIIB (agly-2B6) antibody. Percentages of CD83+ cells are noted.
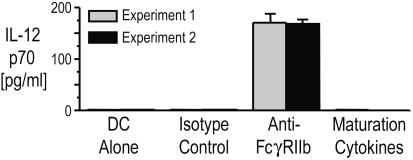
Blocking Inhibitory FcγRIIb on Immature DCs Leads to IL-12 Production. Maturation of DCs by ligands for Toll receptors or by CD40L leads to the secretion of IL-12, a cytokine that plays a major role in driving CD4+ T cells along the T helper 1-type pathway needed for protection against tumors and pathogens. Blockade of FcγRIIb on pure populations of monocyte-derived human DCs led to the production of IL-12p70 (Fig. 3). In contrast, DCs matured by using the inflammatory cytokine mixture that is commonly used in DC vaccination trials were poor IL-12p70 producers, as noted in ref. 31, in the presence or absence of isotype control antibody. These data show that in the presence of activating ligands present in normal human sera, simple blockade of inhibitory FcγRs induces DCs to secrete IL-12p70.
Fig. 3.
FcγRIIb blockade leads to IL-12p70 production. Supernatants of immature monocyte-derived DCs treated overnight with anti-FcγRIIb (2B6, 1 μg/ml), isotype control antibody, or inflammatory cytokines were analyzed for IL-12p70 by ELISA.
Blocking FcγRIIb Enhances the Generation of Tumor-Reactive T Cell Immunity by Tumor-Loaded DCs. DCs can acquire antigen from tumors or virally infected cells and cross-present antigens from them to elicit antigen-specific CD8+ T cells (32). In prior studies, we and others have shown that coating tumor cells with antitumor monoclonal antibodies before uptake by human DCs leads to enhanced cross-presentation (14, 18). This process depends on the engagement of FcγRs on DCs and the addition of a maturation stimulus. However, the uptake of antibody-coated cells likely causes simultaneous engagement of both activating and inhibitory receptors. Therefore, we tested whether blocking the inhibitory FcγR would alter DC maturation and the generation of T cell immunity without further stimuli.
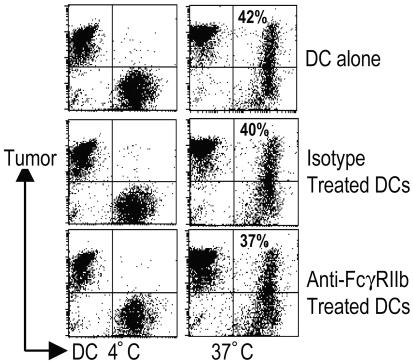
First, we tested the effect of blocking FcγRIIb on uptake of tumor cells by DCs. Myeloma tumor cells coated with anti-syndecan-1 antibody were cultured with immature DCs pretreated with either isotype or anti-FcγRIIb antibody. Blockade of FcγRIIb did not alter the uptake of tumor cells by DCs (Fig. 4). In prior studies, we did not observe phenotypic DC maturation after uptake of antibody-coated myeloma cells by DCs, and we reasoned that this effect might be due to simultaneous engagement of both the activating and inhibitory receptors. In contrast, blocking FcγRIIb led to up-regulation of DC maturation markers (data not shown), consistent with the data presented above (Fig. 2). If an inflammatory cytokine mixture was added, these DCs could be induced to undergo more complete phenotypic maturation with further up-regulation of CD83 (not shown).
Fig. 4.
Effect of FcγRIIb blockade on the uptake of tumor cells by DCs. Myeloma cells were labeled with dye (PKH26), opsonized with anti-syndecan-1 antibody, and cocultured with dye (PKH67)-labeled DCs at 4°C or 37°C. DCs were also pretreated with either isotype control antibody or anti-FcγRIIB antibody (2B6). After 4–8 h of coculture, the percentage of double-positive DCs was evaluated by flow cytometry.
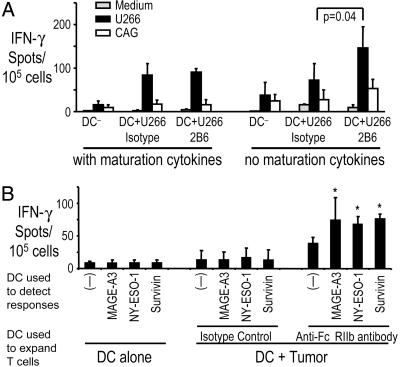
Next, we examined whether blocking FcγRIIb leads to stimulation of tumor-specific T cells. In prior experiments, generation of antitumor immunity by antibody-coated tumor-loaded DCs required the addition of exogenous maturation stimuli (14, 18). In contrast, under these same conditions, FcγRIIb blockade of DCs led to enhanced stimulation of the T cells, as measured by IFN-γ production, even in the absence of additional maturation stimuli (Fig. 5A). Indeed, the presentation of tumor antigens by these DCs was comparable to that elicited by using DCs that had undergone full maturation using a cytokine mixture. To further test whether FcγRIIb blockade enhanced the generation of tumor antigen-specific T cells, immature DCs from HLA-A2+ individuals were loaded with A2– cag myeloma cells and used to stimulate autologous T cells in the presence or absence of anti-FcγRIIb antibody, as described earlier. Cag cells express high levels of the cancer testis antigens MAGE-A3 and NY-ESO-1 (14). Therefore, we could evaluate whether FcγRIIb blockade enhances cross-presentation of these specific antigens by tumor cell-loaded DCs. Stimulation with tumor-loaded DCs treated with anti-FcγRIIb antibody (without any additional maturation stimulus) led to enhanced T cell responses to defined A2 restricted epitopes from MAGE-A3, NY-ESO-1, and an overlapping peptide library derived from a shared tumor antigen, survivin (Fig. 5B).
Fig. 5.
Effect of FcγRIIb blockade on the expansion of myeloma-reactive T cells by tumor-loaded DCs. (A) Monocyte-derived DCs alone or loaded with opsonized U266 tumor cells were either left untreated (“no maturation cytokines”) or matured ex vivo by using a cytokine mixture as a maturation stimulus (“with maturation cytokines”). DCs were also pretreated with either isotype control or anti-FcγRIIb antibody (2B6). The tumor-loaded and unpulsed DCs were each used to stimulate autologous T cells. IFN-γ producers against U266 (A2+) or cag (A2–) cells as control were analyzed by ELISPOT assay. Data shown are mean/SD of three separate experiments. (B) Immature monocyte-derived DCs from HLA-A2+ donors were loaded with opsonized cag (A2–) myeloma cells in the presence of isotype control or anti-FcγRIIB antibody (2B6) and used to stimulate autologous T cells. After 14 days of culture, T cells were stimulated overnight in ELISPOT plates with autologous DCs pulsed with 10 μM A2 restricted peptides derived from MAGE-A3, NY-ESO-1, or 2.5 μMof an overlapping 15-mer peptide library derived from survivin. IFN-γ producers were quantified by an ELISPOT assay. Data shown are mean/SD of four replicates in independent experiments on two blood donors. *, P value for comparison with no antigen control. MAGE-A3, P = 0.048; NYESO-1 and survivin, P < 0.01.
Discussion
We have shown that blockade of the inhibitory FcγR with a specific monoclonal antibody, 2B6, has a major effect on the function of human DCs in vitro, thus suggesting that the inhibitory FcγR is an important component of the regulatory network that prevents DC maturation in response to naturally occurring antibodies and immune complexes (ICs). Under steady-state/physiologic conditions, DCs may encounter such ICs either in the circulation or, more likely, as immobilized ICs in lymphoid tissues (29).
Blockade of the inhibitory FcγR is associated not only with surface remodeling (such as up-regulation of CD80/86 costimulatory molecules) associated with DC maturation, but also with the induction of IL-12p70. This cytokine helps DCs to activate T cell immunity and to polarize responses to the T helper 1 phenotype (2). Indeed, we observed that the blockade of inhibitory FcγRs on DCs leads to both induction of IL-12p70 and enhanced tumor-specific, IFN-γ-producing T cells, without the involvement of other signals from microbial products or innate lymphocytes. These FcγRIIb-blocked DCs are efficient at inducing T cell immunity, even though they have a less mature phenotype (based on the expression of CD83, HLA-DR, CD80, and CD86) compared with DCs cultured in the presence of an inflammatory cytokine mixture.
Our data suggest that immune complexes or naturally occurring autoantibodies in normal human serum (33) are sufficient to activate human DCs unless the control mechanisms mediated by inhibitory FcγRs are intact. Recent studies have shown that B cells with specificities against autoantigens are much more common than previously thought (33). Dysregulation of FcγRs may therefore play an important role in autoimmune diseases characterized by T helper 1-type T cell immunity. Gershwin and colleagues (16) have reported that autoantibody complexes are presented by human DCs to autologous CD8+ T cells. Therefore, a reduction in inhibitory FcγRs may lead to DC maturation, including the production of IL-12 and the induction of immunity, thereby promoting the amplification of the autoreactive state. Indeed, polymorphisms in the transmembrane region of FcγRIIb that may restrict inhibitory signaling, as well as polymorphisms in FcγRIIb's promoter that result in reduced surface expression of FcγRIIb, have been recently linked to an increased risk of lupus (34, 35). Recent studies in the mouse suggest a central role for the expression of this inhibitory receptor on B cells in maintaining peripheral tolerance (36, 37). The relative contribution of altered FcγRIIb expression on B cells or DCs in promoting autoimmunity will need to be clarified in future studies.
Monoclonal antibodies are currently under active investigation for their therapeutic potential in several cancers. Recent studies have emphasized the importance of FcγRs in effector functions engaged in vivo by these antibodies (10). The immunity to antibody-coated tumor cells that follows FcγR-mediated uptake by cultured DCs has suggested the additional possibility that enhanced T cell immunity may contribute to the antitumor effects of these antibodies in vivo (14, 18). In this context, FcγRIII and FcγRIIa polymorphisms may alter the activation/inhibition ratio by increasing binding affinity to activation receptors, thereby augmenting DC maturation and stimulation of antitumor immunity (38, 39). Blockade of FcγRIIb leads to the induction of antitumor immunity without the need for exogenous maturation stimuli and, therefore, may suffice for boosting T cell responses by means of monoclonal antibodies in vivo. Thus, these data provide a rationale for blocking inhibitory human FcγRs to improve the efficacy of monoclonal antibodies and to improve vaccine design, including human DC vaccination against cancer or infections. Likewise, blockade of activating FcγRs could retard both inflammation and immunization components of autoimmune diseases.
Acknowledgments
We thank Judy Adams for help with preparing the manuscript. This work was supported in part by funding from the National Institutes of Health (to M.V.D., K.M.D., R.M.S., and J.V.R.), the Damon Runyon Cancer Research Fund, the Irene Diamond Foundation, the Fund to Cure Myeloma, the Irma T. Hirschl Foundation (to M.V.D.), the American Society of Clinical Oncology Career Development Award, the Program in Human Immunology from the Dana Foundation (to K.M.D.), and the Alliance for Lupus Foundation (to J.V.R.).
Abbreviations: DC, dendritic cell; FcγR, Fcγ receptor; ELISPOT, enzyme-linked immunospot.
References
- 1.Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. (2003) Annu. Rev. Immunol. 21, 685–711. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., Briere, F., Caux, C., Davoust, J., Lebecque, S., Liu, Y. J., Pulendran, B. & Palucka, K. (2000) Annu. Rev. Immunol. 18, 767–811. [DOI] [PubMed] [Google Scholar]
- 3.Mellman, I. & Steinman, R. M. (2001) Cell 106, 255–258. [DOI] [PubMed] [Google Scholar]
- 4.Steinman, R. M. & Nussenzweig, M. C. (2002) Proc. Natl. Acad. Sci. USA 99, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brimnes, M. K., Bonifaz, L., Steinman, R. M. & Moran, T. M. (2003) J. Exp. Med. 198, 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regnault, A., Lankar, D., Lacabanne, V., Rodriguez, A., Thery, C., Rescigno, M., Saito, T., Verbeek, S., Bonnerot, C., Ricciardi-Castagnoli, P. & Amigorena, S. (1999) J. Exp. Med. 189, 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravetch, J. V. & Bolland, S. (2001) Annu. Rev. Immunol. 19, 275–290. [DOI] [PubMed] [Google Scholar]
- 8.Bolland, S., Yim, Y. S., Tus, K., Wakeland, E. K. & Ravetch, J. V. (2002) J. Exp. Med. 195, 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolland, S. & Ravetch, J. V. (2000) Immunity 13, 277–285. [DOI] [PubMed] [Google Scholar]
- 10.Clynes, R. A., Towers, T. L., Presta, L. G. & Ravetch, J. V. (2000) Nat. Med. 6, 443–446. [DOI] [PubMed] [Google Scholar]
- 11.Bolland, S., Pearse, R. N., Kurosaki, T. & Ravetch, J. V. (1998) Immunity 8, 509–516. [DOI] [PubMed] [Google Scholar]
- 12.Bolland, S. & Ravetch, J. V. (1999) Adv. Immunol. 72, 149–177. [DOI] [PubMed] [Google Scholar]
- 13.Amigorena, S. (2002) J. Exp. Med. 195, F1–F3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhodapkar, K., Krasovsky, J., Williamson, B. & Dhodapkar, M. (2002) J. Exp. Med. 195, 125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selenko, N., Majdic, O., Jager, U., Sillaber, C., Stockl, J. & Knapp, W. (2002) J. Clin. Immunol. 22, 124–130. [DOI] [PubMed] [Google Scholar]
- 16.Kita, H., Lian, Z. X., Van de Water, J., He, X. S., Matsumura, S., Kaplan, M., Luketic, V., Coppel, R. L., Ansari, A. A. & Gershwin, M. E. (2002) J. Exp. Med. 195, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata, Y., Ono, S., Matsuo, M., Gnjatic, S., Valmori, D., Ritter, G., Garrett, W., Old, L. J. & Mellman, I. (2002) Proc. Natl. Acad. Sci. USA 99, 10629–10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhodapkar, M. V., Krasovsky, J. & Olson, K. (2002) Proc. Natl. Acad. Sci. 99, 13009–13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rafiq, K., Bergtold, A. & Clynes, R. (2002) J. Clin. Invest. 110, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gil-Torregrosa, B. C., Lennon-Dumenil, A. M., Kessler, B., Guermonprez, P., Ploegh, H. L., Fruci, D., van Endert, P. & Amigorena, S. (2004) Eur. J. Immunol. 34, 398–407. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez, A., Regnault, A., Kleijmeer, M., Ricciardi-Castagnoli, P. & Amigorena, S. (1999) Nat. Cell Biol. 1, 362–368. [DOI] [PubMed] [Google Scholar]
- 22.Sedlik, C., Orbach, D., Veron, P., Schweighoffer, E., Colucci, F., Gamberale, R., Ioan-Facsinay, A., Verbeek, S., Ricciardi-Castagnoli, P., Bonnerot, C., et al. (2003) J. Immunol. 170, 846–852. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama, K., Ebihara, S., Yada, A., Matsumara, K., Aiba, S., Nukiwa, T. & Takai, T. (2003) J. Immunol. 170, 1641–1648. [DOI] [PubMed] [Google Scholar]
- 24.Ravetch, J. V. (2002) J. Clin. Invest. 110, 1759–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalergis, A. M. & Ravetch, J. V. (2002) J. Exp. Med. 195, 1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonuleit, H., Kuhn, U., Muller, G., Steinbrink, K., Paragnik, L., Schmitt, E., Knop, J. & Enk, A. H. (1997) Eur. J. Immunol. 27, 3135–3142. [DOI] [PubMed] [Google Scholar]
- 27.Sallusto, F. & Lanzavecchia, A. (1994) J. Exp. Med. 179, 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bave, U., Magnusson, M., Eloranta, M. L., Perers, A., Alm, G. V. & Ronnblom, L. (2003) J. Immunol. 171, 3296–3302. [DOI] [PubMed] [Google Scholar]
- 29.Schifferli, J. A. & Taylor, R. P. (1989) Kidney Int. 35, 993–1003. [DOI] [PubMed] [Google Scholar]
- 30.Mustafa, A. S. & Godal, T. (1987) Clin. Exp. Immunol. 69, 255–262. [PMC free article] [PubMed] [Google Scholar]
- 31.Kalinski, P., Vieira, P. L., Schuitemaker, J. H., de Jong, E. C. & Kaspenberg, M. L. (2001) Blood 97, 3466–3469. [DOI] [PubMed] [Google Scholar]
- 32.Heath, W. R. & Carbone, F. R. (2001) Annu. Rev. Immunol. 19, 47–64. [DOI] [PubMed] [Google Scholar]
- 33.Wardemann, H., Yurasov, S., Schaefer, A., Young, J. W., Meffre, E. & Nussenzweig, M. C. (2003) Science 301, 1374–1377. [DOI] [PubMed] [Google Scholar]
- 34.Li, X., Wu, J., Carter, R. H., Edberg, J. C., Su, K., Cooper, G. S. & Kimberly, R. P. (2003) Arthritis Rheum. 48, 3242–3252. [DOI] [PubMed] [Google Scholar]
- 35.Su, K., Wu, J., Edberg, J. C., Li, X., Ferguson, P., Cooper, G. S., Langefeld, C. D. & Kimberly, R. P. (2004) J. Immunol. 172, 7186–7191. [DOI] [PubMed] [Google Scholar]
- 36.Fukuyama, H., Nimmerjahn, F. & Ravetch, J. V. (2005) Nat. Immunol. 6, 99–106. [DOI] [PubMed] [Google Scholar]
- 37.McGaha, T. L., Sorrentino, B. & Ravetch, J. V. (2005) Science, in press. [DOI] [PubMed]
- 38.Weng, W. K. & Levy, R. (2003) J. Clin. Oncol. 21, 3940–3947. [DOI] [PubMed] [Google Scholar]
- 39.Weng, W. K., Czerwinski, D., Timmerman, J., Hsu, F. J. & Levy, R. (2004) J. Clin. Oncol. 22, 4665–4672. [DOI] [PubMed] [Google Scholar]